Highly sensitive and colorimetric detection of hydrogen sulphide by in situ formation of Ag2S@Ag nanoparticles in polyelectrolyte multilayer film†
Hongxia Fu and
Xinrui Duan*
Key Laboratory of Analytical Chemistry for Life Science of Shaanxi Province, School of Chemistry and Chemical Engineering, Shaanxi Normal University, Xi’an, Shaanxi 710119, P. R. China. E-mail: duanxr@snnu.edu.cn
First published on 2nd December 2014
Abstract
Ag2S nanoparticles (NPs) were formed by reacting Ag+ ions with H2S gas in a polyelectrolyte multilayer film. Ag2S NPs later catalyse the formation of Ag NPs. H2S (when as low as 10 nM Na2S was used as a donor) was sensitively detected by monitoring the UV-vis absorbance of the newly formed Ag NPs.
H2S was discovered to be a gasotransmitter, along with nitric oxide and carbon monoxide.1 H2S influences cellular events through regulation of intracellular redox homeostasis and post-translational modification of proteins through S-sulfhydration.2 H2S can be endogenously generated by cystathionine beta-synthase (CBS), cystathionine gamma-lyase (CSE), and 3-mercaptopyruvate sulfurtransferase during cysteine metabolism. H2S is mainly produced by CSE in the cardiovascular system, liver, kidney, and pancreas. More importantly, the H2S level is related to Down’s syndrome and Alzheimer’s disease.3
Many methods have been developed for H2S detection, including colorimetry,4 a fluorescence technique,5 electrochemical assays,6 gas chromatography,7 and nanofibers/wires and nanotube based methods.8 A recent report has shown that hydrogen sulphide concentrations are much lower than presently accepted values in blood and tissues (100 pM in blood and 15 nM in tissues, respectively);7 to our best knowledge, only polarography and gas chromatography-based methods have the ability to detect it, which requires the involvement of highly toxic mercury and/or complicated instruments. Recent advances of developing fluorescent probes and a colorimetry assay9 have highlighted the need to develop a sensitive, selective, and rapid method for H2S detection for disease diagnosis and in vitro cultured cell-based inhibitor screening for H2S-related enzymes. To date, the lack of an affordable and sensitive method is still the major obstacle for such applications.
In this work, we would like to develop a sensitive, selective, and rapid method for H2S detection based on Ag2S NP catalysed formation of Ag NPs in a layer-by-layer polyelectrolyte multilayer film. Ag2S NPs have previously been employed in colorimetric H2S sensing.4b With a common microplate reader, the limit of detection is 8.7 μM. Formation of Ag2S on a metallic silver probe caused a light reflection difference enabling concentrations as low as 100 nM of H2S to be detected.4c By using Au@Ag NPs and sophisticated dark-field microscopy, H2S as low as 50 nM could be detected.4d
Su and co-workers extensively studied the in situ formation of Ag NPs in layer-by layer polyelectrolyte multilayer films (PEMs).10 Shannon and his co-workers have prepared Au/CuI and Au/CdS core–shell nanoparticles in poly (diallyl dimethylammonium) (PDDA) by using electrochemical atomic layer deposition.11 Li and his co-workers have prepared CuS NPs in a PVA polymer matrix by embedding copper ions with H2S gas.12 Under the inspiration of these previous works and the fact that PEMs are very stable in aqueous solution, we designed our system as follows.
As shown in Scheme 1, PEMs were prepared by a standard layer-by-layer process in the presence of extra sodium ions. Sodium ions were later exchanged with Ag ions. At physiological pH, the assay takes advantage of the volatility of H2S gas. As H2S is volatilized in a microplate well, it can react with the Ag ions to form Ag2S NPs. Ag NPs can be generated on Ag2S NPs by reducing Ag+ ions with hydroquinone or sodium sulphite in solution.13 UV light irradiation substantially accelerates this process. Because of this catalytic effect of Ag2S NPs, Ag NPs were formed at Ag2S NP sites in the PEMs in the presence of silver nitrate and Na2SO3, which greatly improved the sensitivity of detection. The concentrations of silver nitrate and sodium sulphite, and time of amplification have a great impact on the silver amplification process, thus we chose the previously reported optimized concentrations for our study. Furthermore, we optimized the time of amplification (Fig. S2†). Two hours of amplification produced the best signal/noise ratio.
We used Na2S as the H2S donor to develop our detection method, since the purity (>98%) is much higher than commercially available NaSH, which may contain polysulfides and have a purity of only 60%.14 H2S gas is spontaneously released from Na2S aqueous solution until the HS– and S2− are used up. H2S reacted with the Ag ions embedded in the (PDDA/PSS)5 film coated on the glass slide. After reaction with H2S gas from the Na2S solution, Ag2S NPs formed in the nano-size spaces of the PEMs.10a Scanning electron microscopy (SEM) images and the absorption spectra of (PDDA/PSS)5/Ag+, (PDDA/PSS)5/Ag2S and (PDDA/PSS)5/Ag2S@Ag PEMs are shown in Fig. 1. The magnification of all SEM images is 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000×, except the inset image of Fig. 1b which is 100
000×, except the inset image of Fig. 1b which is 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000×. The (PDDA/PSS)5/Ag+ film does not have any significant morphology under SEM and has a weak absorption in the corresponding wavelength region due to absorption of glass.
000×. The (PDDA/PSS)5/Ag+ film does not have any significant morphology under SEM and has a weak absorption in the corresponding wavelength region due to absorption of glass.
Although the formed Ag2S NPs can barely be seen under SEM, we could observe a roughness increase of the (PDDA/PSS)5/Ag2S film (Fig. 1c). It produces a broad absorption band in UV-vis spectra (Fig. S1,† UV-vis spectra of highly concentrated Ag2S NPs). Although SEM is not the ideal tool for characterization of Ag2S NP morphology, the existence of silver NPs after silver amplification also supports the existence of earlier formed Ag2S NPs. Ag amplification has been extensively studied where silver ions were reduced to metallic silver in the presence of a reducing agent (such as Na2SO3).13 Previous work reported the catalytic effect of Ag2S NPs in solution; in our work we also observed this phenomenon in the PEM films. After the silver amplification process, Ag NPs appear clearly under SEM (Fig. 1b). As for the UV-vis detection in Fig. 1d, a much stronger absorption peak appears around 430 nm after silver amplification. The inset image is an image of PEM films taken by a cell phone camera, which were on top of the PBS solution or the Na2S solution in a 96 well plate after silver amplification. We can see the circular shapes of spots that well preserve the shape of wells. By converting the absorption signal of Ag2S NPs to that of Ag NPs in the PEMs, the sensitivity of H2S detection was greatly improved due to the following reasons: (1) the maximum absorption peak of Ag NPs is higher than 300 nm, which avoids the interference of the glass slide and cell culture plate itself; (2) the Ag2S NPs serve as a catalyst, which results in significant signal amplification.
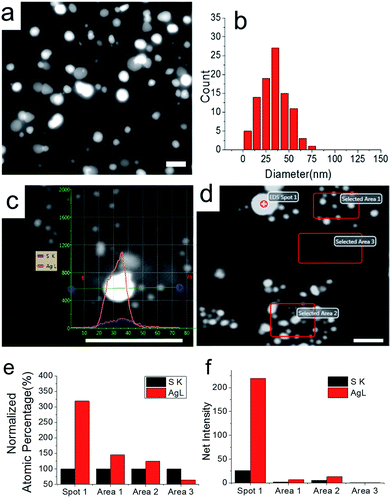
To get a direct view of the morphology and the composition of the Ag2S@Ag NPs in the PEM films, we studied a sample with field emission transmission electron microscopy (FE-TEM) under a scanning TEM (STEM) mode and energy dispersive X-ray analysis (EDX) under STEM of single particles in a PEM film. The results are shown in Fig. 2. Since the NPs are crowded in high concentration samples (as shown in Fig. 1b), we chose a sample from a lower concentration of H2S (500 nM) for all the STEM studies. The PEM film was prepared by scratching it off from the glass surface in water. The film floated on the water surface and later was caught by a TEM copper grid.
Fig. 2a shows the morphology of single NPs under FE-TEM by using a STEM mode. The size distribution of the NPs is shown in Fig. 2b. The majority of particles have a diameter between 20 and 70 nm. It is worth noticing, beside large and bright particles, some very small particles with the diameter of several nanometers can be seen surrounding bigger particles. This is more clearly visible with the higher magnification image in Fig. 2c. We assume that the smaller particles are Ag2S NPS and that the bigger particles are Ag NPs which are newly formed on the Ag2S NP sites. We did the line scan of STEM-EDX analysis to find out the content difference between the two types of particles. As shown in Fig. 2c, the net intensity of silver content is much higher than sulphur content on the bigger NPs. Since the intensity of a single small particle is too weak, we performed area analysis on a crowded area of small particles to get more comparable results with a spot from a bigger particle. The selection of the area and the spot are shown in Fig. 2d. Normalized atomic percentage and net intensities are shown in Fig. 2e and f, respectively. The atomic ratio of silver to sulphur in the 20 nm particle is around 3.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, which confirmed the formation of Ag2S@Ag NPs. The atomic ratio of particles with the diameter of several nanometers is around 1.5, which is very close to the theoretical ratio of Ag2S. This difference of ratio between our observed and theoretical ratios might be due to the low net intensities of area 1 and area 2, which will cause the increase of error. Area 3 was selected to show the background signals; from Fig. 2f, we can see that the background signals are negligible. Spectra from the EDX analysis are presented in Fig. S3.†
1, which confirmed the formation of Ag2S@Ag NPs. The atomic ratio of particles with the diameter of several nanometers is around 1.5, which is very close to the theoretical ratio of Ag2S. This difference of ratio between our observed and theoretical ratios might be due to the low net intensities of area 1 and area 2, which will cause the increase of error. Area 3 was selected to show the background signals; from Fig. 2f, we can see that the background signals are negligible. Spectra from the EDX analysis are presented in Fig. S3.†
Fig. 3 shows a standard curve of various concentrations (from 10 nM to 5 μM) of Na2S solutions vs. Ag2S@Ag NP absorbance. A good linear relationship between the UV absorption at 430 nm and the concentration of Na2S solution (10 nM–5 μM) was observed. The linearity equation is A = 0.03928 + 4.03196C. The R square value is 0.9980.
To test the specificity of our method, various biologically relevant molecules which include reactive sulfur such as dithiothreitol (DTT), cysteine (CYS), and reduced glutathione (GSH), as well as the cell culture medium DMEM and DMEM plus 10% fetal bovine serum (FBS) were studied. As shown in Fig. 4a, under the same reaction conditions, all interference candidates with a 2000-fold higher concentration of Na2S only produce the same UV absorption intensity as the control (PBS buffer solution). These results demonstrate excellent selectivity of our method for H2S gas detection. This high selectivity of our method is similar to previous reported hydrogen sulphide gas-based detection methods,4b,5d in which interference candidates only produce comparable signals to the control solutions.
With the excellent sensitivity and selectivity of our method, we were confident to perform the detection of cellular endogenous H2S gas. Since H2S is mainly produced by CSE in the liver, cardiovascular system, kidney and pancreas, the liver cancer cell line HepG2 was used in this section of the study. Detection cells were pre-treated with cysteine (CYS) and pyridoxal phosphate (PLP) to generate H2S gas. Blanks, which underwent the same treatment without cells, were also included in the study. The CSE inhibitor propargylglycine (PAG) was added to test the inhibitor monitoring ability of our method in live cells.3 PEM-coated glass slides were glued on to a 96 well plate cover and placed on top of a well plate for 2 hours, and after 2 hours the silver amplification process in the glass slides was measured using a microplate reader. As shown in Fig. 4b, 1 × 104 (10k) cells (i.e. about 30% coverage of a 0.32 cm2 area) can be detected in a 96 well plate format, and 2 × 104 (20k) cells produce a higher signal than 10k cells. Since we used the same amount of PAG, absorbance of the sample of 20k cells to which PAG had been added also lightly increased. The signals from cells were significantly higher than those from blank and PAG-treated samples.
A previous report on free H2S gas detection of live cells is based on zinc salts embedded in an agar hydrogel.5a After 12 hours or longer incubation, acid was added to release H2S gas from the formed ZnS precipitation, and the released H2S gas reacted with N,N-dimethyl-p-phenylenediamine chloride and FeCl3 to produce methylene blue. Measuring the absorbance of the produced methylene blue gave the quantity of H2S gas. Our method holds two significant improvements: (1) the high sensitivity of our method shortens the incubation time from 12 hours to 2 hours; (2) in situ formation and silver amplification ensured that reacted spots were well preserved, so results can be readily obtained by using a micro-plate reader.
Conclusions
We reported a sensitive, selective, and rapid colorimetric method for cellular endogenous H2S gas detection based on in situ formation of Ag2S@Ag NPs in layer-by-layer PEMs. When using Na2S solution as the gas source, H2S from 10 nM (3 picomole, calculated by the sulfur) of 300 μL Na2S solution in pH 7.0 buffer solution can be detected effectively. As far as we know, this method is the most sensitive among colorimetric methods and has comparable sensitivity with the most sensitive polarographic and gas chromatographic approaches. Furthermore, our method only required a microplate reader with UV-vis absorbance detecting ability for detection. The reaction between H2S and silver ions generated Ag2S NPs, which catalysed the rapid formation of Ag NPs on the Ag2S NP surfaces, thus the absorption intensity of Ag2S NPs was transferred to the absorbance of newly-formed Ag NPs. This results in signal amplification and high sensitivity. Since our method was designed to only measure H2S gas, potential interferents including common thiols and standard DMEM medium plus 10% FBS do not affect detection. Due to its excellent sensitivity and selectivity, we believe our method is significantly important for disease diagnosis and in vitro cultured cell-based inhibitor screening for H2S-related enzymes.Acknowledgements
We thank the National Natural Science Foundation of China (Grant no. 21305083) and Fundamental Research Funds for the Central Universities (Grant no. GK201303003 and GK201402051) for financial support.Notes and references
- R. Wang, Curr. Opin. Nephrol. Hypertens., 2011, 20, 107–112 CrossRef CAS PubMed.
- (a) J. Pan and K. S. Carroll, Biopolymers, 2013, 101, 165–172 CrossRef PubMed; (b) J. Yang, V. Gupta, K. S. Carroll and D. C. Liebler, Nat. Commun., 2014, 5, 4776 CrossRef CAS PubMed.
- (a) M. Thorson, T. Majtan, J. Kraus and A. Barrios, Angew. Chem., Int. Ed., 2013, 52, 4641–4644 CrossRef CAS PubMed; (b) H. Peng, Y. Cheng, C. Dai, A. King, B. Predmore, D. Lefer and B. Wang, Angew. Chem., Int. Ed., 2011, 50, 9672–9675 CrossRef CAS PubMed.
- (a) R. Kartha, J. Zhou, L. Hovde, B. Cheung and H. Schröder, Anal. Biochem., 2012, 423, 102–108 CrossRef CAS PubMed; (b) A. Jarosz, T. Yep and B. Mutus, Anal. Chem., 2013, 85, 3638–3643 CrossRef CAS PubMed; (c) H. Kong, Z. Ma, S. Wang, X. Gong, S. Zhang and X. Zhang, Anal. Chem., 2014, 86, 7734–7739 CrossRef CAS PubMed; (d) J. Hao, B. Xiong, X. Cheng, Y. He and E. Yeung, Anal. Chem., 2014, 86, 4663–4667 CrossRef CAS PubMed.
- (a) Y. Qian, J. Karpus, O. Kabil, S. Y. Zhang, H. L. Zhu, R. Banerjee, J. Zhao and C. He, Nat. Commun., 2011, 2, 495 CrossRef PubMed; (b) A. R. Lippert, E. J. New and C. J. Chang, J. Am. Chem. Soc., 2011, 133, 10078–10080 CrossRef CAS PubMed; (c) Y. Chen, C. Zhu, Z. Yang, J. Chen, Y. He, Y. Jiao, W. He, L. Qiu, J. Cen and Z. Guo, Angew. Chem., Int. Ed., 2013, 52, 1688–1691 CrossRef CAS PubMed; (d) A. Faccenda, J. Wang and B. Mutus, Anal. Chem., 2012, 84, 5243–5249 CrossRef CAS PubMed; (e) Y. Zhou, J. Yu, X. Lei, J. Wu, Q. Niu, Y. Zhang, H. Liu, P. Christen, H. Gehring and F. Wu, Chem. Commun., 2013, 49, 11782–11784 RSC.
- (a) G. Schiavon, G. Zotti, R. Toniolo and G. Bontempelli, Anal. Chem., 1995, 67, 318–323 CrossRef CAS; (b) J. Doeller, T. Isbell, G. Benavides, J. Koenitzer, H. Patel, R. Patel, J. Lancaster, V. Darley-Usmar and D. Kraus, Anal. Biochem., 2005, 341, 40–51 CrossRef CAS PubMed.
- J. Furne, A. Saeed and M. D. Levitt, Am. J. Physiol., 2008, 295, R1479 CAS.
- (a) W. Zheng, X. Lu, W. Wang, Z. Li, H. Zhang, Z. Wang, X. Xu, S. Li and C. Wang, J. Colloid Interface Sci., 2009, 338, 366–370 CrossRef CAS PubMed; (b) L. Ji, A. Medford and X. Zhang, Polymer, 2009, 50, 605–612 CrossRef CAS PubMed; (c) L. Mai, L. Xu, Q. Gao, C. Han, B. Hu and Y. Pi, Nano Lett., 2010, 10, 2604–2608 CrossRef CAS PubMed; (d) S. Mubeen, T. Zhang, N. Chartuprayoon, Y. Rheem, A. Mulchandani, N. Myung and M. Deshusses, Anal. Chem., 2010, 82, 250–257 CrossRef CAS PubMed.
- (a) J. Zhang, Y. Q. Sun, J. Liu, Y. Shi and W. Gou, Chem. Commun., 2013, 49, 11305–11307 RSC; (b) F. Yu, X. Han and L. Chen, Chem. Commun., 2014, 50, 12234–12249 RSC; (c) Z. Huang, S. Ding, D. Yu, F. Huang and G. Feng, Chem. Commun., 2014, 50, 9185–9187 RSC; (d) T. Ozdemir, F. Sozmen, S. Mamur, T. Tekinay and E. U. Akkaya, Chem. Commun., 2014, 50, 5455–5457 RSC; (e) Y. Zhou, J. Yu, X. Lei, J. Wu, Q. Niu, Y. Zhang, H. Liu, P. Christen, H. Gehringe and F. Wu, Chem. Commun., 2013, 49, 11782–11784 RSC.
- (a) X. Zan and Z. Su, Langmuir, 2009, 25, 12355–12360 CrossRef CAS PubMed; (b) J. Wei, L. Wang, X. Zhang, X. Ma, H. Wang and Z. Su, Langmuir, 2013, 29, 11413–11419 CrossRef CAS PubMed; (c) X. Zan, M. Kozlov, T. McCarthy and Z. Su, Biomacromolecules, 2010, 11, 1082–1088 CrossRef CAS PubMed.
- (a) C. Gu, H. Xu, M. Park and C. Shannon, ECS Trans., 2008, 16, 181–190 CAS; (b) C. Gu, H. Xu, M. Park and C. Shannon, Langmuir, 2009, 25, 410–414 CrossRef CAS PubMed.
- J. Xu, X. Cui, J. Zhang, H. Liang, H. Wang and J. Li, Bull. Mater. Sci., 2008, 31, 189–192 CrossRef CAS PubMed.
- (a) A. Kryukov, A. Stroyuk, N. Zin’chuk, A. Korzhak and S. Kuchmii, J. Mol. Catal. A: Chem., 2004, 221, 209–221 CrossRef CAS PubMed; (b) M. Pang, J. Hu and H. Zeng, J. Am. Chem. Soc., 2010, 132, 10771–10785 CrossRef CAS PubMed.
- M. Whiteman, S. Trionnaire, M. Chopra, B. Fox and J. Whatmore, Clin. Sci., 2011, 121, 459–488 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and UV-vis spectra of (PDDA/PSS)5/Ag2S formed under a high concentration of Na2S, effect of silver amplification time, spectra from EDX analysis. See DOI: 10.1039/c4ra11526k |
| This journal is © The Royal Society of Chemistry 2015 |