Catalytic cracking of jatropha-derived fast pyrolysis oils with VGO and their NMR characterization
Desavath V. Naika,
Vimal Kumar*b,
Basheshwar Prasadb,
Mukesh K. Poddara,
Babita Beheraa,
Rajaram Bala,
Om. P. Khatria,
Dilip K. Adhikaria and
Madhukar O. Garga
aBio-fuels Division, CSIR-Indian Institute of Petroleum, Dehradun 248005, India
bDepartment of Chemical Engineering, Indian Institute of Technology Roorkee, Roorkee 247667, India. E-mail: vksinfch@iitr.ac.in
First published on 6th November 2014
Abstract
Lignocellulosic biomass-derived fast pyrolysis oils are potential second-generation bio-fuels towards the reduction of greenhouse gas (GHG) emissions and carbon foot prints. This study pertains to co-process the Jatropha-derived heavy or tar fraction of fast pyrolysis oil (FPO) with vacuum gas oil (VGO) and hydrodeoxygenated fast pyrolysis oil (HDO) with VGO in a standard refinery fluid catalytic cracking (FCC) unit. The crude fast pyrolysis oil from Jatropha curcas is produced at 530 °C and atmospheric pressure using a bubbling fluidized bed pyrolyzer. The heavy fraction of FPO is hydrodeoxygenated over Pd/Al2O3 catalyst into HDO in an autoclave reactor at 300 °C and pressure of 80 bar. Further, HDO is co-processed with petroleum-derived VGO in an advanced cracking evaluation (ACE-R) unit to convert it into refinery FCC product slate hydrocarbons at a blending ratio of 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 95. FPO and HDO are characterized using 31P NMR, whereas FCC distillates, which are obtained on the co-processing of VGO with fast pyrolysis oil and HDO, are characterized using 1H and 13C NMR spectroscopy techniques. The 31P NMR analysis of crude FPO and HDO indicated that hydroxyl, carboxylic and methoxy groups are reduced during the hydrodeoxygenation of FPO. The experimental results at the iso-conversion level on the co-processing of HDO with VGO indicated a higher yield of liquefied petroleum gases (LPG), while lower yields of gasoline and LCO have been observed as compared to FPO co-processing with VGO and co-processing of pure VGO. Furthermore, the results of co-processing of FPO with VGO indicated that the yields of gasoline and LCO increased from 29 to 35 wt% and 14.8 to 20.4 wt%, respectively, whereas the yields of dry gas and LPG decreased from 2.1 to 1.4 wt% and 38.8 to 23.7 wt%, respectively, for an increase in the blending ratio from 5% to 20%. Therefore, it can be concluded that the co-processing of HDO with VGO in a FCC unit would be feasible in order to achieve a higher yield of LPG.
95. FPO and HDO are characterized using 31P NMR, whereas FCC distillates, which are obtained on the co-processing of VGO with fast pyrolysis oil and HDO, are characterized using 1H and 13C NMR spectroscopy techniques. The 31P NMR analysis of crude FPO and HDO indicated that hydroxyl, carboxylic and methoxy groups are reduced during the hydrodeoxygenation of FPO. The experimental results at the iso-conversion level on the co-processing of HDO with VGO indicated a higher yield of liquefied petroleum gases (LPG), while lower yields of gasoline and LCO have been observed as compared to FPO co-processing with VGO and co-processing of pure VGO. Furthermore, the results of co-processing of FPO with VGO indicated that the yields of gasoline and LCO increased from 29 to 35 wt% and 14.8 to 20.4 wt%, respectively, whereas the yields of dry gas and LPG decreased from 2.1 to 1.4 wt% and 38.8 to 23.7 wt%, respectively, for an increase in the blending ratio from 5% to 20%. Therefore, it can be concluded that the co-processing of HDO with VGO in a FCC unit would be feasible in order to achieve a higher yield of LPG.
1. Introduction
The worldwide consumption of liquid fuels is bound to increase from 87 to 97 million barrels per day from 2010 to 2020, respectively, and it is projected to increase to 115 million barrels per day in 2040.1 The proved world oil reserves were estimated to be ∼1638 billion barrels as of January 1, 2013.2 The world oil reserves could be depleted soon in the coming decades with the present rate of consumption. Hence, research is focused on second generation bio-fuels (besides other resources) for the production of liquid fuels from lignocellulosic biomass. Therefore, the conventional (thermal) fast pyrolysis route is an effective approach to convert biomass into higher yields (50 to 75 wt%) of liquid fraction (crude fast pyrolysis oil) at atmospheric pressure and moderate temperature of ∼500 °C. The crude fast pyrolysis oil as such cannot be used as a liquid fuel due to its lower heating value (15–20 MJ kg−1) and the presence of oxygenated compounds that self-react during handling at ambient temperatures to form larger molecules.3,4 The crude fast pyrolysis oil is a complex mixture of water, carboxylic acids, hydroxy-aldehydes, hydroxy-ketones, phenolics, guaiacols, catechols, syringols, vanillins, sugars, and levoglucosan.5 Therefore, crude fast pyrolysis oil requires further upgrading in order to convert it into usable liquid hydrocarbons.Thus, a number of upgrading technologies have been proposed in the last few decades, such as thermal treatment,6,7 high pressure thermal treatment,8,9 thermal hydrotreating,10 catalytic hydrotreating,11–15 catalytic emulsion,16 and catalytic cracking.17–19 Among the aforementioned upgrading techniques, catalytic cracking seems to be a good option for the effective use of trillion dollars refinery infrastructure as well as the integration of the fast pyrolysis process with refinery.20 A critical review has been published by Talmadge et al.21 on the outlook of how to modify the overall chemistry of biomass-derived pyrolysis liquids in order to integrate the pyrolysis process with standard petroleum refineries. Chen et al.22 reported that the effective hydrogen index (H/Ceff) should be above the inflection point of 1.2 for energy production in FCC process with VGO as a feedstock; the same is applicable for co-processing of fast pyrolysis oil with VGO or LCO. Therefore, it is necessary to partially deoxygenate the fast pyrolysis oil to reduce the oxygen level in order to improve the H/Ceff of the pyrolysis oil for better processing in FCC units. Huber et al.7 proposed that, during the cracking of oxygenated molecules over the FCC catalyst, smaller hydrocarbons and coke can be obtained by dehydration reaction. In addition to dehydration the conventional catalytic cracking reactions such as cracking, hydrogen consuming, hydrogen producing and Diels–Alder (C–C bond formation) reactions also takes place. Fogassy et al.23 also proposed a simplified reaction mechanism for oxygen removal from biomass-derived molecules.
The conventional FCC technology is aimed to improve the gasoline yield; however, while co-processing the fast pyrolysis oil with VGO, it is extremely essential to look into the product characterization and the causes of coke formation. Samolada et al.10 co-processed the hydrotreated flash pyrolysis oil (a heavy fraction) with light cycle oil (LCO) for 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 85 blending ratio in a modified MAT fixed bed reactor system (MAT, ASTM D3907-80) over FCC (ReUSY2) catalyst. An increase in coke and gasoline production by 32% and 56%, respectively, was reported while co-processing hydrotreated flash pyrolysis oil (a heavy fraction) with LCO as compared to the pure LCO processing. Fogassy et al.23 reported higher dry gas and coke yields, lower LPG yields, similar yields of gasoline and LCO while co-processing HDO with VGO in 20
85 blending ratio in a modified MAT fixed bed reactor system (MAT, ASTM D3907-80) over FCC (ReUSY2) catalyst. An increase in coke and gasoline production by 32% and 56%, respectively, was reported while co-processing hydrotreated flash pyrolysis oil (a heavy fraction) with LCO as compared to the pure LCO processing. Fogassy et al.23 reported higher dry gas and coke yields, lower LPG yields, similar yields of gasoline and LCO while co-processing HDO with VGO in 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80 blending ratio as compared to the processing of pure VGO. They carried out the catalytic cracking reaction in a validated micro-activity test reactor (fixed bed quartz reactor) for VGO cracking over equilibrium FCC catalyst. They further extended the co-processing of HDO with VGO over various types of FCC catalysts in terms of structural parameters of zeolites.24 It was mentioned that most of the lignin-derived molecules on the co-processing of HDO are partially cracked into smaller methoxyphenols over FCC, HY and HZSM-5 catalysts, and it was reported that very few oxygenated molecules enter into the pores of the zeolite.
80 blending ratio as compared to the processing of pure VGO. They carried out the catalytic cracking reaction in a validated micro-activity test reactor (fixed bed quartz reactor) for VGO cracking over equilibrium FCC catalyst. They further extended the co-processing of HDO with VGO over various types of FCC catalysts in terms of structural parameters of zeolites.24 It was mentioned that most of the lignin-derived molecules on the co-processing of HDO are partially cracked into smaller methoxyphenols over FCC, HY and HZSM-5 catalysts, and it was reported that very few oxygenated molecules enter into the pores of the zeolite.
Mercader et al.15 carried out the co-processing of HDO with long residue in a fluidized bed MAT-5000 reactor over equilibrium FCC catalyst and reported near normal FCC gasoline (44–46 wt%) and LCO (23–25 wt%) products without an excessive increase in undesired coke and dry gases, as compared to the base feed. They further reported that high levels of oxygen can be allowed in upgraded HDO (up to 28 wt%) for co-processing in the FCC unit without deterioration of the yield structure.25 These studies were further extended for the co-processing of catalytic pyrolysis oil (CPO) with VGO and compared with the results of the co-processing of HDO with VGO.26 An increase in alkyl phenols, in addition to an increase in coke, olefins, and aromatics were reported, while co-processing CPO with VGO as compared to the HDO with VGO. In addition, several researchers17–19,27,28 studied the effect of fast pyrolysis oil representative model compounds on product yields while co-processing them with VGO/LCO; however, all these studies are limited to product yields.
Furthermore, 31P NMR is a powerful analytical technique for the identification and quantification of organic oxy-functional groups using the derivatization method, and it has a unique advantage over 1H and 13C NMR for the measurement of oxy components in biomass. It provides the quantitative information for various types of major hydroxyl groups in a relatively short experimental time with small amounts of sample. Compared to 1H NMR, the large range of chemical shifts reported for the 31P nucleus generates a better separation and resolution of signals. In addition, the 100% natural abundance of 31P and its high sensitivity renders 31P NMR a rapid analytical tool in comparison with 13C NMR. Among trivalent and pentavalent derivatization agents, trivalent phosphorous reagents provide a large difference in chemical shifts, which helps to carry out identification and quantification. Wroblewski et al.29 examined five trivalent reagents to derivatize organic model compounds, including phenols, aliphatic alcohols, and aromatic and aliphatic acids, with 2 chloro-4,4,5,5-tetramethyl-1,3,2-dioxaphospholane (TMDP). This method may have broad applicability in biomass conversion to second generation bio-fuels.30
The present investigation discusses the product distribution patterns of FCC on the co-processing of VGO with FPO at different blending ratios. The product profile at the iso-conversion level catalytic cracking of VGO, VGO with FPO, and VGO with HDO have been compared. Furthermore, 31P NMR spectroscopic technique has been employed to characterize FPO and HDO during the pretreatment of feed, while 1H and 13C has been used for the characterization of products.
2. Materials and methods
2.1 Materials
Expelled Jatropha curcas cake (average particle size of ∼1.4 mm) was used as a biomass feedstock for fast pyrolysis experiments. Palladium (99.9%, Sigma-Aldrich Chemicals) and γ-alumina Al2O3 (97%, Sigma-Aldrich Chemicals) were chosen as active and support materials for the preparation of the hydrodeoxygenating catalyst. The commercially available VGO was used for the co-processing studies, and its characteristics are given in our previous paper.38 The catalyst used in the advanced cracking evaluation unit was also an industrially available equilibrium fluid catalytic cracking (FCC) catalyst, i.e. E-CAT. The physicochemical characteristics of E-CAT are listed in our previous paper.38 E-CAT contains synthetic faujacite zeolite (USY or REUSY), silica-alumina matrix, clay (e.g. Kaolin clay) with binder and special additives. The H/Ceff of FPO and HDO were found to be greater than the inflection point of 1.2 and are shown in Table 2. Particularly, the feedstock having H/Ceff ≥ 1.2 can be easily processed in the fluid catalytic cracking unit for energy production.222.2 HDO catalyst preparation
Mesoporous alumina was prepared using the method proposed by Ray et al.31 Pd was loaded over alumina by the incipient wetness impregnation method. In a typical preparation method, 1.0 g of palladium(II) nitrate dihydrate was dissolved in 30 ml of water and 20 ml of ethanol. Subsequently, 20 g of γ-alumina (Al2O3) (surface area = 243 m2 g−1) was added. The mixture was stirred constantly at 80 °C for 5 hours to dry the sample. The dried sample was further dried at 120 °C overnight in an oven. Finally, the Pd metal loaded on alumina was calcined at 500 °C in a furnace for 8 h to prepare the Pd/Al2O3 catalyst (2% Pd on alumina). The typical pore diameter, of the mesoporous alumina, which is measured using the BET apparatus, was around 5 nm.2.3 Fast pyrolysis
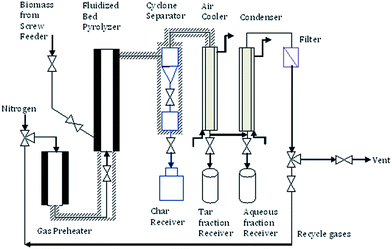
The continuous electrically heated bubbling fluidized bed fast pyrolyzer with sand as the fluidizing media was used for the fast pyrolysis of biomass at ∼530 °C and atmospheric pressure. The fluidizing gas (nitrogen) was preheated to 400 °C using an electric furnace. The biomass was fed into the reactor by a screw feeder system in continuous mode at a feed rate of 300 g h−1. The char was separated by a cyclone separator next to the reactor, vapors were condensed and separated as crude fast pyrolysis oil in a series of condensers, and the non-condensable gases were vented off to the atmosphere. The schematic diagram of the fast pyrolysis unit is shown in Fig. 1.2.4 Hydrodeoxygenation
The experiment aimed to produce partially hydrodeoxygenated fast pyrolysis oil, which is suitable for co-processing in a petroleum refinery fluid catalytic cracking unit. A known amount (2 wt%) of palladium on alumina catalyst was used in a 100 ml batch high pressure stirred reactor (USA made autoclave) to hydrogenate the heavy fraction of FPO. Initially, the reactor was purged with hydrogen gas for a period of 5 min, and then it was pressurized up to 80 bar. A constant speed of stirrer was maintained at 700 rpm. The reactor temperature was raised to reaction temperature from ambient temperature with a heating rate of 5 °C min−1. The reaction temperature was maintained for a period of 4 h. The reactor was then cooled down to ambient temperature. The liquid products were collected and analyzed separately using NMR spectroscopy and are listed in Table 5. The water content in the liquid product was measured with a Mitsubishi MCI moisture meter using the Karl Fischer technique.2.5 Catalytic cracking
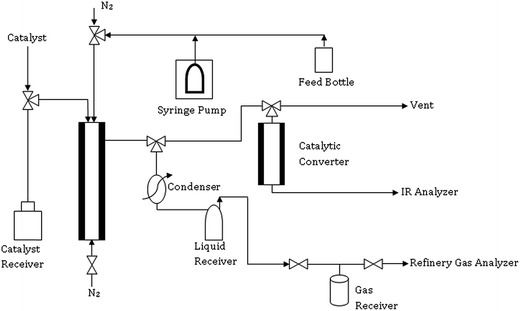
Advanced Cracking Evaluation (ACE-R™) unit, M/s. Kayser Technology Texas (USA), was used for the catalytic cracking of the heavy fraction of the fast pyrolysis oil and HDO, which was equipped with an automated fixed-fluidized bed reactor. The schematic diagram of the ACE-R unit is shown in Fig. 2. A constant amount of catalyst (9 g) was loaded for each experiment and a constant C/O ratio of 5 was maintained by keeping a constant time on-stream (t) of 90 s, feed rate of 1.2 g min−1 and weight hourly space velocity (WHSV) of 8 h−1. The reaction was performed at atmospheric pressure and 530 °C. The catalyst was stripped off by nitrogen for a period of multiple of 7 times of the injection time. During the catalytic cracking and stripping steps, the liquid products were collected and maintained at −10 °C in a glass receiver, which was located at the end of the reactor exit. Moreover, the gaseous products were collected in a gas receiver by the water displacement method. After the cracking and stripping steps, the reactor was operated in regeneration mode, in which the coke deposited on the catalyst surface during the cracking reaction was burnt off with air at a temperature of 700 °C. The flue gases generated during the regeneration process were sent to the catalytic converter/furnace packed with cuprous oxide, where carbon monoxide was converted into carbon dioxide at 540 °C. The step of regeneration process/mode was continued till the amount of carbon dioxide formation became nil in the flue gases. The reactor effluent gases were measured on-line, which were used to estimate the amount of carbon deposited on E-CAT during cracking (coke).2.6 Product analysis
The product gases were analyzed with a Varian CP-3800 gas chromatograph equipped with three detectors, a flame ionization detector (FID) and two thermal conductivity detectors. The coke deposited on the catalyst was burned with air in regeneration mode, and the resulting total carbon dioxide was analyzed using IR spectroscopy. The liquid products were analyzed by a chromatographic simulated procedure described by the ASTM D-2887 method with an Agilent 6890 gas chromatograph, using a HP-1 methyl silicon column and a flame ionization detector. As in petroleum refinery practice, the product distribution was quantified by their boiling point range: dry gas (H2 and C1–C2 hydrocarbons), LPG (C3–C4 hydrocarbons), gasoline (IBP – 216 °C), light cycle oil [LCO (216–370 °C)], heavy cycle oil [HCO (>370 °C)] and coke. The conversion was estimated using the following equation:| Conversion, wt% = 100 − (LCO wt% + HCO wt%) |
For 31P NMR analysis, the solvents used with the bio-oil sample were usually a mixture of anhydrous pyridine and deuterated chloroform (1.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0, v/v) containing a relaxation agent (i.e., chromium(III) acetylacetonate) and an internal standard. 20 mg of FPO was dissolved in pyridine CDCl3 solvent of 0.5 ml. TMDP reagent (0.05–0.10 ml) was added, stirred and transferred into a 5 mm NMR tube for 31P NMR recording. Quantitative 31P NMR spectra were recorded with a long pulse delay of 10 s using a 90° 31P pulse. The number of transients recorded in inverse gated decoupling mode on a Bruker Avance III 500 MHz spectrometer at room temperature was 128. Chemical shifts are usually calibrated relative to the phosphitylation product of TMDP with water (sample moisture), which gives a sharp and stable signal at 132.2 ppm in pyridine-CDCl3 solvent.
1.0, v/v) containing a relaxation agent (i.e., chromium(III) acetylacetonate) and an internal standard. 20 mg of FPO was dissolved in pyridine CDCl3 solvent of 0.5 ml. TMDP reagent (0.05–0.10 ml) was added, stirred and transferred into a 5 mm NMR tube for 31P NMR recording. Quantitative 31P NMR spectra were recorded with a long pulse delay of 10 s using a 90° 31P pulse. The number of transients recorded in inverse gated decoupling mode on a Bruker Avance III 500 MHz spectrometer at room temperature was 128. Chemical shifts are usually calibrated relative to the phosphitylation product of TMDP with water (sample moisture), which gives a sharp and stable signal at 132.2 ppm in pyridine-CDCl3 solvent.
1H and 13C NMR spectra of FCC liquid distillates, produced from the co-processing of FPO or HDO with VGO, were recorded on a Bruker Avance III NMR spectrometer equipped with a 5 mm BBFO probe resonating at the frequency of 500.13 and 125.7 MHz, for 1H and 13C, respectively. The conventional 1H spectra were recorded using 5% w/v sample solutions in CDCl3 containing 0.03% TMS (99.8% Merck) with a sweep width of 6 kHz, 16 scans, 13.4 μs π/2 proton pulse and 2 s relaxation delay. The 13C NMR spectra of the sample were recorded using 30% (w/v) in CDCl3 solutions. Quantitative 13C spectra were acquired using the nuclear overhauser effect (NOE) suppressed, inverse gated proton decoupled technique (Waltz-16), with a sweep width of 19 kHz. The number of scans collected was 8k, using a 5 s relaxation delay. All the 13C spectra were processed with 1.0 Hz line broadening prior to Fourier transform (FT). All the 1H and 13C NMR spectra were referenced to TMS at 0 ppm. Before starting the analysis, the spectra obtained were corrected for phase and baseline, and then each of them was separated into different regions, which correspond to different types of protons and carbons according to their position in the molecule. Later, each spectrum was integrated thrice and averaged within the indicated regions.
3. Results and discussion
This section has been divided into two parts. In the first part, the discussion is restricted to the process, which includes an approach of char removal from the heavy fraction of Jatropha-derived fast pyrolysis oil and hydrodeoxygenation of FPO in order to convert it into HDO over Pd/Al2O3 catalyst (i.e. Section 3.1.1). The discussion is further extended to reactions such as the co-processing of FPO with VGO in an ACE-R FCC unit with the blending ratios of 5%, 10%, 15%, 17% and 20%. Furthermore, the product distribution pattern at an iso-conversion level of around 66% is discussed in Section 3.1.2 for the direct processing of VGO, co-processing of VGO with FPO at 17% blending ratio and co-processing of VGO with HDO at a blending ratio of 5%. The second part is highlighted with the discussion on NMR characterization of feed and product (i.e., Section 3.2).3.1 Process
Therefore, in the first step of the pretreatment of fast pyrolysis oil, a chemical treatment method was applied to free the char particles from the fast pyrolysis oil. Here, the Jatropha-derived fast pyrolysis oil obtained from the bubbling fluidized bed pyrolysis reactor is found to have a large concentration of char particles (nano-to-micro scale). Therefore, the heavy fraction of fast pyrolysis oil (in semi-solid form) is diluted with ethanol, and then larger size particles (>200 nm) were separated by membrane filtration under vacuum. Subsequently, the filtrate containing small particles was centrifuged at 8000 rpm for 20 minutes. As a result, the filtrate component was separated into two phases: an upper liquid phase containing a blend of fast pyrolysis oil and ethanol, and the deposited char particles at the bottom of the centrifuge tubes. The ethanol present in the residual pyrolysis oil was then recovered by vacuum distillation. After the process, the residual pyrolysis oil is thinner as compared to the earlier semi-solid like phase, and is termed as fast pyrolysis oil (FPO). The FPO was used for further hydrodeoxygenation, followed by catalytic cracking.
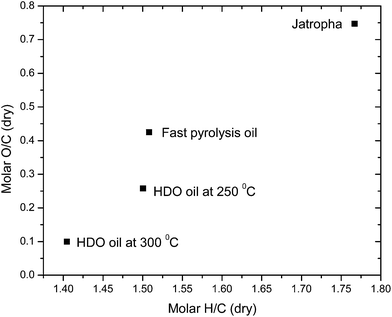
In the second step of the pretreatment of FPO, a hydrodeoxygenation method was applied to reduce the oxygen content of the FPO. The obtained FPO, containing 32 wt% of oxygen, was subjected to hydrodeoxygenation with the Pd/Al2O3 catalyst in a batch stirred reactor at 80 bar pressure. Increase in the reactor pressure from 80 to 105 and 120 bars was observed with increase in temperature from ambient to 250 and 300 °C, respectively. The gas analysis indicated that the bound oxygen was removed in the form of carbon dioxide by decarboxylation reaction, which is higher in yield, i.e. 45 and 51 wt% at 250 and 300 °C, respectively. The respective CHNO elemental analyses of feed Jatropha curcas cake, FPO and HDO are shown in Table 1. From the elemental analysis (Table 1), it was found that the amount of oxygen content is reduced from 32 wt% to 22 and 10 wt% for 250 and 300 °C, respectively. If the oxygen content could not be removed, the deep or high deoxygenation level of >95% is needed to match the specifications of the pyrolysis oil with standard crude oil in terms of carbon–hydrogen ratio, oxygen content and density.10 The van Krevelen diagram for the dry H/C and O/C ratios of the FPO and HDO is shown in Fig. 3. The O/C atomic ratio of HDO is drastically decreased to 0.257 and 0.099, respectively, as compared to FPO (0.424), whereas a relatively minor change and decline in the H/C ratio at 250 °C and 300 °C, respectively, was observed. A similar trend was observed by Mercader et al.36 From CCR analysis (Table 2), the carbon residue was found to be higher (∼16 wt%) in FPO, whereas it decreased to about 8 wt% on hydrodeoxygenation at 300 °C.
| Sample name | C, wt% | H, wt% | N, wt% | O, wt% | S, wt% | H/C | O/C |
|---|---|---|---|---|---|---|---|
| Jatropha curcas cake | 45.50 | 6.70 | 2.43 | 45.33 | 0.04 | 1.767 | 0.747 |
| Fast pyrolysis oil | 56.50 | 7.10 | 4.308 | 32.0 | 0.092 | 1.507 | 0.424 |
| HDO at 250 °C | 64.98 | 8.0 | 4.91 | 22.0 | 0.11 | 1.500 | 0.257 |
| HDO at 300 °C | 76.18 | 8.8 | 4.91 | 10.0 | 0.11 | 1.404 | 0.099 |
| Feedstock | Blending ratio | Density at 15 °C, g cm−3 | CCR, wt% | H/Ceff | Boiling point range, °C | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mass recovery, wt% | ||||||||||||
| IBP | 10% | 30% | 50% | 70% | 90% | FBP | ||||||
| VGO | 100 | 0.919 | 3.64 | 1.725 | 350 | 369 | 400 | 441 | 489 | 550 | 550 | |
| FPO | 100 | 1.18 | 16.26 | — | 36 | 162 | 259 | 328 | 357 | 445 | 592 | |
VGO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) FPO FPO |
95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
0.932 | 4.27 | 1.65 | 36 | 359 | 393 | 435 | 482 | 545 | 592 | |
90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
0.945 | 4.90 | 1.59 | 36 | 348 | 386 | 430 | 476 | 539 | 592 | ||
85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15 |
0.958 | 5.53 | 1.53 | 36 | 337 | 379 | 424 | 469 | 534 | 592 | ||
80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
0.971 | 6.16 | 1.47 | 36 | 327 | 372 | 418 | 462 | 529 | 592 | ||
| HDO | 100 | 1.04 | 8.6 | — | 36 | 159 | 270 | 344 | 405 | 499 | 597 | |
VGO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HDO HDO |
95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
0.925 | 3.88 | 1.68 | 36 | 358 | 394 | 436 | 485 | 548 | 597 | |
Initially, the blending ratio of FPO with VGO was varied at 5%, 10%, 15%, 17%, and 20% in order to see its effect and optimize the same for obtaining a similar FCC conversion. The FCC conversion of different feeds and their product yields of dry gas, LPG, gasoline, LCO, HCO, and coke are shown in Table 3. The mass balance obtained was more than 98%. From Table 3, it can be seen that the conversion decreases from 75% to 64% with an increase in the blending ratio of FPO from 5% to 20%. The decrease in conversion is due to the decrease in the yield of dry gases and LPG from 2.1 to 1.4 and 38 to 23 wt%, respectively, whereas the yields of gasoline, LCO and HCO were increased from 29 to 35 wt%, 14 to 20 wt%, and 8 to 14 wt%, respectively, with an increase in the blending ratio of the FPO with VGO from 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 95 to 20
95 to 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80. However, the results of co-processing at a lower blending ratio (5
80. However, the results of co-processing at a lower blending ratio (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 95) indicated a higher conversion (around 9 wt%) as compared to the direct catalytic cracking of pure VGO at a constant C/O ratio and temperature. This was due to a higher yield (38 wt%) of the LPG fraction as compared to 15 wt% in the case of catalytic cracking of pure VGO. The increase in LPG yield was observed at the cost of gasoline yield, which was 29 wt%; however, the gasoline yield obtained from the catalytic cracking of pure VGO was 44 wt%. Further, there was also a decrease in the LCO and HCO yields by 5 and 4 wt%, respectively.
95) indicated a higher conversion (around 9 wt%) as compared to the direct catalytic cracking of pure VGO at a constant C/O ratio and temperature. This was due to a higher yield (38 wt%) of the LPG fraction as compared to 15 wt% in the case of catalytic cracking of pure VGO. The increase in LPG yield was observed at the cost of gasoline yield, which was 29 wt%; however, the gasoline yield obtained from the catalytic cracking of pure VGO was 44 wt%. Further, there was also a decrease in the LCO and HCO yields by 5 and 4 wt%, respectively.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) FPO, VGO
FPO, VGO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HDO and pure VGO at different blending ratios
HDO and pure VGO at different blending ratios
| Feedstocks | VGO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) FPO FPO |
VGO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HDO HDO |
VGO | VGO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) FPO FPO |
|||
|---|---|---|---|---|---|---|---|
| Blending ratio | 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15 |
80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
100 | 83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17 17 |
| FCC conversion | 75.68 | 74.69 | 69.35 | 64.39 | 66.96 | 66.89 | 66.08 |
| Yield, wt% | |||||||
| Dry gas | 2.182 | 2.05 | 1.43 | 1.41 | 1.507 | 1.798 | 1.42 |
| LPG | 38.876 | 35.70 | 28.69 | 23.77 | 28.78 | 15.5 | 25.44 |
| Gasoline | 29.038 | 31.14 | 35.11 | 35.04 | 32.50 | 44.02 | 35.08 |
| LCO | 14.885 | 15.43 | 17.99 | 20.49 | 18.98 | 19.84 | 19.11 |
| HCO | 8.054 | 8.48 | 10.67 | 14.08 | 13.27 | 12.4 | 12.31 |
| Coke | 5.48 | 5.21 | 4.23 | 4.16 | 4.17 | 5.58 | 4.14 |
However, with an increase in the blending ratio from 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 95 to 10
95 to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90, there was a decrease in the LPG yield by ∼3 wt%; increase in the gasoline yield by ∼2 wt%; increase in the LCO yield by ∼1 wt%; and a slight increase in the HCO yield by ∼0.5%. Clearly, these results indicate that the FPO could be co-processed with VGO at lower blending ratios of 5
90, there was a decrease in the LPG yield by ∼3 wt%; increase in the gasoline yield by ∼2 wt%; increase in the LCO yield by ∼1 wt%; and a slight increase in the HCO yield by ∼0.5%. Clearly, these results indicate that the FPO could be co-processed with VGO at lower blending ratios of 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 95 and 10
95 and 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90 for LPG production at the cost of gasoline, followed by LCO and HCO range hydrocarbons.
90 for LPG production at the cost of gasoline, followed by LCO and HCO range hydrocarbons.
Furthermore, with an increase in the blending ratio from 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90 to 15
90 to 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 85, a similar (as 10
85, a similar (as 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90 blending studies) trend of LPG (decreased by 7 wt%), gasoline (increased by 4 wt%), LCO (increased by 2.5 wt%), HCO (increased by 2 wt%) yields were observed. Moreover, with an increase in the blending ratio FPO
90 blending studies) trend of LPG (decreased by 7 wt%), gasoline (increased by 4 wt%), LCO (increased by 2.5 wt%), HCO (increased by 2 wt%) yields were observed. Moreover, with an increase in the blending ratio FPO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VGO to 17%, the FCC conversion of ∼66% was observed. At this particular blending ratio, the dry gas, gasoline, and coke yields were lowered by 0.4, 9, and 1.4 wt%, respectively, whereas the yield of LPG was increased by ∼10 wt%, and the yields of LCO and HCO were found to be almost constant.
VGO to 17%, the FCC conversion of ∼66% was observed. At this particular blending ratio, the dry gas, gasoline, and coke yields were lowered by 0.4, 9, and 1.4 wt%, respectively, whereas the yield of LPG was increased by ∼10 wt%, and the yields of LCO and HCO were found to be almost constant.
A similar trend in product yields was observed with an increase in the blending ratio from 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 83 to 20
83 to 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80. From a general perspective, C5+ liquid hydrocarbons increase with an increase in H/Ceff, and a similar observation could be made in the present study: there was a decrease in C5+ hydrocarbons with an increase in H/Ceff. From the abovementioned results, it is also believed that in addition to H/Ceff, the type of oxygenated molecules present in FPO also plays a major role in the distribution of the FCC product profile. However, the increase in the yield of gasoline with the increase in the blending ratio of FPO with VGO is observed even with the decrease in H/Ceff. This was due to the presence of lignin monomers in FPO, and the same is discussed with the help of NMR analysis in the following section.
80. From a general perspective, C5+ liquid hydrocarbons increase with an increase in H/Ceff, and a similar observation could be made in the present study: there was a decrease in C5+ hydrocarbons with an increase in H/Ceff. From the abovementioned results, it is also believed that in addition to H/Ceff, the type of oxygenated molecules present in FPO also plays a major role in the distribution of the FCC product profile. However, the increase in the yield of gasoline with the increase in the blending ratio of FPO with VGO is observed even with the decrease in H/Ceff. This was due to the presence of lignin monomers in FPO, and the same is discussed with the help of NMR analysis in the following section.
The coke yield for all blending ratios is within limits and is lower as compared to pure VGO processing. The previous studies on the co-processing of aliphatic oxygenates like acetic acid, hydroxyacetone and glycolaldehyde with VGO for similar conditions also indicated the coke yield to be within the limits, except on the co-processing of lignin-derived monomer (guaiacol) with VGO.32,38 Water formation was also observed on the co-processing of FPO with VGO; however, the yield is not shown.
Furthermore, an attempt has been made to co-process HDO, obtained on the hydrodeoxygenation of FPO at 300 °C and 80 bar pressure with VGO in a blending ratio of 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 95 in an ACE-R unit. The conversion was found to be 66.96%, which is approximately equivalent to the conversion obtained on catalytic cracking pure VGO or co-processing of FPO with VGO for similar operating parameters. This shows that the highest conversion is possible with the co-processing of HDO with VGO as compared to pure VGO catalytic cracking and co-processing of FPO with VGO. The increase in conversion is due to increase in the yields of LPG and gasoline. However, the yields of LCO and HCO were observed in similar for all cases. The increase in the effective hydrogen index from 1.65 to 1.68 on the addition of HDO instead of FPO resulted in an increase in the yield of C5+ liquid hydrocarbons.
95 in an ACE-R unit. The conversion was found to be 66.96%, which is approximately equivalent to the conversion obtained on catalytic cracking pure VGO or co-processing of FPO with VGO for similar operating parameters. This shows that the highest conversion is possible with the co-processing of HDO with VGO as compared to pure VGO catalytic cracking and co-processing of FPO with VGO. The increase in conversion is due to increase in the yields of LPG and gasoline. However, the yields of LCO and HCO were observed in similar for all cases. The increase in the effective hydrogen index from 1.65 to 1.68 on the addition of HDO instead of FPO resulted in an increase in the yield of C5+ liquid hydrocarbons.
3.2 NMR characterization
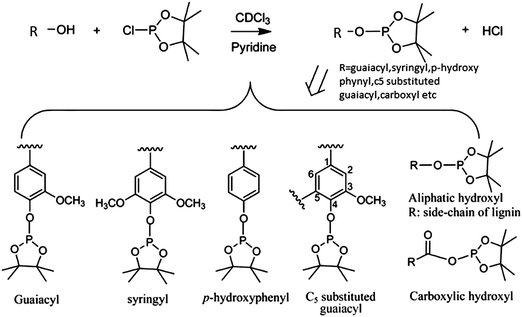 | ||
| Fig. 4 A reaction scheme for the phosphitylation of hydroxyl groups of the lignin structural units with TMDP. | ||
| Sr. no. | Functional group | Integration region, ppm | FPO | HDO, 250 °C | HDO, 300 °C | |
|---|---|---|---|---|---|---|
| Ben et al.39 | Present study | |||||
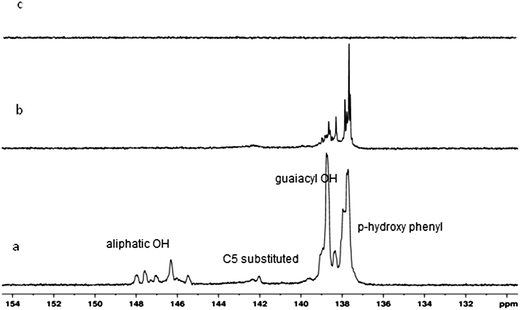
| 1 | Aliphatic OH | 150.0 to 145.5 | 150.02 to 145.07 | 2.37 | 0.27 | ab |
| 2 | C5 substituted β-5 | 144.7 to 142.8 | 145.07 to 140.42 | 1.23 | 0.6 | ab |
| 3 | Guaiacyl phenolic OH | 140.0 to 139.0 | 140.42 to 138.2 | 6.2 | 1.51 | ab |
| 4 | p-Hydroxy-phenyl OH | 138.2 to 137.3 | 138.2 to 136.96 | 5.75 | 1.89 | ab |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 95 ratio) as compared to the co-processing of HDO (at 5
95 ratio) as compared to the co-processing of HDO (at 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 95 ratio). The finding is also reflected from the higher value of branchiness index (BI) in oil (at 5
95 ratio). The finding is also reflected from the higher value of branchiness index (BI) in oil (at 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
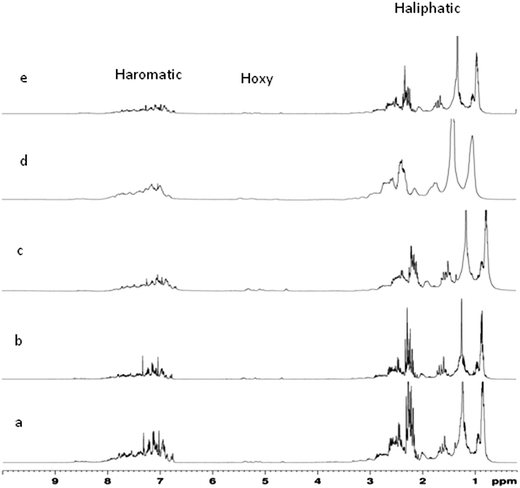
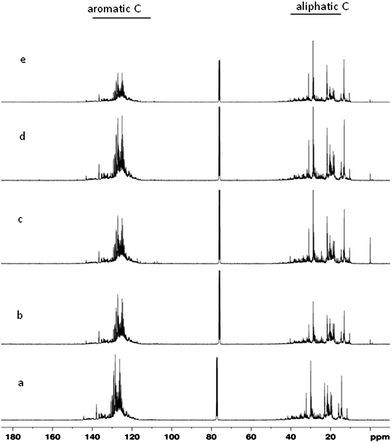
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 95). This indicates that the product of FPO, co-processing with VGO, contains more iso-paraffinic CH3 substructure, and the product of HDO, co-processing with VGO, contains more paraffinic CH3 substructure. Furthermore, the fraction of substituted aromatics fsa shows the fraction of aromatics substituted per molecule. In the feeds, H/Ceff is found to vary from 1.47 to 1.725. The aromatic protons vary from 15.5 to 21.69, with higher di-aromatic and poly aromatic protons in products for the blending ratios of 5
95). This indicates that the product of FPO, co-processing with VGO, contains more iso-paraffinic CH3 substructure, and the product of HDO, co-processing with VGO, contains more paraffinic CH3 substructure. Furthermore, the fraction of substituted aromatics fsa shows the fraction of aromatics substituted per molecule. In the feeds, H/Ceff is found to vary from 1.47 to 1.725. The aromatic protons vary from 15.5 to 21.69, with higher di-aromatic and poly aromatic protons in products for the blending ratios of 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 95 and 10
95 and 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90. This indicates that the product of HDO, co-processing with VGO, contains more paraffinic CH3 substructure, and the product of FPO, co-processing with VGO, contains more iso-paraffinic CH3 substructure. Furthermore, the normalized average percentage of protonated aromatic carbons varies from 37.2 to 44.2, that of bridgehead aromatic carbons varies from 2.9 to 3.3, and that of substituted aromatics varies from 6.3 to 7.8. The branchiness index shows the percentage of branching within the alkyl side chains. Higher the Branchiness Index, more is the branched side chains to aromatics. Table 5 also shows that the side chains are more branched in the blending ratio of 20
90. This indicates that the product of HDO, co-processing with VGO, contains more paraffinic CH3 substructure, and the product of FPO, co-processing with VGO, contains more iso-paraffinic CH3 substructure. Furthermore, the normalized average percentage of protonated aromatic carbons varies from 37.2 to 44.2, that of bridgehead aromatic carbons varies from 2.9 to 3.3, and that of substituted aromatics varies from 6.3 to 7.8. The branchiness index shows the percentage of branching within the alkyl side chains. Higher the Branchiness Index, more is the branched side chains to aromatics. Table 5 also shows that the side chains are more branched in the blending ratio of 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80.
80.
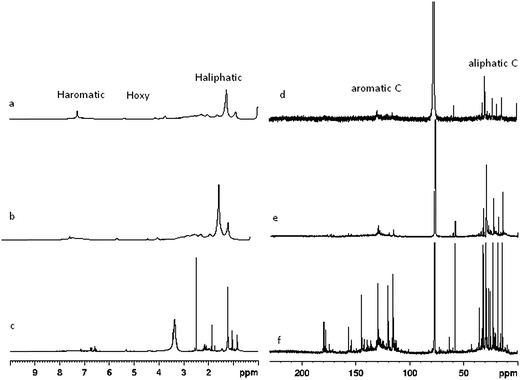 | ||
| Fig. 6 (a) 1H NMR of HDO at 300 °C; (b) 1H NMR of HDO at 250 °C; (c) 1H NMR of FPO; (d) 13C NMR of HDO at 300 °C; (e) 13C NMR of HDO at 250 °C; (f) 13C NMR of FPO. | ||
| Feedstock | Blending Ratio | n | fa | Ch | Cb | ARq | BI | fsa | m-a | d-a | p-a |
|---|---|---|---|---|---|---|---|---|---|---|---|
| VGO | 100 | 18 | 0.13 | 4.90 | 1.36 | 5.70 | 0.35 | 0.44 | 2.33 | 1.6 | 0.55 |
| VGO* | 6 | 0.48 | 37.27 | 3.19 | 7.32 | — | 0.15 | 9.2 | 7.63 | 2.15 | |
FPO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VGO VGO |
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 95 95 |
0.13 | |||||||||
FPO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VGO* VGO* |
3 | 0.55 | 43.5 | 3.3 | 7.8 | 0.47 | 0.14 | 10.56 | 8.66 | 2.47 | |
FPO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VGO VGO |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90 90 |
0.13 | |||||||||
FPO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VGO* VGO* |
3 | 0.54 | 44.2 | 3.1 | 6.4 | 0.53 | 0.12 | 10.00 | 8.51 | 2.58 | |
FPO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VGO VGO |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 85 85 |
0.14 | |||||||||
FPO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VGO* VGO* |
3 | 0.52 | 41.7 | 3.0 | 6.8 | 0.47 | 0.13 | 10.46 | 6.84 | 1.05 | |
FPO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VGO VGO |
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80 80 |
0.14 | |||||||||
FPO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VGO* VGO* |
3 | 0.49 | 39.5 | 2.9 | 6.7 | 0.6 | 0.14 | 10.05 | 5.26 | 0.19 | |
HDO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VGO VGO |
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 95 95 |
0.15 | |||||||||
HDO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VGO* VGO* |
3 | 0.47 | 37.2 | 3.0 | 6.3 | 0.53 | 0.13 | 9.67 | 5.51 | 0.79 |
4. Conclusion
The petroleum-derived pure VGO and mixtures of the heavy fraction of Jatropha curcas cake-derived fast pyrolysis oil and its HDO with VGO were used as the feedstocks for the present co-processing studies. The FPO containing 32 wt% of oxygen seems to be unsuitable for co-processing with petroleum-derived VGO at optimized process conditions of the FCC unit for higher gasoline, as the FPO containing the lignin-derived monomers cannot be cracked with the FCC catalysts. However, the lower blending ratios of 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 95 and 10
95 and 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90 of FPO with VGO is extremely suitable for the production of light olefins, mainly LPG, at the loss of gasoline range hydrocarbons, whereas the decrease in dry gas yield and increase in liquid hydrocarbons were observed with an increase in the FPO blending ratio with VGO. The hydrodeoxygenating Pd/Al2O3 catalyst seemed to be very effective in order to reduce the oxygen content of FPO from 32 to 10 wt% at the lower operating pressure of 80 bar and 300 °C. The co-processing of HDO with VGO resulted in an increase in the yields of gasoline and LCO as compared to the co-processing of FPO with VGO, at the similar blending ratio of 5
90 of FPO with VGO is extremely suitable for the production of light olefins, mainly LPG, at the loss of gasoline range hydrocarbons, whereas the decrease in dry gas yield and increase in liquid hydrocarbons were observed with an increase in the FPO blending ratio with VGO. The hydrodeoxygenating Pd/Al2O3 catalyst seemed to be very effective in order to reduce the oxygen content of FPO from 32 to 10 wt% at the lower operating pressure of 80 bar and 300 °C. The co-processing of HDO with VGO resulted in an increase in the yields of gasoline and LCO as compared to the co-processing of FPO with VGO, at the similar blending ratio of 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 95. The FCC distillate on the co-processing of FPO with VGO contains more iso-paraffinic CH3 substructure components, whereas the liquid on co-processing HDO with VGO contains more paraffinic CH3 substructure. The coke yield was found to be within the limit, and in fact, lower than the pure VGO processing over the same equilibrium FCC catalyst. Based on the results of the present experimental investigations, HDO may be co-processed instead of FPO with VGO at a lower blending ratio of up to 5
95. The FCC distillate on the co-processing of FPO with VGO contains more iso-paraffinic CH3 substructure components, whereas the liquid on co-processing HDO with VGO contains more paraffinic CH3 substructure. The coke yield was found to be within the limit, and in fact, lower than the pure VGO processing over the same equilibrium FCC catalyst. Based on the results of the present experimental investigations, HDO may be co-processed instead of FPO with VGO at a lower blending ratio of up to 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 95 in the FCC unit without many modifications in the process configuration and catalyst if the demand for LPG is more, as is the case in India.
95 in the FCC unit without many modifications in the process configuration and catalyst if the demand for LPG is more, as is the case in India.
Abbreviations
| LPG | Liquefied petroleum gas |
| LCO | Light cycle oil |
| HCO | Heavy cycle oil |
| FPO | Heavy fraction of Jatropha-derived char free fast pyrolysis oil |
| H/Ceff | Effective hydrogen index based on elemental analysis |
| C/O | Catalyst-to-oil ratio |
| n | Average chain length |
| fa | Fraction of aromaticity |
| Ch | Protonated aromatic carbon |
| Cb | Bridgehead aromatic carbon |
| ARq | Substituted aromatic carbon |
| BI | Branchiness Index |
| fsa | Fraction of substituted aromatics |
| m-a | Mono-aromatic protons |
| d-a | Di-aromatic protons |
| p-a | Poly-aromatic protons |
| ab | Absent |
| H/C | Hydrogen-to-carbon atomic ratio |
| O/C | Oxygen-to-carbon atomic ratio |
| E-CAT | Equilibrium FCC catalyst |
| IBP | Initial boiling point, °C |
| FBP | Final boiling point, °C |
| CCR | Conradson carbon residue, wt% |
Acknowledgements
The authors would like to acknowledge Mr Shiv Prasad Nautiyal of CSIR-Indian Institute of Petroleum for his unconditional support/participation in the continuous operation of the biomass fast pyrolysis unit. Furthermore, the authors there are thankful to the FCC group of CSIR-IIP for allowing them to carry out the catalytic cracking studies in the ACE-R unit.References
- C. John, H. Paul, J. A. Beamon, S. Naolitano, A. M. Schaal, J. T. Turnure and L. Westfall, International Energy Outlook, 2013, Report number: DOE/EIA-0484 2013 Search PubMed.
- Worldwide look at reserves and production, Oil & Gas Journal, 2012, 110, 28–31, http://www.ogj.com, subscription site.
- D. C. Elliott and E. G. Baker, Upgrading biomass liquefaction products through hydrodeoxygenation, Biotechnol. Bioeng. Symp., 1984, 14, 159–174 CAS.
- D. C. Elliott and G. F. Schiefelbein, Liquid hydrocarbon fuels from biomass, Amer. Chem. Soc., Fuel Chem. Preprts., 1989, 34(4), 1160–1166 CAS.
- A. Demirbas, Biorefineries: For biomass upgrading facilities, Green Energy and Technology, 2010, 75–92 Search PubMed.
- Approach to refining processes, http://petrofed.winwinhosting.net/upload/25-28May10/S_Bose.pdf, accessed on 23rd May 2014.
- G. W. Huber and A. Corma, Synergies between Bio- and Oil Refineries for the Production of Fuels from Biomass, Angew. Chem., Int. Ed., 2007, 46, 7184–7201 CrossRef CAS PubMed.
- R. H. Venderbosch, A. R. Ardiyanti, J. Wildschut, A. Oasmaa and H. J. Heeres, Stabilization of biomass-derived pyrolysis oils, 2010, http://onlinelibrary.wiley.com/doi/10.1002/jctb.2354/pdf, accessed on 23rd May 2014 Search PubMed.
- F. M. Mercader, M. J. Groeneveld, S. R. A. Kersten, R. H. Venderbosch and J. A. Hogendoorn, Pyrolysis oil upgrading by high pressure thermal treatment, Fuel, 2010, 89, 2829–2837 CrossRef CAS PubMed.
- M. C. Samolada, W. Baldauf and I. A. Vasalos, Production of a bio-gasoline by upgrading biomass flash pyrolysis liquids via hydrogen processing and catalytic cracking, Fuel, 1998, 77, 1667–1675 CrossRef CAS.
- J. Wildschut, F. H. Mahfud, R. H. Venderbosch and H. J. Heeres, Hydrotreatment of Fast Pyrolysis Oil Using Heterogeneous Noble-Metal Catalysts, Ind. Eng. Chem. Res., 2009, 48, 10324–10334 CrossRef CAS.
- E. Churin, P. Grange and B. Delmon, Quality Improvement of Pyrolysis Oils, Report number: EUR 12441 EN, Commission of the European Communities, 1989 Search PubMed.
- R. J. French, J. Stunkel and R. M. Baldwin, Mild Hydrotreating of Bio-Oil: Effect of Reaction Severity and Fate of Oxygenated Species, Energy Fuels, 2011, 25, 3266–3274 CrossRef CAS.
- P. Grange, E. Laurent, R. Maggi, A. Centeno and B. Delmon, Hydrotreatment of pyrolysis oils from biomass: reactivity of the various categories of oxygenated compounds and preliminary techno-economical study, Catal. Today, 1996, 29, 297–301 CrossRef CAS.
- F. M. Mercader, M. J. Groeneveld, S. R. A. Kersten, N. W. J. Way, C. J. Schaverien and J. A. Hogendoorn, Production of advanced biofuels: Co -processing of upgraded pyrolysis oil in standard refinery units, Appl. Catal., B, 2010, 96, 57–66 CrossRef PubMed.
- P. A. Zapata, J. Faria, M. P. Ruiz and D. E. Resasco, Condensation/Hydrogenation of Biomass-Derived Oxygenates in Water/Oil Emulsions Stabilized by Nanohybrid Catalysts, Top. Catal., 2012, 55, 38–52 CrossRef CAS.
- I. Graça, F. R. Ribeiro, H. S. Cerqueira, Y. L. Lam and M. B. B. de Almeida, Catalytic cracking of mixtures of model bio-oil compounds and gasoil, Appl. Catal., B, 2009, 90, 556–563 CrossRef PubMed.
- A. Corma, G. W. Huber, L. Sauvanaud and P. O. Connor, Processing biomass-derived oxygenates in the oil refinery: Catalytic cracking (FCC) reaction pathways and role of catalyst, J. Catal., 2007, 247, 307–327 CrossRef CAS PubMed.
- G. Fogassy, N. Thegarid, G. Toussaint, A. C. Van veen, Y. Schuurman and C. Mirodatos, Biomass derived feedstock co-processing with vacuum gas oil for second-generation fuel production in FCC units, Appl. Catal., B, 2010, 96, 476–485 CrossRef CAS PubMed.
- S. B. Jones, J. E. Holladay, C. Valkenburg, D. J. Stevens, C. W. Walton, C. Kinchin, D. C. Elliott, and S. Czernik, Production of Gasoline and Diesel from Biomass via Fast Pyrolysis, Hydrotreating and Hydrocracking: A Design Case, US Department of Energy, February 2009, PNNL Report no. 18284 Search PubMed.
- M. S. Talmadge, R. M. Baldwin, M. J. Biddy, R. L. McCormick, G. T. Beckham, G. A. Ferguson, S. Czernik, K. A. Magrini-Bair, T. D. Foust, P. D. Metelski, C. Hetrick and M. R. Nimlos, A perspective on oxygenated species in the refinery integration of pyrolysis oil, Green Chem., 2014, 16, 407–453 RSC.
- N. Y. Chen, J. T. F. Degnan and L. R. Koenig, Liquid Fuel from Carbohydrates, Chem. Tech., 1986, 16, 506–511 CAS.
- G. Fogassy, N. Thegarid, G. Toussaint, A. C. van Veen, Y. Schuurman and C. Mirodatos, Biomass derived feedstock co-processing with vacuum gas oil for second-generation fuel production in FCC units, Appl. Catal., B, 2010, 96, 476–485 CrossRef CAS PubMed.
- G. Fogassy, N. Thegarid, Y. Schuurman and C. Mirodatos, From biomass to bio-gasoline by FCC co-processing: effect of feed composition and catalyst structure on product quality, Energy Environ. Sci., 2011, 4, 5068–5076 CAS.
- F. M. Mercader, Pyrolysis oil upgrading for co-processing in standard refinery units, Ph.D. thesis, University of Twente, Netherlands, 2010.
- N. Thegarid, G. Fogassy, Y. Schuurman, C. Mirodatos, S. Stefanidis, E. F. Iliopoulou, K. Kalogiannis and A. A. Lappas, Second-generation biofuels by co-processing catalytic pyrolysis oil in FCC units, Appl. Catal., B, 2013, 145, 161–166 CrossRef PubMed.
- I. Graça, J. M. Lopes, M. F. Ribeiro, F. R. Ribeiro, H. S. Cerqueira and M. B. B. de Almeida, Catalytic cracking in the presence of guaiacol, Appl. Catal., B, 2011, 101, 613–621 CrossRef PubMed.
- A. A. Lappas, S. Bezergianni and I. A. Vasalos, Production of biofuels via co-processing in conventional refining processes, Catal. Today, 2009, 145, 55–62 CrossRef CAS PubMed.
- A. E. Wroblewski, C. Lensink, R. Markuszewski and J. G. Verkade, Phosphorus-31 NMR spectroscopic analysis of coal pyrolysis condensates and extracts for heteroatom functionalities possessing labile hydrogen, Energy Fuels, 1988, 2, 765–774 CrossRef CAS.
- A. Majhi, Y. K. Sharma, R. Bal, B. Behera and J. Kumar, Upgrading of Bio-oils over PdO/Al2O3 Catalyst and Fractionation, Fuel, 2013, 107, 131–137 CrossRef CAS PubMed.
- J. C. Ray, K.-S. You, J.-W. Ahn and W.-S. Ahn, Mesoporous alumina(I): comparison of synthesis schemes using anionic, cationic, and non-ionic surfactants, Microporous Mesoporous Mater., 2007, 100, 183–190 CrossRef CAS PubMed.
- D. V. Naik, V. Kumar, B. Prasad, B. Behera, N. Atheya, K. K. Singh, D. K. Adhikari and M. O. Garg, Catalytic cracking of pyrolysis oil oxygenates (aliphatic and aromatic) with vacuum gas oil and their characterization, Chem. Eng. Res. Des., 2014, 92(8), 1579–1590 CrossRef CAS PubMed.
- M. Ringer, V. Putsche, and J. Scahill, Large-Scale Pyrolysis Oil Production: A Technology Assessment and Economic Analysis. National Renewable Energy Laboratory Technical Report, November 2006, NREL/TP-510–37779 Search PubMed.
- F. A. Agblevor, S. Besler and A. E. Wiselogel, Fast Pyrolysis of Stored Biomass Feedstocks, Energy Fuels, 1995, 4, 635–640 CrossRef.
- K. Raveendran, A. Ganesh and C. K. Kartic, Influence of mineral matter on biomass pyrolysis characteristics, Fuel, 1995, 12, 1812–1822 CrossRef.
- F. M. Mercader, M. J. Groeneveld, S. R. A. Kersten, C. Geantet, G. Toussaint, N. W. J. Way, C. J. Schaverien and K. J. A. Hogendoorn, Hydrodeoxygenation of pyrolysis oil fractions: process understanding and quality assessment through co-processing in refinery units, Energy Environ. Sci., 2011, 4, 985–997 CAS.
- Y. Pu, S. Cao and A. J. Ragauskas, Application of quantitative 31P NMR in biomass lignin and biofuel precursors Characterization, Energy Environ. Sci., 2011, 4, 3154–3166 CAS.
- D. V. Naik, V. Kumar, B. Prasad, B. Behera, N. Atheya, D. K. Adhikari, K. D. P. Nigam and M. O. Garg, Catalytic cracking of C2–C3 carbonyls with vacuum gas oil, Ind. Eng. Chem. Res., 2014 DOI:10.1021/ie501331b.
- H. Ben and A.J. Ragauskas, One step thermal conversion of lignin to the gasoline range liquid products by using zeolites as additives, RSC Advances, 2012, 2, 12892–12898 RSC.
| This journal is © The Royal Society of Chemistry 2015 |