A novel sorbent for lanthanide adsorption based on tetraoctyldiglycolamide, modified carbon inverse opals
Alexander N. Turanova,
Vasilii K. Karandashevb,
Nadezhda S. Sukhininaa,
Vladimir M. Masalova,
Andrey A. Zhokhova and
Gennadi A. Emelchenko*a
aInstitute of Solid State Phisics, Russian Academy of Sciences, Chernogolovka 142432, Russia. E-mail: emelch@issp.ac.ru
bInstitute of Microelectronics Technology and High Purity Materials, Russian Academy of Sciences, Chernogolovka 142432, Russia
First published on 1st December 2014
Abstract
Carbon inverse opals (C-IOP) were noncovalently modified with tetraoctyldiglycolamide (TODGA). The effect of HNO3 concentration in the aqueous phase and that of the TODGA concentration in the sorbent phase on the adsorption of microquantities of Y, La, Ce, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb and Lu nitrates from HNO3 solutions by CIOP modified with TODGA was considered. The stoichiometry of the sorbed complexes has been determined by the slope analysis method. The efficiency of lanthanides(III) adsorption from moderate-concentration HNO3 solutions increases with increasing atomic number of the element. The process of lanthanides(III) adsorption is exothermic, spontaneous and followed a pseudo-second-order kinetic model.
Introduction
Separation and recovery of actinides and lanthanides from highly active wastes of nuclear fuel cycles are of great significance for sustainable development of nuclear energy as well as environment protection. Several solvent extraction processes have been developed for separation on a laboratory as well as an industrial scale for reprocessing of spent fuel using bidentate neutral organophosphorus compounds such as aryl substituted methylene diphosphine dioxides and diaryl- or alkyl(aryl)[dialkylcarbamoylmethyl]phosphine oxides.1,2 The newly developed alkyl-substituted diglycolamides, Alk2NC(O)CH2OCH2C(O)NAlk2, have been reported to exhibit high extraction ability towards actinides and lanthanides in strong nitric acid solutions.3–7 Diglycolamides (DGA) contain three oxygen atoms which vigorously capture the metal ions, so that they act as tridentate ligands.7,8 The hydrophobicity of DGA extractants is controlled by the length of the carbon chains attached to the amidic N atoms.5 In the case of N,N,N′,N′-tetraoctyldiglycolamide (TODGA), the octyl chains attached to the nitrogen atoms give sufficient lipophilicity to the TODGA molecule.5 Diglycolamides have advantages before the bidentate neutral organophosphorus compounds such as easy synthesis, milder stripping requirements, and harmless chemical compositions of fully incinerable elements (i.e. no P present).It is now widely accepted that the use of the solid-phase extraction technique in metal recovery offers numerous advantages for application of the liquid–liquid extraction technique. The most important of these advantages is the simplicity of equipment and operation, high enrichment factors, absence of emulsion, low consumption of reagent especially organic solvent, minimum waste generation and the possibility of using a solid adsorbent for many extraction cycles without losses in the metal extraction capacity. Therefore, the concept of using solvent impregnated sorbents was put forward and developed in.9–11 This is a very simple and in many cases the only way to prepare ion exchange sorbents containing reactive groups with special properties, which can not be immobilized by chemical bonding. This concept includes incorporation of an extractant by a physical impregnation technique into a solid matrix. The use of macroporous polymeric sorbents9–11 and hydrophobized silica gels12 impregnated with extractants of various nature for the extraction of metal ions from aqueous solutions was described earlier. Carbonaceous materials might be most promising for use as matrices due to their restively high radiation, thermal and chemical stability, especially acid resistance.13–15 In previous works, we have found that carbon materials with a high specific surface area such as fullerene black, an amorphous product of electric arc graphite vaporization after extraction of fullerenes16 and carbon nanotubes17 can be used as a matrix for the preparation of impregnated sorbents.
We have recently synthesized and studied SiC/C and C-inverse opal nanostructures with novel functional properties.18–20 Further studies of the nanostructures will expand the area of their potential use and are, therefore, highly desirable. C-inverse opals (C-IOP) have a 3D ordered meso- and macroporous structure consisting of interconnected spherical pores whose size corresponds to the template particles. In previous work, we found that C-IOP noncovalently modified with tetraphenylmethylenediphosphine dioxide possesses a high adsorption ability towards U(VI), Th(IV) and lanthanides(III) in nitric acid media.21
The aim of this work is to study the adsorption of lanthanides(III) from nitric acid solutions by C-IOP modified with TODGA. The sorption properties of the above material are compared with those of nonionic polymeric resin Amberlite XAD7HP and multi-walled carbon nanotubes impregnated with TODGA.
Experimental
C-IOP structures were synthesized by the template method using an opal matrix composed of spherical globules of amorphous silicon dioxide 260 nm in diameter which was used as a template in accordance with20 the technique of preparation of opal matrices is detailed in work.22 The use of carbon inverse opals widens significantly the possibilities of modification of their structure. The inverse opal structure contains voids of direct opal filled with organic or inorganic material which make up a frame of an opal-like structure, but it contains no SiO2 globules. Newly formed structures are replicas of regular voids of the opal matrix which contain an interconnected system of micro-, meso- and micropores with a high specific surface area.The preparation of the C-IOP samples can be briefly described as follows: flakes of an empty opal matrix were placed into an aqueous solution (1.25 g C12H22O11, 0.14 g H2SO4 and 5 g H2O per 1 g SiO2) and together with the solution kept at 100 °C for about 5 h, then dried at 160 °C for 18 h. Then the samples were again soaked in the solution (1 g SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.8 g C12H22O11 : 0.09 g H2SO4
0.8 g C12H22O11 : 0.09 g H2SO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 g H2O) and the thermal treatment was repeated. Next the samples were carbonized by annealing in an argon flow at 1200 K, 3 h.
5 g H2O) and the thermal treatment was repeated. Next the samples were carbonized by annealing in an argon flow at 1200 K, 3 h.
The carbon–silica composite obtained by pyrolysis was etched with 40 wt% hydrofluoric acid for 24–120 h at room temperature to remove the silica. The template-free carbon product was washed with distilled water and dried at 400 K. The morphology and structure of the samples were investigated using a high resolution scanning electron microscope (Zeiss Supra 50 VP).
Fig. 1 shows the C-IOP microstructure obtained by the above method.
 | ||
| Fig. 1 C-IOP structures (SEM): (a) typical view of C-IOP at small magnification, (b) empty carbon semispheres upon etching of SiO2 globules. | ||
Low magnification reveals that the sample consists of agglomerates of tens of microns size (Fig. 1a). The agglomerate surface is covered with a cellular structure. High magnification reveals the ordered structure of the void hemispheres formed by etching of SiO2 globules (Fig. 1b). The carbon shell surrounding the empty spheres is 10–15 nm thick. The diameter of the spheres corresponds to that of the silica globules (∼260 nm). The characteristics (properties) of the porous structure were determined by gas adsorption–desorption (N2, 77 K, Quantachrome QuadraSorb SI). The specific surface area was 250 m2 g−1. The fraction of micropores (up to 2 nm) was 48%, that of mesopores (from 2 to 50 nm) 45% and that of macropores 7%. The main contribution (80%) to the specific surface area of the samples is made by pores sized ∼1 nm, ∼2 nm, ∼3.5 nm and 4.7 nm.
Multi-walled carbon nanotubes (Taunit) were obtained with the use of NanoTekhTsentr, Russia. Amberlite XAD7HP was purchased from Supelco Analytical, USA. Tetraoctyldiglycolamide was synthesized by the known method.5
C-IOP–TODGA sorbents were prepared in accordance with the principles of the dry impregnation method.10 An appropriate amount of C-IOP (0.5–1 g) was placed into a round-bottom flask and dichloromethane containing TODGA of different concentrations was added. The mixture was equilibrated for 12 h on a rotary evaporator without applying a vacuum. Then, dichloromethane was removed by applying a controlled vacuum and the sorbent was further dried to constant weight. The amount of TODGA in the sorbent phase was calculated from the difference between the weights of the C-IOP sample before and after impregnation. In some cases TODGA was stripped from the sorbent with pure ethanol to verify the mass balance. The concentrations of TODGA in the sorbent were varied from 0.1 to 0.5 mmol g−1. The same procedure was followed to prepare Amberlite XAD7HP and multi-walled carbon nanotubes impregnated with TODGA.
Stock solutions of lanthanide nitrates were prepared by dissolving the corresponding oxides in concentrated nitric acid and diluting them with distilled water to required volume. The distribution of Y, La, Ce, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb and Lu in the adsorption systems was studied in model solutions of nitric acid of variable concentrations at the initial metal concentration 4 × 10−6 M for each element.
The experiments on adsorption of the metal ions were performed in the static mode at 20 ± 3 °C. The ratio of the aqueous solution volume to the sorbent weight (V/m) was varied from 200 to 5000 mL g−1. A weighed sample of the sorbent was mixed with the aqueous solution for 3 h; this time was found earlier to be sufficient for the system to reach equilibrium. Then the sorbent was separated from the aqueous solutions by filtration through a filter paper. Preliminary experiments showed that the adsorption of lanthanides(III) from HNO3 solutions in the absence of TODGA is negligible.
The metal ion concentrations in the initial and equilibrium aqueous solutions were determined by inductively coupled plasma mass-spectrometry (ICP-MS) on a X-7 mass spectrometer with quadrupole mass analyzer (Thermo Scientific, USA) following the procedure described in ref. 23. The concentration of metal ions in the sorbent phase was found by the material balance equation. The distribution ratios of the metal ions (Kd, mL g−1) were calculated as the ratio of the concentrations in the equilibrium solid and equilibrium aqueous solutions. Duplicate experiments showed that the reproducibility of the Kd measurements was generally within 10%. The nitric acid concentration in the equilibrium aqueous solutions was determined by potentiometric titration with KOH solution.
Results and discussion
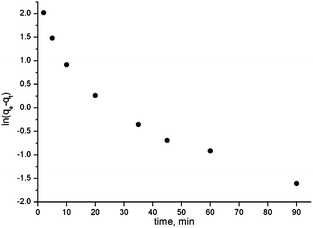
In order to establish equilibrium time for maximum Ln(III) uptake and to uncover the kinetics of the adsorption process, Eu(III) adsorption with C-IOP–TODGA from 3 M HNO3 solutions was studied as a function of contact time. Fig. 2 shows that the adsorption rate of Eu(III) increased rapidly during the first 10 min and then gradually tended to equilibrium. In this system the adsorption of Eu(III) reaches equilibrium after 180 min. The time in which C-IOP–TODGA attains 50% saturation with Eu(III), i.e., loading half-time, was 2.5 min. It is worth noting that approximately 90% of the maximum adsorption capacity was achieved within 20 min, indicating that the Eu(III) adsorption is a rapid process.To evaluate the controlling mechanism of the adsorption process, two different kinetic models, namely, the pseudo-first-order and pseudo-second-order models were employed to describe the adsorption process. The linear form of the two models could be expressed as follows.24
The pseudo-first-order model equation:
ln(qe − qt) = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe − k1t qe − k1t
| (1) |
The pseudo-second-order model equation:
| t/qt = 1/k2qe2 + t/qe | (2) |
Plots of ln(qe − qt) vs. t and t/qt vs. t are shown in Fig. 3. The absence of a linear plot of ln(qe − qt) vs. t indicates that the mechanism of Eu(III) adsorption on C-IOP–TODGA does not follow the pseudo-first-order kinetic model. In contrast, the linear plot of t/qt vs. t was obtained and the values of k2 = 0.035 g mg−1 min−1 and qe = 14.58 mg g−1 were calculated. The calculated qe value was in good agreement with the experimental data. Therefore, the adsorption of Eu(III) on C-IOP–TODGA is consistent with the pseudo-second-order kinetic model. The results obtained suggested that the adsorption process might be regarded as chemisorption, which could be the rate-controlling step.25
Adsorption of lanthanide(III) ions from nitric acid solution on C-IOP–TODGA involves the interaction of Ln(III) ions with TODGA molecules via tridentate coordination8 and can be presented by the following equation:
| Ln(aq)3+ + 3NO3(aq)− + nL(s) = [LnLn(NO3)m](NO3)3−m(s) | (3) |
| Keq = Kd[NO3−]−3[L]f−n | (4) |
| [L]f = ([L]t − [L]Ln)/(1 + K[H+][NO3−]) | (5) |
| [L]f = [L]t (1 + K[H+][NO3−])−1 | (6) |
| Keq = Kd[NO3−]−3[L]t−n(1 + K[H+][NO3−])n | (7) |
Taking the logarithmic results in the linear equation we obtain
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kd = log Kd = log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Keq + 3 Keq + 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log[NO3−] − n log[NO3−] − n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log(1 + K[H+][NO3−]) + n log(1 + K[H+][NO3−]) + n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log[L]t log[L]t
| (8) |
At a constant HNO3 concentration in the aqueous phase, the efficiency of the Ln(III) adsorption increases with an increase in the total TODGA concentration in the sorbent phase (Fig. 4). The slope of the log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kd − log[L] dependences is close to 3 (Fig. 4), which corresponds to the adsorption of Ln(III) with C-IOP–TODGA in the form [LnL3](NO3)3. It was shown earlier that Ln(III) are extracted with TODGA solutions in organic diluents as complexes of the same stoichiometry.7,26
Kd − log[L] dependences is close to 3 (Fig. 4), which corresponds to the adsorption of Ln(III) with C-IOP–TODGA in the form [LnL3](NO3)3. It was shown earlier that Ln(III) are extracted with TODGA solutions in organic diluents as complexes of the same stoichiometry.7,26
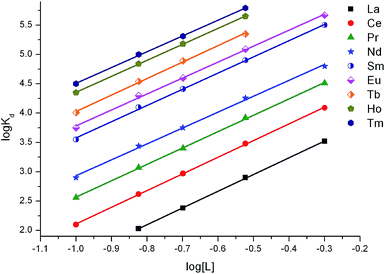 | ||
| Fig. 4 Effect of total TODGA concentration in the sorbent phase (mmol g−1) on adsorption of Ln(III) from 3 M HNO3 solutions. V/m = 500 mL g−1. | ||
The effect of HNO3 concentration in the aqueous phase on the lanthanides(III) adsorption with C-IOP–TODGA has been studied (Fig. 5). At a constant total TODGA concentration in the sorbent phase, the efficiency of the Ln(III) adsorption increases as the concentration of the nitric acid in the aqueous phase grows. This can be related to the shift of equilibrium (3) to the right as the NO3− ion concentration increases. When [HNO3] > 3 M, further growth of Kd slows down which is due to a decrease in the concentration of the free TODGA in the sorbent phase as a result of protonation of this ligand. A similar Kd vs. [HNO3] dependence was observed for the Ln(III) adsorption on polymeric resin Amberchrom CG-71C impregnated with TODGA27 and on celite diatomaceous silica Chromosorb W impregnated with TODGA.28 The data in Fig. 5 show that the adsorption of Ln(III) from moderate-concentration HNO3 solutions increases from La(III) to Lu(III). A similar lanthanide pattern was observed for solvent extraction of Ln(III) with TODGA7 and other diglycolamide extractants.4 These extractants behave as hard ligands due to their carbonyl and ether oxygen atoms. In general, the stability constant of Ln(III)-hard ligand complexes increases across the lanthanide series with the increasing atomic number of the element as the ionic radii decrease, which corresponds to the increase in the positive charge density of the lanthanide ion.1 On the other hand, the increase in the positive charge density leads to an increase in the energy required to dehydrate the cation. Such factors reflect the lanthanide pattern. Extraction of Ln(III) from HNO3 solutions with neutral bidentate organophosphorus ligands, such as carbamoylmethylphosphine oxides29,30 and substituted methylenediphosphine dioxides31,32 decreases from La(III) to Lu(III). In contrast, extraction of Ln(III) tridentate diglycolamide ligands4,7 as well as tridentate phosphoryl-containing podands33 increases with increasing atomic number. Apparently, the electrostatic interaction between the metal ions and TODGA is the dominant factor governing the lanthanide pattern.
 | ||
| Fig. 5 Effect of HNO3 concentration in the aqueous phase on adsorption of Ln(III) by C-IOP–TODGA sorbent. Total TODGA concentration in the sorbent phase 0.5 mmol g−1; V/m = 500 mL g−1. | ||
Fig. 6 shows that the efficiency of Eu(III) adsorption from nitric acid solutions on C-IOP–TODGA increases with increasing m/V ratio. The amount of C-IOP–TODGA required to achieve almost complete (>99%) removal of Eu(III) from a solution containing 15 mg L−1 Eu(III) was 2 g L−1. The data indicate clearly that C-IOP–TODGA can be used for preconcentration of lanthnide(III) ions from HNO3 solutions.
The isotherm for the Eu(III) adsorption from 3 M HNO3 solutions was studied by batch equilibrium experiments at a fixed temperature (25 °C) and at a range of Eu(III) initial concentrations from 7 to 46 mg L−1. The maximum adsorption capacity was found to be 18.0 mg g−1 under the current experimental conditions (Fig. 7).
 | ||
| Fig. 7 Equilibrium isotherm for Eu(III) adsorption by C-IOP–TODGA from 3 M HNO3 solutions. Total TODGA concentration in the sorbent phase 0.5 mmol g−1, V/m = 500 mL g−1, temperature 25 °C. | ||
The adsorption data were fitted using the Langmuir equation
| qe = bLqmCe(1 + bLCe)−1 | (6) |
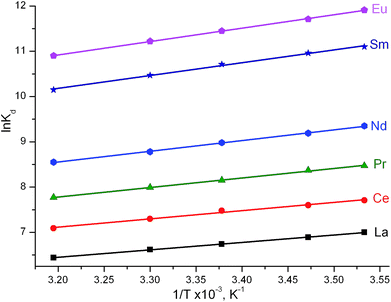 | ||
| Fig. 8 Effect of temperature on Ln(III) adsorption by C-IOP–TODGA from 1 M HNO3 solutions. Total TODGA concentration in the sorbent phase 0.5 mmol g−1, initial Ln(III) concentration 4 × 10−6 mol L−1. | ||
The effect of temperature on the adsorption of Ln(III) ions was also investigated. The data in Fig. 8 show that the efficiency of Ln(III) adsorption decreases with increasing temperature, which indicates that the process is exothermic in nature. Three basic thermodynamic parameters, free energy change (ΔG), enthalpy change (ΔH) and entropy change (ΔS) were calculated using the following equations:
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kd = −ΔH/RT + ΔS/R Kd = −ΔH/RT + ΔS/R
| (7) |
| ΔG = −ΔH − TΔS | (8) |
| Ln(III) | ΔH, kJ mol−1 | ΔS, J mol−1 | ΔG, kJ mol−1 for 25 °C |
|---|---|---|---|
| La(III) | −13.6 | 11.2 | −17.0 |
| Ce(III) | −15.2 | 10.2 | −18.3 |
| Pr(III) | −17.2 | 9.81 | −20.1 |
| Nd(III) | −19.7 | 9.51 | −22.5 |
| Sm(III) | −23.4 | 10.1 | −26.4 |
| Eu(III) | −24.9 | 10.6 | −28.1 |
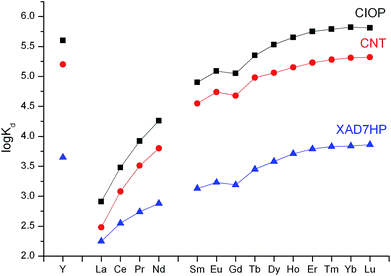
The negative values of ΔG and ΔH point to the spontaneous and exothermic nature of Ln(III) adsorption. The overall change in enthalpy is dependent on numerous factors including energy required to dehydrate the metal ion, energy release during formation of the Ln(III)–TODGA complex, energy required for deprotonation of TODGA H+ cations. The exothermic effect indicates that Ln(III) adsorption on C-IOP–TODGA is driven by formation of TODGA–metal ion complexes. Simultaneous adsorption of lanthanides(III) from 3 M HNO3 solutions by C-IOP, multi-walled carbon nanotubes (CNT) and acrylate resin Amberlite XAD7HP modified with TODGA were used to compare the matrix effect on the efficiency of Ln(III) adsorption. The data in Fig. 9 suggest that the adsorption of Ln(III) increases in the order XAD7HP < CNT < C-IOP. The values of the Lu/La separation factor (SFLu/La = Kd,Lu/Kd,La) increase in the same order XAD7HP (41) < CNT (690) < C-IOP (794). Therefore, carbon materials, C-IOP and CNT, possess higher Lu/La selectivity than polymeric resin XAD7HP. However, the cause of this phenomenon requires further investigation.
For desorption of lanthanide(III) ions, we used a solution of 0.1 M 1-hydroxyethane-1,1-diphosphonic acid forming with those ions very stable complexes in the aqueous phase.34 After three adsorption–desorption cycles, the adsorption efficiency of C-IOP–TODGA did not diminish. Therefore, this material can be used for lanthanide(III) ions preconcentration from nitric acid solutions.
Conclusions
Carbon inverse opals noncovalently modified with tetraoctyldiglycolamide have a high adsorption ability towards lanthanide(III) ions in the nitric acid media and can be used for preconcentration of metal ions from HNO3 solutions. The efficiency of metal ion adsorption increases with increasing concentration of TODGA in the sorbent phase. The distribution ratio of lanthanides(III) in moderate-concentration solutions of HNO3 increases with increasing element atomic number. The process of lanthanides(III) adsorption is fast, exothermic, spontaneous and followed the pseudo-second-order kinetic model. The novel C-IOP–TODGA sorbent has some advantages over bidentate neutral organophosphorus compounds, such as easy synthesis, milder stripping requirements, and harmless chemical compositions of fully incinerable elements. Modification of carbon inverse opal structure with tetraoctyldiglycolamide extends the functional properties of materials used as sorbents of various metal ions.Acknowledgements
The work was partly supported by the Russian Foundation for Basic Research (Project no. 13-02-00777).Notes and references
- K. L. Nash and M. P. Jensen, in Handbook on the Physics and Chemistry of Rare Earths, ed. K. A. Gschnedner Jr and L. Eyring, Elsevier Scince B.V., 2000, vol. 28, pp. 311–371 Search PubMed.
- A. M. Rozen, Z. I. Nikolotova and N. A. Kartasheva, Radiokhimiya, 1986, 28, 407 CAS (in Russian).
- H. Narita, T. Yaita, K. Tamura and S. Tachimori, J. Radioanal. Nucl. Chem., 1999, 329, 381 CrossRef.
- H. Narita, T. Yaita, K. Tamura and S. Tachimori, Radiochim. Acta, 1998, 81, 223 CAS.
- Y. Sasaki, Y. Sugo, S. Suzuki and S. Tachimori, Solvent Extr. Ion Exch., 2001, 19, 91 CrossRef CAS PubMed.
- S. Tachimori, Y. Sasaki and S. Suzuki, Solvent Extr. Ion Exch., 2002, 20, 687 CrossRef CAS PubMed.
- Y. Sasaki, P. Rapold, M. Arisaka, M. Hirata, T. Kimura, K. Hill and G. Cote, Solvent Extr. Ion Exch., 2007, 25, 187 CrossRef CAS.
- Y. Sasaki and G. R. Choppin, J. Radioanal. Nucl. Chem., 1996, 207, 383 CrossRef CAS.
- A. Warshawsky, Trans. Inst. Min. Metall., Sect. A, 1974, 83, 101 Search PubMed.
- A. Warshawsky, in Ion Exchange and Solvent Extraction, ed. J. A. Marinsky and Y. Marcus, Marcel Dekker Inc., New York, 1981, vol. 8, p. 229 Search PubMed.
- J. L. Cortina and A. Warshawsky, in Ion Exchange and Solvent Extraction, ed. J. A. Marinsky and Y. Marcus, Marcel Dekker Inc., New York, 1997, vol. 13, p. 195 Search PubMed.
- Y. A. Zolotov, G. I. Tsisin, S. G. Dmitrienko and E. I. Morosanova, Sorption preconcentration of microcomponents from solutions, Nauka, Moscow, 2007 Search PubMed.
- A. Mellah, S. Chegrouche and M. Barkat, J. Colloid Interface Sci., 2006, 296, 434 CrossRef CAS PubMed.
- D. Shao, Z. Jiang, X. Wang, J. Li and Y. Meng, J. Phys. Chem. B, 2009, 113, 860 CrossRef CAS PubMed.
- Y. Jung, S. Kim, S. J. Park and J. M. Kim, Colloids Surf., A, 2008, 313–314, 292 CrossRef PubMed.
- A. N. Turanov, V. K. Karandashev and N. K. Evseeva, Russ. J. Phys. Chem., 2006, 80, 260 CrossRef CAS.
- A. N. Turanov, V. K. Karandashev, N. K. Evseeva, N. N. Kolesnikov and D. N. Borisenko, Russ. J. Phys. Chem., 2008, 82, 2223 CrossRef CAS.
- G. A. Emelchenko, A. A. Zhokhov, V. M. Masalov, M. Y. Maximuk, T. N. Fursova, A. V. Bazhenov, I. I. Zverkova, S. S. Khasanov, E. A. Steinman and A. N. Tereshenko, Nanotechnology, 2010, 21, 475604 CrossRef CAS PubMed.
- A. N. Tereshenko, V. I. Zinenko, I. I. Khodos, Y. A. Agafonov, A. A. Zhokhov, V. M. Masalov, E. A. Steinman and G. A. Emelchenko, Phys. Solid State, 2012, 54(5), 586 CrossRef.
- G. A. Emelchenko, V. M. Masalov, A. A. Zhokhov and I. I. Khodos, Phys. Solid State, 2013, 55(5), 1105 CrossRef CAS.
- A. N. Turanov, V. K. Karandashev, V. M. Masalov, A. A. Zhokhov and G. A. Emelchenko, J. Colloid Interface Sci., 2013, 405, 183 CrossRef CAS PubMed.
- I. I. Bardyshev, A. D. Mokrushin, A. A. Pribylov, E. N. Samarov, V. M. Masalov, I. A. Karpov and G. A. Emelchenko, Colloid J., 2006, 68, 20 CrossRef CAS.
- A. N. Turanov, V. K. Karandashev and V. E. Baulin, Solvent Extr. Ion Exch., 1996, 14, 227 CrossRef CAS.
- M. Yurdakoc, Y. Scki and S. K. Yuedakoc, J. Colloid Interface Sci., 2005, 286, 440 CrossRef CAS PubMed.
- Q. Song, L. Ma, J. Liu, C. Bai, J. Geng, H. Wang, B. Li, L. Wang and S. Li, J. Colloid Interface Sci., 2012, 386, 291 CrossRef CAS PubMed.
- M. Arisaka and T. Kimura, Solvent Extr. Ion Exch., 2011, 29, 72 CrossRef CAS.
- K. van Hecke, G. Modolo and J. Radioanal, Nucl. Chem., 2004, 261, 269 CrossRef CAS.
- S. A. Ansari, P. N. Pathak, M. Husain, A. K. Prasad, V. S. Parmar and V. K. Manchanda, Talanta, 2006, 68, 1273 CrossRef CAS PubMed.
- E. P. Horwitz, K. A. Martin, H. Diamond and L. Kaplan, Solvent Extr. Ion Exch., 1986, 4, 449 CrossRef CAS.
- A. N. Turanov, V. K. Karandashev, E. V. Sharova, O. I. Artyushin and I. L. Odinets, Solvent Extr. Ion Exch., 2012, 30, 604 CrossRef CAS.
- T. Yaita and S. Tachimori, Radiochim. Acta, 1996, 73, 27 CAS.
- A. N. Turanov, V. K. Karandashev, A. N. Yarkevich and Z. V. Safronova, Solvent Extr. Ion Exch., 2004, 22, 391 CrossRef CAS PubMed.
- A. N. Turanov, V. K. Karandashev, V. E. Baulin, A. N. Yarkevich and Z. V. Safronova, Solvent Extr. Ion Exch., 2009, 27, 551 CrossRef CAS.
- K. L. Nash, Radiochim. Acta, 1991, 54, 171 CAS.
| This journal is © The Royal Society of Chemistry 2015 |