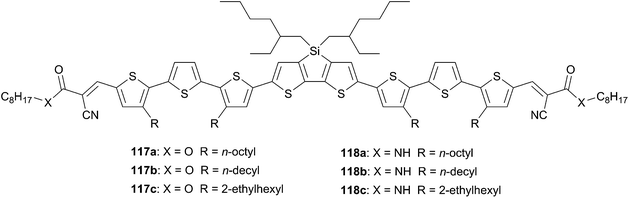
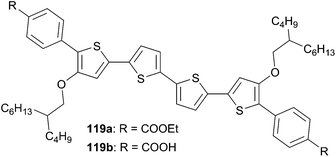
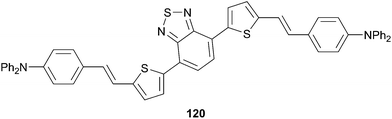
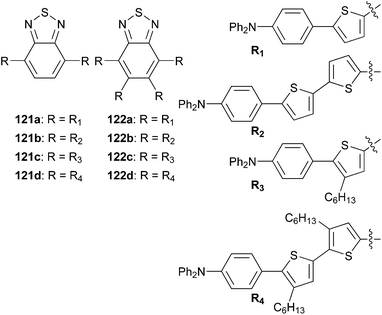
Thiophene-based push–pull chromophores for small molecule organic solar cells (SMOSCs)
Volodymyr Malytskyi
abc,
Jean-Jacques Simon
b,
Lionel Patrone
*bc and
Jean-Manuel Raimundo
*a
aCentre Interdisciplinaire de Nanoscience de Marseille, Aix-Marseille Université, CNRS, CINaM UMR 7325, 163 Ave de Luminy case 913, 13288 Marseille Cedex 09, France. E-mail: raimundo@cinam.univ-mrs.fr; Fax: +33 491 418 916; Tel: +33 629 413 925
bInstitut Matériaux Microélectronique Nanosciences de Provence, Aix-Marseille Université, CNRS, Université de Toulon, IM2NP UMR 7334, Domaine Universitaire de St Jérôme, Services 151 & 231, 13397, Marseille Cedex 20, France
cInstitut Supérieur de l'Electronique et du Numérique (ISEN-Toulon), CNRS, IM2NP UMR 7334, Maison des Technologies, Place Georges Pompidou, F-83000, Toulon, France. Tel: +33 494 038 950/+33 483 361 984
First published on 13th November 2014
Abstract
The last decade has witnessed rapid progress in organic photovoltaics boosted by the design and synthesis of novel π-conjugated small donor–acceptor molecules (mainly thiophene-based chromophores) and by the control and optimization of both device processing and fabrication. Although some important progress has been reached, current challenges remain to further improve their efficiency, durability and cost-effectiveness in order to compete with silicon-based solar cells. This review will provide the scientific community with both general and in depth information on the structure–property relationships related to the photocurrent efficiencies comprising detailed I/V characteristics. It will highlight guidelines for designing new efficient and emerging alternatives to conjugated polymers on the basis of thiophenic chromophores representing, to date, the most widely used class of organic materials for such a purpose as well as important information on device processing or fabrication factors that could influence their performances.
Introduction
Organic solar cells (OSCs) constitute third generation solar cell technology and are nowadays of crucial interest because they can offer a good alternative to high cost solar cells based on silicon. Indeed, organic materials display several advantages compared to their inorganic counterpart, such as for instance a lighter weight, facile chemical tailoring to fine tune their optoelectronic properties and an ease of processability, opening thus the possibilities of developing large-area and cost-effective solar cells based on ecofriendly technologies.The first attempts to fabricate organic solar cell were described in the early 80's by Chamberlain from thin organic layers sandwiched between two dissimilar electrodes. Such devices have shown very low solar power conversion efficiency up to 0.3% (PCE)1 that strongly depends on the nature of the electrodes. A major breakthrough was achieved in 1986 by Tang2 with the possibility of producing efficient solar cells based on organic semiconductors. A two-layer device fabricated from a copper phthalocyanine (CuPc, as donor material) and perylene tetracarboxylic derivate (PV, as acceptor), end-capped respectively by an ITO and Ag electrodes, led to a PCE value near 1%. Interestingly, in such devices, the charge generation efficiency was found to be relatively independent of the bias voltage. The donor–acceptor interface (p–n junction) provides efficient exciton dissociation in comparison to single layer solar cells. These findings stimulated new works in the field of organic and polymer-based solar cells. A new milestone was reached with the discovery of C60 as a highly efficient acceptor.3 Although the development of new acceptor materials is strongly requested and seems to be promising,4 fullerenes C60, C70 and their analogues PC61BM, PC71BM ([6,6]-phenyl-C61-butyric acid methyl ester and [6,6]-phenyl-C71-butyric acid methyl ester) respectively are readily used and became a traditional acceptor part in OPV cells.
In spite of achieving good photovoltaic conversion efficiencies with the so-called planar heterojunction cells (PHJ), their performances are rather limited due to the small area (interface) contributing to the photovoltaic effect. To circumvent this dilemma, researchers have turned out their attention to the development of bulk heterojunction (BHJ). In such structures the p-type and n-type materials are co-deposited, in a determined ratio, to ensure a greater dissociation irrespectively to the thickness. Nevertheless, despite to be a very attractive solution, numerous problematic originate and need to be solved to ensure perfect reproducibility of the devices structures.
Among them, batch-to-batch reproducible synthesis is somewhat difficult to implement, that is why since very recently scientists have paid more and more attention in the use of small organic molecules.5 Using this approach new OPV devices have been fabricated and fast-optimized leading to power conversion efficiency (PCE) values as high as 11%.6
For characterizing overall performance of solar cell devices it is appropriate to use few parameters. First of them is an open-circuit voltage, VOC, which shows the maximum possible voltage which could be extracted from a device. In first approximation VOC is determined by an energy difference between LUMO level of an acceptor molecule and HOMO of donor one. Keeping in mind that in most cases fullerene derivatives are used as an acceptor, a low HOMO level of donor is responsible for high VOC. The maximum current delivered by a device in short circuit conditions, ISC, characterizes the possibility of charge generation by the cell. In consequence, it depends from other factors, namely light absorption properties and effectiveness of exciton separation. While amelioration of optical properties usually demands a low bandgap of a donor component, the effective charge separation could not occur if LUMO of donor is less than ≈0.3 eV higher than LUMO of acceptor. So, obviously, there is a competition to find an optimal donor molecule with a low enough HOMO level, a high enough LUMO and at the same time not a large bandgap between them. In OC or SC conditions photovoltaic cell does not produce any power (W = V × I). The operating point is a condition with the maximum power and values of Vm and Im lower then VOC and ISC respectively. A ratio between products Vm × Im (means maximum achieved power) and VOC × ISC (means maximum possible power) is called a fill factor, FF, and in generally characterizes the efficacy of the cell structure. It could depend on very various factors among which the active layer morphology and charge carrier mobilities are very important. So, solubility properties of active compounds and their solid state electrical properties should also be taken in consideration during a molecular engineering of new organic materials for photovoltaic application.
In this article, we will focus on various criteria that influence power conversion efficiencies in small organic molecules, mainly based on the thiophene skeleton, used in organic solar cells from a chemist's viewpoint with the aim to introduce both a variety of exotic organic materials and insights that are potentially applicable to small molecule organic solar cells.
D–A type
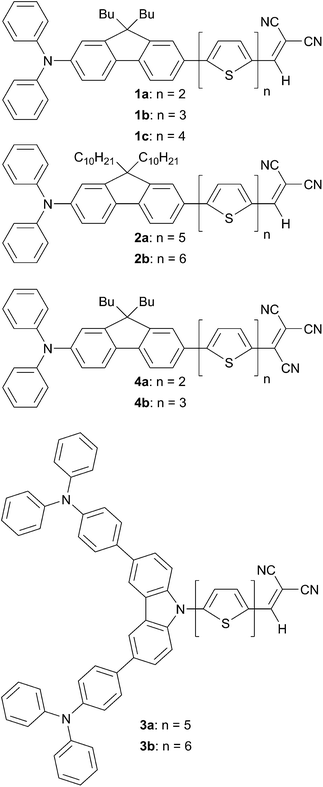
Despite their extensive uses as keycomponents in the field of Graëtzel solar cells (i.e. DSSC), D–A structures have gained only recently a strong interest for OPVs devices.Among them, triarylamine-based conjugated chromophores end-capped with acceptors, such as di- or tri-cyanovinyl groups, are some representative D–A structures that have been largely tested.7,8 Elongation of the π-conjugated system between the donor and the dicyanovinyl acceptor edge substituents by the increment of thiophene units (from 3 to 6 units) does not affect significantly the λmax on such structures. Interestingly unexpected, the highest molar extinction coefficient was attained for the tetrathiophene (4T) analogous derivative. As anticipated consecutive change of the acceptor moiety from di- to tri-cyanovinyl (DCV, TCV) leads to a significant bathochromic shift, due to an increase of the electron withdrawing strength, (from 514 to 629 nm and 524 to 643 nm for 2T and 3T derivatives, respectively)9 associated with a reduced molar extinction coefficient. Modification of the donor unit with a fluorene or heterofluorene such as carbazole constitutes an attractive way to tailor and enhance optical properties. Thus, the substitution of a benzene ring on the triphenylamine (TPA) fragment by a fluorene leads to a slight bathochromic shift with a concomitant increase of the PCE values (Scheme 1).
Modification of the donor part by a dendritic-like structure based on two TPA units (3a and 3b) has a dramatic effect on electrochemical properties. It was shown that such changes reduce the HOMO energy level to 0.15–0.16 V as well the LUMO to 0.11–0.13 V compared to their linear analogs 3a relied on the donor strength. This fact results in a higher energy band gap estimated from cyclic voltammetry and correlates well with the observed optical gap. The highest PCE value (2.67%) was obtained for the device based on the compound 1c prepared by bilayer vacuum deposition and annealed for 20 min at 100 °C.
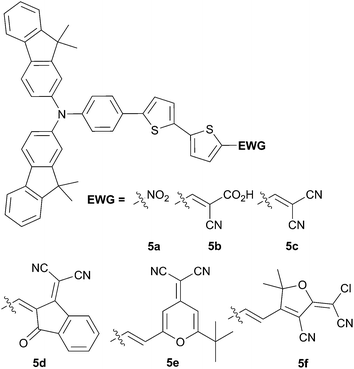
Further substitutions on the TPA part were envisioned by Ko et al.10,11 in order to fine tune the optical and electrochemical properties of the donating part (Scheme 2).
It can be noticed herein that shorter π-conjugated spacer lengths provide, in that case, comparable conversion efficiencies up to 2.46% for 5c, which can be attributed to higher JSC value (8.91 mA cm−2) compared to 1a (4.92 mA cm−2) due to an optimized performance architecture in the BHJ blend with PC71BM compared to the PHJ with C60 for 1a. Further optimization of the phase segregation upon addition of 1-chloronaphthalene (CN) as an additive, leads to a significant enhancement of the conversion efficiency up to 3.66%.
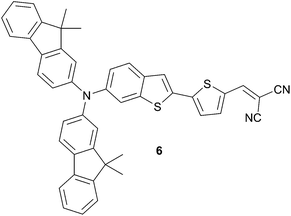
Additional improvement of the photophysical properties has been achieved with the fused benzothiophene 6 (Scheme 3) instead of 2-thienylbenzene as spacer.12 Therefore, compared to 5c, better optical and hole-mobility properties are attained. Subsequent device made from a blend of 6 and PC71BM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) with TiOx layer affords 4% conversion efficiency.
3) with TiOx layer affords 4% conversion efficiency.
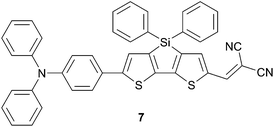
Silole bridged bithiophene (DTS) spacer has been used in order to gain further insights for the design of SMOSCs in the above TPA compounds series.13 Thus, introduction of the DTS moiety along the π-conjugated spacer (7) does not change significantly the optical properties (λmax = 537 nm ε = 45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 M−1 cm−1) compared to 5c (λmax = 536 nm ε = 32
800 M−1 cm−1) compared to 5c (λmax = 536 nm ε = 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 174 M−1 cm−1) but results in a refinement of the HOMO and LUMO energy levels which are lowered by almost 0.5 V (Scheme 4).
174 M−1 cm−1) but results in a refinement of the HOMO and LUMO energy levels which are lowered by almost 0.5 V (Scheme 4).
Fabrication of a planar-mixed heterojunction (PMHJ), by vacuum deposition, with 7 and fullerenes derivatives in the molar ratio (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) achieves conversion efficiency up to 2.69% and 3.82% with C60 and C70 respectively. The higher PCE originates from the high VOC potentials achieved in these devices (0.88 V and 0.83 V respectively compared to 0.774 V for 5c) as a consequence of the energy levels optimization.
1) achieves conversion efficiency up to 2.69% and 3.82% with C60 and C70 respectively. The higher PCE originates from the high VOC potentials achieved in these devices (0.88 V and 0.83 V respectively compared to 0.774 V for 5c) as a consequence of the energy levels optimization.
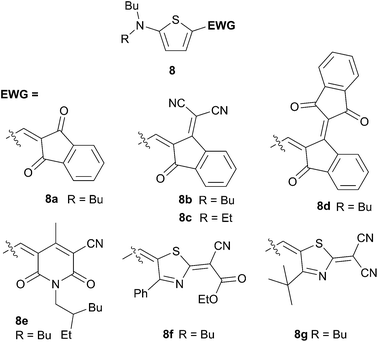
Besides the common covalent bridging strategy, the design of “dwarf” chromophores (i.e. minimizing the size of the chromophore) has recently emerged to be a possible alternative for controlling the batch-to-batch reproducibility as well as to get low cost systems associated with high purity and optimized photophysical properties.14,15 Therefore, both Roncali's group and Würthner's group have demonstrated the validity of this concept throughout the synthesis of the smallest push–pull dyes based on thiophene rings (Schemes 5 and 6).
Optimized chemical structures have been obtained by varying only the acceptor edge substituent. On the basis of this series the best performances were achieved with the compound 8c leading to PCE up to 3.0% in blended films with PC61BM 55 wt%. Further devices optimizations were made, for instance replacing PC61BM by PC71BM, and the hole-collecting PEDOT:PSS layer by MoO3, and using a Ba/Ag metal electrode leading to attain remarkable PCE value of 4.5%.
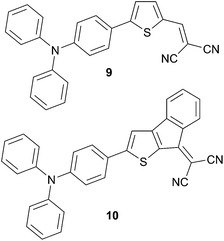
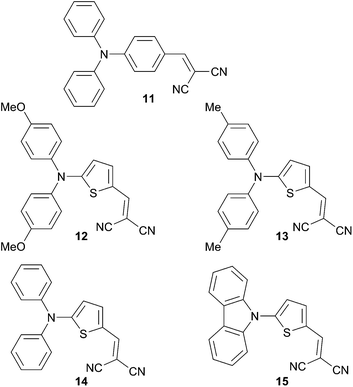
However, by changing the donor part from dialkylamino to TPA, Roncali et al. have obtained a reasonable value of 2.53% of conversion efficiency on un-optimized architectures devices (Scheme 6). In furtherance of their continuous efforts on the understanding of structure–properties relationships, the covalent bridging strategy was applied on the acceptor part in order to optimize the light harvesting properties (Scheme 7). Thus substitution of the dicyanovinyl (9) by the thieno-fused-dicyanoindene (10) moiety leads to a 110 nm red shift, as expected. Although this bathochromic shift is accompanied by a two-fold decrease of the molar extinction coefficient an enhanced PCE (2.97%) was clearly evidenced. Additional work on fine-tuning properties of the donating group has been done in order to give a better accuracy of the HOMO levels and bandgap control as well as to gain further information on the design of such chromophores (Scheme 7).15 Shortening the π-conjugated spacer results in a 55 nm hypsochromic shift (from 9 to 11) while an average shift of 27 nm was observed for compounds 12 to 13 because of the lower resonance energy of the thienyl ring. As a consequence, higher FF and JSC characteristics were obtained beside a decrease of the VOC leading to an increase of the photocurrent (1.60–1.70%). Interestingly, the best performances were obtained for compound 14 evidenced by the highest JSC (6.80 mA cm−2) and PCE (1.92%) values, whereas the bridged carbazole analogous 15 did not improve the photocurrent.16
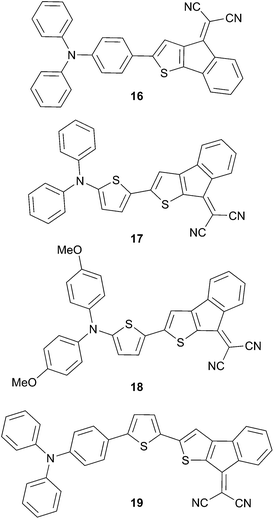
Additionally it has been demonstrated that 16, a structural isomer of 10, exhibits a higher dihedral angle leading to a less planar π-conjugated system. According to the structural modification of the chromophores general behaviors can be deduced that are consistent with the optical and electrochemical experiments (Scheme 8).17 For instance, bridging the dicyano group leads to a positive shift of the reduction potential (from 9 to 10 and 16–19) due to a better stabilization of the radical anion upon reduction attributed to the formation of the cyclopentadienyl anion radical on those structures. Replacement (from 10 to 17 and 18) of the phenyl ring by thienyl or introduction of methoxy groups significantly increases the HOMO levels. Optical observations were in agreement with the cyclic voltammetry experiments, thus the narrow bandgap for 17 and 18 compared to 10 is related to an increase of the HOMO level. Evaluation of the performances of these chromophores has been achieved using a bilayer planar heterojunction with the following architecture ITO/PEDOT:PSS (40 nm)/10, 16–19 (35 nm)/C60 (30 nm)/Al (100 nm). The highest performance gives a PCE of 2.97% for 10 (VOC = 0.97 V, JSC = 5.32 mA cm−2 and FF = 0.52) while 16 exhibits the lowest PCE according to the optical and electrochemical data (0.57%, VOC = 0.71 V, JSC = 2.07 mA cm−2 and FF = 0.35).
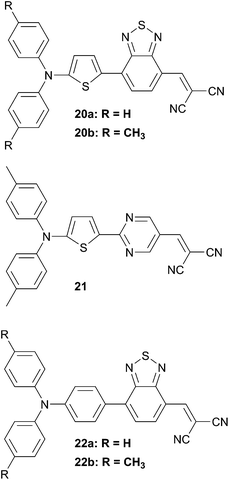
Further interesting insights were obtained from the previous D–A series by the insertion of a second acceptor along the π-conjugated spacer. The elegant combination of two different acceptors gives rise to chromophores with the D–A–A configuration.18–20 These D–A–A structures exhibit both narrow bandgap and lower HOMO levels compared to their D–A counterparts (Scheme 9). Thus, insertion of the benzothiadiazole (BT) unit in the D–A structures fosters the creation of quinoidal mesomeric forms over the π-conjugated skeleton resulting to a bathochromic shift of the maximum of absorption. Moreover, judicious choices of the substituents as well as the aromatic rings over the π-conjugated backbone are key parameters to control both energy levels and bandgap. For instance, substitution of the phenyl ring by a thienyl leads to a substantial red shift of 90 nm of the maximum of absorption (i.e. 22a → 20a and 22b → 20b). Effective PMHJ solar cells (AM 1.5–00 mW cm−2) have been obtained by vacuum-deposition of compounds 20 to 22 as donors and C70 as acceptor (blended films in ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.6) with photocurrent characteristics ranging from 5.00 to 6.60%. By varying the nature of the inserted acceptor from BT to pyrimidine (20b → 21) a lowered HOMO level and an increased VOC where attained. Hence, solar cell performances of PMHJ devices (21
1.6) with photocurrent characteristics ranging from 5.00 to 6.60%. By varying the nature of the inserted acceptor from BT to pyrimidine (20b → 21) a lowered HOMO level and an increased VOC where attained. Hence, solar cell performances of PMHJ devices (21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C70, in ratio 1
C70, in ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) have shown enhanced efficiency up to 6.40%.
1) have shown enhanced efficiency up to 6.40%.
Precise adjustments of electrical parameters can be also implemented to improve the generated photocurrent. Therefore, accurate balance control between the photovoltage and the photocurrent causes a notable enhancement of the PCE up to 6.80% for 22b. Optimization of the cell structure is also critical for boosting cell performances. As exemplified hereby it was shown that an ultrathin layer of 1 nm of Ca before the Ag cathode leads to an increase of the conversion efficiency from 4.41 to 5.28% for a PMHJ solar cell based on 20b and PC61BM.21
A3D type
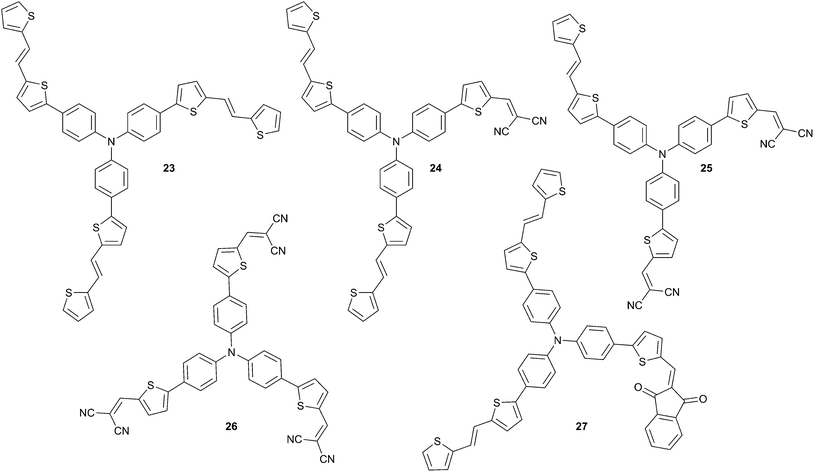
Optimization of the light harvesting properties of the chromophores is a crucial point that needs to be addressed in order to improve the conversion efficiencies. An elegant solution to tackle this problem has been developed by several groups, from 3D-structures mainly based on the TPA core. Indeed, TPA structures are of peculiar interest for this problematic, as they have already demonstrated their ability to generate efficient linear chromophores and allow the possibility to introduce various acceptor edge groups on the same skeleton. Hence, through an iterative pathway, a star-shaped series of TPA has been synthetized by Roncali et al. in which the π-conjugated arms offer variability in term of length, nature and acceptor edge groups. Using this strategy, dissymmetric structures are therefore attainable with both a controlled acceptor load and morphology structures (Scheme 10).As demonstrated on bulk heterojunction devices, from the structures 23 to 25, a noticeably linear improvement in power conversion efficiencies is observed ranging from 0.46 to 1.17% respectively.22 Explanations of this observation originate from (i) the fact that there is a linear dependency of the intramolecular charge transfer (ICT) with the number of acceptors present over the π-conjugated backbone and (ii) an enhanced VOC (23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.48 V; 24
0.48 V; 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.72 V; 25
0.72 V; 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.89 V; i.e. lower HOMO levels). Moreover increasing the number of acceptor groups promotes different spatial arrangement in the solid-state with strong intermolecular D–A interactions that can affect also the PCE. As expected, the highest VOC (0.96 V) was obtained for the compound 26 containing three dicyanovinyl end-groups, however, reduced PCE (1.02%) is achieved due to a lower filing factor compared to 25. Furthermore, optimizations of these rudimentary devices were undertaken to boost the power conversion efficiencies. For instance, addition of a thin film of LiF between the C60 acceptor layer and the Al electrode provides an impressive increase of the PCE from ∼1 to 1.85% (with VOC = 1.15 V and ISC = 4.59 mA cm−2)23 for the device made from 26.
0.89 V; i.e. lower HOMO levels). Moreover increasing the number of acceptor groups promotes different spatial arrangement in the solid-state with strong intermolecular D–A interactions that can affect also the PCE. As expected, the highest VOC (0.96 V) was obtained for the compound 26 containing three dicyanovinyl end-groups, however, reduced PCE (1.02%) is achieved due to a lower filing factor compared to 25. Furthermore, optimizations of these rudimentary devices were undertaken to boost the power conversion efficiencies. For instance, addition of a thin film of LiF between the C60 acceptor layer and the Al electrode provides an impressive increase of the PCE from ∼1 to 1.85% (with VOC = 1.15 V and ISC = 4.59 mA cm−2)23 for the device made from 26.
Taking into account these aspects the strategy is valid and worth pursuing as the observed synergistic effects lead to considerable improvement of the PCE.
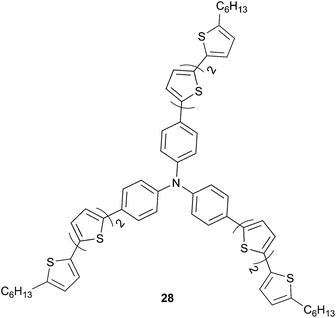
With the aim to enhance the photovoltaic output signals, several structural modifications have been conducted on the TPA derivatives based on the previous obtained results. Interestingly, contrary to polymeric or polycrystalline organic structures, amorphous TPA based chromophores have the advantage not to require control of the molecular orientation. First attempts have been focused basically on the hole-transport properties by engineering TPA derivatives with linear p-type organic semiconductors acting as the donor material in PV devices. Thus, structure-like 28 (Scheme 11) has been synthetized and tested as donor material in a bilayer heterojunction with C60 as the acceptor. PCE not exceeding 0.32% (VOC = 0.67 V; JSC = 1.70 mA cm−2)24 has been attained with such compound as mainly a consequence of a low filing factor. Although low PV efficiency has been obtained, 28 demonstrated good hole-mobility properties up μh = 1.10 10−2 cm2 V−1 s−1 which is very promising for PV applications.
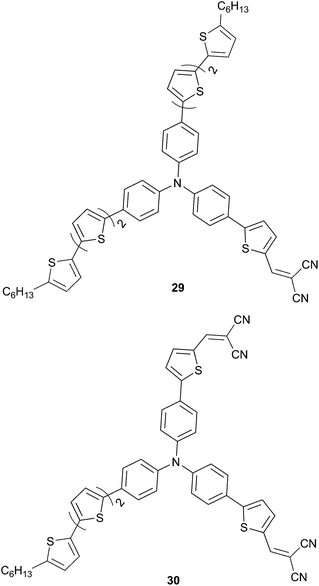
Subsequent replacement of one or two arms by unit end-capped with DCV (29 and 30 respectively, Scheme 12) as acceptor has led to a drastic improvement of the PCE (1.80% and 2.02% respectively) due to a large increase of the short-circuit current density and VOC (29: VOC = 0.85 V; JSC = 5.30 mA cm−2; 30: VOC = 1.07 V; JSC = 5.83 mA cm−2) that are intrinsically correlated to the ICT.25
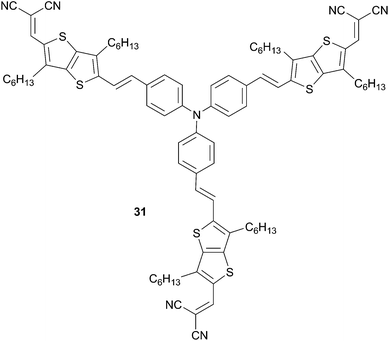
To achieve improved results and gain further insights into structure–properties relationships, bridged-thienyl spacers have been introduced in the TPA molecular skeleton. Covalently bridged-spacers are well-known to exalt photophysical properties compared to their un-bridged analogues.26 For instance, Li27 and co-workers used 3,6-dihexyl-thieno[3,2-b]thiophene as electron relay between the TPA donor part and the dicyanovinyl end groups for the construction of star-shaped structures (Scheme 13). Devices made from a blend of 31 and PC61BM or PC71BM in weight ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 with a Al/Ca as cathode leads to high-performance samples under standard illumination conditions (respectively 1.63% and 2.87%) associated with exceptional high JSC values (4.66 to 6.80 mA cm−2) and VOC (0.90 V and 0.96 V) that are among to the best reported in literature.
2 with a Al/Ca as cathode leads to high-performance samples under standard illumination conditions (respectively 1.63% and 2.87%) associated with exceptional high JSC values (4.66 to 6.80 mA cm−2) and VOC (0.90 V and 0.96 V) that are among to the best reported in literature.
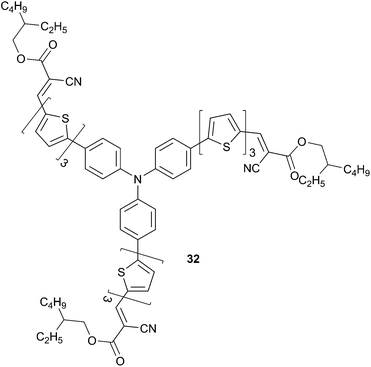
To achieve readily processable active materials, Lin et al. have synthetized star-shaped structures based on the TPA scaffold using alkyl-cyanoacetate (ACA) as acceptor.28 Incorporation of ACA aims at introducing simultaneously solubilizing and acceptor groups on the molecular π-conjugated backbone and avoids tedious multistep reactions. As a result, successful synthesis of 32 has been achieved associated with a good solubility in common solvents and high molar extinction coefficient (7.3 × 104 M−1 cm−1) (Scheme 14). Absorption maximum is red-shifted by 65 nm compared to the unsubstituted analogue 28 because of the intramolecular charge transfer. Additionally, 32 retains relatively high hole-mobility, measured by the space charge limited current (SCLC) method, up to 1.5 × 10−3 cm2 V−1 s−1 guarantied effective charge carrier transport and reduced photocurrent loss. Optimized BHJ solar cells made from 33 in conjunction with PC71BM (best weight ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
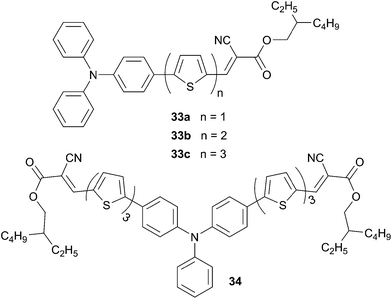
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 respectively) as the electron acceptor gave under standard illumination condition AM1.5 a power conversion efficiency, without any post-treatments, of 3.60% (VOC = 0.89 V, JSC = 7.30 mA cm−2). Furthermore, the photophysical properties of the star-shaped chromophores 32 were compared with linear and 2D analogous chromophores (33 and 34, Scheme 15) in order to investigate the structural effects on the above properties.29
2 respectively) as the electron acceptor gave under standard illumination condition AM1.5 a power conversion efficiency, without any post-treatments, of 3.60% (VOC = 0.89 V, JSC = 7.30 mA cm−2). Furthermore, the photophysical properties of the star-shaped chromophores 32 were compared with linear and 2D analogous chromophores (33 and 34, Scheme 15) in order to investigate the structural effects on the above properties.29
As expected, on the linear analogous derivatives, increment of the number of thiophene unit from 1 to 3 (33a to 33c) led to a 20 nm red shift of the maximum of absorption whereas increasing the number of arms around the TPA core it remains almost unchanged (32, 33c and 34 494, 498, 494 nm respectively). In addition, the energy differences between the HOMO levels of the chromophores and the LUMO level of PC71BM are sufficient to ensure the exciton dissociation and lead to a high VOC (from 0.87 to 0.99 V). Relative to 33a–c, chromophores 34 and 32 exhibit the highest PCEs (at same weight ratio with PC71BM 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), attaining 4.03% for 34 (JSC = 9.03 mA cm−2), due to stronger absorption and higher mobilities.
2), attaining 4.03% for 34 (JSC = 9.03 mA cm−2), due to stronger absorption and higher mobilities.
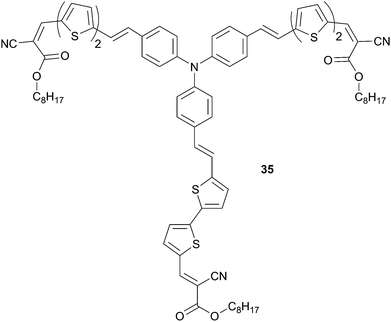
Replacing the thienyl unit by an ethylenic bond can be used to reduce the aromatic character of the π-conjugated arms in order to design more efficient chromophores. The ternary substituted TPA-based compound 35, end-capped with an octyl cyanoacetate (CA) group, was synthesized and evaluated by Li et al. in BHJ solar cells and compared to its analogue 32 (Scheme 16).30 Due to the less aromatic character of the arms better charge transfer occurs between the central donor and external acceptor groups. As expected, compound 35 possesses broad and strong visible absorption band which is red-shifted by 24 nm related to 32 and lower HOMO energy level. The photovoltaic performance was collected from a BHJ solar cell device with the following architecture ITO/PEDOT:PSS (30 nm)/35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM (70 nm)/Ca/Al (120 nm). The performances were recorded after a thermal annealing of the photosensitive layer at 80 °C (10 min.) at different blend weight ratios (from 1
PC71BM (70 nm)/Ca/Al (120 nm). The performances were recorded after a thermal annealing of the photosensitive layer at 80 °C (10 min.) at different blend weight ratios (from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 1
1 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, w/w) under AM1.5 conditions. The best device, based on a blend at 1
3, w/w) under AM1.5 conditions. The best device, based on a blend at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 ratio, exhibited a PCE of 2.1% (VOC = 0.91 V, JSC = 4.64 mA cm−2 and FF = 0.50). The reduced photoconversion compared to 32 was ascribed to a decrease of the short-circuit current due to lower hole-mobility (μh = 2.03 × 10−4 cm2 V−1 s−1 vs. μh = 1.5 × 10−3 cm2 V−1 s−1).
3 ratio, exhibited a PCE of 2.1% (VOC = 0.91 V, JSC = 4.64 mA cm−2 and FF = 0.50). The reduced photoconversion compared to 32 was ascribed to a decrease of the short-circuit current due to lower hole-mobility (μh = 2.03 × 10−4 cm2 V−1 s−1 vs. μh = 1.5 × 10−3 cm2 V−1 s−1).
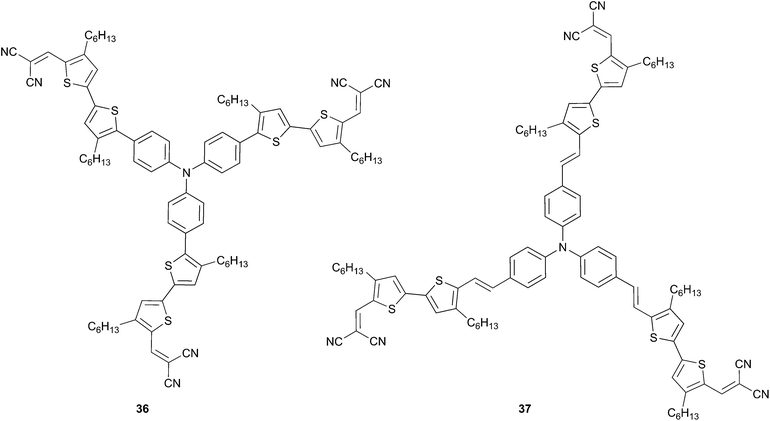
Motivated by the obtained results and continuing their efforts on the structure–properties relationships of star-shaped chromophores Li et al. investigated a novel class of trisubstituted TPA. Those are based on 5-vinyl-4,4′-dihexylbithiophene as π-conjugated spacer, in order to afford efficient solution-processable OSCs materials associated with good film forming properties and are end-capped with a dicyanovinyl group.31 Compared properties of 36 vs. 37 show that the latter displays enhanced photophysical properties caused by the insertion of the vinyl group onto the π-conjugated backbone. For instance 37 exhibits red shift by 40 nm of the maximum of absorption and lower HOMO level (−5.03 vs. −5.22 eV for 36) likewise the bandgap (1.61 vs. 1.88 eV for 36). Consequently, spin-coated films of either 36 or 37 were used to fabricate BHJ with PC71BM as acceptor (with ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 w/w respectively). Improved performances devices were obtained as anticipated for 37 exhibiting a power conversion efficiency of 3.00% (VOC = 0.88 V, JSC = 7.76 mA cm−2) which is twofold higher than 36 (Scheme 17).
2 w/w respectively). Improved performances devices were obtained as anticipated for 37 exhibiting a power conversion efficiency of 3.00% (VOC = 0.88 V, JSC = 7.76 mA cm−2) which is twofold higher than 36 (Scheme 17).
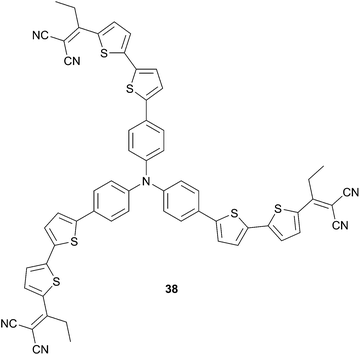
Besides processability, stability of chromophores is a crucial parameter that needs to be considered. Regarding dicyanovinyl acceptors, acidic hydrogen could be problematic for long-term stability of the active layers. In this context, Min et al.32 have designed a new star-shaped TPA 38 possessing an ethyl-dicyanovinyl group as acceptor (Scheme 18), which avoids the presence of acidic hydrogen. Additionally, 38 shows reduced torsional interactions compared to 36 because of lack of steric hindrance of the hexyl chains (leading consequently to lower HOMO level) and presents good solubility. Thus, BHJ made from 38 and PC71BM has resulted in a twofold improvement of the PCE (3.10%) relative to 36 (1.40%) even without post-treatments. Moreover, as already stated, device performances could be enhanced by optimizing the processing conditions such as thermal annealing methods or solvent additives. Therefore, the previous device treated, during the processing step, with 2% of 4-bromoanisole as additive has attained a remarkable efficiency of 3.60% (VOC = 0.96 V, JSC = 7.60 mA cm−2) related to morphology optimization and larger sized-domains.
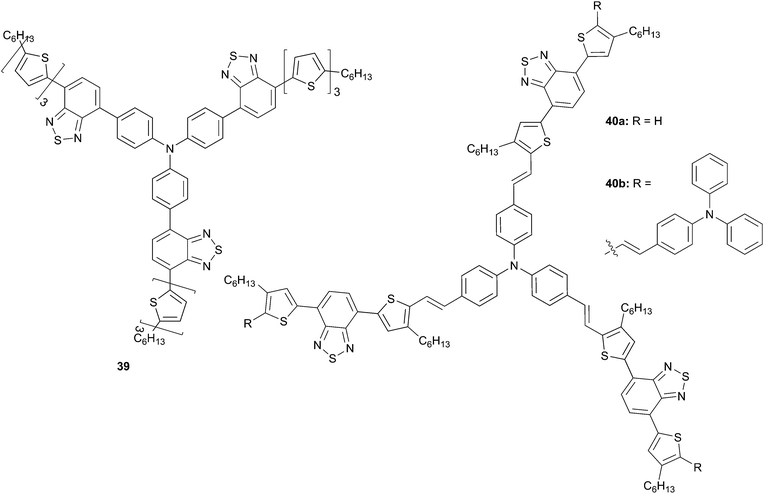
Additional improvements have been achieved regarding the absorption properties compared to 28 by insertion of benzothiadiazole (BT) moieties into the π-conjugated systems of the TPA starburst molecular structures (Scheme 19). Indeed, introduction of BT lead, not only, to a broadening absorption spectrum and also to an increase of the thermal stability probably due to the S–N intramolecular bondings.33 Moreover, 39 displayed typical p-type semiconductor behavior in air associated with a hole-mobility of μh = 4.90 10−4 cm2 V−1 s−1 which is almost 50 times higher than the reported analog 28.
All these features, associated to good solubility properties, make 39 to be a donor of choice for fabricating solution-processed OSCs. Consequently, a blend of 39 and PC71BM has been deposited by spin coating exhibiting a PCEs as high as 4.3% without any post-treatment (device structure: ITO/PEDOT:PSS/39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM (1
PC71BM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, w/w)/Ca/Al, (VOC = 0.87 V, JSC = 9.51 mA cm−2)). The high value obtained has been correlated to the surface morphology displaying nano-aggregated domains that are suitable and beneficial to the charge separation. In order to expand the scope of this compounds family, He et al. have developed and tested in BHJ OSCs the derivatives 40a and 40b (40a or 40b
2, w/w)/Ca/Al, (VOC = 0.87 V, JSC = 9.51 mA cm−2)). The high value obtained has been correlated to the surface morphology displaying nano-aggregated domains that are suitable and beneficial to the charge separation. In order to expand the scope of this compounds family, He et al. have developed and tested in BHJ OSCs the derivatives 40a and 40b (40a or 40b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM (1
PC71BM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, w/w)).34 Extended π-conjugated backbone was obtained by inserting the vinylene groups and TPA end-groups in order to achieve broader absorption. Although similar optical properties to 28 were gained lower photovoltaic performances (PCEs of 1.90% and 1.23% with VOC = 0.75 V, JSC = 6.41 mA cm−2; VOC = 0.71 V, JSC = 5.41 mA cm−2 respectively) were attained mainly due to poorer film-forming properties (large molecular size, lower solubility) and weaker ICTs.
3, w/w)).34 Extended π-conjugated backbone was obtained by inserting the vinylene groups and TPA end-groups in order to achieve broader absorption. Although similar optical properties to 28 were gained lower photovoltaic performances (PCEs of 1.90% and 1.23% with VOC = 0.75 V, JSC = 6.41 mA cm−2; VOC = 0.71 V, JSC = 5.41 mA cm−2 respectively) were attained mainly due to poorer film-forming properties (large molecular size, lower solubility) and weaker ICTs.
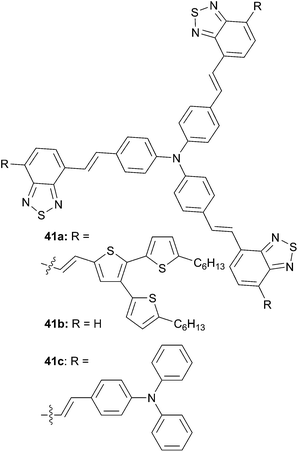
As was demonstrated for structure-like 39, thiophene or polythiophene structures can play not only a linker role but can also act as a donor. The 41a star-shaped conjugated molecule possesses a D–A structure with TPA as the donor core unit, benzo[1,2,5]thiadiazole (BT) as the acceptor unit and terthiophene (TT) donor end groups.35 Comparatively to the corresponding star-shaped molecules 41b36 and 41c,37 41a shows a red-shifted absorption with a maximum at 538 nm and an absorption edge extending to 650 nm associated to higher hole mobility up μh = 1.1 × 10−4 cm2 V−1 s−1. The BHJ solar cells based on the device structure ITO/PEDOT:PSS/41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM (70 nm)/Ca/Al, with a blend 41
PC71BM (70 nm)/Ca/Al, with a blend 41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM at a 1
PC71BM at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 ratio (w/w), exhibited a power conversion efficiency of 2.28% (VOC = 0.82 V, JSC = 6.39 mA cm−2 and FF = 0.43) under standard illumination AM1.5 conditions. Compounds 41b and 41c exhibit lower efficiencies, 0.61% (VOC = 0.91 V, JSC = 1.54 mA cm−2 and FF = 0.43, with a Ba (10 nm)/Al (100 nm) cathode) and 1.33% (VOC = 0.81 V, JSC = 4.18 mA cm−2 and FF = 0.39) respectively incriminated to lower hole-mobility as well as less π–π interactions in the solid state due to the less planar TPA scaffold related to TT (Scheme 20).
3 ratio (w/w), exhibited a power conversion efficiency of 2.28% (VOC = 0.82 V, JSC = 6.39 mA cm−2 and FF = 0.43) under standard illumination AM1.5 conditions. Compounds 41b and 41c exhibit lower efficiencies, 0.61% (VOC = 0.91 V, JSC = 1.54 mA cm−2 and FF = 0.43, with a Ba (10 nm)/Al (100 nm) cathode) and 1.33% (VOC = 0.81 V, JSC = 4.18 mA cm−2 and FF = 0.39) respectively incriminated to lower hole-mobility as well as less π–π interactions in the solid state due to the less planar TPA scaffold related to TT (Scheme 20).
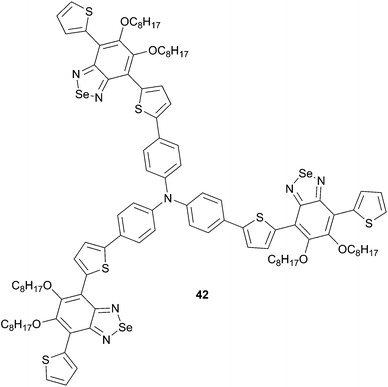
Replacing benzothiadiazole by benzoselenadiazole (BS) into TPA star-shaped skeleton was described recently (Scheme 21).38 Selenium atom was used because of its more electron rich character compared to sulfur and induced a red shift in π-conjugated systems relative to their sulfur counterparts. Thereby, compound 42 was designed and synthesized displaying both a good solubility and a broad absorption from 300 to 650 nm similar to that of 39. BHJ OSCs based on blend films of 42 and PC71BM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, w/w) have given rise to photovoltaic performances up to 1.54% (VOC = 0.91 V, JSC = 4.54 mA cm−2, FF = 0.37).
2, w/w) have given rise to photovoltaic performances up to 1.54% (VOC = 0.91 V, JSC = 4.54 mA cm−2, FF = 0.37).
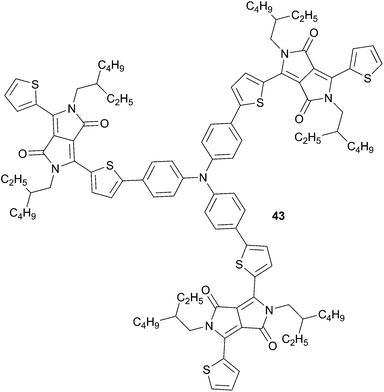
Due to their intrinsic attractive properties, diketopyrrolopyrrole (DPP) have been proven promising materials for the design of efficient OSCs. Thus changing BT or BS acceptors by a DPP moiety in TPA based-materials has led to the design of novel materials that can be used as non-fullerene acceptors for solution-processed OSCs. To this end, Zhan et al.39 have synthesized a soluble chromophore 43 and have therefore demonstrated its potential application in OSCs (Scheme 22). A device structure ITO/PEDOT:PSS/P3HT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 43 (1
43 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, w/w)/Ca/Al leads to an impressive increase of the VOC = 1.18 V (JSC = 2.68 mA cm−2) associated to a conversion efficiency as high as 1.20%.
1, w/w)/Ca/Al leads to an impressive increase of the VOC = 1.18 V (JSC = 2.68 mA cm−2) associated to a conversion efficiency as high as 1.20%.
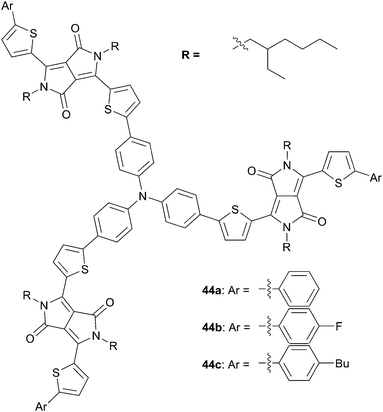
Shi and co-workers have recently optimized the photophysical properties of the above DPP architectures by end-capping with substituted or unsubstituted benzene rings (compounds 44a–c – Scheme 23).40 The latter were investigated as π-conjugated donors in blend films with PC61BM as the acceptor at ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (w/w) (for 44a and 44b) or 1
1 (w/w) (for 44a and 44b) or 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (for 44c). Best performances were obtained after improving the film morphologies by adding 2% of DIO (1,8-diiodooctane) during the solution-process step. Generally, the synthesized chromophores exhibit roughly similar optical and electrochemical behaviors with slight differences relative to the endgroup that influences mainly the π–π stacking in the solid state. The device structures demonstrated that the highest PCE (2.98%) was obtained for 44a and was correlated to its most efficient charge transport properties and higher hole-mobility among the others (44a: μh = 8.63 10−5 cm2 V−1 s−1, VOC = 0.72 V, JSC = 7.94 mA cm−2 vs. 44b: μh = 2.42 10−5 cm2 V−1 s−1, VOC = 0.76 V, JSC = 5.14 mA cm−2 and 44c: μh = 4.43 10−5 cm2 V−1 s−1, VOc = 0.80 V, JSC = 5.83 mA cm−2) as well as a smoother film morphology and an higher exciton dissociation efficiency.
2 (for 44c). Best performances were obtained after improving the film morphologies by adding 2% of DIO (1,8-diiodooctane) during the solution-process step. Generally, the synthesized chromophores exhibit roughly similar optical and electrochemical behaviors with slight differences relative to the endgroup that influences mainly the π–π stacking in the solid state. The device structures demonstrated that the highest PCE (2.98%) was obtained for 44a and was correlated to its most efficient charge transport properties and higher hole-mobility among the others (44a: μh = 8.63 10−5 cm2 V−1 s−1, VOC = 0.72 V, JSC = 7.94 mA cm−2 vs. 44b: μh = 2.42 10−5 cm2 V−1 s−1, VOC = 0.76 V, JSC = 5.14 mA cm−2 and 44c: μh = 4.43 10−5 cm2 V−1 s−1, VOc = 0.80 V, JSC = 5.83 mA cm−2) as well as a smoother film morphology and an higher exciton dissociation efficiency.
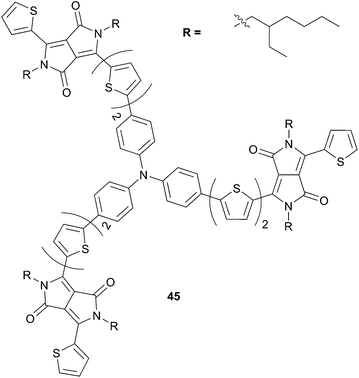
Expanding the π-conjugated system of 43 by embedding additional thienyl ring has demonstrated to be beneficial to improve the light harvesting properties on the TPA–DPP based structures. Indeed, improvement of the photophysical properties were ascribed to an increase of the molecular ordering that inter alia impacts on the absorption properties and on the charge mobilities (Scheme 24).41 Consequently, a dramatic enhancement of the photovoltaic performances, more than two fold magnitude, was obtained in favor to 45 (2.95%, VOC = 0.81 V, JSC = 10.02 mA cm−2) compared to 43 (1.38%, VOC = 0.77 V, JSC = 5.08 mA cm−2) in conventional device structures with PC71BM as acceptor (at ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 and 1
4 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (w/w) respectively). Similar results were obtained when PC61BM was used as acceptor (1.81%, VOC = 0.83 V, JSC = 5.60 mA cm−2 vs. 1.13%, VOC = 0.84 V, JSC = 4.19 mA cm−2). Moreover, the HOMO (−5.13 eV) and LUMO (−3.32 eV) energy levels suggest that 45 could be used as an acceptor in blended films with P3HT yielding a PCE of 0.63% with an impressive VOC = 1.14 V, (JSC = 1.33 mA cm−2).
2 (w/w) respectively). Similar results were obtained when PC61BM was used as acceptor (1.81%, VOC = 0.83 V, JSC = 5.60 mA cm−2 vs. 1.13%, VOC = 0.84 V, JSC = 4.19 mA cm−2). Moreover, the HOMO (−5.13 eV) and LUMO (−3.32 eV) energy levels suggest that 45 could be used as an acceptor in blended films with P3HT yielding a PCE of 0.63% with an impressive VOC = 1.14 V, (JSC = 1.33 mA cm−2).
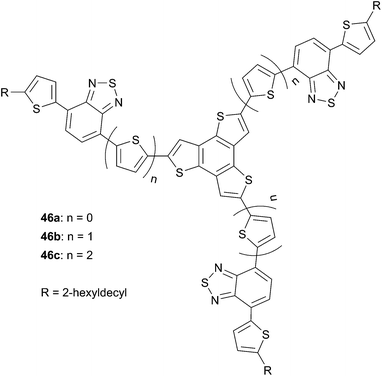
Original star-shaped chromophores based on non-TPA cores, with a C3h symmetry, are of interest due to the possibility to fine-tune the properties of such structures by modifying for instance the planarity and controlling the solid-state interactions. Based on these considerations, Xiao et al.42 and Bo et al.43 have envisioned the use of planar structures such as benzotrithiophene (BTT) and benzotrisindole (BTI) respectively to develop new star-shaped structures (Schemes 25 and 26).
Fine-tuning the properties of molecules 46a–c by a selective increase of the thiophenic units leads to a control of the HOMO level while the LUMO level remains almost unaffected.
Compounds 46a–c exhibit broad optical absorption with similar behaviors; the main difference came from the highest energy band due to the increase of the number of thienyl units. BHJ solar cells were fabricated with the device structure ITO/PEDOT:PSS (45 nm)/46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC61BM or 46
PC61BM or 46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM/Ca (20 nm)/Al (80 nm). Increasing the number of thienyl units led to a significant enhancement of the PCE from 0.16% for 46a (VOC = 0.54 V, JSC = 0.95 mA cm−2 and FF = 0.31) to 0.74% for 46c (VOC = 0.69 V, JSC = 2.93 mA cm−2 and FF = 0.7) at a 46
PC71BM/Ca (20 nm)/Al (80 nm). Increasing the number of thienyl units led to a significant enhancement of the PCE from 0.16% for 46a (VOC = 0.54 V, JSC = 0.95 mA cm−2 and FF = 0.31) to 0.74% for 46c (VOC = 0.69 V, JSC = 2.93 mA cm−2 and FF = 0.7) at a 46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC61BM 1
PC61BM 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. Changing the blend to 46c
1 ratio. Changing the blend to 46c![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM (1
PC71BM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio) improves the conversion efficiency up to 1.36% (VOC = 0.72 V, JSC = 4.13 mA cm−2 and FF = 0.46) due to a better intimate donor acceptor mixing and smoother surface.
2 ratio) improves the conversion efficiency up to 1.36% (VOC = 0.72 V, JSC = 4.13 mA cm−2 and FF = 0.46) due to a better intimate donor acceptor mixing and smoother surface.
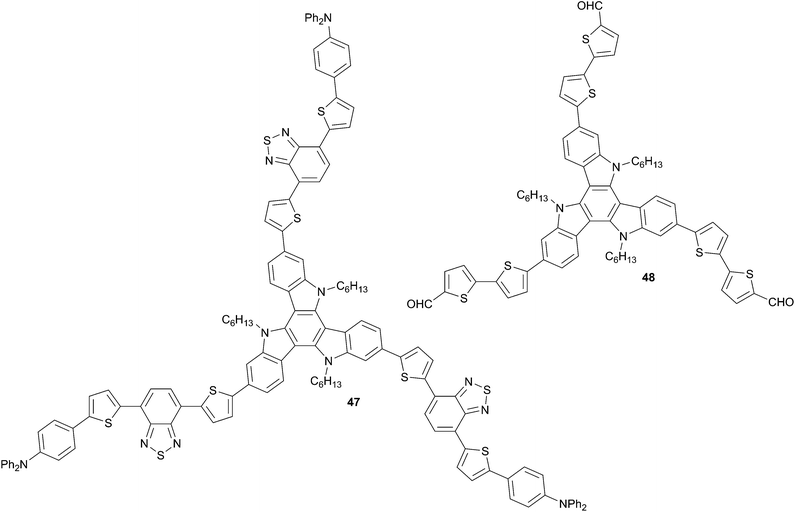
BTI core has been used for the synthesis of chromophores 47 and 48 and due to presence of the N-atom it should display a higher donor character relative to BTT. The films made from 47 and 48 display a broad absorption in the visible associated to an optical bandgap of 1.82 eV and 2.07 eV respectively. From the electrochemical experiments the HOMO and LUMO energy levels have been calculated to be −5.12 eV and −3.30 eV for 47 and −5.07 eV and −3.00 eV for 48 attesting their ability to be used as donors in BHJ in conjunction with PC61BM or PC71BM as acceptors. Hence, solar cells devices with the architecture ITO/PEDOT:PSS (40 nm)/47–48![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM/LiF (0.5 nm)/Al (100 nm) were prepared and evaluated. The photovoltaic performances were found to be dependent to the ratio of donor to acceptor. The best results were obtained for a ratio of 1
PC71BM/LiF (0.5 nm)/Al (100 nm) were prepared and evaluated. The photovoltaic performances were found to be dependent to the ratio of donor to acceptor. The best results were obtained for a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 with a thickness of the photoactive layer of about 105 nm. Under these conditions, PCEs of 1.95% for 47 (VOC = 0.82 V, JSC = 5.31 mA cm−2 and FF = 0.45) and 1.73% for 48 (VOC = 0.65 V, JSC = 8.13 mA cm−2 and FF = 0.33) were achieved. However, a post-deposition thermal annealing at 130 °C for 5 min. for 48 increases the conversion efficiency up to 2.05% (VOC = 0.72 V, JSC = 6.41 mA cm−2 and FF = 0.44) while an improvement for 47 is obtained when 0.5% of CN is used as solvent additive leading to a PCE of 2.29% (VOC = 0.82 V, JSC = 6.00 mA cm−2 and FF = 0.47). The enhancement has been incriminated to marked changes of the film morphologies when the post-treatments were performed as attested by the surface and morphology analysis. The results obtained from these structures make them very promising candidates for the elaboration of SMOSCs and need to be further explored.
3 with a thickness of the photoactive layer of about 105 nm. Under these conditions, PCEs of 1.95% for 47 (VOC = 0.82 V, JSC = 5.31 mA cm−2 and FF = 0.45) and 1.73% for 48 (VOC = 0.65 V, JSC = 8.13 mA cm−2 and FF = 0.33) were achieved. However, a post-deposition thermal annealing at 130 °C for 5 min. for 48 increases the conversion efficiency up to 2.05% (VOC = 0.72 V, JSC = 6.41 mA cm−2 and FF = 0.44) while an improvement for 47 is obtained when 0.5% of CN is used as solvent additive leading to a PCE of 2.29% (VOC = 0.82 V, JSC = 6.00 mA cm−2 and FF = 0.47). The enhancement has been incriminated to marked changes of the film morphologies when the post-treatments were performed as attested by the surface and morphology analysis. The results obtained from these structures make them very promising candidates for the elaboration of SMOSCs and need to be further explored.
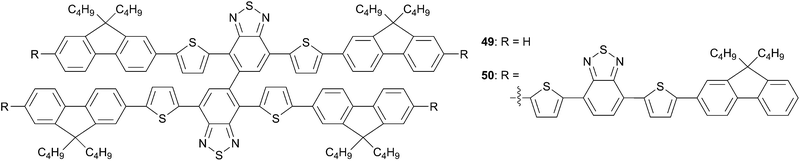
Recently Wu et al. have envisioned the synthesis of cruciform-like structures originating from the link of two linear chromophores (Scheme 27).44 Thus, two new structures 49 and 50 have been designed and synthesized in order to be investigated as potential new efficient donor for organic solar cells. They differ one to each other by the length of the arms. As expected, 50 exhibiting the longest π-conjugated system led to the highest maximum absorption (λmax = 525 nm). Interestingly, from solution (λmax = 495 nm) to thin film (λmax = 499 nm) 49 shown similar spectra suggesting weak intermolecular contacts in the solid-state while 50 displays a 13 nm red shift due to better π–π interactions. From the solid-state spectra the optical bandgap can be deduced to be 2.07 eV and 1.82 eV respectively to 49 and 50. The low-lying HOMO energy levels of 49 and 50 are appropriate to generate large VOC in the devices.
Conventional structure of ITO/PEDOT:PSS (30 nm)/49 or 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM (1
PC71BM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, w/w, 90 nm)/Ca (15 nm)/Al (100 nm) have been used for the fabrication of BHJ solar cells. Photovoltaic performances were determined under standard illumination conditions AM1.5. Conversion efficiencies of 0.74% for 49 (VOC = 1.09 V, JSC = 2.47 mA cm−2 and FF = 0.27) and 3.14% for 50 (VOC = 1.08 V, JSC = 6.75 mA cm−2 and FF = 0.43) were obtained without any treatments. The significant differences of the PCEs in favor to 50 came from the improved short-circuit current and fill factor related to a better phase separation and broader absorption spectrum.
3, w/w, 90 nm)/Ca (15 nm)/Al (100 nm) have been used for the fabrication of BHJ solar cells. Photovoltaic performances were determined under standard illumination conditions AM1.5. Conversion efficiencies of 0.74% for 49 (VOC = 1.09 V, JSC = 2.47 mA cm−2 and FF = 0.27) and 3.14% for 50 (VOC = 1.08 V, JSC = 6.75 mA cm−2 and FF = 0.43) were obtained without any treatments. The significant differences of the PCEs in favor to 50 came from the improved short-circuit current and fill factor related to a better phase separation and broader absorption spectrum.
A – D – A type
Besides the recently developed TPA-based chromophores most efforts have been devoted to the linear oligomeric thiophene π-conjugated systems. On such derivatives the thiophene π-conjugated backbone, is routinely combined with selected acceptors in order to fine-tune their photophysical properties. From a given donor (D) and acceptor (A) different combinations are possible leading to several defined architectures namely D–A, A–D–A, D–A-D etc… Symmetric and dissymmetric structures can be easily obtained using iterative synthetic pathways and offer most advantages than polymers because of their higher solubility, monodisperse character and the possibility to better fine tune their properties.In addition, as evidenced on TPA star-shaped structures, combination of a donor with a given strength acceptor broadens the absorption spectrum and optimizes the light-harvesting properties. Consequently a reduction of Eg was produced concomitantly with an increase of the VOC due to a decrease of the HOMO level. All these phenomena are in favor of optimizing the photovoltaic performances.
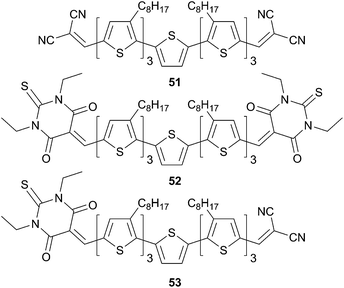
In this context, association of a septithiophene with two different acceptors namely the dicyanovinyl (DCN) and the diethylthiobarbituric (DETA) acid affords two symmetrical (A–D–A) and one asymmetrical (A–D–A′) structures (Scheme 28). In those systems the oligothiophene π-backbone acts as the hole-transporting material whereas the acceptor expands the absorption spectrum to higher wavelengths, via an ICT, allowing a better match with the solar spectrum.
As expected, the replacement of one DCN by DETA (51 to 53) leads to a 31 nm red shift and subsequent exchange of the second DCN by DETA (51 to 52) leads to another 21 nm red shift of the λmax. Surprisingly, it can be noticed that from 51 to 52 a reduction of the band gap is attained while a concomitant diminution of the power conversion efficiency is obtained as the number of DETA increase in a PHJ configuration with a spin-coated donor layer and a vacuum deposited C60 layer (1.64% 51, 1.21% 53 and 0.36% 52 respectively).45 From these observations it appears that the molecule desymmetrization affects both the optical properties as well as the packing arrangement in the solid state, which has the dramatic effect on the PCE. Further investigations in BHJ solar cells based on a blend of 51 and PC61MB (in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.4 (w/w) ratio) led to an improvement of the PCE value up to 2.45%46 and 3.7%.47
1.4 (w/w) ratio) led to an improvement of the PCE value up to 2.45%46 and 3.7%.47
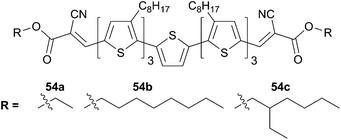
Chen's group further studied an analogous series by changing the nature of the acceptor. Interestingly, it was found that the alkyl-cyanoacetate (ACA) end-group acts as a quite effective acceptor group with the benefit to improve the film morphologies qualities by changing the nature of the alkyl chain (Scheme 29).
Thus, compounds 54a, 54b and 54c display similar optical properties in CHCl3 (absorption maxima ca. 492 nm and absorption coefficients are 7.6 × 104, 6.3 × 104, 6.0 × 104 M−1 cm−1, respectively). PCE were evaluated on a BHJ devices, made from a blend of each of these donors and PC61BM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 w/w), leading to high efficiency, up to 4.46%, 5.08% and 4.52% for 54a, 54b and 54c respectively.48 The observed variations can be incriminated to the formation of different grain-sized domains organizations in the blend as evidenced by AFM. For instance, microphotographs of 54b, exhibit well separated domains (assigned to the acceptor-rich and donor-rich parts) associated to a higher roughness (4.05 nm compared to 2.51 nm for 54a and 54c). Comparison of the volume occupied by the side chains (linear or hyperbranched alkyl chains) is of importance on the final properties (54b and 54c) and has to be considered in the molecular design of linear systems.
0.5 w/w), leading to high efficiency, up to 4.46%, 5.08% and 4.52% for 54a, 54b and 54c respectively.48 The observed variations can be incriminated to the formation of different grain-sized domains organizations in the blend as evidenced by AFM. For instance, microphotographs of 54b, exhibit well separated domains (assigned to the acceptor-rich and donor-rich parts) associated to a higher roughness (4.05 nm compared to 2.51 nm for 54a and 54c). Comparison of the volume occupied by the side chains (linear or hyperbranched alkyl chains) is of importance on the final properties (54b and 54c) and has to be considered in the molecular design of linear systems.
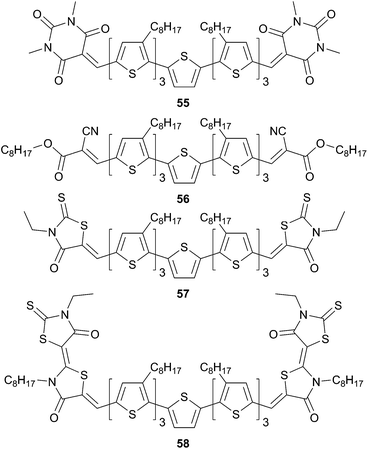
Continuing their studies on the structure–property relationships modulation of the properties have been achieved by varying the nature of the acceptor on symmetrical structures49,50 (Scheme 30).
As expected the energy levels and bandgap can be easily fine-tuned on these architectures by changing the strength of the acceptors. Nonetheless, the nature of the terminal acceptors will affect not only the optical properties but also dramatically the solid-state packing as well as on the solubility. That might impact both on the hole and electron mobilities and on the grain size domains. Hence, hole-mobilities were measured by the space charge limited current method (SCLC) on films made from the compounds 55 to 58 giving the following mobilities μh = 0.47 × 10−4 cm2 V−1 s−1, μh = 3.26 × 10−4 cm2 V−1 s−1, μh = 1.50 × 10−4 cm2 V−1 s−1 and μh = 0.24 × 10−4 cm2 V−1 s−1 respectively. Bulk heterojunction devices fabricated with a blend of each donors and PC61BM in the ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 (w/w) (except for 55
0.5 (w/w) (except for 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.8 (w/w)) led moderate to high PCE as high as 4.05%, 5.08%, 6.10% and 2.46% for 55–58 respectively. The enhancement of PCE for 57 compared to 56 is attributed to the higher JSC value (JSC = 13.98 mA cm−2 versus JSC = 10.74 mA cm−2) due to a stronger red shift of the maximum of absorption.
0.8 (w/w)) led moderate to high PCE as high as 4.05%, 5.08%, 6.10% and 2.46% for 55–58 respectively. The enhancement of PCE for 57 compared to 56 is attributed to the higher JSC value (JSC = 13.98 mA cm−2 versus JSC = 10.74 mA cm−2) due to a stronger red shift of the maximum of absorption.
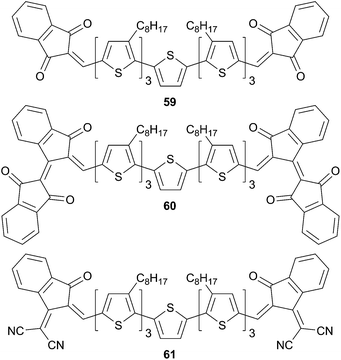
Changing the previous acceptors by the 1,3-indanedione analogous in compound 61 leads to a reduced band gap and should improve their photovoltaic conversion efficiencies (Scheme 31). Nevertheless, the poor packing in the solid state (as revealed by XRD analysis) and its low LUMO level (−3.86 eV) provide a relatively low PCE for 60 that not exceed 0.66%. The rather low PCE could be assigned to the lack of solubility for those chromophores, which turns to be prohibitive for fabrication solution processes (i.e. for 61). However, considering the better solubility of 59, the 59-based structure gives rise to an efficient device with a PCE of 4.93% and I/V characteristics close to that of 56 and 57.51
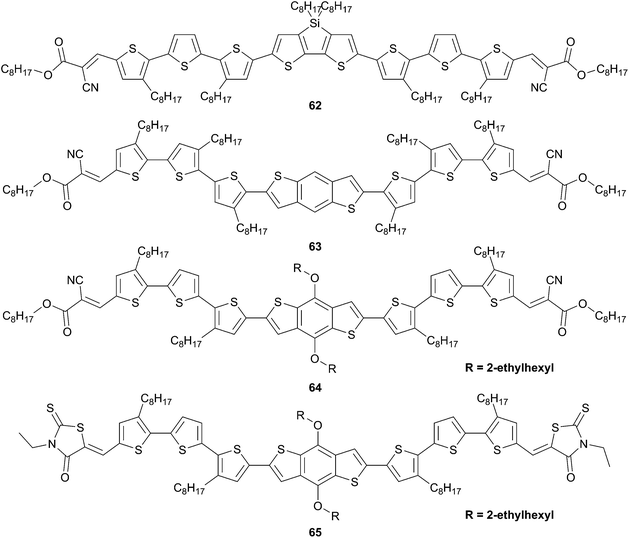
Further modulations were achieved from these linear systems by inserting into the π-conjugated systems various unsubstituted or substituted rigid blocks (Schemes 32and 33) that can help to adjust the photovoltaic characteristics as well as their intrinsic optoelectronic properties. For instance, the dithienosilole derivative 62 demonstrated greater light harvesting efficiency. BHJ devices based on this chromophore showed a PCE of 5.84% along with a noticeably high fill factor of 0.64.52 As an archetype structure benzodithiophene (BDT) 63 was first investigated and used for the preparation of an efficient BHJ cell (PCE = 5.44%) with a high VOC = 0.93 V.53 Encouraged by these results further optimization were achieved by changing the nature of the acceptors (to have greater light absorption) and also by increasing their solubility by the introduction of alkyl side chains (64 and 65).54 Hence, an optimized BHJ device made from a blend of 65, PC71BM and PDMS as additive in the active layer led to date to one of the highest PCE value (7.38%) with an average 7.18% obtained from a hundred devices.
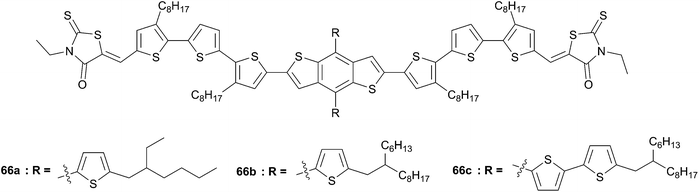
With the aim to enlarge the scope of this series, chromophore 65 was further optimized into 2D structures by expanding laterally the π-conjugated system with different thienyl substituents (66a–c, Scheme 33).6 Solution-processed BHJ devices were fabricated from these chromophores and PC71BM as electron acceptor. Similarly to 65, the optimal weight ratio D–A was selected to be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.8, and the device performances were improved by addition of PDMS as an additive (0.2 mg mL−1) (PDMS). Additionally, when other additives such as 1,8-diiodooctane and 1-chloronaphthalene were used no significant improvement was attained. For instance, optimized device performance based on 66a showed an exceptional PCE value of 8.12% with a VOC = 0.93 V, JSC = 13.17 mA cm−2 and a fill factor of 0.66.
0.8, and the device performances were improved by addition of PDMS as an additive (0.2 mg mL−1) (PDMS). Additionally, when other additives such as 1,8-diiodooctane and 1-chloronaphthalene were used no significant improvement was attained. For instance, optimized device performance based on 66a showed an exceptional PCE value of 8.12% with a VOC = 0.93 V, JSC = 13.17 mA cm−2 and a fill factor of 0.66.
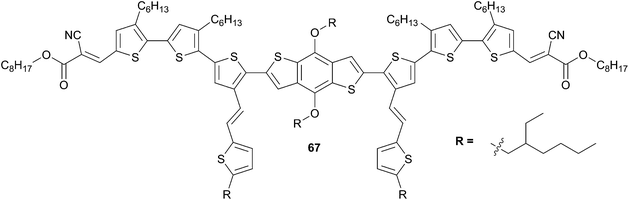
Cui et al.55 prepared the analogous 2D molecule 67 of derivative 64 with an A–D–A framework (Scheme 34). Although optical properties were not modified significantly compared to 64, the BHJ OSC device based on 67![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM (1
PC71BM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5, w/w) leads to an increase of the PCE 4.0% (which almost twofold magnitude higher than for 64) associated to a notable fill factor of 0.63 without any specific treatments.
0.5, w/w) leads to an increase of the PCE 4.0% (which almost twofold magnitude higher than for 64) associated to a notable fill factor of 0.63 without any specific treatments.
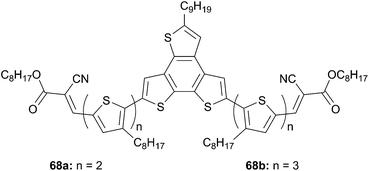
The use of benzotrithiophene (BTT) as central core instead of BDT was investigated by Patra et al. (Scheme 35).56 The first attempts to make solar cells devices from 68b and PC71BM (in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.75 ratio (w/w)) led to a respectably power conversion efficiency of 3.61% with VOC = 0.88 V, JSC = 7.39 mA cm−2 and a fill factor of 0.57. Improvements during the fabrication process by adding 0.25 vol% of 1-chloronaphthalene, as an additive, during the formation of the blend film of 68b
0.75 ratio (w/w)) led to a respectably power conversion efficiency of 3.61% with VOC = 0.88 V, JSC = 7.39 mA cm−2 and a fill factor of 0.57. Improvements during the fabrication process by adding 0.25 vol% of 1-chloronaphthalene, as an additive, during the formation of the blend film of 68b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM, increased significantly the PCE to 5.05% with VOC = 0.86 V, JSC = 9.94 mA cm−2 and a fill factor of 0.59. In such devices, the enhancement was attributed to the suppression of the nanophase molecular aggregation.
PC71BM, increased significantly the PCE to 5.05% with VOC = 0.86 V, JSC = 9.94 mA cm−2 and a fill factor of 0.59. In such devices, the enhancement was attributed to the suppression of the nanophase molecular aggregation.
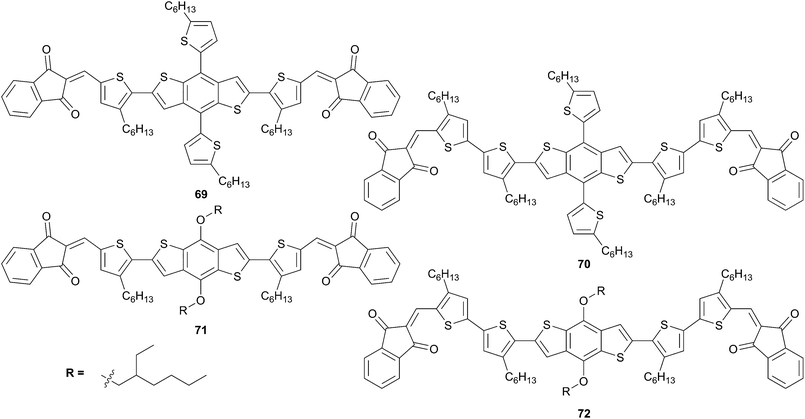
Pursuing their efforts on the optimization of the molecular structures led Shen et al. to develop shorten derivatives of compounds 63–67 that are depicted in Scheme 36.57 Thus, four new solution-processable A–D–A chromophores (69–72) were designed and synthetized with a BDT as central core end-capped with indenedione (ID) as acceptor and connected by thiophene or bithiophene as π-conjugated spacers. The photovoltaic conversion efficiencies were determined for each compound in presence of PC71BM (1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio (w/w)). The results indicate that in such structures the π-conjugated spacer is of importance, as well as the side chain on BDT, because the highest efficiencies were obtained for the longest spacer and correlate perfectly with the results for the series 63–67. Indeed, the PCE are respectively 5.67% for 69, 6.75% for 70, 4.15% for 71 and 5.11% for 72.
1 ratio (w/w)). The results indicate that in such structures the π-conjugated spacer is of importance, as well as the side chain on BDT, because the highest efficiencies were obtained for the longest spacer and correlate perfectly with the results for the series 63–67. Indeed, the PCE are respectively 5.67% for 69, 6.75% for 70, 4.15% for 71 and 5.11% for 72.
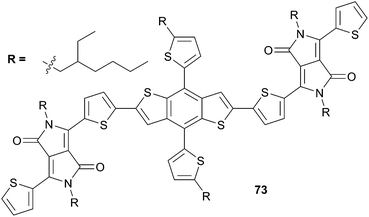
Since recently new interesting acceptors have been commonly used for the synthesis of advanced chromophores in optoelectronic applications namely the diketopyrrolopyrrole core (DPP). Thus, Yao et al.58 end-capped the previous DBT core with DPP and fabricate an efficient device with PC71BM as acceptor (Scheme 37). Indeed, when compound 73 was deposited as a blend by spin-casting from an o-dichlorobenzene solution in presence of an optimized percentage of DIO (0.70%), used as additive, a continuous interpenetrating network film was formed associated to a photovoltaic efficiency up to 5.29% and gathering the following I/V characteristics VOC = 0.72 V, JSC = 11.86 mA cm−2 and a fill factor of 0.62. The lowest value obtained for 73 compared to 69 was incriminated to a reduction of the VOC. Slight enhancements have been achieved, from the work of Zhian et al.,59 by changing the nature of the acceptor, from PC71BM to PC61BM, and applying a thermal annealing at 110 °C during 10 min. Consequently a PCE of 5.79% was attained.
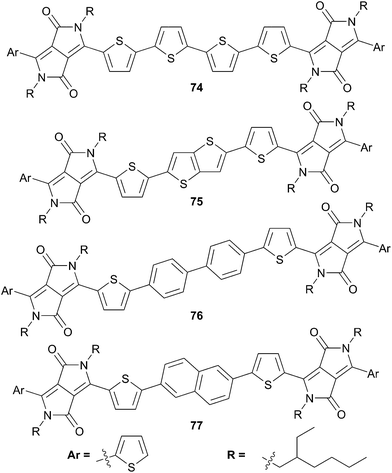
Besides this example, the DPP unit has been used in conjunction with several other π-conjugated spacers in order to investigate the influence of the spacer on the electroptical properties of the synthetized chromophores. Thereby, Jo and Choi60 have studied and compared the influence of electro-donating character and planarity of different spacers on the properties (Scheme 38).
From this study it appears that the insertion of weak π-donating spacers, such as biphenyl (76) and naphthalene (77), contribute to higher VOC inversely to stronger systems like dithienyl (74), or fused-bithiophene (75) due to a lower HOMO energy level. In addition, when more rigid and planar π-conjugated spacers were used it was observed an increase of the short-circuit current density JSC (75 and 77) owing to a higher hole-mobility. Therefore the use of naphthalene in this series corresponds to the best compromise owing its enhanced VOC and JSC characteristics leading to a device with a power conversion efficiency of 4.40%.
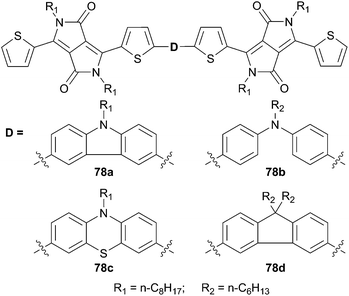
Y. Li et al. have done further investigation by replacing the central core by other rigid and non-rigid nitrogen aromatic π-conjugated structures (Scheme 39).61 Owing their light harvesting properties and appropriate energy levels the new compounds have been tested in a device having the following structure ITO/PEDOT:PSS/78![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC61BM/LiF/Al. Nevertheless among the examined structures only 78a gave satisfactory performance with a PCE of 1.50%, along the I/V characteristics VOC = 0.66 V, JSC = 4.12 mA cm−2 and a FF of 0.44.
PC61BM/LiF/Al. Nevertheless among the examined structures only 78a gave satisfactory performance with a PCE of 1.50%, along the I/V characteristics VOC = 0.66 V, JSC = 4.12 mA cm−2 and a FF of 0.44.
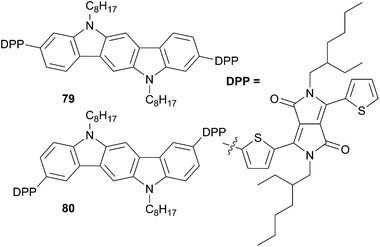
Extension of the carbazole π-conjugated spacer of the compound 78a, based on the two regioisomeric indolo[3,2-b]carbazoles cores (compounds 79 and 80, Scheme 40), has been carried out by Yagai et al.62 Interestingly, depending on the regiochemistry of the backbone linkages (3,9- or 2,8-) two different solid-state behaviors are found (crystalline vs. amorphous). As a result, significant dissimilarities in phase segregation are obtained from 79 and 80 blended films with PC61BM that affect dramatically the photophysical properties. For instance, compound 79 presents a linear structure (crystalline) whereas 80 displays a S-shape structure (amorphous). These differences imply that thermal annealing would decrease or increase respectively the degree of phase separation and would modify their molecular packing. Consequently, although the compounds display the same optical and electrochemical properties their PCE are quite different upon thermal annealing due to a difference on the degree of phase separation. The best results (PCE = 1.80%) were obtained for 79 after thermal annealing at 130 °C (VOC = 0.77 V, JSC = 5.91 mA cm−2 and a FF of 0.40) while 80 presents under the same condition a PCE of 1.40% (VOC = 0.72 V, JSC = 3.92 mA cm−2 and a FF of 0.50).
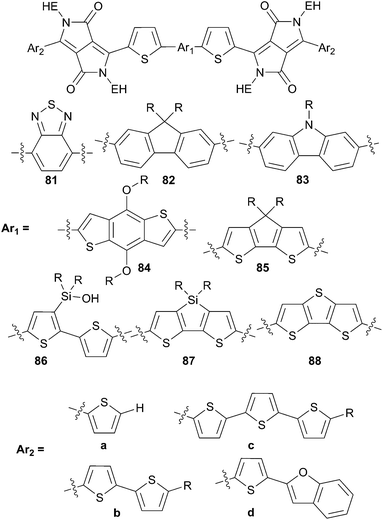
Thereafter, a novel series of bis-DPP derivatives bridged with various π-conjugated spacers were also investigated by Nguyen and co-workers in order to elucidate and understand more deeply the molecular features that can be helpful for the construction of novel effective molecular structures (Scheme 41).63 Additionally, their studies have been correlated with DFT calculations that have been proven to be very effective as an aide prediction tools. A rapid screening of compounds 81–88 has revealed that the structure 86d constitutes one of the most promising structures and was chosen for further optimization. Therefore, the best performances (PCE up to 4.20% with VOC = 0.85 V, JSC = 9.90 mA cm−2 and FF = 0.49) for 86d have been achieved for a blend of 86d![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM (1
PC71BM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w) annealed at 80 °C in presence of chloronaphthlene (0.50%) as solvent additive.
1 w/w) annealed at 80 °C in presence of chloronaphthlene (0.50%) as solvent additive.
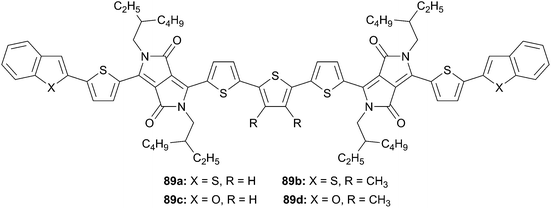
In a similarly work, R. A. J. Janssen et al.64 have studied four novel tetrathiophene derivatives flanked with DKKP end-capped either with benzothiophene or benzofuran moieties (Scheme 42). Interestingly, all structures (89a–d) possess comparable electro-optical properties but display quite different solubility behaviors. During the device fabrication, careful adaptation of the processing conditions (concentration, solvent, temperature, additive) for each compounds in presence of PC71BM as acceptor have given cell performances ranking from 3.6 to 4.6%. This experiments point out that small structural changes will strongly affect the solubility properties and consequently the photovoltaic performances.
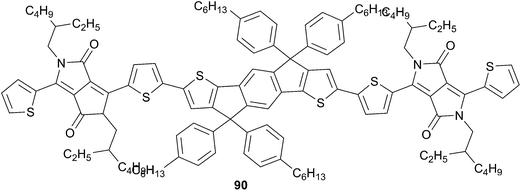
An analogous derivative 90 (Scheme 43) has been recently described by Zhu et al.65 showing good optical properties and optimum energy levels for OPV applications. Solution processed BHJ solar cells have been fabricated from 90 and PC71BM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5, w/w) leading to devices with PCE of 2.82% after thermal annealing. The lowered values obtained from this device compared to the previous, based on tetrathiophene core, might be relied on a decrease of the molecular packing. More interestingly, compound 90 has been successfully used as an acceptor in a BHJ device in a blend film with P3HT (1
1.5, w/w) leading to devices with PCE of 2.82% after thermal annealing. The lowered values obtained from this device compared to the previous, based on tetrathiophene core, might be relied on a decrease of the molecular packing. More interestingly, compound 90 has been successfully used as an acceptor in a BHJ device in a blend film with P3HT (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2, w/w respectively) yielding a PCE of 0.83% with a VOC = 1.17 V after thermal annealing. This evidences an ambipolar character for 90 with the possibilities to use such compounds either as donor or acceptor in the construction of solar cells.
1.2, w/w respectively) yielding a PCE of 0.83% with a VOC = 1.17 V after thermal annealing. This evidences an ambipolar character for 90 with the possibilities to use such compounds either as donor or acceptor in the construction of solar cells.
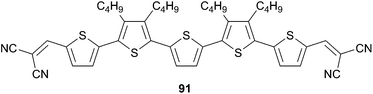
As already mentioned the possibilities to boost the photo-absorption properties as well as the electron and hole-mobilities can be achieved both by chemical design and engineering, but drive to more and more complicated structures. In addition, the aim to use solution-processed technologies requires the use of large solubilizing units that can affect at the meantime the solid-state arrangement. To circumvent such difficulties Bäuerle et al. studied, using vacuum deposition process, some oligothiophenic structures substituted with shorter alkyl-chains, based on the α-quinquethiophene core, and end-capped with dicyanovinyl groups (Scheme 44) in OPV devices.
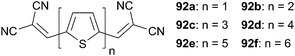
Thus, optimized planar heterojunctions made from compounds 91 and C60 demonstrated PCE of 3.4% with VOC = 0.98 V and JSC = 10.60 mA cm−2.66 Based on those promising results a series of unsubstituted oligothiophene 92 (n = 1–6) end-capped with DCV was further investigated (Scheme 45).67
For instance 92a showed a well-structured absorption band centered at 406 nm. Increasing the number of thiophene units leads to a bathochromic shift of the maximum of absorption from 406 nm (92a) to 532 nm for 92f associated to a hyperchromic effect (92a: ε = 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 L mol−1 cm−1 to 92e: ε = 73
000 L mol−1 cm−1 to 92e: ε = 73![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 L mol−1 cm−1). Devices made by vacuum deposition of 15 nm of a C60 layer and 6 nm of 92d–f, showed a high values of PCE = 1.20%, 2,.60% and 2.80% respectively. Beside, BHJ cells based on 92e and C60 were also prepared leading to PCE as high as 5.2% after device fabrication optimizations.
300 L mol−1 cm−1). Devices made by vacuum deposition of 15 nm of a C60 layer and 6 nm of 92d–f, showed a high values of PCE = 1.20%, 2,.60% and 2.80% respectively. Beside, BHJ cells based on 92e and C60 were also prepared leading to PCE as high as 5.2% after device fabrication optimizations.
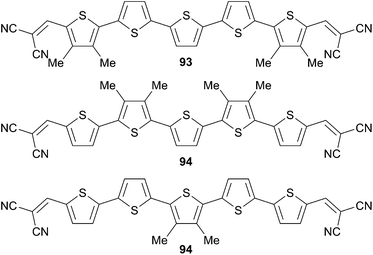
Solid-state packing was also studied on the methyl substituted derivatives 92e along the π-conjugated backbone. Methyl groups were introduced at different positions of the π-conjugated skeleton (Scheme 46). These modified oligomers (93–95) were incorporated by vacuum deposition process in BHJ solar cells exhibiting PCEs ranking from 4.80 to 6.10%.
Subsequent optimization of the deposition process and changes of the fabrication conditions have led to an enhanced PCE up to 6.90%.68 This result suggested the existence of strong specific intermolecular interactions within the organic layer associated to effective π–π overlap and multidirectional electronic coupling evidenced by single-crystal X-ray structure analysis of 94.
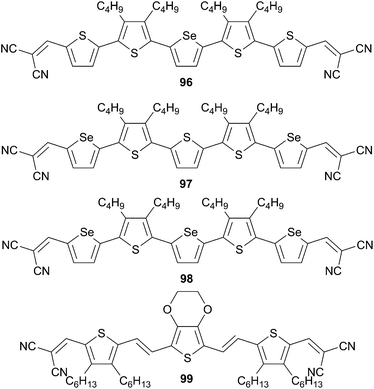
Replacement of thiophene units by analogous selenophene or ethylenedioxythiophene (EDOT) core were also investigated (Scheme 47).69
Nevertheless, despite a broader and more intense absorption properties selenium-containing oligomers (96–98) have shown lower performances compared to 92e with PCEs ranging from 2.50 to 3.10%. The lowest obtained values might be attributed to lowered solid-state packing due to the longer alkyl chains used along the π-conjugated backbone. In addition increasing the number of selenophene decreases also the photovoltaic characteristics. Later on Palomares et al. have changed the thiophene or selenium containing oligomers by a more electron rich unit such as EDOT (99). A BHJ device structure based on 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC70BM gave rise to an enhancement of the PCE up to 3.75% associated to good annealing properties and high open-circuit voltage and fill-factor (VOC = 1.01 V, JSC = 6.00 mA cm−2 and FF = 0.63).69b
PC70BM gave rise to an enhancement of the PCE up to 3.75% associated to good annealing properties and high open-circuit voltage and fill-factor (VOC = 1.01 V, JSC = 6.00 mA cm−2 and FF = 0.63).69b
Benzothiadiazole derivatives have been also examined as terminal acceptors in oligothiophene. Thus compounds 100 and 101 have been synthetized (Scheme 48) using a terthiophene as electron relay between the acceptors.
Combination of the dyes 100 or 101 with C60 have been tested both in PHJ and BHJ solar cells.70 The obtained devices showed high VOC values (VOC = 0.98–1.05 V), and the performances of a PHJ device based on 101 and C60 bilayer have given rise to a PCE up to 3.15%.
Combination of benzothiadiazole with 2,7-carbazole end-capped with oligothiophene have been explored par Li et al.71 in order to readjust HOMO levels compared to molecules 100 or 101 and optimized the band gap (Scheme 49). Thus, solution processed organic solar cell based on a blend 102a/PC71BM exhibit a PCE of 2.26% associated with a high open-circuit voltage close to that of 101 (VOC = 0.98–1.05 V). Expanding the terminal π-conjugated system by an oligothiophene moiety (102b and 102c) leads to a slight decrease of the VOC (VOC = 0.92 and 0.86 V respectively) on devices having the same configuration accompanied with higher PCE values up to 3.44% and 3.90% respectively without any post-treatment due to an enhancement of the JSC and FF.
Different types of s-tetrazine derivatives have been investigated and tested as end-groups in an oligothiophene series synthetized by Tu et al.72 Thus, active solar cells obtained from the series of the compound 103 (Scheme 50) show, by AFM and TEM experiments, that on blended films of 103![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC61BM (1
PC61BM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w ratio) well-ordered packing structure of 103 in the crystalline state leads to higher photovoltaic performances. In addition, replacement of PC61BM by PC71BM in presence of 1% of DIO as additive affords a device with a power conversion efficiency reaching 3.24% (JSC = 7.87 mA cm−2).
1 w/w ratio) well-ordered packing structure of 103 in the crystalline state leads to higher photovoltaic performances. In addition, replacement of PC61BM by PC71BM in presence of 1% of DIO as additive affords a device with a power conversion efficiency reaching 3.24% (JSC = 7.87 mA cm−2).
The strategy has been applied toward the synthesis of extended A–D–A–D–A chromophores incorporating two different acceptors at central and terminal positions along the π-conjugated backbone namely the benzothiadiazole and trifluoroacetyl (Scheme 51). Using vacuum deposition technique PHJ solar cells were prepared from the compounds 104–106. The presence of these acceptors lower the HOMO level, providing an unusually high VOC (VOC = 1.17 V (105) and 1.10 V (106)) associated to moderate PCE of 1.56% and 1.45% respectively.73
Similar studies have been conducted by Y. Chen et al.74 on the solution processable chromophore 107 incorporated in BHJ solar cells (Scheme 52) through a spin coating process. An optimized donor/acceptor weight ratio was found to be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.8 for 107
0.8 for 107![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC61BM affording a PCE of 2.43% without any post-treatment (VOC = 0.76 V, JSC = 5.20 mA cm−2 and FF = 0.61) and using LiF/Al as cathode. Thermal annealing at 150 °C for 10 min improved the PCE up to 3.07% (VOC = 0.78 V, JSC = 7.10 mA cm−2 and FF = 0.55), suggesting that the enhancement can be attributed to a better molecular packing at the solid state associated with an improvement of the absorption intensity and hence the shunt current circuit density from 5.20 to 7.10 mA cm−2
PC61BM affording a PCE of 2.43% without any post-treatment (VOC = 0.76 V, JSC = 5.20 mA cm−2 and FF = 0.61) and using LiF/Al as cathode. Thermal annealing at 150 °C for 10 min improved the PCE up to 3.07% (VOC = 0.78 V, JSC = 7.10 mA cm−2 and FF = 0.55), suggesting that the enhancement can be attributed to a better molecular packing at the solid state associated with an improvement of the absorption intensity and hence the shunt current circuit density from 5.20 to 7.10 mA cm−2
Thienothiadiazole and thienopyrazine have been also examined as central acceptors in oligothiophene series end-capped with DCV or trifluoroaceyl groups (Scheme 53). Insertion of such central acceptors leads to a red shift of the maximum of absorption compared to their analogous parent compounds with benzothiadiazole (707 nm, 675 nm, 620 nm for 108, 109 and 110 respectively). Nevertheless, the different attempts to optimize the solar cells during the process of fabrication have not permitted to enhance efficiently the photovoltaic characteristics. Indeed, only moderate PCE values were attained in the range of 0.8–1.3% ascribed to the lack of transport of the photo-generated electrons and holes (JSC are not exceeding 3.20 mA cm−2), the low molar absorptivity and the low-lying LUMO energy levels of the donor.75
With the aim to enhance the electron withdrawing efficiency properties of DCV and to reduce the optical band gap, a nitrobenzene ring can be used in place of one of the cyano groups. Mikroyannidis et al. have successfully applied this strategy during the synthesis of the phenylenevinylene-based chromophore 111 (Scheme 54).76 Replacement of one of the cyano groups leads to a reduction of the optical band gap, with a maximum of absorption around 640 nm, compared to the unsubstituted DCV compound. BHJ solar cells device were fabricated using 111![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PCBM as active material sandwiched between ITO/Al electrodes. The low PCE, 1.36%, is mainly attributed to the low JSC (VOC = 0.73 V, JSC = 0.056 mA cm−2 and FF = 0.31) due to the small exciton diffusion lengths as well as low mobility of hole. Thermal annealing of the blend affords an improvement of the PCE up to 2.33% induced by an increase of the packing density facilitating both the exciton dissociation and charge transportation.
PCBM as active material sandwiched between ITO/Al electrodes. The low PCE, 1.36%, is mainly attributed to the low JSC (VOC = 0.73 V, JSC = 0.056 mA cm−2 and FF = 0.31) due to the small exciton diffusion lengths as well as low mobility of hole. Thermal annealing of the blend affords an improvement of the PCE up to 2.33% induced by an increase of the packing density facilitating both the exciton dissociation and charge transportation.
In addition, changing the PC61BM acceptor by perylene diimide PDI in the previous device leads to a remarkable PCE of 1.87%. Further improvement, notably by insertion of ZnO in between the active layer and the Al-cathode followed by a thermal annealing (5 min., 100 °C) gives rise to an exceptional PCE of 3.17%.77
With the aim to enhance the light-harvesting abilities and optimize the optical bandgap compared to A–D–A counterparts, Wong et al. have envisioned the combination of two strong electron-withdrawing groups leading to the A–A–D–A–A structures.78 Hence, in the following examples 112 and 113 (Scheme 55) the dithienosilole (DTS) core has been selected as p-type building block in respect with its better packing abilities and higher hole-mobilities compared to their carbon-based homologues.
Elaboration of planar mixed heterojunction devices based on 112 and fullerene acceptors (C60 or C70) leads to decent power conversion efficiencies from 2.3–3.7% respectively (VOC = 1.05 V, JSC = 5.80 mA cm−2 and FF = 0.38, VOC = 1.01 V, JSC = 9.79 mA cm−2 and FF = 0.38) after tuning the donor–acceptor ratio (from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 1
1 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5) without any post-treatments such as thermal of solvent annealing processes.
1.5) without any post-treatments such as thermal of solvent annealing processes.
Further investigations have been done with the DTS core incorporating solubilizing chains (Scheme 56). The roles of such chains are to improve the solubility of chromophores in organic solvents for solution casting processes as well as their packing structure and their solid-state miscibility with fullerenes. A careful attention on the molecular design, chain position, and electron density has to be paid to ensure the best properties. In this context, Wang et al. described in a recent work the impact of such molecular modification on the photoconversion properties.79
The best results were obtained for 116 and PC61BM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.8 ratio) with a PCE up to 3.81% (VOC = 0.92 V, JSC = 8.73 mA cm−2 and FF = 0.49). AFM microphotographs of spin-coated films from chloroform solutions show better interpenetrating network for 116 compared to 114 and 115 associated to higher roughness and better optical absorption properties. These results highlight again the importance to control the size, nature, length and position of the alkyl chains that strongly impact the optical absorption, energy levels, packing and film morphology.
0.8 ratio) with a PCE up to 3.81% (VOC = 0.92 V, JSC = 8.73 mA cm−2 and FF = 0.49). AFM microphotographs of spin-coated films from chloroform solutions show better interpenetrating network for 116 compared to 114 and 115 associated to higher roughness and better optical absorption properties. These results highlight again the importance to control the size, nature, length and position of the alkyl chains that strongly impact the optical absorption, energy levels, packing and film morphology.
To gain further insights, Kim et al. have introduced on the terminal alkyl substituents an amide function (compounds 118) that would favor intermolecular interactions via a hydrogen bonding network80 compared to 117 structures (Scheme 57). This hydrogen bonding will affect dramatically the interfacial interactions, optical properties, the film morphology and therefore the photovoltaic properties. Thus, it has been demonstrated in this work that the introduction of intermolecular interaction induces well-ordered molecular packing that is not suited for the optimization of the photovoltaic performance. Indeed, the best result was obtained for 117b with a PCE exceeding 4.30% (VOC = 0.82 V, JSC = 9.79 mA cm−2 and FF = 0.54) while its counterpart 118b leads only a PCE of 3.22% under the same conditions (VOC = 0.87 V, JSC = 7.94 mA cm−2 and FF = 0.47).
An analogous strategy was adopted by Lam and co-workers but using a terminal acid group (CO2H) (Scheme 58).81 Interestingly, in this case an improvement of the photovoltaic efficiency was observed for the derivative 119b compared to its ester analogue (119a). The enhanced PCE value was mainly attributed to a better hydrogen-bonding network that induces strong π–π interactions and highly ordered films with a vertical alignment. This behavior leads to higher hole-mobilities in favor of 119b as measured by the SCLC method (μh = 2.83 10−5 cm2 V−1 s−1 (119b) μh = 6.91 10−6 cm2 V−1 s−1 (119a)) that directly impact on the photovoltaic conversion efficiency (0.91% (VOC = 0.71 V, JSC = 2.86 mA cm−2, FF = 0.45) and 0.06% (VOC = 0.53 V, JSC = 0.45 mA cm−2, FF = 0.26) respectively). In addition, it is noted that the formation of nanofibers as-cast films prevents the use of post-treatments or solvent additives.
D–A–D type
Besides the A–D–A structures, several groups have developed in parallel D–A–D architectures with the same aim. For instance, one can cite the work of Li86 who has widely used the benzo[1,2,5]thiadiazole core as central acceptor part flanked with TPA donors and connected together by thiophenic linkers (Scheme 59).Preliminary studies based on the derivative 120 have been performed with different device architectures made from pristine 120, a PHJ structure (120 and C60) and a BHJ from a blend of 120 and PC61BM. In a typical device, the active layer was sandwiched between a ITO/PEDOT:PSS as anode and LiF/Al as cathode. From the obtained results it has been demonstrated herein that the configuration BHJ shows the highest photovoltaic performances with a PCE 0.19%. Further optimization of cathode material by using Ba/Al instead of LiF/Al gave rise to an enhancement of the PCE up to 0.26%.82
In order to increase the photovoltaic performances of such derivatives, multi-arms architectures were built up as shown in Scheme 60 and compared with their linear D–A–D counterparts.83
All structures are well soluble in dichlorobenzene, which makes them suitable to elaborate organic solar cells by spin-coating processes. Thus, in devices with structure of ITO/PEDOT:PSS/active layer/LiF/Al and optimized conditions (thickness (70–90 nm), donor–acceptor (PC71BM) ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (w/w)) the highest PCE was obtained for the four-arms derivatives 122c (1.80%, VOC = 0.92 V, JSC = 4.90 mA cm−2 and FF = 0.41). Nevertheless for all compounds tested the PCE values are comprised between 1.10 to 1.80% demonstrating that other parameters have to be controlled and taken into account to optimize the devices.
3 (w/w)) the highest PCE was obtained for the four-arms derivatives 122c (1.80%, VOC = 0.92 V, JSC = 4.90 mA cm−2 and FF = 0.41). Nevertheless for all compounds tested the PCE values are comprised between 1.10 to 1.80% demonstrating that other parameters have to be controlled and taken into account to optimize the devices.
Further investigations were done on compounds 123 and 124 (Scheme 61). To ensure sufficient solubility alkyl chains have been introduced along the π-conjugated backbone (123) or at the terminal position (124). The derivative 123 is crystalline while its counterpart 124 is amorphous, this difference will certainly affect the performances of the devices. As expected, 124 exhibits a bathochromic shift (λmax = 456 nm and λmax = 353 respectively) and a higher HOMO level compared to 123 due to its extended π-conjugated system. Organic solar cells were fabricated and the device based on 123![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC61BM (1
PC61BM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 ratio (w/w)) exhibits higher VOC and PCE than that for 124
3 ratio (w/w)) exhibits higher VOC and PCE than that for 124![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC61BM (0.56% (VOC = 0.80, JSC = 1.80 mA cm−2, FF = 0.39) and 0.42% (VOC = 0.62 V, JSC = 1.89 mA cm−2, FF = 0.36) respectively) attributed to the lower HOMO energy level of 123. Changing the nature of the acceptor (123
PC61BM (0.56% (VOC = 0.80, JSC = 1.80 mA cm−2, FF = 0.39) and 0.42% (VOC = 0.62 V, JSC = 1.89 mA cm−2, FF = 0.36) respectively) attributed to the lower HOMO energy level of 123. Changing the nature of the acceptor (123![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM) yielded to a higher PCE up to 1.23% (VOC = 0.86, JSC = 3.50 mA cm−2, FF = 0.41), which is not improved by thermal annealing.84
PC71BM) yielded to a higher PCE up to 1.23% (VOC = 0.86, JSC = 3.50 mA cm−2, FF = 0.41), which is not improved by thermal annealing.84
In the following example Zhen et al. have investigated the star-shaped bis-(tri-substituted)TPA 12585 end-capped at two of its extremities by an hexyl-thiophene and connected through a benzothiadiazole core (Scheme 62). Organic solar cells based on 125![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM (donor–acceptor ratio 1
PC71BM (donor–acceptor ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) yielded, without any solvent additives or thermal post-treatments, a PCE of 3.18% (VOC = 0.96 V, JSC = 6.83 mA cm−2, FF = 0.48). This example demonstrates that the connection pathway of the different building blocks along the π-conjugated system is of crucial of importance to control the photoconversion efficiencies.
3) yielded, without any solvent additives or thermal post-treatments, a PCE of 3.18% (VOC = 0.96 V, JSC = 6.83 mA cm−2, FF = 0.48). This example demonstrates that the connection pathway of the different building blocks along the π-conjugated system is of crucial of importance to control the photoconversion efficiencies.
The nature of the π-conjugated linker plays also an essential role in the achieved properties. In this context, Li and coll. have structurally modified the archetype chromophore 120 in order to improve its efficiency in BHJ solar cells (Scheme 63).86 Thus the insertion of hexyl side chains (126) helps in getting better blend morphology. As a consequence a PCE up to 1.44% was attained. (126![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM (1
PC71BM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 ratio) VOC = 0.79 V, JSC = 4.84 mA cm−2, FF = 0.37). Extending the π-conjugated system with thieno[3,2-b]thiophene (127) instead of thiophene allows significantly to broaden the absorption spectra. However, even if an increase of the shunt current circuit density is noticed (from JSC = 4.84 mA cm−2 to JSC = 5.71 mA cm−2) the PCE for 126 still the same (1.44%) as the VOC has decreased (from VOC = 0.79 V to VOC = 0.74 V) due to a higher HOMO energy level. The introduction of bulky alkyl chains at the periphery (128) reduced significantly the PCE from 1.44% to 0.75% related to poorer miscibility with PC71BM as evidenced by the AFM microphotographs and the lower hole-mobilities (from μh = 1.48 10−4 cm2 V−1 s−1 (127) to μh = 2.78 10−5 cm2 V−1 s−1 (128)) determined by the SCLC (space-charge-limited-current) method.
3 ratio) VOC = 0.79 V, JSC = 4.84 mA cm−2, FF = 0.37). Extending the π-conjugated system with thieno[3,2-b]thiophene (127) instead of thiophene allows significantly to broaden the absorption spectra. However, even if an increase of the shunt current circuit density is noticed (from JSC = 4.84 mA cm−2 to JSC = 5.71 mA cm−2) the PCE for 126 still the same (1.44%) as the VOC has decreased (from VOC = 0.79 V to VOC = 0.74 V) due to a higher HOMO energy level. The introduction of bulky alkyl chains at the periphery (128) reduced significantly the PCE from 1.44% to 0.75% related to poorer miscibility with PC71BM as evidenced by the AFM microphotographs and the lower hole-mobilities (from μh = 1.48 10−4 cm2 V−1 s−1 (127) to μh = 2.78 10−5 cm2 V−1 s−1 (128)) determined by the SCLC (space-charge-limited-current) method.
The results demonstrate that broadening the absorption spectra with remaining the lower HOMO energy level have to be considered in the synthesis and design of high performance organic chromophores.
Pursuing their efforts in the understanding and in the design/synthesis of efficient chromophores for photovoltaic applications, Li et al. have replaced the benzothiadiazole core by a (dicyanomethylene)-pyran unit in chromophores 129 to 131 (Scheme 64). Organic solar cell based on 128![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM (1
PC71BM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 ratio w/w) exhibits a PCE of 1.40% (VOC = 0.71 V, JSC = 5.07 mA cm−2, FF = 0.38) which corresponds to almost twice the value obtained from that based on 129
3 ratio w/w) exhibits a PCE of 1.40% (VOC = 0.71 V, JSC = 5.07 mA cm−2, FF = 0.38) which corresponds to almost twice the value obtained from that based on 129![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC61BM (PCE = 0.79%) due to the stronger absorption properties of PC71BM in the wavelength range 350–550 nm. Modifying the linker from benzene (129) to thiophene (130) alters the photophysical properties. For instance a red shift of the maximum of absorption was observed as well as a lower bandgap. Therefore, under the same conditions an improvement up to 2.06% of the photovoltaic efficiency was attained with 129
PC61BM (PCE = 0.79%) due to the stronger absorption properties of PC71BM in the wavelength range 350–550 nm. Modifying the linker from benzene (129) to thiophene (130) alters the photophysical properties. For instance a red shift of the maximum of absorption was observed as well as a lower bandgap. Therefore, under the same conditions an improvement up to 2.06% of the photovoltaic efficiency was attained with 129![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM (VOC = 0.79 V, JSC = 5.94 mA cm−2, FF = 0.44). Introduction of solubilizing chains along the π-conjugated system (131) leads to a better miscibility with PC71BM, without altering the optical properties, associated to an enhancement of the PCE to 2.10% (VOC = 0.78 V, JSC = 6.74 mA cm−2, FF = 0.39).87
PC71BM (VOC = 0.79 V, JSC = 5.94 mA cm−2, FF = 0.44). Introduction of solubilizing chains along the π-conjugated system (131) leads to a better miscibility with PC71BM, without altering the optical properties, associated to an enhancement of the PCE to 2.10% (VOC = 0.78 V, JSC = 6.74 mA cm−2, FF = 0.39).87
Further work has been done by Zhan and coll. with other central acceptors such as thieno[3,4-c]pyrrole-4,6-dione (132a–c)88 and bisthiazole (133a–c)89 that are widely used to synthetize low-bandgap polymers due to their strong electron withdrawing abilities (Scheme 65).
Photovoltaic cells were fabricated with a structure of ITO/PEDOT:PSS/132a–c![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM/Al. Interestingly the use of thieno[3,4-c]pyrrole-4,6-dione in 132 as acceptor appears to be a promising candidate as its photovoltaic efficiency was higher than its counterparts 121a or 123. Indeed, under identical conditions 121a and 123 have a PCE of 1.30% (VOC = 0.71 V, JSC = 4.80 mA cm−2, FF = 0.38) and 1.70% (VOC = 0.87 V, JSC = 4.80 mA cm−2, FF = 0.40) respectively while 132 exhibits values of PCE ranking from 1.91% to 2.41% (VOC = 0.85 V, JSC = 5.52 mA cm−2, FF = 0.41 and VOC = 0.86 V, JSC = 6.51 mA cm−2, FF = 0.43). By changing the donor–acceptor ratio from 1
PC71BM/Al. Interestingly the use of thieno[3,4-c]pyrrole-4,6-dione in 132 as acceptor appears to be a promising candidate as its photovoltaic efficiency was higher than its counterparts 121a or 123. Indeed, under identical conditions 121a and 123 have a PCE of 1.30% (VOC = 0.71 V, JSC = 4.80 mA cm−2, FF = 0.38) and 1.70% (VOC = 0.87 V, JSC = 4.80 mA cm−2, FF = 0.40) respectively while 132 exhibits values of PCE ranking from 1.91% to 2.41% (VOC = 0.85 V, JSC = 5.52 mA cm−2, FF = 0.41 and VOC = 0.86 V, JSC = 6.51 mA cm−2, FF = 0.43). By changing the donor–acceptor ratio from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (w/w) to 1
3 (w/w) to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 (w/w) enhanced the PCE up to 2.87% (VOC = 0.94 V, JSC = 6.94 mA cm−2, FF = 0.44) and 2.90% (VOC = 0.88 V, JSC = 7.73 mA cm−2, FF = 0.43) respectively. Finally, thermal annealing of the blend 132b
4 (w/w) enhanced the PCE up to 2.87% (VOC = 0.94 V, JSC = 6.94 mA cm−2, FF = 0.44) and 2.90% (VOC = 0.88 V, JSC = 7.73 mA cm−2, FF = 0.43) respectively. Finally, thermal annealing of the blend 132b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM (1
PC71BM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 w/w) led to a PCE of 3.31% (VOC = 0.91 V, JSC = 7.70 mA cm−2, FF = 0.47). Substitution of thieno[3,4-c]pyrrole-4,6-dione by bisthiazole leads to the chromophores 133a–c. Increasing the π-conjugated system from 133a to 133c provides better intermolecular interactions in the solid-sate and generates strong tendency to absorb the low energy photons as evidenced by the UV-Vis spectroscopy. The chromophores show typical p-type semi-conductor behavior in air with hole-mobilities as high as μh = 3.60 10−4 cm2 V−1 s−1 (133c). BHJ solar cells based on 133a–c
4 w/w) led to a PCE of 3.31% (VOC = 0.91 V, JSC = 7.70 mA cm−2, FF = 0.47). Substitution of thieno[3,4-c]pyrrole-4,6-dione by bisthiazole leads to the chromophores 133a–c. Increasing the π-conjugated system from 133a to 133c provides better intermolecular interactions in the solid-sate and generates strong tendency to absorb the low energy photons as evidenced by the UV-Vis spectroscopy. The chromophores show typical p-type semi-conductor behavior in air with hole-mobilities as high as μh = 3.60 10−4 cm2 V−1 s−1 (133c). BHJ solar cells based on 133a–c![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM (1
PC71BM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4; w/w) blend films showed increasing PCE (up to 2.61%) due to broader and stronger absorption as well as higher hole-mobility as expanding the π-conjugated system (spin-cast solutions: 133a: VOC = 0.71 V, JSC = 3.42 mA cm−2, FF = 0.30, 133b: VOC = 0.81 V, JSC = 4.69 mA cm−2, FF = 0.33 and 133c: VOC = 0.84 V, JSC = 7.72 mA cm−2, FF = 0.40).
4; w/w) blend films showed increasing PCE (up to 2.61%) due to broader and stronger absorption as well as higher hole-mobility as expanding the π-conjugated system (spin-cast solutions: 133a: VOC = 0.71 V, JSC = 3.42 mA cm−2, FF = 0.30, 133b: VOC = 0.81 V, JSC = 4.69 mA cm−2, FF = 0.33 and 133c: VOC = 0.84 V, JSC = 7.72 mA cm−2, FF = 0.40).
A narrow band gap chromophore 134 based on triphenylamine and isoindigo was further investigated by Yang and coll. (Scheme 66).90a due its efficiency previously demonstrated in DSSCs devices.90b AFM microphotographs evidenced a good miscibility between 134 and PC61BM with interpenetrating structures, which would facilitate charge separation in the solid state, associated to a broad absorption spectrum from 300 to 750 nm. BHJ OSCs devices with a layered structure of ITO/PEDOT:PSS/134![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC61BM (1
PC61BM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio w/w)/(80 nm)/Ca (10 nm)/Al (100 nm) were fabricated and led to a PCE of 0.61% (VOC = 1.00 V, JSC = 2.35 mA cm−2, FF = 0.26). VOC is among the highest values in all-small molecules or polymers highlighting the importance of such acceptor in future development of SMOSCs due to the high VOC attained.
1 ratio w/w)/(80 nm)/Ca (10 nm)/Al (100 nm) were fabricated and led to a PCE of 0.61% (VOC = 1.00 V, JSC = 2.35 mA cm−2, FF = 0.26). VOC is among the highest values in all-small molecules or polymers highlighting the importance of such acceptor in future development of SMOSCs due to the high VOC attained.
α-Heptathiophene series of D–A–D-type oligomers 135, 136, and 137 based on TPD (thieno[3,4-c]-pyrrole-4,6-dione), TPN (thienoperylene naphthalene) and TIN (thienoimidazole naphthalene) as central acceptor core respectively have been developed by H.-Y. Wang and coll. (Scheme 67).91 Modification of the diimide unit of 135 by perylene or imidiazole analogues in 136 and 137 has the benefits to enhance the coplanarity and to tune the energy levels due to a better intermolecular stacking in the solid-state. The photovoltaic performances were determined by fabricating BHJ devices (ITO/PEDOT:PSS/135–137![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM/LiF/Al) with a donor–acceptor ratio (2
PC71BM/LiF/Al) with a donor–acceptor ratio (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, w/w). The device 135
1, w/w). The device 135![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM blends showed the best PCE of 1.87% (VOC = 0.87 V, JSC = 4.93 mA cm−2, FF = 0.44) while 136
PC71BM blends showed the best PCE of 1.87% (VOC = 0.87 V, JSC = 4.93 mA cm−2, FF = 0.44) while 136![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM and 137
PC71BM and 137![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM blends in the same conditions led only to PCE of 0.77% and 0.11% respectively (VOC = 0.76 V, JSC = 2.22 mA cm−2, FF = 0.45, VOC = 0.75 V, JSC = 0.58 mA cm−2, FF = 0.25). However, for 136 and 137 efficiencies can be improved in blends device structures with a weight ratio of 1
PC71BM blends in the same conditions led only to PCE of 0.77% and 0.11% respectively (VOC = 0.76 V, JSC = 2.22 mA cm−2, FF = 0.45, VOC = 0.75 V, JSC = 0.58 mA cm−2, FF = 0.25). However, for 136 and 137 efficiencies can be improved in blends device structures with a weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (w/w) respectively to 1.24% and 0.34% (VOC = 0.77 V, JSC = 2.75 mA cm−2, FF = 0.59, VOC = 0.69 V, JSC = 1.91 mA cm−2, FF = 0.26). The lower PCE are mainly attributed to the different morphology of the blend films and the presence of high crystalline domains.
1 (w/w) respectively to 1.24% and 0.34% (VOC = 0.77 V, JSC = 2.75 mA cm−2, FF = 0.59, VOC = 0.69 V, JSC = 1.91 mA cm−2, FF = 0.26). The lower PCE are mainly attributed to the different morphology of the blend films and the presence of high crystalline domains.
By comparison of the work done by Zhan (Scheme 65), Suh et al. have developed a new series 138a and 138b based on the bithiophene imide (BTI) as an electron deficient unit (Scheme 68).92 The BTI is unit of choice due to its strong electron-withdrawing character, planar architecture, good solubility, and close π–π stacking in the solid state. In addition, BTI presents the advantage to decrease the steric hindrance between the alkyl chains of the electron rich unit and the imide group and changes the shape of the oligomers (as banana-like structure) compared to the 132 analogues. J–V characteristics obtained from devices with the geometry ITO/PEDOT:PSS (40 nm)/138![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM (80 nm)/Al (100 nm) led to the best performances in favour of 138a with a PCE of 1.36% (1
PC71BM (80 nm)/Al (100 nm) led to the best performances in favour of 138a with a PCE of 1.36% (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 ratio, VOC = 0.91 V, JSC = 3.93 mA cm−2, FF = 0.38) after a thermal annealing a 130 °C. Extension of the π-conjugated system for 138b did not induced any improvement of the photovoltaic characteristics, as the best PCE attained is only of 1.08% (1
3 ratio, VOC = 0.91 V, JSC = 3.93 mA cm−2, FF = 0.38) after a thermal annealing a 130 °C. Extension of the π-conjugated system for 138b did not induced any improvement of the photovoltaic characteristics, as the best PCE attained is only of 1.08% (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 ratio, thermal annealing at 120 °C, VOC = 0.80 V, JSC = 3.79 mA cm−2, FF = 0.36) even after addition of solvent additives such as 1,8-octanedithiol (ODT) or 1,8-diiodooctane (DIO).
4 ratio, thermal annealing at 120 °C, VOC = 0.80 V, JSC = 3.79 mA cm−2, FF = 0.36) even after addition of solvent additives such as 1,8-octanedithiol (ODT) or 1,8-diiodooctane (DIO).
Cao et al. have investigated the influence of the extension of the π-conjugated system of the chromophores 121, using fused fluorenyl TPA-based units, on the photovoltaic properties (Scheme 69).93,94 BHJ solar cells were fabricated with the device configuration ITO/PEDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS/139
PSS/139![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC61BM or 140
PC61BM or 140![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC61BM/Ba/Al. Several weight ratios were studied to improve the efficiencies (1
PC61BM/Ba/Al. Several weight ratios were studied to improve the efficiencies (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 1
2 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4). As demonstrated improvement of the device characteristics were obtained by increasing the PC61BM content in the blend films. In both examples the best PCE achieved were 0.75% and 0.22% (1
4). As demonstrated improvement of the device characteristics were obtained by increasing the PC61BM content in the blend films. In both examples the best PCE achieved were 0.75% and 0.22% (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 ratio, VOC = 0.75 V, JSC = 1.95 mA cm−2, FF = 0.34, 1
4 ratio, VOC = 0.75 V, JSC = 1.95 mA cm−2, FF = 0.34, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 ratio, VOC = 0.70 V, JSC = 1.59 mA cm−2, FF = 0.22) respectively. Despite an extended photosensitivity response to near-infrared region for those chromophores and good film forming properties, the PCE was still low. The low PCE was incriminated to a less efficient charge separation due to LUMO levels close to the LUMO level of PC61BM.
4 ratio, VOC = 0.70 V, JSC = 1.59 mA cm−2, FF = 0.22) respectively. Despite an extended photosensitivity response to near-infrared region for those chromophores and good film forming properties, the PCE was still low. The low PCE was incriminated to a less efficient charge separation due to LUMO levels close to the LUMO level of PC61BM.
Similarly to 139, Hyun et al. have evaluated the photovoltaic properties of thiadiazoloquinoxaline-based units95 by varying the nature of the π-conjugated backbone as well as the substituents on the quinoxaline moiety (for instance isobutyl and thiophene) (Scheme 70). The nature of the substituents on the quinoxaline part has a dramatic influence on the final optical properties; from isobutyl to thiophene the absorption onset was shifted from 900 to 1100 nm in the solid state. Device structures with the configuration ITO/PEDOT:PSS/141 and 142![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM (1
PC71BM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 w/w)/TiOx/Al have been fabricated and the performances evaluated under the AM1.5 G conditions. 141
3 w/w)/TiOx/Al have been fabricated and the performances evaluated under the AM1.5 G conditions. 141![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM blend films showed better PCE (0.24%, VOC = 0.42 V, JSC = 2.42 mA cm−2, FF = 0.24) than 142
PC71BM blend films showed better PCE (0.24%, VOC = 0.42 V, JSC = 2.42 mA cm−2, FF = 0.24) than 142![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM (0.14%, VOC = 0.38 V, JSC = 1.40 mA cm−2, FF = 0.36) even if 142 possesses a broader absorption up to 1100 nm. The lowest PCE for 142 was attributed to a reduced charge separation and a relatively rougher surface. Compared to its counterpart 139 the lower PCE attained was also due to the weakness of the donor used.
PC71BM (0.14%, VOC = 0.38 V, JSC = 1.40 mA cm−2, FF = 0.36) even if 142 possesses a broader absorption up to 1100 nm. The lowest PCE for 142 was attributed to a reduced charge separation and a relatively rougher surface. Compared to its counterpart 139 the lower PCE attained was also due to the weakness of the donor used.
Cyano substituents have been introduced along the π-conjugated systems in order to take advantage of additional intramolecular charge transfer to reduce the bandgap and to extend the absorption spectrum of the chromophores (Scheme 71). Hence, Li et al. have conducted preliminary investigations on compounds 143 bearing two different acceptors namely BX (benzoxadiazole 143a) and BT (benzothiadiazole 143b).96 BX was used to lower the HOMO level of molecular donors, compared to BT, and thus to increase the open-circuit voltage. Devices fabricated with the configuration ITO/PEDOT:PSS/143![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC61BM/Al showed PCE of 0.33% for 143a (VOC = 0.58 V, JSC = 2.39 mA cm−2, FF = 0.24) while 143b exhibited a PCE of 0.50% (VOC = 0.60 V, JSC = 2.86 mA cm−2, FF = 0.29). Interestingly, the introduction of alkoxy side chains on the BT core has a dramatic effect on the photovoltaic properties (144 and 145). The photovoltaic performances were evaluated on devices with the structure ITO/PEDOT:PSS/144 and 145
PC61BM/Al showed PCE of 0.33% for 143a (VOC = 0.58 V, JSC = 2.39 mA cm−2, FF = 0.24) while 143b exhibited a PCE of 0.50% (VOC = 0.60 V, JSC = 2.86 mA cm−2, FF = 0.29). Interestingly, the introduction of alkoxy side chains on the BT core has a dramatic effect on the photovoltaic properties (144 and 145). The photovoltaic performances were evaluated on devices with the structure ITO/PEDOT:PSS/144 and 145![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC61BM (1
PC61BM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio, w/w)/Al. The BHJ fabricated with 145
2 ratio, w/w)/Al. The BHJ fabricated with 145![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC61BM as a blend film provided an impressive PCE of 3.85% (VOC = 1.04 V, JSC = 11.20 mA cm−2, FF = 0.33) while 144
PC61BM as a blend film provided an impressive PCE of 3.85% (VOC = 1.04 V, JSC = 11.20 mA cm−2, FF = 0.33) while 144![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC61BM did not exceed 2% (VOC = 0.94 V, JSC = 6.33 mA cm−2, FF = 0.33).97 The highest PCE was consistent with a deeper low-lying HOMO level induced by the cyano groups and better film morphology compared to 143b.
PC61BM did not exceed 2% (VOC = 0.94 V, JSC = 6.33 mA cm−2, FF = 0.33).97 The highest PCE was consistent with a deeper low-lying HOMO level induced by the cyano groups and better film morphology compared to 143b.
To get further insights on molecular design of efficient chromophores Ko et al. have studied the unsymmetrical D–A–D 146 chromophores based on a benzo[1,2,5]thiadiazole central core (Scheme 72).98
Their photovoltaic performances were assessed with the devices configuration ITO/PEDOT:PSS (35 nm)/146![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM (55–65 nm) (1
PC71BM (55–65 nm) (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 ratio, w/w)/Al (100 nm). Importantly, it was observed that fluorination increased the short-circuit current JSC and the VOC induced by improved hole-mobility and reduced HOMO levels, respectively. The best performance was obtained for 146c affording a PCE up to 3.24% (VOC = 0.84 V, JSC = 9.45 mA cm−2, FF = 0.41) while 146b and 146a afforded 3.05% (VOC = 0.81 V, JSC = 9.37 mA cm−2, FF = 0.40) and 2.70% (VOC = 0.80 V, JSC = 9.29 mA cm−2, FF = 0.38) respectively. In addition, insertion of a thin layer of TiOx as an optical spacer enhanced the photovoltaic properties by reducing the interfacial resistance between the active layer and the Al metal electrode. Performances were enhanced in all cases reaching a PCE of 4.24% for 146c (VOC = 0.88 V, JSC = 10.76 mA cm−2, FF = 0.44), 3.73% for 146b (VOC = 0.85 V, JSC = 10.55 mA cm−2, FF = 0.44) and 3.54% for 146a (VOC = 0.82 V, JSC = 10.52 mA cm−2, FF = 0.42).
5 ratio, w/w)/Al (100 nm). Importantly, it was observed that fluorination increased the short-circuit current JSC and the VOC induced by improved hole-mobility and reduced HOMO levels, respectively. The best performance was obtained for 146c affording a PCE up to 3.24% (VOC = 0.84 V, JSC = 9.45 mA cm−2, FF = 0.41) while 146b and 146a afforded 3.05% (VOC = 0.81 V, JSC = 9.37 mA cm−2, FF = 0.40) and 2.70% (VOC = 0.80 V, JSC = 9.29 mA cm−2, FF = 0.38) respectively. In addition, insertion of a thin layer of TiOx as an optical spacer enhanced the photovoltaic properties by reducing the interfacial resistance between the active layer and the Al metal electrode. Performances were enhanced in all cases reaching a PCE of 4.24% for 146c (VOC = 0.88 V, JSC = 10.76 mA cm−2, FF = 0.44), 3.73% for 146b (VOC = 0.85 V, JSC = 10.55 mA cm−2, FF = 0.44) and 3.54% for 146a (VOC = 0.82 V, JSC = 10.52 mA cm−2, FF = 0.42).
Fused bisthiazole, namely thiazolothiazole, has served to build up D–A–D architectures because it possesses a rigid coplanar structure and the presence of the imine bonds confers to it an electron-accepting character. Thus, linear chromophores 147 flanked with oligothiophenic units have been investigated by Shin et al.99 The terminal hexyl side chains ensure their solubility in common organic solvents and induce liquid crystalline properties that can be beneficial to the the supramolecular arrangement in the solid state (Scheme 73). Solution processed BHJ have been fabricated with the structure ITO/PEDOT:PSS (50 nm)/147![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC61BM (100 nm, 1
PC61BM (100 nm, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio, w/w)/Al (80–100 nm) and evaluated. Reasonably high PCE of 1.57% were achieved (VOC = 0.65 V, JSC = 7.85 mA cm−2, FF = 0.31). Furthermore, increasing the donor–acceptor ratio to 1
1 ratio, w/w)/Al (80–100 nm) and evaluated. Reasonably high PCE of 1.57% were achieved (VOC = 0.65 V, JSC = 7.85 mA cm−2, FF = 0.31). Furthermore, increasing the donor–acceptor ratio to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 has not led to an improvement of the efficiency and the device has exhibited a PCE of 1.20% (VOC = 0.42 V, JSC = 7.59 mA cm−2, FF = 0.37).
2 has not led to an improvement of the efficiency and the device has exhibited a PCE of 1.20% (VOC = 0.42 V, JSC = 7.59 mA cm−2, FF = 0.37).
To improve the efficiencies of thiazolothiazole-based chromophores, Zhan et al. have used of triphenylamine as end-capped unit associated to different length of the π-conjugated bridge (Scheme 74).100 As expected, linear extension of the π-conjugated system, from 148 to 150, induced a bathochromic shift of the maximum of absorption and an enhancement of the molar absorptivity.
Organic solar cells were fabricated with the configuration ITO/PEDOT:PSS (35 nm)/148–150![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM (70–80 nm, 1
PC71BM (70–80 nm, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 1
2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 or 1
3 or 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 ratio, w/w)/Ca (15 nm)/Al (60 nm) and tested. The best performances were obtained for a donor–acceptor ratio of 1
4 ratio, w/w)/Ca (15 nm)/Al (60 nm) and tested. The best performances were obtained for a donor–acceptor ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 and thermal annealing at 110 °C (148, 149) or 120 °C (150) affording a PCE of 2.17% (VOC = 0.86 V, JSC = 6.08 mA cm−2, FF = 0.415) for 148, 3.61% for 149 (VOC = 0.90 V, JSC = 9.38 mA cm−2, FF = 0.428) and 3.89% (VOC = 0.85 V, JSC = 9.74 mA cm−2, FF = 0.47) for 150. Particularly, the thermal annealing has affected the electron mobility and hole-transport contributing into their balance (for instance for 150 μh = 1.70 10−6 cm2 V−1 s−1 and μe = 1.13 10−4 cm2 V−1 s−1 (μe/μh = 66.47) and after the thermal annealing μh = 1.01 10−5 cm2 V−1 s−1 and μe = 1.89 10−5 cm2 V−1 s−1 (μe/μh = 1.89)) and increased the miscibility of PC71BM facilitating the phase separation.
4 and thermal annealing at 110 °C (148, 149) or 120 °C (150) affording a PCE of 2.17% (VOC = 0.86 V, JSC = 6.08 mA cm−2, FF = 0.415) for 148, 3.61% for 149 (VOC = 0.90 V, JSC = 9.38 mA cm−2, FF = 0.428) and 3.89% (VOC = 0.85 V, JSC = 9.74 mA cm−2, FF = 0.47) for 150. Particularly, the thermal annealing has affected the electron mobility and hole-transport contributing into their balance (for instance for 150 μh = 1.70 10−6 cm2 V−1 s−1 and μe = 1.13 10−4 cm2 V−1 s−1 (μe/μh = 66.47) and after the thermal annealing μh = 1.01 10−5 cm2 V−1 s−1 and μe = 1.89 10−5 cm2 V−1 s−1 (μe/μh = 1.89)) and increased the miscibility of PC71BM facilitating the phase separation.
Analogously to 147 Nguyen et al. have designed and synthetized the chromophore 151 having a diketopyrrolopyrrole (DPP) as central electron-withdrawing core (Scheme 75).101
Replacement of the thiazolothiazole core by DPP has led to a strong red shift of 169 nm in chloroform solution (447 nm to 616 nm). In thin film, 151 exhibited two absorption bands centered at 660 nm and 742 nm shifting the absorption band edge from 1.72 eV to 1.51 eV. This significant change was ascribed to an improved molecular ordering as evidenced by AFM microphotographs and hole-mobility measurement (μh = 3.00 10−6 cm2 V−1 s−1). BHJ solar cells were fabricated with the structure ITO/PEDOT:PSS (40 nm)/151![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC61BM (100 nm, blend ratios 30/70, 50/50, 70/30)/Al (100 nm). Photovoltaic performances were evaluated for each donor–acceptor ratios. The best PCE of 2.33% (VOC = 0.67 V, JSC = 8.42 mA cm−2, FF = 0.45) was obtained for a 70
PC61BM (100 nm, blend ratios 30/70, 50/50, 70/30)/Al (100 nm). Photovoltaic performances were evaluated for each donor–acceptor ratios. The best PCE of 2.33% (VOC = 0.67 V, JSC = 8.42 mA cm−2, FF = 0.45) was obtained for a 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 ratio without any thermal post-treatment to prevent the BOC group elimination.
30 ratio without any thermal post-treatment to prevent the BOC group elimination.
Adashi et al. also have investigated mesomorphic DPP-based chromophores, in order to achieve optimized photovoltaic efficiencies taking into account their intrinsic self-organizing properties (Scheme 76).102 Effect of the nature of the alkyl chains, with the appropriate ratio, on the photovoltaic performances was studied. As expected, compounds 152 exhibit liquid-crystalline properties and form well-developed nanostructured bulk heterojunction (BHJ) architectures with a fullerene derivative (PC71BM). The organic solar cells have been fabricated with the standard device structure ITO/PEDOT:PSS (40 nm)/152![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM blend (90–120 nm, 1
PC71BM blend (90–120 nm, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1.5
1, 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and 2
1, and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratios, w/w)/LiF (1 nm)/Al (100 nm) and evaluated under AM 1.5 G illumination. The best performances were achieved with a 1
1 ratios, w/w)/LiF (1 nm)/Al (100 nm) and evaluated under AM 1.5 G illumination. The best performances were achieved with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio after a thermal annealing at 140 °C for 152a. As evidenced on UV-Vis spectra, upon thermal post-treatment major structural changes were obtained in the solid state for the blend 152a
1 ratio after a thermal annealing at 140 °C for 152a. As evidenced on UV-Vis spectra, upon thermal post-treatment major structural changes were obtained in the solid state for the blend 152a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM highlighting the formation of H-aggregates at the expense of J-aggregates that are predominant for 152b even after heating. This thermo-responsive organization was fully in agreement with the LC state of 152a occurring from 117 °C upon heating. The self-assembling into ordered domains strongly affects the stacking in the solid state, the charge carriers mobilities and the solar cell performances. As anticipated 152a exhibited the highest PCE of 4.30% (VOC = 0.93 V, JSC = 8.37 mA cm−2, FF = 0.55) after thermal annealing (at 140 °C) without any additives while 152a gave only a PCE of 1.20% (VOC = 0.93 V, JSC = 3.90 mA cm−2, FF = 0.36) (with a thermal post treatment at 120 °C).
PC71BM highlighting the formation of H-aggregates at the expense of J-aggregates that are predominant for 152b even after heating. This thermo-responsive organization was fully in agreement with the LC state of 152a occurring from 117 °C upon heating. The self-assembling into ordered domains strongly affects the stacking in the solid state, the charge carriers mobilities and the solar cell performances. As anticipated 152a exhibited the highest PCE of 4.30% (VOC = 0.93 V, JSC = 8.37 mA cm−2, FF = 0.55) after thermal annealing (at 140 °C) without any additives while 152a gave only a PCE of 1.20% (VOC = 0.93 V, JSC = 3.90 mA cm−2, FF = 0.36) (with a thermal post treatment at 120 °C).
To favor the π-π arrangement and the intermolecular contacts in the solid state DPP 153 end-capped with benzofuran units (Scheme 77) has been synthetized and tested by Nguyen et al.103 Thin film properties have been investigated from pure 153 to blends films of 153![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM at various ratios (30
PC71BM at various ratios (30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70, 50
70, 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, 60
50, 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40, 30
40, 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70) and temperatures (from room temperature to 150 °C). From these experiments, it has been shown that changing the annealing temperature one can control and adjust the surface domains sizes for a given blend. These changes in morphologies are fully in agreement with the absorption and XRD experiments and correlated with the charge carrier mobility measured. Hence, from the understanding thin-films properties, the best conditions are obtained for a given donor–acceptor ratio of 60
70) and temperatures (from room temperature to 150 °C). From these experiments, it has been shown that changing the annealing temperature one can control and adjust the surface domains sizes for a given blend. These changes in morphologies are fully in agreement with the absorption and XRD experiments and correlated with the charge carrier mobility measured. Hence, from the understanding thin-films properties, the best conditions are obtained for a given donor–acceptor ratio of 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 annealed at 110 °C. Using these criterions a PCE as high as 4.40% was achieved (VOC = 0.92 V, JSC = 10.00 mA cm−2, FF = 0.48) in BHJ solar cells.
40 annealed at 110 °C. Using these criterions a PCE as high as 4.40% was achieved (VOC = 0.92 V, JSC = 10.00 mA cm−2, FF = 0.48) in BHJ solar cells.
Pursuing their efforts in structure–properties relationships in the DPP chromophores family, T.-Q. Nguyen et al. have studied the effects of stereoisomerism of 153 on the crystallization properties.104 Typically, 2-ethylhexyl chains are widely used to improve the solution processability of the synthesized chromophore but possess the disadvantage to present an asymmetric carbon (R or S configuration) affording a mixture of stereoisomers that induces different solid-state properties. Thus, to elucidate the influence of the nature of the stereocenters on the optoelectrical properties the three stereoisomers, namely 153-RR, 153-SS and 153-RS (mesomer), were synthesized and purified by a preparative chiral HPLC. As expected, from crystallographic data 153-RR and 153-SS exhibit the same parameters (as they are enantiomers) while the mesomer 153-RS exhibits two additional structures. In addition, due to its centrosymmetry 153-RS adopts a coplanar structure with a π–π stacking of 3.28 Å unlike 153-RR or 153-SS exhibit a twisted structure associated to inferior intermolecular stacking (3.47 Å). Among these stereoisomers 153-RS possesses the highest crystallinity and carrier mobility (μh = 2.80 10−3 cm2 V−1 s−1 (after annealing at 100 °C for 10 min.), for 153-RR μh = 8.60 10−4 cm2 V−1 s−1 and 153-SS μh = 9.10 10−4 cm2 V−1 s−1). Unlike to 153, 153-RS exhibits larger crystalline domain size and better intermolecular contacts demonstrating the significant effect of the isomeric purity on the material's optoelectrical properties that could be beneficial to improve the photovoltaic performances.
As seen in the previous sections, the most reported BHJ solar cells combine a small molecule (p-type) and exclusively a fullerene derivative (n-type). However, there is still need to develop other n-type materials that can be used as alternatives of fullerene derivatives. For this purpose, Nguyen et al.105 envisioned to investigate a novel heterojunction based on their DPP chromophores, such as 153 and/or 154 (ref. 106) and the dicyanoimidazole 155 (vinazene) acceptor (Scheme 78).
BHJ solar cells were fabricated with the architecture ITO/PEDOT:PSS/153 or 154![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 155a or 155b/Al. Current-density characteristics were determined with different blend ratios and at different annealing temperatures and compared with devices made from PC61BM as acceptor. As expected, the nature of the alkyl chains on vinazene derivatives 155 influences the optoelectrical properties: the less hindered and bulky hexyl chain led to more favorable morphology than its 2-ethylhexyl counterpart. Upon thermal annealing marginal improvement was observed. Nevertheless the best photovoltaic performances were obtained with a blend 154
155a or 155b/Al. Current-density characteristics were determined with different blend ratios and at different annealing temperatures and compared with devices made from PC61BM as acceptor. As expected, the nature of the alkyl chains on vinazene derivatives 155 influences the optoelectrical properties: the less hindered and bulky hexyl chain led to more favorable morphology than its 2-ethylhexyl counterpart. Upon thermal annealing marginal improvement was observed. Nevertheless the best photovoltaic performances were obtained with a blend 154![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 155a (1
155a (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio, 80 °C) 0.80% (VOC = 1.23 V, JSC = 2.30 mA cm−2, FF = 0.28) and 154
1 ratio, 80 °C) 0.80% (VOC = 1.23 V, JSC = 2.30 mA cm−2, FF = 0.28) and 154![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 155b (1
155b (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio, 110 °C) 1.10% (VOC = 1.08 V, JSC = 2.76 mA cm−2, FF = 0.35). Higher PCE values were achieved when PC61BM was used as acceptor up to 3.72% in the case of the blend 154
1 ratio, 110 °C) 1.10% (VOC = 1.08 V, JSC = 2.76 mA cm−2, FF = 0.35). Higher PCE values were achieved when PC61BM was used as acceptor up to 3.72% in the case of the blend 154![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC61BM (1
PC61BM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 110 °C, VOC = 0.84 V, JSC = 9.26 mA cm−2, FF = 0.48) but with lower VOC.
1, 110 °C, VOC = 0.84 V, JSC = 9.26 mA cm−2, FF = 0.48) but with lower VOC.
A more deeply structure–properties relationships on 153 analogues have been conducted by Roncali et al. using a combination of benzothiophene or benzofuran with thienyl or furanyl-DPP-based D–A–D molecules (153, 156) as well the replacement of the carbonyl groups of DPP by thioketo (157) (Scheme 79).107 Interestingly, within the series 156 the composition of the side chains has no effect on the HOMO–LUMO levels as evidenced by the absorption spectroscopy and the cyclic voltammetry. Furthermore and surprisingly using a planar heterojunction device structure ITO/PEDOT:PSS (40 nm)/153, 156 (20 nm)/C60 (50 nm)/Al (100 nm), only molecule 153 gave rise to a reasonable PCE of 2.50% (VOC = 0.72 V, JSC = 7.90 mA cm−2, FF = 0.39) while for the other structures the PCE did not exceed 0.80%. Hence, minor structural modifications may cause dramatic changes on the optoelectrical properties. Additionally, although the replacement of the carbonyl of DPP by thioketo groups produces a significant reduction of the bandgap there is a complete attenuation of the photovoltaic conversion efficiency. The best structure from 153 was then used in a BHJ device structure with PC61BM affording a PCE of 3.13% (VOC = 0.81 V, JSC = 10.01 mA cm−2, FF = 0.35) after a thermal annealing at 70 °C.
To fine tune the device performances Li et al. have investigated and synthesized highly polarizable DPP-based chromophores 158 using thiophene (158a), selenophene (157b) and/or fused thienothiophene (158c) as π-conjugated side-chains (Scheme 80).108 Electro-optical properties and films properties were analyzed revealing that blends of 158b and PC61BM exhibited the best film-forming characteristics and 158c the poorer packing order. ITO/PEDOT:PSS/158![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC61BM/Ca/Al devices were fabricated and evaluated. Increasing the donor–acceptor ratio (1
PC61BM/Ca/Al devices were fabricated and evaluated. Increasing the donor–acceptor ratio (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2
1, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 3
1, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) leads to an improvement of the photovoltaic performances in all cases. As expected from the solid-state properties analysis the best performance was achieved for 158b with a PCE of 2.30% (3
1) leads to an improvement of the photovoltaic performances in all cases. As expected from the solid-state properties analysis the best performance was achieved for 158b with a PCE of 2.30% (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, VOC = 0.86 V, JSC = 5.81 mA cm−2, FF = 0.46) while 160c exhibits the lowest PCE (1.25% (3
1, VOC = 0.86 V, JSC = 5.81 mA cm−2, FF = 0.46) while 160c exhibits the lowest PCE (1.25% (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), VOC = 0.79 V, JSC = 4.51 mA cm−2, FF = 0.36).
1), VOC = 0.79 V, JSC = 4.51 mA cm−2, FF = 0.36).
To get further insights various terminal modifications have been explored by Frechet et al. in order to promote their solid-state self-assembling via end-to-end π–π interactions (Scheme 81).109 End-groups were selected for their tendency to induce strong π-stacks like pyrene (derivatives 161 and 162) and compared with non-planar analogues (159) and planar ones bearing non-coplanar substituents (160). Thin-film BHJ solar cells were fabricated with the following architecture ITO/PEDOT:PSS (30–40 nm)/159–162![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM/Al (100 nm). Devices prepared from 159 to 161 where optimized at a 1
PC71BM/Al (100 nm). Devices prepared from 159 to 161 where optimized at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 donor–PC71BM ratio while a blend ratio of 2
4 donor–PC71BM ratio while a blend ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was used for 162. Increasing the donor part in the devices comprising compounds 159–161 leads to poorer conversion efficiencies even though an enhancement of the hole-mobilities was gained. In contrast in devices made from 162 higher concentration of donor up to the optimized 2
1 was used for 162. Increasing the donor part in the devices comprising compounds 159–161 leads to poorer conversion efficiencies even though an enhancement of the hole-mobilities was gained. In contrast in devices made from 162 higher concentration of donor up to the optimized 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio enhanced both the fill factor and the hole-mobilities suggesting that pyrene promotes the molecular packing and film morphology in the solid-state that are favorable to an increase of the PCE. Indeed, the highest PCE of 3.7% (VOC = 0.76 V, JSC = 8.30 mA cm−2, FF = 0.58) was achieved for 162b after thermal annealing. Interestingly, 161 as only a PCE of 0.70% (VOC = 0.73 V, JSC = 3.20 mA cm−2, FF = 0.29) compared to 162a (2.40%, VOC = 0.77 V, JSC = 5.70 mA cm−2, FF = 0.55) indicating that photovoltaic performances are strongly dependent both on end-group planarity and symmetry.
1 ratio enhanced both the fill factor and the hole-mobilities suggesting that pyrene promotes the molecular packing and film morphology in the solid-state that are favorable to an increase of the PCE. Indeed, the highest PCE of 3.7% (VOC = 0.76 V, JSC = 8.30 mA cm−2, FF = 0.58) was achieved for 162b after thermal annealing. Interestingly, 161 as only a PCE of 0.70% (VOC = 0.73 V, JSC = 3.20 mA cm−2, FF = 0.29) compared to 162a (2.40%, VOC = 0.77 V, JSC = 5.70 mA cm−2, FF = 0.55) indicating that photovoltaic performances are strongly dependent both on end-group planarity and symmetry.
Extension of the π-conjugated system, by the insertion of an acetylene linkage between the pyrene end-group and the DPP (163), and its consequences on PCE was investigated by Park et al. (Scheme 82).110 The insertion of the linkage favors a more planar geometry of the π-conjugated backbone for derivatives 163, as supported by DFT calculation. This behavior should be beneficial to increase the PCE relative to 161. BHJ with the structure ITO/PEDOT:PSS (30–40 nm)/161 or 163![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM (7
PC71BM (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 ratio, 130 nm)/Al (100 nm) were prepared according to reported procedures. The performances of the solar cells were recorded under standard illumination conditions AM1.5 giving a PCE of 2.95% (VOC = 0.85 V, JSC = 8.89 mA cm−2, FF = 0.42) that is almost 6.5 times greater than 161 measured in the same conditions (0.46% (0.70%),109 VOC = 0.79 V, JSC = 2.38 mA cm−2, FF = 0.27). The improved performances were unambiguously assigned to the acetylene linkage that induced a more planar backbone facilitating the intermolecular contacts and increased absorption.
3 ratio, 130 nm)/Al (100 nm) were prepared according to reported procedures. The performances of the solar cells were recorded under standard illumination conditions AM1.5 giving a PCE of 2.95% (VOC = 0.85 V, JSC = 8.89 mA cm−2, FF = 0.42) that is almost 6.5 times greater than 161 measured in the same conditions (0.46% (0.70%),109 VOC = 0.79 V, JSC = 2.38 mA cm−2, FF = 0.27). The improved performances were unambiguously assigned to the acetylene linkage that induced a more planar backbone facilitating the intermolecular contacts and increased absorption.
Controlling the morphology of the donor/acceptor domains in the active layer is of crucial importance to optimize the performance of the solar cells. Stupp et al. proposed recently an elegant strategy by combining synergistically different weak interactions within the active layer (namely H-bonds and π–π interactions) to control the supramolecular organization of each component upon proper and optimized conditions. Thus, they have combined a trans-1,2-diaminocyclohexane building block, that possesses the capability to engender H-bonding, with the DPP chromophore 164 affording the hairpin-shaped chromophore 165 (Scheme 83).111 Upon a careful stepwise cooling process associated to a minimal solution stirring led to the formation of a supramolecular nanowire driven by H-bonds and π–π stacking. This supramolecular arrangement should be helpful to improve the electroptical properties. Additionally, the shaped of 165 is also favorable to create cavities allowing better cofacials interfaces between donor and acceptor. Solar cells were fabricated using 165 as donor and PC71BM as acceptor under different conditions (systematically donor–acceptor ratio, temperature and solvent). Hence, a blend of disrupted assembly of 165![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM affords only a PCE of 1.40 10−3% while a 400-fold greater PCE (0.53%, VOC = 0.66 V, JSC = 2.98 mA cm−2, FF = 0.27) was achieved with devices fabricated with assembled 165 solutions. If 164 is used under the same conditions a PCE of 0.24% (VOC = 0.57 V, JSC = 1.43 mA cm−2, FF = 0.29) was obtained, nevertheless in this case the concentration of the active component is higher attesting de facto that the self-assembling contributes significantly to enhance the device performances.
PC71BM affords only a PCE of 1.40 10−3% while a 400-fold greater PCE (0.53%, VOC = 0.66 V, JSC = 2.98 mA cm−2, FF = 0.27) was achieved with devices fabricated with assembled 165 solutions. If 164 is used under the same conditions a PCE of 0.24% (VOC = 0.57 V, JSC = 1.43 mA cm−2, FF = 0.29) was obtained, nevertheless in this case the concentration of the active component is higher attesting de facto that the self-assembling contributes significantly to enhance the device performances.
Besides DPP, other interesting acceptors could be used for the synthesis of novel D–A–D structures. Among them, fluorenone has been widely used in this context. To study the role of fluorenone on photovoltaic performances, symmetrically chromophores bearing different π-conjugated side chains have been synthesized by Demadrille et al. (Scheme 84).112 Solar cells were fabricated with the following device structure ITO/PEDOT:PSS/166–169![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC61BM (1
PC61BM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 or 1
2 or 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio, 100 ± 20 nm)/LiF (0.80 nm)/Al. As expected the molecules possessing the shortest π-conjugated systems have exhibited the lowest conversion efficiencies. The highest PCE reported for these compounds reaches 1.19%, for 169, (VOC = 0.82 V, JSC = 3.61 mA cm−2, FF = 0.40) after post-thermal treatment at 70 °C at a 1
1 ratio, 100 ± 20 nm)/LiF (0.80 nm)/Al. As expected the molecules possessing the shortest π-conjugated systems have exhibited the lowest conversion efficiencies. The highest PCE reported for these compounds reaches 1.19%, for 169, (VOC = 0.82 V, JSC = 3.61 mA cm−2, FF = 0.40) after post-thermal treatment at 70 °C at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. For compounds 166–168 PCEs are ranging from 0.14% to 0.58% (VOC = 0.72 V, JSC = 0.73 mA cm−2, FF = 0.27 (166)), (VOC = 0.52 V, JSC = 0.91 mA cm−2, FF = 0.29, (167)), (VOC = 0.68 V, JSC = 2.67 mA cm−2, FF = 0.32 (168)) under the same conditions and without thermal annealing. Furthermore, for 166 and 167 increasing the ratio from 1
1 ratio. For compounds 166–168 PCEs are ranging from 0.14% to 0.58% (VOC = 0.72 V, JSC = 0.73 mA cm−2, FF = 0.27 (166)), (VOC = 0.52 V, JSC = 0.91 mA cm−2, FF = 0.29, (167)), (VOC = 0.68 V, JSC = 2.67 mA cm−2, FF = 0.32 (168)) under the same conditions and without thermal annealing. Furthermore, for 166 and 167 increasing the ratio from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 1
1 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 gives a substantial improvement of the PCE up to 0.37% (VOC = 0.67 V, JSC = 1.85 mA cm−2, FF = 0.30) and 0.42% (VOC = 0.75 V, JSC = 1.86 mA cm−2, FF = 0.30) respectively. Further enhancement can be achieved by thermal annealing at 80 °C.
2 gives a substantial improvement of the PCE up to 0.37% (VOC = 0.67 V, JSC = 1.85 mA cm−2, FF = 0.30) and 0.42% (VOC = 0.75 V, JSC = 1.86 mA cm−2, FF = 0.30) respectively. Further enhancement can be achieved by thermal annealing at 80 °C.
Encouraged by the results reported on DPP oligothiophenes by Nguyen, isoindigo-based chromophores in conjunction with bithiophene as π-conjugated donor were developed and evaluated by Reynolds et al. (Scheme 85).113 Compounds 170 and 171 present remarkable broad spectra in the solid-state with estimated optical band-gaps of 1.67 eV and 1.72 eV, respectively. BHJ solar cells were made from spin-coated chlorobenzene solution of 170 and 171![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC61BM blends onto ITO/PEDOT:PSS glass substrate. Various parameters have been optimized (comprising the solution concentrations, blends ratio, annealing temperature) in order to achieve the highest PCEs. For instance, optimized blend ratios were found to be 1
PC61BM blends onto ITO/PEDOT:PSS glass substrate. Various parameters have been optimized (comprising the solution concentrations, blends ratio, annealing temperature) in order to achieve the highest PCEs. For instance, optimized blend ratios were found to be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for 170 and 1.5
1 for 170 and 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for 171. After thermal annealing at 100 °C, the device fabricated from 170 showed significant better photovoltaic performances (PCE = 1.76%) than the solar cell made from 171 (0.55%) (VOC = 0.74 V, JSC = 6.30 mA cm−2, FF = 0.38 and VOC = 0.66 V, JSC = 2.40 mA cm−2, FF = 0.36, respectively). The higher PCE for 170 was assigned to a better solid-state ordering, greater film morphology and lower energy gap.
1 for 171. After thermal annealing at 100 °C, the device fabricated from 170 showed significant better photovoltaic performances (PCE = 1.76%) than the solar cell made from 171 (0.55%) (VOC = 0.74 V, JSC = 6.30 mA cm−2, FF = 0.38 and VOC = 0.66 V, JSC = 2.40 mA cm−2, FF = 0.36, respectively). The higher PCE for 170 was assigned to a better solid-state ordering, greater film morphology and lower energy gap.
Recently, tetracyanobuta-1,3-diene (TCBD) has been developed as an efficient electron-withdrawing group for the synthesis of NLOphores with improved nonlinear properties and thermal stabilities. Thanks to its intrinsic properties, Roncali et al. have envisioned to use such TCBD for the design of active materials in photovoltaic devices.114 Thus centrosymmetric and non-centrosymmetric D–A–D structures have been designed and synthesized (Scheme 86). Compounds exhibit non-planar structures (restricting π-electron delocalization in the TCBD core) associated to good solubility and reduced aggregation in solution, high oxidation potentials and strong absorption properties. Bilayer heterojunction solar cells have been fabricated with the following structure ITO/PEDOT:PSS (40 nm)/172–174 (25 nm)/C60 (20 nm)/Al (100 nm) and PCEs were determined under AM1.5 conditions at 90 mW cm−2. As spin-casted from chloroform solution at 10 mM, chromophore 172 led to a PCE of 1.08% (VOC = 0.97 V, JSC = 3.06 mA cm−2, FF = 0.33) while this PCE is reduced down to 0.56% when the chromophore is deposited by vacuum as a film of 13 nm. These encouraging results demonstrate the interest to use such acceptor for developing BHJ solar cells but improvements have to be pursued.
D–A–D–A–D type
Further improvements can be achieved by inserting additionally D–A π-conjugated chromophores to D–A–D architectures leading to D–A–D–A–D structures that should exhibit higher performances due to the extended π-conjugated backbone. Nevertheless, careful disposition and selection of the D and A have to be taken into account in order to optimize the optoelectrical properties.Recently, Lee et al. have studied and compared the properties of D–A–D having the bithiazole (175) and the fused bithiazole (176, analogous of 149) (Scheme 87). BHJ solar cells have been fabricated with the following structure ITO/PEDOT:PSS/175 and 176![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM (65–75 nm)/Al and evaluated. The best performances, after device optimizations, were found to be 1.30% (1
PC71BM (65–75 nm)/Al and evaluated. The best performances, after device optimizations, were found to be 1.30% (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 ratio, VOC = 0.97 V, JSC = 4.63 mA cm−2, FF = 0.29) and 2.39% (1
3 ratio, VOC = 0.97 V, JSC = 4.63 mA cm−2, FF = 0.29) and 2.39% (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 ratio, VOC = 0.94 V, JSC = 6.49 mA cm−2, FF = 0.39). These performances might be improved by further optimization of the device morphology using processing additives, annealing, and so on. Additionally, it was speculated that the design of new molecular structures with these thiazole derivatives might also provide better tunings for seeking desirable band gap and energy levels and thereby enable better performance.115
3 ratio, VOC = 0.94 V, JSC = 6.49 mA cm−2, FF = 0.39). These performances might be improved by further optimization of the device morphology using processing additives, annealing, and so on. Additionally, it was speculated that the design of new molecular structures with these thiazole derivatives might also provide better tunings for seeking desirable band gap and energy levels and thereby enable better performance.115
Hence, from these thiazolo-based chromophores were designed and synthesized the extended π-conjugated systems 177–179 depicted in the Scheme 87.116,117 Incorporation of fused π-conjugated aromatic units into small molecules have the benefit to stabilize the quinoidal structure and to increase both the rigidity and coplanarity of the molecular backbone. In turn, improved charge transport characteristics, reduction of the bandgap and enhancement of the absorption properties are expected that might all contribute to greater device performances.
BHJ organic solar cells were prepared as follow glass/ITO/PEDOT:PSS (40 nm)/177–179![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM (60–70 nm)/LiF (0.50 nm)/Al (120 nm). Compounds 177 and 178 exhibited similar absorption profiles in solution, however in the solid state 177 showed a better well-defined vibronic splitting than 178 indicating a greater efficient π-stacking and strong intermolecular interaction in the film confirmed by thin-film XRD. Furthermore, their electrochemical properties are consistent with their optical properties and molecular structures. The device performances were evaluated under AM1.5 conditions at different weight ratios (1
PC71BM (60–70 nm)/LiF (0.50 nm)/Al (120 nm). Compounds 177 and 178 exhibited similar absorption profiles in solution, however in the solid state 177 showed a better well-defined vibronic splitting than 178 indicating a greater efficient π-stacking and strong intermolecular interaction in the film confirmed by thin-film XRD. Furthermore, their electrochemical properties are consistent with their optical properties and molecular structures. The device performances were evaluated under AM1.5 conditions at different weight ratios (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 1
1 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) and using chloroform (CF) or chlorobenzene (CB) as solvents. The best performances resulting from the optimization of the conditions led to a PCE of 1.09% for 177 (1
4) and using chloroform (CF) or chlorobenzene (CB) as solvents. The best performances resulting from the optimization of the conditions led to a PCE of 1.09% for 177 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 ratio in CF, VOC = 0.81 V, JSC = 3.35 mA cm−2, FF = 0.41) and 1.62% for 178 (1
3 ratio in CF, VOC = 0.81 V, JSC = 3.35 mA cm−2, FF = 0.41) and 1.62% for 178 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio in CF, VOC = 0.76 V, JSC = 4.47 mA cm−2, FF = 0.48). Despite to better solid-state properties of 178, the greater PCE attained for 179 than 178, even when similar conditions were used (PCE is only 0.76% for 178 when 1
1 ratio in CF, VOC = 0.76 V, JSC = 4.47 mA cm−2, FF = 0.48). Despite to better solid-state properties of 178, the greater PCE attained for 179 than 178, even when similar conditions were used (PCE is only 0.76% for 178 when 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio is used), is ascribed to the finest film morphology for 178. Analogously to 175 and 176, changing the acceptor from 177 to 179 should lead to enhanced performances in favor of 179. However, the device characteristics gave for the best BHJ solar cell a PCE of 1.44% (1
1 ratio is used), is ascribed to the finest film morphology for 178. Analogously to 175 and 176, changing the acceptor from 177 to 179 should lead to enhanced performances in favor of 179. However, the device characteristics gave for the best BHJ solar cell a PCE of 1.44% (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio in CB, VOC = 0.75 V, JSC = 5.10 mA cm−2, FF = 0.38) that is inferior to what is expected and obtained for 178. Nevertheless under the same conditions, i.e. 1
2 ratio in CB, VOC = 0.75 V, JSC = 5.10 mA cm−2, FF = 0.38) that is inferior to what is expected and obtained for 178. Nevertheless under the same conditions, i.e. 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio in CB, 178 led to a PCE of 0.65% (VOC = 0.73 V, JSC = 3.01 mA cm−2, FF = 0.29) confirming that fused bithiazole is a better acceptor than bithiazole.
2 ratio in CB, 178 led to a PCE of 0.65% (VOC = 0.73 V, JSC = 3.01 mA cm−2, FF = 0.29) confirming that fused bithiazole is a better acceptor than bithiazole.
Following the same strategy Marks et al. have studied the influence of the shape of the naphthodithiophene core, i.e. linear (NDT) vs. zig-zag (zNDT), on the photovoltaic performances of D–A–D–A–D comprising a DPP group as acceptor (Scheme 88).118,119 Interestingly, by changing the shape of the central core from NDT to zNDT, both 180 and 181 exhibit the same optical properties and the same optical bandgap (1.70 eV). However 181 exhibits a predicable reduced oxidation potential compared to 180 that should affect in turn the VOC. BHJ solar cells were fabricated with the structure ITO/PEDOT:PSS/180 and 181/PC61BM/LiF/Al and evaluated. The device performances after optimizing the donor–acceptor ratio and the thermal annealing (1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 110 °C for 180 and 1.2
1, 110 °C for 180 and 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 140 °C for 181) led to a PCE of 4.00% (VOC = 0.84 V, JSC = 11.20 mA cm−2, FF = 0.43) and 4.40% (VOC = 0.75 V, JSC = 11.70 mA cm−2, FF = 0.50) respectively. The results are consistent with the greater hole-mobility for 181 (μh = 1.00 10−4 cm2 V−1 s−1) than 180 (μh = 2.50 10−7 cm2 V−1 s−1) leading to a substantial enhancement of the short-circuit current in favor of 181 due to a more efficient charge separation and reduced recombination process.
1, 140 °C for 181) led to a PCE of 4.00% (VOC = 0.84 V, JSC = 11.20 mA cm−2, FF = 0.43) and 4.40% (VOC = 0.75 V, JSC = 11.70 mA cm−2, FF = 0.50) respectively. The results are consistent with the greater hole-mobility for 181 (μh = 1.00 10−4 cm2 V−1 s−1) than 180 (μh = 2.50 10−7 cm2 V−1 s−1) leading to a substantial enhancement of the short-circuit current in favor of 181 due to a more efficient charge separation and reduced recombination process.
Recently, the indacenodithiophene (IDT) has sparked great interest as donor molecular building block due to its intrinsic properties, for instance a planar rigid structure, beneficial to the fabrication of SMOSCs. Thus, Hou et al. have designed and synthesized small IDT-based chromophores with the structure 182 depicted on Scheme 89.120 The synthesized chromophore presents optoelectronic properties that make it suitable for the fabrication of organic solar cells (Eg = 1.80 eV, broad absorption in the visible region, low-lying HOMO energy, high thermal stability).
Hence, BHJ solar cells based on the blend 182![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM have been fabricated with the following architecture ITO/PEDOT:PSS (30 nm)/182
PC71BM have been fabricated with the following architecture ITO/PEDOT:PSS (30 nm)/182![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM (80–100 nm)/Ca/Al. Several conditions have been tested by varying the donor–acceptor ratio (from 1
PC71BM (80–100 nm)/Ca/Al. Several conditions have been tested by varying the donor–acceptor ratio (from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 1
1 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) leading to the highest PCE of 4.25% (VOC = 0.93 V, JSC = 9.42 mA cm−2, FF = 0.48) for a 1
4) leading to the highest PCE of 4.25% (VOC = 0.93 V, JSC = 9.42 mA cm−2, FF = 0.48) for a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 ratio. No further improvements have been achieved by the addition of DIO as solvent additive or even by thermal annealing. The results indicated that chromophores based on IDT core are promising materials for the fabrication of SMOSCs.
3 ratio. No further improvements have been achieved by the addition of DIO as solvent additive or even by thermal annealing. The results indicated that chromophores based on IDT core are promising materials for the fabrication of SMOSCs.
Alongside the work of Marks based on NDT derivatives, Lee et al. have design new molecular D–A–D–A–D architectures based on the BDT unit, analogously to those described in Scheme 32. Chromophore 183 has been synthesized following known procedures and evaluated as donor in BHJ solar cells (Scheme 90).121 The solar cells based on the blend 183![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM have fabricated at different weight ratios, from 1
PC71BM have fabricated at different weight ratios, from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 to 1
0.5 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, with the structure ITO/PEDOT:PSS (40 nm)/183
4, with the structure ITO/PEDOT:PSS (40 nm)/183![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM (50–55 nm)/LiF (0.5 nm)/Al (120 nm). The best performance achieved without any post-treatment gave a PCE of 1.18% (VOC = 0.83 V, JSC = 4.80 mA cm−2, FF = 0.29) at a 1
PC71BM (50–55 nm)/LiF (0.5 nm)/Al (120 nm). The best performance achieved without any post-treatment gave a PCE of 1.18% (VOC = 0.83 V, JSC = 4.80 mA cm−2, FF = 0.29) at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 weight ratio. Impressive enhancement of the PCE up to 2.83% (VOC = 0.85 V, JSC = 7.81 mA cm−2, FF = 0.39) was produced upon thermal annealing at 180 °C due to a higher π-stacking order and increased hole-mobility up to μh = 2.70 10−6 cm2 V−1 s−1 (untreated film μh = 1.40 10−6 cm2 V−1 s−1) corroborating the solid-state analysis. In addition the alkyloxy side-chains are helpful to ensure good film morphology and phase separation.
3 weight ratio. Impressive enhancement of the PCE up to 2.83% (VOC = 0.85 V, JSC = 7.81 mA cm−2, FF = 0.39) was produced upon thermal annealing at 180 °C due to a higher π-stacking order and increased hole-mobility up to μh = 2.70 10−6 cm2 V−1 s−1 (untreated film μh = 1.40 10−6 cm2 V−1 s−1) corroborating the solid-state analysis. In addition the alkyloxy side-chains are helpful to ensure good film morphology and phase separation.
DTS core has also demonstrated to be an important and useful molecular scaffold for the construction of active component for SMOSCs (see for example Scheme 32). To get further insights on the molecular design of new active donors based on the DTS unit, Bazan et al. have synthesized news D–A–D–A–D structures in association with thiadiazolo pyridine (PT) as acceptor and deeply studied the parameters affecting their photovoltaic performances (Scheme 91).122 From the solution to the solid-state the chromophore showed a broadening and a red shift of the optical absorption along with the emergence of a vibronic splitting coherent with a more rigid and ordered structure in the film (Eg = 1.51 eV). Such features lead to greater π-electron delocalization across the molecular backbone and enhanced inter-chromophore interactions. Upon thermal annealing up to and below 110 °C the lowest energy absorption peak is enhanced consistently with a better π-stacking. In agreement with the opto-elelectrochemical properties of 184, SM-BHJ photovoltaic devices were made using a standard device architecture of ITO/Plexcore® OC AQ/184![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM (85–105 nm)/Al. The active layers were optimized by varying the 184
PC71BM (85–105 nm)/Al. The active layers were optimized by varying the 184![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM weight ratios at 3 different thickness layers and thermal annealing 2 min. at 110 °C. From this set of experiments the best performance was achieved for a 60
PC71BM weight ratios at 3 different thickness layers and thermal annealing 2 min. at 110 °C. From this set of experiments the best performance was achieved for a 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 ratio with a blend thickness of 85 nm leading to a PCE of 3.20% (VOC = 0.70 V, JSC = 10.90 mA cm−2, FF = 0.42). Determination of the hole and electron mobilities (μh = 1.50 10−6 cm2 V−1 s−1, μe = 5.30 10−5 cm2 V−1 s−1, respectively) leads to unbalanced charge carrier transport and may be responsible for the lower fill factor.
40 ratio with a blend thickness of 85 nm leading to a PCE of 3.20% (VOC = 0.70 V, JSC = 10.90 mA cm−2, FF = 0.42). Determination of the hole and electron mobilities (μh = 1.50 10−6 cm2 V−1 s−1, μe = 5.30 10−5 cm2 V−1 s−1, respectively) leads to unbalanced charge carrier transport and may be responsible for the lower fill factor.
In close collaboration with A.J. Heeger, mechanistic studies have been performed on the effect of solvent additives processed active layers during the film morphology formation and their impact on the photovoltaic efficiencies.123,124 During this study it has been clearly demonstrated that the use of DIO improves, beyond doubts, the interfacial charge transfer between donor and acceptor domains. This effect was also investigated theoretically. It was also demonstrated that Si-based chromophores are more efficient than their carbon counterparts.125 Carbon analogue of 184 showed only a PCE of 0.2% (as cast, VOC = 0.66 V, JSC = 1.00 mA cm−2, FF = 0.26) and 0.09% (annealed, VOC = 0.50 V, JSC = 0.60 mA cm−2, FF = 0.28) vs. a PCE of 0.9% (as cast, VOC = 0.67 V, JSC = 3.60 mA cm−2, FF = 0.29) and 3.3% (annealed, VOC = 0.70 V, JSC = 9.90 mA cm−2, FF = 0.47) respectively for the blend 184![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM without additives. The lower efficiency for the carbon analogue is assigned to the presence of larger crystalline domains.
PC71BM without additives. The lower efficiency for the carbon analogue is assigned to the presence of larger crystalline domains.
Heeger et al. have conducted additional structural modification on chromophore 184. Thus, chromophore 185 corresponding to an isomer of 184 has been designed and synthesized (Scheme 92).126 The difference came only from the position of the N atom on the PT scaffold. Different weight ratios were evaluated and it was found that at a 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 ratio a remarkable PCE of 4.52% (VOC = 0.80 V, JSC = 12.50 mA cm−2, FF = 0.45) was achieved for the blend 185
3 ratio a remarkable PCE of 4.52% (VOC = 0.80 V, JSC = 12.50 mA cm−2, FF = 0.45) was achieved for the blend 185![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM with a device structure ITO/MoOx/185
PC71BM with a device structure ITO/MoOx/185![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM/Al. Indeed, it has been shown that ITO/PEDOT:PSS/185
PC71BM/Al. Indeed, it has been shown that ITO/PEDOT:PSS/185![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM/Al is not a suitable structure to test the performance of 185 due to the acidic character of PEDOT:PSS diminishing the conversion efficiencies and in particular a loss in VOC. Interestingly, solar cells processed with 0.25% v/v DIO exhibit significant enhancement of the photocurrent generation leading to a PCE of 6.70% (VOC = 0.78 V, JSC = 14.40 mA cm−2, FF = 0.59). Higher concentrations of additive lead to a decrease of the photovoltaic efficiencies. It is evidenced that through a rational molecular design associated to controlled active-layer processing methodologies it was possible to achieve highly efficient small organic solar cells.
PC71BM/Al is not a suitable structure to test the performance of 185 due to the acidic character of PEDOT:PSS diminishing the conversion efficiencies and in particular a loss in VOC. Interestingly, solar cells processed with 0.25% v/v DIO exhibit significant enhancement of the photocurrent generation leading to a PCE of 6.70% (VOC = 0.78 V, JSC = 14.40 mA cm−2, FF = 0.59). Higher concentrations of additive lead to a decrease of the photovoltaic efficiencies. It is evidenced that through a rational molecular design associated to controlled active-layer processing methodologies it was possible to achieve highly efficient small organic solar cells.
The impact of the N atom and its position on 184 analogues was deeply investigated in a further work of Bazan (Scheme 93) and compared to the non-nitrogen analogue 187.127 The position of the N-atom affects the self-assembling properties in the BHJ film and might be correlated to the dipole–dipole induced-orientation in the solid-state. Chromophores 184–186 exhibit similar optical behaviors in solution and in the solid-state with a pronounced vibronic splitting compared to 187. Optical band gap are determined to be 1.50 eV for 185 and 186, 1.52 eV for 184 and 1.58 eV for 187. The small molecules BHJ solar cells were fabricated with the structure of ITO/MoOx/184–187![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM/Al and tested under the same conditions and same donor–acceptor weight ratios. The devices were evaluated without any treatment giving PCEs of 4.52% for 185 (VOC = 0.80 V, JSC = 12.50 mA cm−2, FF = 0.45), 2.47% for 186 (VOC = 0.75 V, JSC = 8.90 mA cm−2, FF = 0.37), 1.78% for 184 (VOC = 0.73 V, JSC = 7.40 mA cm−2, FF = 0.33) and 0.19% for 187 (VOC = 0.83 V, JSC = 0.90 mA cm−2, FF = 0.26). Using 0.25% v/v DIO as solvent additive an increase of the photovoltaics efficiencies were obtained in most cases leading to the following PCEs: 6.70% for 185 (VOC = 0.78 V, JSC = 14.40 mA cm−2, FF = 0.59), 2.47% for 186 (VOC = 0.73 V, JSC = 12.70 mA cm−2, FF = 0.60), 3.16% for 184 (VOC = 0.72 V, JSC = 9.80 mA cm−2, FF = 0.45) and 0.18% for 187 (VOC = 0.78 V, JSC = 0.90 mA cm−2, FF = 0.25). These results imply that the design of new organic materials for SMOSCs needs to consider not only the electronic structure and the nature of the side-chains but to also to take into account other interactions that can enhance/drive the self-assembly in the film.
PC71BM/Al and tested under the same conditions and same donor–acceptor weight ratios. The devices were evaluated without any treatment giving PCEs of 4.52% for 185 (VOC = 0.80 V, JSC = 12.50 mA cm−2, FF = 0.45), 2.47% for 186 (VOC = 0.75 V, JSC = 8.90 mA cm−2, FF = 0.37), 1.78% for 184 (VOC = 0.73 V, JSC = 7.40 mA cm−2, FF = 0.33) and 0.19% for 187 (VOC = 0.83 V, JSC = 0.90 mA cm−2, FF = 0.26). Using 0.25% v/v DIO as solvent additive an increase of the photovoltaics efficiencies were obtained in most cases leading to the following PCEs: 6.70% for 185 (VOC = 0.78 V, JSC = 14.40 mA cm−2, FF = 0.59), 2.47% for 186 (VOC = 0.73 V, JSC = 12.70 mA cm−2, FF = 0.60), 3.16% for 184 (VOC = 0.72 V, JSC = 9.80 mA cm−2, FF = 0.45) and 0.18% for 187 (VOC = 0.78 V, JSC = 0.90 mA cm−2, FF = 0.25). These results imply that the design of new organic materials for SMOSCs needs to consider not only the electronic structure and the nature of the side-chains but to also to take into account other interactions that can enhance/drive the self-assembly in the film.
Further improvement has been achieved by the introduction of a fluorine atom in place of the N-atom (Scheme 94). This structural modification renders the chromophore less sensitive to acidic medium and may increase its stability. Photovoltaic devices were fabricated using the general structure of ITO/PEDOT:PSS/188![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM/Ca/Al and evaluated.128
PC71BM/Ca/Al and evaluated.128
As a cast film without any post-treatment the device delivers a moderate photocurrent with a PCE of 1.80% (VOC = 0.78 V, JSC = 6.60 mA cm−2, FF = 0.36). To improve the above efficiency, post-deposition thermal annealing were investigated and it was shown that heating 10 min. at 130 °C gives an increase of the PCE up to 5.80% (VOC = 0.82 V, JSC = 10.80 mA cm−2, FF = 0.65). Encouraged by these results further investigations in order to improve the device performances were pursued notably by using solvent additives. Progressive increase of DIO up to 0.4 v/v% followed by a thermal treatment at 70 °C led to an impressive PCE of 7.00% (VOC = 0.81 V, JSC = 12.80 mA cm−2, FF = 0.68).
Interestingly, a non-fullerene acceptor, namely the perylene diimide PDI, has been used for the fabrication of BHJ solar cells in combination with 188 as donor.129 PDI exhibits complementary absorption spectra to that of 187 leading to a broad coverage of the visible spectrum. Screenings of the parameters that can influence the performances such as blend ratios, solvent additives and thermal annealing have been examined. Optimized devices were obtained for a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 blend ratio dissolved in CB (30 mg mL−1) with 0.4% v/v of DIO. BHJ solar cells were fabricated with the standard architecture of ITO/PEDOT:PSS (45 nm)/188
1 blend ratio dissolved in CB (30 mg mL−1) with 0.4% v/v of DIO. BHJ solar cells were fabricated with the standard architecture of ITO/PEDOT:PSS (45 nm)/188![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PDI (1
PDI (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 110 nm)/Ca (5 nm)/Al (100 nm) and evaluated. The highest PCE achieved under these conditions was 3.00% (VOC = 0.78 V, JSC = 7.40 mA cm−2, FF = 0.52). Compared to 188
1, 110 nm)/Ca (5 nm)/Al (100 nm) and evaluated. The highest PCE achieved under these conditions was 3.00% (VOC = 0.78 V, JSC = 7.40 mA cm−2, FF = 0.52). Compared to 188![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM the lower IQE and reduced FF for 188
PC71BM the lower IQE and reduced FF for 188![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PDI arise from the lower hole/electron mobilities and higher charge recombination as evidenced by experiments.
PDI arise from the lower hole/electron mobilities and higher charge recombination as evidenced by experiments.
Finally, extended π-conjugated systems of 185 have been investigated regarding their electroptical properties in order to obtain insights into the role of the molecular size on the relevant properties that can be used for the fabrication of SM BHJ solar cells (Scheme 95).130
As expected compounds 189 and 190, that are oligomeric analogues of 185, exhibit a red-shift absorption spectra in solution and in the solid-state. From the absorption onsets, the optical band-gaps have been determined to be 1.44 eV and 1.41 eV respectively which agrees with the HOMO (−5.17 eV, −5.04 eV respectively) and LUMO energy levels determined by cyclic voltammetry. In addition, the presence of a vibronic fine structure is associated to specific intermolecular interactions resulting from π–π overlaps in the thin film. Moreover, a thermal annealing (100 °C) has shown to be benefit for improving the molecular organization as more intense π–π interactions were observed after a thermal treatment, which is more pronounced for 189. These features are important to ensure efficient charge transport within the film. Hence, relatively high hole-mobilities were measured from the films μh = 1.00 × 10−2 cm2 V−1 s−1 (189) and μh = 6.00 × 10−3 cm2 V−1 s−1 (190) even after thermal treatment beyond 200 °C moderate hole-mobilities are maintained μh = 3.00 × 10−3 cm2 V−1 s−1 at 230 °C (189) and μh = 2.60 × 10−3 cm2 V−1 s−1 at 250 °C (190).
Owing to these considerations, BHJ solar cells have been prepared with the standard structure ITO/MoOx/189 or 190![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC61BM/Al and tested. An optimal weight ratio of 60
PC61BM/Al and tested. An optimal weight ratio of 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 for 189
40 for 189![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC61BM blend followed by a thermal treatment at 100 °C (10 min.) affords a PCE of 5.80% (Voc = 0.71 V, JSC = 13.60 mA cm−2, FF = 0.60) while a PCE of 6.50% (Voc = 0.66 V, JSC = 15.20 mA cm−2, FF = 0.65) is reached for 190
PC61BM blend followed by a thermal treatment at 100 °C (10 min.) affords a PCE of 5.80% (Voc = 0.71 V, JSC = 13.60 mA cm−2, FF = 0.60) while a PCE of 6.50% (Voc = 0.66 V, JSC = 15.20 mA cm−2, FF = 0.65) is reached for 190![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC61BM blend at ratio 50
PC61BM blend at ratio 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 without any post-deposition treatment. Indeed, it is worth noticing that for 190
50 without any post-deposition treatment. Indeed, it is worth noticing that for 190![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC61BM blends thermal annealing does not affect the photovoltaic performances, which are also quite insensitive to the blend ratio composition. The general trends observed for 189 and 190 will be useful to design other materials with improved performances that can withstand major environmental changes.
PC61BM blends thermal annealing does not affect the photovoltaic performances, which are also quite insensitive to the blend ratio composition. The general trends observed for 189 and 190 will be useful to design other materials with improved performances that can withstand major environmental changes.
Conclusions
The last decade has witnessed a rapid progress of organic photovoltaics boosted by the design and synthesis of novel π-conjugated small donor molecules, the control and optimization of both device processing and fabrication. Although some important progress has been reached, current challenges remain to further improve efficiency, durability and cost-effectiveness in order to compete with silicon-based solar cells. Within this article we aimed at suggesting some guiding principles for designing new efficient and emerging alternatives to π-conjugated polymers that are solution-processable, facile to synthesize, simple to purify and monodisperse for a batch-to-batch reproducibility. To date, efficiencies up to 8% have been reported and important understandings were found. The main approaches consist in either a modification of the photoactive layer itself or addition of extra transport/blocking layers to facilitate the contacts. As the injection and the photovoltage critically depend on the interface, molecular modifications or physical treatments have been proposed to appropriately modulate the injection barriers either at the electron or hole collecting electrodes (for instance, LiF or PEDOT:PSS respectively). As seen, optimized light harvesting material is also required to match the solar spectrum and to enhance the photoconversion efficiency. Substantial increases of the solar photon harvesting have led to the design and synthesis of suitable organic low band-gaps materials with appropriate side-groups. Another way is to replace the PC61BM by PC71BM or by changing the nature of the acceptor with the aim to increase the absorption range. In addition, novel solutions can be applied to achieve the same effect, for instance the combination of small molecules with complementary absorption spectrum might be tested in the future. More importantly, the nanoscale morphology of the blend dramatically influences the photovoltaic properties. Hence, in order to increase the efficiency it is crucial to improve the miscibility of the mixed materials leading to finer phase segregation. This can be achieved by adding plasticizer additive, choosing the best solvents, controlling the evaporation time, their wettability and surface interactions, applying thermal annealing and varying the D–A composition. It is of crucial interest to develop also new methodologies and concepts to control the nanomorphology of the bulk heterojunction based on our supramolecular chemistry knowledge, crystallisation processes and solid-sate controlled orientation.Acknowledgements
This work was supported by the Centre National de la Recherche Scientifique, the Ministère de l'Enseignement Supérieur et de la Recherche (MESR) and Aix-Marseille Université throughout their financial support. Financial support from ANR program (SAGe III-V project ANR-11-BS10-012) and “Solutions Communicantes Sécurisées” (SCS) competitive cluster are also acknowledged. V.M. thanks also the Ministère de l'Enseignement Supérieur et de la Recherche for its doctoral financial support.Notes and references
- G. A. Chamberlain, Sol. Cells, 1983, 8, 47–83 CrossRef CAS.
- C. W. Tang, Appl. Phys. Lett., 1986, 48, 183–185 CrossRef CAS PubMed.
- N. S. Sariciftci, L. Smilowitz, A. J. Heeger and F. Wudi, Science, 1992, 258, 1474–1476 CAS.
- (a) C. Uhrich, R. Schueppel, A. Petrich, M. Pfeiffer, K. Leo, E. Brier, P. Kilickiran and P. Baeuerle, Adv. Funct. Mater., 2007, 17, 2991–2999 CrossRef CAS; (b) P. Huhomme, EPJ Photovoltaics, 2013, 4, 40401 CrossRef PubMed.
- (a) J. Roncali, Acc. Chem. Res., 2009, 42, 1719–1730 CrossRef CAS PubMed; (b) B. Walker, C. Kim and T.-Q. Nguyen, Chem. Mater., 2011, 23, 470–482 CrossRef CAS; (c) A. Mishra and P. Bauerle, Angew. Chem., Int. Ed., 2012, 51, 2020–2067 CrossRef CAS PubMed; (d) Y. Lin, Y. Li and X. Zhan, Chem. Soc. Rev., 2012, 41, 4245–4270 RSC; (e) J. Roncali, P. Leriche and P. Blanchard, Adv. Mater., 2014, 26, 3821–3838 CrossRef CAS PubMed; (f) J. E. Coughlin, Z. B. Henson, G. C. Welch and G. C. Bazan, Acc. Chem. Res., 2014, 47, 257–270 CrossRef CAS PubMed.
- (a) M. A. Green, K. Emery, Y. Hishikawa, W. Warta and E. D. Dunlop, Progress in Photovoltaics: Research and Applications, 2013, 21, 827–837 CrossRef; (b) R. F. Service, Science, 2011, 332, 293 CrossRef CAS PubMed.
- P. F. Xia, X. J. Feng, J. Lu, S.-W. Tsang, R. Movileanu, Y. Tao and M. S. Wong, Adv. Mater., 2008, 20, 4810–4815 CrossRef CAS.
- W. Zhang, S. C. Tse, J. Lu, Y. Tao and M. S. Wong, J. Mater. Chem., 2010, 20, 2182–2189 RSC.
- P. F. Xia, X. J. Feng, J. Lu, R. Movileanu, Y. Tao, J.-M. Baribeau and M. S. Wong, J. Phys. Chem. C, 2008, 112, 16714–16720 CAS.
- H. M. Ko, H. Choi, S. Paek, K. Kim, K. Song, J. K. Lee and J. Ko, J. Mater. Chem., 2011, 21, 7248–7253 RSC.
- N. Cho, J. Kim, K. Song, J. K. Lee and J. Ko, Tetrahedron, 2012, 68, 4029–4036 CrossRef CAS PubMed.
- J. Kim, H. M. Ko, N. Cho, S. Paek, J. K. Lee and J. Ko, RSC Adv., 2012, 2, 2692–2695 RSC.
- H.-W. Lin, L.-Y. Lin, Y.-H. Chen, C.-W. Chen, Y. Lin, S.-W. Chiu and K.-T. Wong, Chem. Commun., 2011, 47, 7872–7874 RSC.
- H. Bürckstümmer, E. V. Tulyakova, M. Deppisch, M. R. Lenze, N. M. Kronenberg, M. Gsänger, M. Stolte, K. Meerholz and F. Würthner, Angew. Chem., 2011, 123, 11832–11836 CrossRef.
- A. Leliège, C.-H. Le Régent, M. Allain, P. Blanchard and J. Roncali, Chem. Commun., 2012, 48, 8907–8909 RSC.
- V. Jeux, D. Demeter, P. Leriche and J. Roncali, RSC Adv., 2013, 3, 5811–5814 RSC.
- A. Leliège, J. Grolleau, M. Allain, P. Blanchard, D. Demeter, T. Rousseau and J. Roncali, Chem.–Eur. J., 2013, 19, 9948–9960 CrossRef PubMed.
- L.-Y. Lin, Y.-H. Chen, Z.-Y. Huang, H.-W. Lin, S.-H. Chou, F. Lin, C.-W. Chen, Y.-H. Liu and K.-T. Wong, J. Am. Chem. Soc., 2011, 133, 15822–15825 CrossRef CAS PubMed.
- S.-W. Chiu, L.-Y. Lin, H.-W. Lin, Y.-H. Chen, Z.-Y. Huang, Y.-T. Lin, F. Lin, Y.-H. Liu and K.-T. Wong, Chem. Commun., 2012, 48, 1857–1859 RSC.
- Y.-H. Chen, L.-Y. Lin, C.-W. Lu, F. Lin, Z.-Y. Huang, H.-W. Lin, P.-H. Wang, Y.-H. Liu, K.-T. Wong, J. Wen, D. J. Miller and S. B. Darling, J. Am. Chem. Soc., 2012, 134, 13616–13623 CrossRef CAS PubMed.
- H.-W. Lin, H.-W. Kang, Z.-Y. Huang, C.-W. Chen, Y.-H. Chen, L.-Y. Lin, F. Lin and K.-T. Wong, Org. Electron., 2012, 13, 1925–1929 CrossRef CAS PubMed.
- S. Roquet, A. Cravino, P. Leriche, O. Alévêque, P. Frère and J. Roncali, J. Am. Chem. Soc., 2006, 128, 3459–3466 CrossRef CAS PubMed.
- A. Cravino, P. Leriche, O. Alévêque, S. Roquet and J. Roncali, Adv. Mater., 2006, 18, 3033–3037 CrossRef CAS.
- A. Cravino, S. Roquet, O. Alévêque, P. Leriche, P. Frère and J. Roncali, Chem. Mater., 2006, 18, 2584–2590 CrossRef CAS.
- E. Ripaud, T. Rousseau, P. Leriche and J. Roncali, Adv. Energy Mater., 2011, 1, 540–545 CrossRef CAS.
- O. Kim, A. Fort, M. Barzoukas, M. Blanchard-Desce and J.-M. Lehn, J. Mater. Chem., 1999, 9, 2227–2232 RSC.
- D. Deng, S. Shen, J. Zhang, C. He, Z. Zhang and Y. Li, Org. Electron., 2012, 13, 2546–2552 CrossRef CAS PubMed.
- Y. Lin, Z.-G. Zhang, H. Bai, Y. Li and X. Zhan, Chem. Commun., 2012, 48, 9655–9657 RSC.
- Y. Lin, Z.-G. Zhang, Y. Li, D. Zhu and X. Zhan, J. Mater. Chem. A, 2013, 1, 5128–5135 CAS.
- S. Shen, L. Gao, C. He, Z. Zhang, Q. Sun and Y. Li, Org. Electron., 2013, 14, 875–881 CrossRef CAS PubMed.
- J. Zhang, D. Deng, C. He, Y. He, M. Zhang, Z.-G. Zhang, Z. Zhang and Y. Li, Chem. Mater., 2011, 23, 817–822 CrossRef CAS.
- J. Min, Y. N. Luponosov, T. Ameri, A. Elschner, S. M. Peregudova, D. Baran, T. Heumüller, N. Li, F. Machui, S. Ponomarenko and C. J. Brabec, Org. Electron., 2013, 14, 219–229 CrossRef CAS PubMed.
- H. Shang, H. Fan, Y. Liu, W. Hu, Y. Li and X. Zhan, Adv. Mater., 2011, 23, 1554–1557 CrossRef CAS PubMed.
- J. Zhang, J. Yu, C. He, D. Deng, Z.-G. Zhang, M. Zhang, Z. Li and Y. Li, Org. Electron., 2012, 13, 166–172 CrossRef CAS PubMed.
- L. Gao, J. Zhang, C. He, S. Shen, Y. Zhang, H. Liu, Q. Sun and Y. Li, Sci. China Chem., 2013, 56, 997–1003 CrossRef CAS.
- G. Wu, G. Zhao, C. He, J. Zhang, Q. He, X. Chen and Y. Li, Sol. Energy Mater. Sol. Cells, 2009, 93, 108–113 CrossRef CAS PubMed.
- C. He, Q. He, Y. Yi, G. Wu, F. Bai, Z. Shuai and Y. Li, J. Mater. Chem., 2008, 18, 4085–4090 RSC.
- X. Guo, L. Xiao, W. Tang, B. Liu, R. Cui and Y. Zou, J. Mater. Sci., 2013, 48, 5833–5839 CrossRef CAS.
- Y. Lin, P. Cheng, Y. Li and X. Zhan, Chem. Commun., 2012, 48, 4773–4775 RSC.
- J.-Y. Pan, L.-J. Zuo, X.-L. Hu, W.-F. Fu, M.-R. Chen, L. Fu, X. Gu, H.-Q. Shi, M.-M. Shi, H.-Y. Li and H.-Z. Chen, ACS Appl. Mater. Interfaces, 2013, 5, 972–980 CAS.
- A. Tang, L. Li, Z. Lu, J. Huang, H. Jia, C. Zhan, Z. Tan, Y. Li and J. Yao, J. Mater. Chem. A, 2013, 1, 5747–5757 CAS.
- Y. Jiang, D. Yu, L. Lu, C. Zhan, D. Wu, W. You, Z. Xie and S. Xiao, J. Mater. Chem. A, 2013, 1, 8270–8279 CAS.
- Z. Lu, C. Li, T. Fang, G. Li and Z. Bo, J. Mater. Chem. A, 2013, 1, 7657–7665 CAS.
- D. Ni, B. Zhao, T. Shi, S. Ma, G. Tu and H. Wu, ACS Macro Lett., 2013, 2, 621–624 CrossRef CAS.
- D. Demeter, T. Rousseau, P. Leriche, T. Cauchy, R. Po and J. Roncali, Adv. Funct. Mater., 2011, 21, 4379–4387 CrossRef CAS.
- Y. Liu, X. Wan, B. Yin, J. Zhou, G. Long, S. Yin and Y. Chen, J. Mater. Chem., 2010, 20, 2464–2468 RSC.
- B. Yin, L. Yang, Y. Liu, Y. Chen, Q. Qi, F. Zhang and S. Yin, Appl. Phys. Lett., 2010, 97, 023303 CrossRef PubMed.
- Y. Liu, X. Wan, F. Wang, J. Zhou, G. Long, J. Tian, J. You, Y. Yang and Y. Chen, Adv. Energy Mater., 2011, 1, 771–775 CrossRef CAS.
- Z. Li, G. He, X. Wan, Y. Liu, J. Zhou, G. Long, Y. Zuo, M. Zhang and Y. Chen, Adv. Energy Mater., 2012, 2, 74–77 CrossRef CAS.
- G. He, Z. Li, X. Wan, Y. Liu, J. Zhou, G. Long, M. Zhang and Y. Chen, J. Mater. Chem., 2012, 22, 9173–9180 RSC.
- G. He, Z. Li, X. Wan, J. Zhou, G. Long, S. Zhang, M. Zhang and Y. Chen, J. Mater. Chem. A, 2013, 1, 1801–1809 CAS.
- J. Zhou, X. Wan, Y. Liu, G. Long, F. Wang, Z. Li, Y. Zuo, C. Li and Y. Chen, Chem. Mater., 2011, 23, 4666–4668 CrossRef CAS.
- Y. Liu, X. Wan, F. Wang, J. Zhou, G. Long, J. Tian and Y. Chen, Adv. Mater., 2011, 23, 5387–5391 CrossRef CAS PubMed.
- J. Zhou, X. Wan, Y. Liu, Y. Zuo, Z. Li, G. He, G. Long, W. Ni, C. Li, X. Su and Y. Chen, J. Am. Chem. Soc., 2012, 134, 16345–16351 CrossRef CAS PubMed.
- C. Cui, J. Min, C.-L. Ho, T. Ameri, P. Yang, J. Zhao, C. J. Brabec and W.-Y. Wong, Chem. Commun., 2013, 49, 4409–4411 RSC.
- D. Patra, C.-C. Chiang, W.-A. Chen, K.-H. Wei, M.-C. Wu and C.-W. Chu, J. Mater. Chem. A, 2013, 1, 7767–7774 CAS.
- S. Shen, P. Jiang, C. He, J. Zhang, P. Shen, Y. Zhang, Y. Yi, Z. Zhang, Z. Li and Y. Li, Chem. Mater., 2013, 25, 2274–2281 CrossRef CAS.
- J. Huang, C. Zhan, X. Zhang, Y. Zhao, Z. Lu, H. Jia, B. Jiang, J. Ye, S. Zhang, A. Tang, Y. Liu, Q. Pei and J. Yao, ACS Appl. Mater. Interfaces, 2013, 5, 2033–2039 CAS.
- Y. Lin, L. Ma, Y. Li, Y. Liu, D. Zhu and X. Zhan, Adv. Energy Mater., 2013, 3, 1166–1170 CrossRef CAS.
- Y. S. Choi and W. H. Jo, Org. Electron., 2013, 14, 1621–1628 CrossRef CAS PubMed.
- L. Zhang, S. Zeng, L. Yin, C. Ji, K. Li, Y. Li and Y. Wang, New J. Chem., 2013, 37, 632–639 RSC.
- X. Lin, Y. Tani, R. Kanda, K. Nakayama and S. Yagai, J. Mater. Chem. A, 2013, 1, 14686–14691 CAS.
- B. Walker, J. Liu, C. Kim, G. C. Welch, J. K. Park, J. Lin, P. Zalar, C. M. Proctor, J. H. Seo, G. C. Bazan and T.-Q. Nguyen, Energy Environ. Sci., 2013, 6, 952–962 CAS.
- W. Li, M. Kelchtermans, M. M. Wienk and R. a. J. Janssen, J. Mater. Chem. A, 2013, 1, 15150–15157 CAS.
- H. Bai, P. Cheng, Y. Wang, L. Ma, Y. Li, D. Zhu and X. Zhan, J. Mater. Chem. A, 2014, 2, 778–784 CAS.
- K. Schulze, C. Uhrich, R. Schüppel, K. Leo, M. Pfeiffer, E. Brier, E. Reinold and P. Bäuerle, Adv. Mater., 2006, 18, 2872–2875 CrossRef CAS.
- R. Fitzner, E. Reinold, A. Mishra, E. Mena-Osteritz, H. Ziehlke, C. Körner, K. Leo, M. Riede, M. Weil, O. Tsaryova, A. Weiß, C. Uhrich, M. Pfeiffer and P. Bäuerle, Adv. Funct. Mater., 2011, 21, 897–910 CrossRef CAS.
- R. Fitzner, E. Mena-Osteritz, A. Mishra, G. Schulz, E. Reinold, M. Weil, C. Körner, H. Ziehlke, C. Elschner, K. Leo, M. Riede, M. Pfeiffer, C. Uhrich and P. Bäuerle, J. Am. Chem. Soc., 2012, 134, 11064–11067 CrossRef CAS PubMed.
- (a) S. Haid, A. Mishra, C. Uhrich, M. Pfeiffer and P. Bäuerle, Chem. Mater., 2011, 23, 4435–4444 CrossRef CAS; (b) N. F. Montcada, B. Pelado, A. Viterisi, J. Albero, J. Coro, P. de la Cruz, F. Langa and E. Palomares, Org. Electron., 2013, 14, 2826–2832 CrossRef CAS PubMed.
- S. Steinberger, A. Mishra, E. Reinold, J. Levichkov, C. Uhrich, M. Pfeiffer and P. Bäuerle, Chem. Commun., 2011, 47, 1982–1984 RSC.
- P. Li, H. Tong, J. Ding, Z. Xie and L. Wang, J. Mater. Chem. A, 2013, 1, 8805–8812 CAS.
- Y. Chen, C. Li, P. Zhang, Y. Li, X. Yang, L. Chen and Y. Tu, Org. Electron., 2013, 14, 1424–1434 CrossRef CAS PubMed.
- S. Steinberger, A. Mishra, E. Reinold, C. M. Müller, C. Uhrich, M. Pfeiffer and P. Bäuerle, Org. Lett., 2011, 13, 90–93 CrossRef CAS PubMed.
- X. Wan, Y. Liu, F. Wang, J. Zhou, G. Long and Y. Chen, Org. Electron., 2013, 14, 1562–1569 CrossRef CAS PubMed.
- S. Steinberger, A. Mishra, E. Reinold, E. Mena-Osteritz, H. Müller, C. Uhrich, M. Pfeiffer and P. Bäuerle, J. Mater. Chem., 2012, 22, 2701–2712 RSC.
- J. a. Mikroyannidis, M. M. Stylianakis, P. Balraju, P. Suresh and G. D. Sharma, ACS Appl. Mater. Interfaces, 2009, 1, 1711–1718 CAS.
- G. D. Sharma, P. Suresh, J. a. Mikroyannidis and M. M. Stylianakis, J. Mater. Chem., 2010, 20, 561–567 RSC.
- L.-Y. Lin, C. Lu, W. Huang, Y. Chen, H. Lin and K.-T. Wong, Org. Lett., 2011, 13, 4962–4965 CrossRef CAS PubMed.
- D. Ye, X. Li, L. Yan, W. Zhang, Z. Hu, Y. Liang, J. Fang, W.-Y. Wong and X. Wang, J. Mater. Chem. A, 2013, 1, 7622–7629 CAS.
- K.-H. Kim, H. Yu, H. Kang, D. J. Kang, C.-H. Cho, H.-H. Cho, J. H. Oh and B. J. Kim, J. Mater. Chem. A, 2013, 1, 14538–14547 CAS.
- K. H. Lam, T. R. B. Foong, Z. E. Ooi, J. Zhang, A. C. Grimsdale and Y. M. Lam, ACS Appl. Mater. Interfaces, 2013, 5, 13265–13274 CAS.
- C. He, Q. He, Y. He, Y. Li, F. Bai, C. Yang, Y. Ding, L. Wang and J. Ye, Sol. Energy Mater. Sol. Cells, 2006, 90, 1815–1827 CrossRef CAS PubMed.
- W. Li, C. Du, F. Li, Y. Zhou, M. Fahlman, Z. Bo and F. Zhang, Chem. Mater., 2009, 21, 5327–5334 CrossRef CAS.
- H. Shang, H. Fan, Q. Shi, S. Li, Y. Li and X. Zhan, Sol. Energy Mater. Sol. Cells, 2010, 94, 457–464 CrossRef CAS PubMed.
- Q. Hou, Y. Chen, H. Zhen, Z. Ma, W. Hong, G. Shi and F. Zhang, J. Mater. Chem. A, 2013, 1, 4937–4940 CAS.
- D. Deng, Y. Yang, J. Zhang, C. He, M. Zhang, Z.-G. Zhang, Z. Zhang and Y. Li, Org. Electron., 2011, 12, 614–622 CrossRef CAS PubMed.
- J. Zhang, G. Wu, C. He, D. Deng and Y. Li, J. Mater. Chem., 2011, 21, 3768–3774 RSC.
- Y. Lin, P. Cheng, Y. Liu, X. Zhao, D. Li, J. Tan, W. Hu, Y. Li and X. Zhan, Sol. Energy Mater. Sol. Cells, 2012, 99, 301–307 CrossRef CAS PubMed.
- Y. Lin, P. Cheng, Y. Liu, Q. Shi, W. Hu, Y. Li and X. Zhan, Org. Electron., 2012, 13, 673–680 CrossRef CAS PubMed.
- (a) Q. Liu, Z. Du, W. Chen, L. Sun, Y. Chen, M. Sun and R. Yang, Synth. Met., 2013, 178, 38–43 CrossRef CAS PubMed; (b) W. Ying, F. Guo, J. Li, Q. Zhang, W. Wu, H. Tian and J. Hua, ACS Appl. Mater. Interfaces, 2012, 4, 4215–4224 CrossRef CAS PubMed.
- H.-Y. Wang, J. Gao, L.-J. Gu, J.-H. Wan, W. Wei and F. Liu, J. Mater. Chem. A, 2013, 1, 5875–5885 CAS.
- S. Song, T. Kim, H. Park, Y. Jin, I. Kim, J. Y. Kim and H. Suh, Synth. Met., 2013, 183, 16–23 CrossRef CAS PubMed.
- M. Sun, L. Wang, X. Zhu, B. Du, R. Liu, W. Yang and Y. Cao, Sol. Energy Mater. Sol. Cells, 2007, 91, 1681–1687 CrossRef CAS PubMed.
- M. Sun, L. Wang, B. Du, Y. Xiong, R. Liu and Y. Cao, Synth. Met., 2008, 158, 125–129 CrossRef CAS PubMed.
- V. Tamilavan, M. Song, S.-H. Jin and M. H. Hyun, Bull. Korean Chem. Soc., 2013, 34, 661–664 CrossRef CAS.
- S. Zeng, L. Yin, X. Jiang, Y. Li and K. Li, Dyes Pigm., 2012, 95, 229–235 CrossRef CAS PubMed.
- S. Zeng, L. Yin, C. Ji, X. Jiang, K. Li, Y. Li and Y. Wang, Chem. Commun., 2012, 48, 10627–10629 RSC.
- S. Paek, N. Cho, K. Song, M.-J. Jun, J. K. Lee and J. Ko, J. Phys. Chem. C, 2012, 116, 23205–23213 CAS.
- M. Nazim, S. Ameen, M. S. Akhtar, Y.-S. Lee and H.-S. Shin, Chem. Phys. Lett., 2013, 574, 89–93 CrossRef CAS PubMed.
- P. Cheng, Q. Shi, Y. Lin, Y. Li and X. Zhan, Org. Electron., 2013, 14, 599–606 CrossRef CAS PubMed.
- A. B. Tamayo, B. Walker and T.-Q. Nguyen, J. Phys. Chem. C, 2008, 112, 11545–11551 CAS.
- W. Shin, T. Yasuda, G. Watanabe, Y. S. Yang and C. Adachi, Chem. Mater., 2013, 25, 2549–2556 CrossRef CAS.
- B. Walker, A. B. Tamayo, X.-D. Dang, P. Zalar, J. H. Seo, A. Garcia, M. Tantiwiwat and T.-Q. Nguyen, Adv. Funct. Mater., 2009, 19, 3063–3069 CrossRef CAS.
- J. Liu, Y. Zhang, H. Phan, A. Sharenko, P. Moonsin, B. Walker, V. Promarak and T.-Q. Nguyen, Adv. Mater., 2013, 25, 3645–3650 CrossRef CAS PubMed.
- B. Walker, X. Han, C. Kim, A. Sellinger and T.-Q. Nguyen, ACS Appl. Mater. Interfaces, 2012, 4, 244–250 CAS.
- C. Kim, J. Liu, J. Lin, A. B. Tamayo, B. Walker, G. Wu and T.-Q. Nguyen, Chem. Mater., 2012, 24, 1699–1709 CrossRef CAS.
- E. Ripaud, D. Demeter, T. Rousseau, E. Boucard-Cétol, M. Allain, R. Po, P. Leriche and J. Roncali, Dyes Pigm., 2012, 95, 126–133 CrossRef CAS PubMed.
- J. Huang, H. Jia, L. Li, Z. Lu, W. Zhang, W. He, B. Jiang, A. Tang, Z. Tan, C. Zhan, Y. Li and J. Yao, Phys. Chem. Chem. Phys., 2012, 14, 14238–14242 RSC.
- O. P. Lee, A. T. Yiu, P. M. Beaujuge, C. H. Woo, T. W. Holcombe, J. E. Millstone, J. D. Douglas, M. S. Chen and J. M. J. Fréchet, Adv. Mater., 2011, 23, 5359–5363 CrossRef CAS PubMed.
- J.-W. Mun, I. Cho, D. Lee, W. S. Yoon, O. K. Kwon, C. Lee and S. Y. Park, Org. Electron., 2013, 14, 2341–2347 CrossRef CAS PubMed.
- A. Ruiz-Carretero, T. Aytun, C. J. Bruns, C. J. Newcomb, W.-W. Tsai and S. I. Stupp, J. Mater. Chem. A, 2013, 1, 11674–11681 CAS.
- F. Lincker, N. Delbosc, S. Bailly, R. De Bettignies, M. Billon, A. Pron and R. Demadrille, Adv. Funct. Mater., 2008, 18, 3444–3453 CrossRef CAS.
- J. Mei, K. R. Graham, R. Stalder and J. R. Reynolds, Org. Lett., 2010, 12, 660–663 CrossRef CAS PubMed.
- A. Leliège, P. Blanchard, T. Rousseau and J. Roncali, Org. Lett., 2011, 13, 3098–3101 CrossRef PubMed.
- P. Dutta, W. Yang, S. H. Eom and S.-H. Lee, Org. Electron., 2012, 13, 273–282 CrossRef CAS PubMed.
- P. Dutta, H. Park, W.-H. Lee, I.-N. Kang and S.-H. Lee, Org. Electron., 2012, 13, 3183–3194 CrossRef CAS PubMed.
- P. Dutta, W. Yang, W.-H. Lee, I. N. Kang and S.-H. Lee, J. Mater. Chem., 2012, 22, 10840–10851 RSC.
- S. Loser, C. J. Bruns, H. Miyauchi, R. P. Ortiz, A. Facchetti, S. I. Stupp and T. J. Marks, J. Am. Chem. Soc., 2011, 133, 8142–8145 CrossRef CAS PubMed.
- S. Loser, H. Miyauchi, J. W. Hennek, J. Smith, C. Huang, A. Facchetti and T. J. Marks, Chem. Commun., 2012, 48, 8511–8513 RSC.
- W. Yong, M. Zhang, X. Xin, Z. Li, Y. Wu, X. Guo, Z. Yang and J. Hou, J. Mater. Chem. A, 2013, 1, 14214–14220 CAS.
- P. Dutta, J. Kim, S. H. Eom, W.-H. Lee, I. N. Kang and S. Lee, ACS Appl. Mater. Interfaces, 2012, 4, 6669–6675 CAS.
- G. C. Welch, L. a. Perez, C. V. Hoven, Y. Zhang, X.-D. Dang, A. Sharenko, M. F. Toney, E. J. Kramer, T.-Q. Nguyen and G. C. Bazan, J. Mater. Chem., 2011, 21, 12700–12709 RSC.
- L. G. Kaake, G. C. Welch, D. Moses, G. C. Bazan and A. J. Heeger, J. Phys. Chem. Lett., 2012, 3, 1253–1257 CrossRef CAS.
- J. J. Jasieniak, B. B. Y. Hsu, C. J. Takacs, G. C. Welch, G. C. Bazan, D. Moses and A. J. Heeger, ACS Nano, 2012, 6, 8735–8745 CrossRef CAS PubMed.
- N. D. Eisenmenger, G. M. Su, G. C. Welch, C. J. Takacs, G. C. Bazan, E. J. Kramer and M. L. Chabinyc, Chem. Mater., 2013, 25, 1688–1698 CrossRef CAS.
- Y. Sun, G. C. Welch, W. L. Leong, C. J. Takacs, G. C. Bazan and A. J. Heeger, Nat. Mater., 2012, 11, 44–48 CrossRef CAS PubMed.
- C. J. Takacs, Y. Sun, G. C. Welch, L. A. Perez, X. Liu, W. Wen, G. C. Bazan and A. J. Heeger, J. Am. Chem. Soc., 2012, 134, 16597–16606 CrossRef CAS PubMed.
- T. S. van der Poll, J. A. Love, T.-Q. Nguyen and G. C. Bazan, Adv. Mater., 2012, 24, 3646–3649 CrossRef CAS PubMed.
- A. Sharenko, C. M. Proctor, T. S. van der Poll, Z. B. Henson, T.-Q. Nguyen and G. C. Bazan, Adv. Mater., 2013, 25, 4403–4406 CrossRef CAS PubMed.
- X. Liu, Y. Sun, L. a. Perez, W. Wen, M. F. Toney, A. J. Heeger and G. C. Bazan, J. Am. Chem. Soc., 2012, 134, 20609–20612 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |