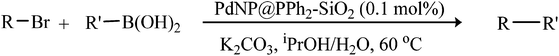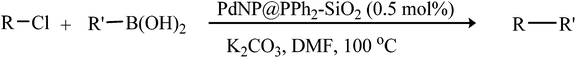Phosphine-stabilized Pd nanoparticles supported on silica as a highly active catalyst for the Suzuki–Miyaura cross-coupling reaction†
Debojeet Sahu and
Pankaj Das*
Department of Chemistry, Dibrugarh University, Dibrugarh, Assam, India. E-mail: pankajd29@yahoo.com; Fax: +91-373-2370323; Tel: +91-373-2370210
First published on 3rd December 2014
Abstract
We have prepared a silica supported palladium nanocatalyst (PdNP@PPh2–SiO2) with high dispersion via a one-step reaction between commercially available 2-diphenylphosphinoethyl-functionalized silica gel (PPh2–SiO2) and PdCl2 without the assistance of an external reductant or stabilizer. The catalyst was well characterized by N2-adsorption, XRD, HRTEM, SEM-EDX and ICP analyses. The supported catalyst exhibited excellent activity for the Suzuki–Miyaura cross-coupling reactions of aryl halides (including aryl chlorides) with arylboronic acids. The facile synthesis of the catalyst combined with high product yields at relatively low catalyst loading (<0.5 mol%) represents one of the most efficient silica supported heterogeneous systems for the Suzuki coupling of aryl chlorides. The catalyst could be reused several times without compromising its activity.
Introduction
Remarkable developments have been achieved in palladium (Pd) mediated organic synthesis during the past two decades.1 Among the various Pd catalyzed reactions, the Suzuki–Miyaura reaction of aryl halides with arylboronic acids forming biaryls has emerged as one of the most intense areas of research both in industry and in the laboratory.2 These biaryl compounds have a wide range of applications in pharmaceutical, material and agrochemical industries.2b,c Over the last two decades, significant advancements have been made in developing efficient Pd catalysts for this application.3 Unfortunately, the majority of the successful catalysts for Suzuki reactions are still homogeneous in nature where catalyst separation and reusability are often problematic. Compared to homogeneous protocols, heterogeneous systems possess several advantages which include easy separation, better recyclability, cost-effectiveness, etc. As a result, during the past few years, numerous efforts have been made to design novel heterogeneous catalysts4 by immobilizing Pd salts or complexes into/onto various supports such as silica,5 MCM-41,6 SBA-15/SBA-16,7 alumina,8 Zeolite,9 etc. Among the supports, silica remains one of the most preferred choices because of its multiple advantages that include low cost, wide accessibility, high thermal stability, excellent porosity and high surface reactivity which facilitate grafting of various ligand functionalities (such as –NH2, –PPh2, –SH, etc.) onto its surfaces.4b,10 Although a significant progress has already been achieved in terms of development of stable and reusable silica supported Pd catalysts of high efficiency,5 majority of such catalysts showed good activities only with aryl bromides/iodides and failed when aryl chlorides were used as substrates. As an example, Costa et al., few years back, developed a silica supported Pd catalyst that showed moderate to good activities with aryl bromides but completely failed when aryl chlorides were used as coupling partner with arylboronic acids.5e In a similar way, Chen et al. reported a silica-bound Pd phosphine catalyst that showed excellent result with aryl bromides under mild condition but very poor activity with aryl chlorides.5g Very recently, we have developed two silica-supported Schiff-base derived Pd catalysts that showed excellent activities for the Suzuki reactions of aryl bromides and iodides under mild condition but they miserably failed when aryl chlorides were used as substrates.11 It needs to mention that aryl chlorides are the most desirable substrates in Suzuki reaction as these chlorides are easily accessible and less expensive compared to corresponding bromides or iodides. Hence, the development of new silica-supported Pd catalysts for efficient activation of aryl chlorides, preferably with low Pd loading, still considered as one of the most challenging works in Suzuki reaction.Recent examples show that Pd nanoparticles (NPs) either in their colloidal form12 or immobilized on solid supports13 are highly active catalysts for activation of aryl halides in Suzuki reaction. As a normal practice, such NPs are synthesized via reduction of a Pd(II) species with an external reducing agent (such as hydrazine hydrate, NaBH4), in presence of a surfactant or ligand-based stabilizer as capping agent.13c,14 Compared to surfactant, the ligand-based stabilizers are preferable as removal of residual surfactants from the reaction mixture is a troublesome affair and thus making the system more expensive and industrially unacceptable.
Herein, we report facile synthesis of a new silica-supported Pd nanocatalyst (PdNP@PPh2–SiO2) stabilized by an anchored phosphine and its application in the Suzuki–Miyaura reactions of aryl halides (including chlorides). To the best of our knowledge, this is the first example for preparation of highly dispersed PdNPs on silica gel without using any external reductant or stabilizer. Noteworthy to mention that although, there are some reports on the use of silica-supported PdNPs as active catalysts for Suzuki–Miyaura reaction, until now their activities were limited only with aryl bromides and iodides5e,13b–e and very little is known about their performance in activating aryl chlorides.
Experimental
General information
2-Diphenylphosphinoethyl-functionalized silica gel (PPh2–SiO2: PPh2 loading, 1.05 mmol g−1 of silica gel; 200–400 mesh) was purchased from Sigma-Aldrich. PdCl2 was purchased from Acros Organics. The substrates used in the Suzuki–Miyaura reactions were purchased from Sigma-Aldrich, Spectrochem and Rankem. All other chemicals and solvents were purchased from different Indian firms. The solvents were distilled and dried prior to use. Powder X-ray diffractions (XRD) were performed on a Bruker AXS D8 Advance Diffractometer with Cu-Kα (1.541 Å) radiation. The SEM images were recorded in JEOL Model JSM-6390 LV and the EDX peaks were obtained from JEOL Model JED-2300. TEM images were recorded on a JEM-2100 operating at 200 kV. The palladium content was determined using ICP-AES of model Thermo Electron IRIS INTREPID II XSP DUO. Adsorption of nitrogen was carried out at 77 K using a Quantacrome, ASIQA 2010 analyzer for analyzing surface area and pore-size distributions of the materials. Prior to analysis the samples were degassed by heating at 200 °C for 4 h under vacuum. Specific surface area was calculated following the Brunauer–Emmett–Teller (BET) calculation and pore-size distribution was obtained by using the Barrett–Joyner–Halenda (BJH) pore analysis method applied to the desorption branch of the nitrogen adsorption–desorption isotherms. The Gas chromatographic studies of the products were carried out using Agilent 7820A GC system.Synthesis of the palladium catalyst
1.90 g of PPh2–SiO2 was added to a solution of 1.00 mmol of PdCl2 in 40 mL of acetonitrile and the reaction mixture was stirred at 70 °C for 6 h under nitrogen atmosphere. The resulting solution was filtered off and the yellow solids were washed several times through Soxhlet extraction with acetonitrile (until the extracted solvent become colorless). The material was designated as PdNP@PPh2–SiO2.General procedure for the Suzuki–Miyaura reactions of aryl halides using Pd-catalyst
A 50 mL round bottomed flask was charged with a mixture of aryl halide (0.5 mmol), arylboronic acid (0.75 mmol), K2CO3 (1.5 mmol), PdNP@PPh2–SiO2 (0.1–0.5 mol%), solvent (6 mL). The mixture was heated under stirring condition at appropriate temperature for the required time. The reaction progress was monitored by thin layer chromatography using aluminum coated TLC plates (Merck) under UV light or in some cases by GC. After completion, the catalyst was separated by filtration and washed the residual solid with the same reaction solvent. The filtrate was diluted with water (20 mL) and extracted with ether (3 × 20 mL). The combined extract was washed with brine (3 × 20 mL) and dried over Na2SO4. After evaporation of the solvent under reduced pressure, the residue was chromatographed (silica gel, ethyl acetate–hexane, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) to obtain the desired product. For recycling experiments, after each cycle of utilization, the catalyst was washed several times under refluxing condition with iPrOH–H2O followed by DCM to remove any occluded reactants and products physically attached with the catalyst surface. After drying the recovered catalyst at 80 °C overnight, transferred it to a reaction vessel containing substrates, base and solvent and performed the reusability experiments under identical condition. The various products separated were characterized 1H NMR and mass spectra.
9) to obtain the desired product. For recycling experiments, after each cycle of utilization, the catalyst was washed several times under refluxing condition with iPrOH–H2O followed by DCM to remove any occluded reactants and products physically attached with the catalyst surface. After drying the recovered catalyst at 80 °C overnight, transferred it to a reaction vessel containing substrates, base and solvent and performed the reusability experiments under identical condition. The various products separated were characterized 1H NMR and mass spectra.
Results and discussion
Synthesis and characterization of the catalyst
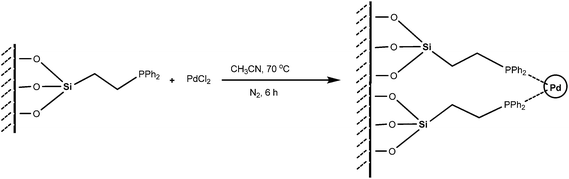
The silica supported Pd catalyst was prepared by reacting commercially available 2-diphenylphosphinoethyl-functionalized silica gel (PPh2–SiO2) with PdCl2 in acetonitrile at 70 °C (Scheme 1) without taking the assistance of any external reductant or stabilizer. The TEM image of the fresh catalyst is shown in Fig. 1a and reveals formation of discrete spherical palladium NPs without any signs of aggregations. The particle size histogram shows that the particles sizes vary from 5–25 nm with majority of the particles are within the range of 10–15 nm (Fig. 1a). Since we have not used any reducing or capping agent, it is reasonable to conclude that the phosphine ligand bound to silica would perform the two important roles: firstly, reduction of Pd2+ → Pd0; secondly, stabilization of NPs to promote good dispersions. The roles of phosphines in stabilizing metal NPs are well documented in literature, however synthesizing such NPs are always assisted by an external reducing agent.15 On the other hand, reducing properties of phosphines are also well known,16 but the utilities of phosphines to act as reducing and stabilizing agent simultaneously in a supported system are not common. The selected area electron diffraction (SAED) pattern of a single NP (Fig. 1b inset) shows the reticular plan extended throughout the nanocrystal without any fault or defect. The spacing between two crystal planes is found to be about 0.21 nm (Fig. 1b), which corresponds to the face centre cubic (fcc) arrangement of the metal nanocrystals.17 The SEM image (Fig. 2a) of the catalyst material shows silica particles are of different dimensions. The SEM-EDX spectra of the material (Fig. 2b) show the presence of Pd. From ICP-AES analysis, the Pd content was found to be 0.072 mmol g−1 of silica gel.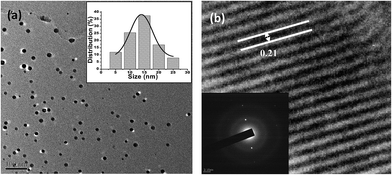 | ||
| Fig. 1 (a) TEM micrographs of PdNP@SiO2–PPh2 and particle size histograms with a Gaussian curve fitting (inset); (b) HRTEM image and SAED pattern (inset) of PdNP@SiO2–PPh2. | ||
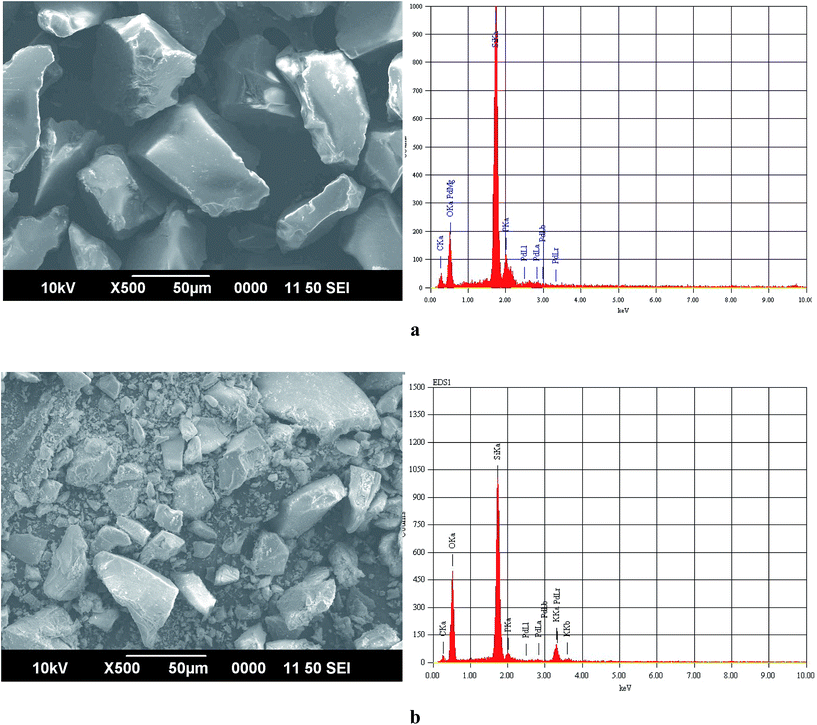 | ||
| Fig. 2 (a) SEM-EDX spectra of PdNP@SiO2–PPh2 (before catalysis). (b) SEM-EDX spectra of PdNP@SiO2–PPh2 (after 6th use). | ||
The N2 adsorption–desorption isotherm measured at 77 K, of the PdNP@PPh2–SiO2 shows a type IV pattern with a sharp hysteresis loop, which is characteristics of mesoporous materials18 (Fig. 3b). A specific surface area (ABET) of 100 m2 g−1 and pore volume of 0.19 cm3 g−1 has been obtained for the catalyst material. Compared to the supported ligand these values are smaller indicating immobilization of Pd onto silica matrix (ESI: Table S1†). The BJH pore size distribution of PdNP@PPh2–SiO2 shows that the pore diameter slightly shifts towards the lower value in comparison with the parent supported ligand indicating generation of NPs inside the pores resulting in decrease of pore volume and pore sizes (Fig. 3b inset).
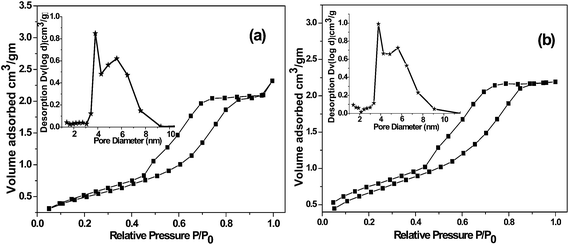 | ||
| Fig. 3 N2 adsorption–desorption isotherms and BJH pore size distribution curves (inset) of (a) PPh2–SiO2 (b) PdNP@PPh2–SiO2. | ||
The powder X-ray diffraction patterns (ESI: Fig. S1†) of phosphine functionalized silica gel, PPh2–SiO2, exhibits a broad peak at 2θ = 22.403° which upon grafting of Pd shows almost no significant change indicating that silica maintains its mesoporous structure even after palladium loading. The PdNP@PPh2–SiO2 material also shows three additional low intensity diffraction peaks corresponds to (111), (200) and (220) planes of the Pd(0) nanoparticles.19
Catalytic activity
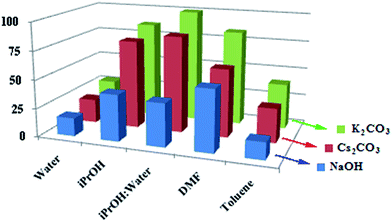
The Suzuki–Miyaura activity of the silica-based material, PdNP@PPh2–SiO2, was first evaluated for aryl bromides. The screening reactions were performed using p-bromoanisole and phenylboronic acid as model substrates in presence of 0.5 mol% catalyst at 100 °C, as this condition was found to give best results with other reported heterogeneous catalysts.5e,6b Initial solvent and base optimization study (Fig. 3) with selected bases and solvents revealed that iPrOH–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was the best solvent and K2CO3 was the best base. In fact, under the condition defined above, quantitative conversion of p-methoxybiphenyl was obtained by GC after 2 h of reaction time (ESI: Table S2,† entry 1). Interestingly, this excellent yield was maintained even after reducing the catalyst loading up to 0.1 mol% (entry 6). Further, the temperature optimization study revealed that the catalyst was virtually inactive at room temperature producing only 16% yield after 6 h (entry 4). The optimum temperature for the model reaction was found to be 60 °C (ESI: Table S2,† entry 3). With the optimized condition (iPrOH–H2O, K2CO3, 0.1 mol% Pd, 60 °C), we examined the scope of our catalyst for several representative coupling reactions with a range of aryl bromides and the results are summarized in Table 1. Our result shows that steric and electronic natures of the substrates have a little influence on our system. Aryl bromides bearing electron-deficient (p-CHO), electron-neutral and electron-rich (p-OCH3) groups reacted efficiently with phenylboronic acid producing corresponding biaryls in excellent yields (Table 1, entries 1–3). This high yields were also maintained with different arylboronic acids (Table 1, entries 10 and 11). Under slightly extended reaction time, our catalyst also tolerates sterically demanding substrates such as o-bromobenzaldehyde and 1-bromo-2,5-dimethoxybenzene and gave good yields (Table 1, entries 5 and 6). It is interesting to note that the heteroaromatic halides are considered to be difficult substrates to activate in Suzuki–Miyaura reaction and often they gave low yields.20 However, in our case, heteroaryl bromides such as 5-bromopyrimidine and 2-bromo-3-methylthiophene gave moderate yields at the present condition. Nevertheless, increasing the reaction temperature (80 °C) and catalyst loading (0.2 mol%) substantial increase in yields were achieved (Table 1, entries 8 and 9) (Fig. 4).
1) was the best solvent and K2CO3 was the best base. In fact, under the condition defined above, quantitative conversion of p-methoxybiphenyl was obtained by GC after 2 h of reaction time (ESI: Table S2,† entry 1). Interestingly, this excellent yield was maintained even after reducing the catalyst loading up to 0.1 mol% (entry 6). Further, the temperature optimization study revealed that the catalyst was virtually inactive at room temperature producing only 16% yield after 6 h (entry 4). The optimum temperature for the model reaction was found to be 60 °C (ESI: Table S2,† entry 3). With the optimized condition (iPrOH–H2O, K2CO3, 0.1 mol% Pd, 60 °C), we examined the scope of our catalyst for several representative coupling reactions with a range of aryl bromides and the results are summarized in Table 1. Our result shows that steric and electronic natures of the substrates have a little influence on our system. Aryl bromides bearing electron-deficient (p-CHO), electron-neutral and electron-rich (p-OCH3) groups reacted efficiently with phenylboronic acid producing corresponding biaryls in excellent yields (Table 1, entries 1–3). This high yields were also maintained with different arylboronic acids (Table 1, entries 10 and 11). Under slightly extended reaction time, our catalyst also tolerates sterically demanding substrates such as o-bromobenzaldehyde and 1-bromo-2,5-dimethoxybenzene and gave good yields (Table 1, entries 5 and 6). It is interesting to note that the heteroaromatic halides are considered to be difficult substrates to activate in Suzuki–Miyaura reaction and often they gave low yields.20 However, in our case, heteroaryl bromides such as 5-bromopyrimidine and 2-bromo-3-methylthiophene gave moderate yields at the present condition. Nevertheless, increasing the reaction temperature (80 °C) and catalyst loading (0.2 mol%) substantial increase in yields were achieved (Table 1, entries 8 and 9) (Fig. 4).
| Entry | R–Br | R′–B(OH)2 | Time (h) | Yieldb,c (%) | TONd |
|---|---|---|---|---|---|
a Reaction conditions: 0.5 mmol aryl bromide, 0.75 mmol arylboronic acid, 1.5 mmol K2CO3, 6 mL iPrOH–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).b Isolated Yield.c Yields are average of two runs.d TON, product mole/Pd mole.e Values in parenthesis are yields for reaction with catalyst: 0.2 mol%; T = 80 °C. 1).b Isolated Yield.c Yields are average of two runs.d TON, product mole/Pd mole.e Values in parenthesis are yields for reaction with catalyst: 0.2 mol%; T = 80 °C. |
|||||
| 1 | 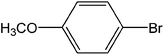 |
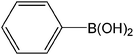 |
3 | 98 | 980 |
| 2 | 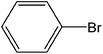 |
 |
3 | 94 | 940 |
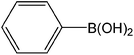
| 3 | 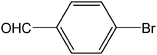 |
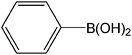 |
3 | 96 | 960 |
| 4 | 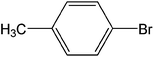 |
 |
3 | 98 | 980 |
| 5 | 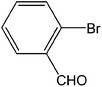 |
 |
4 | 88 | 880 |
| 6 |  |
 |
4 | 92 | 920 |
| 7 |  |
 |
4 | 91 | 910 |
| 8 |  |
 |
4 | 58 (92)e | 580 (460) |
| 9 |  |
 |
4 | 66 (95)e | 660 (475) |
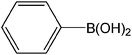
| 10 |  |
 |
4 | 98 | 980 |
| 11 |  |
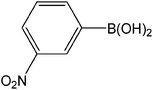 |
4 | 92 | 920 |
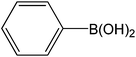
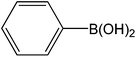
Encouraged by the results of our catalyst with aryl bromides, we turned our attention to the use of aryl chlorides as substrates. It needs to mention that there are several reports of the use of silica-supported Pd catalysts for Suzuki–Miyaura reaction but, only very few of them are successful for promoting aryl chlorides.5i,j Thus, in order to expand the scope of our catalyst for aryl chlorides, initially we have conducted the screening reaction with p-chloronitrobenzene and phenylboronic acid as coupling partners using the same optimum condition used for aryl bromides. Our result suggests that this experimental condition is virtually ineffective for the coupling reaction of aryl chlorides as only 17% biaryl was achieved after 6 h of reaction time (ESI: Table S3,† entry 1). However, search for an alternative condition revealed that changing the solvent to DMF, increasing the temperature to 100 °C and increasing the catalyst loading to 0.5 mol% almost quantitative conversion of biaryl has been achieved (ESI: Table S3,† entry 5). Our study reveals that 0.5 mol% catalyst loading is the lower limit of our catalyst for completion of the reaction. On decreasing the catalyst loading to 0.2 mol%, only 63% (ESI: Table S3,† entry 9) nitrobiophenyl was formed. A further decrease in catalyst loading to 0.1 mol%, only 43% product formation was achieved (ESI: Table S3,† entry 10). Under the same set of experimental condition, we have examined the cross-coupling reactions of a range of electronically and sterically diverse aryl chlorides with phenylboronic acid and the results are depicted in Table 2. Our result establishes that aryl chlorides bearing electron-withdrawing group such as p-chloronitrobenzene/p-chloroacetophenone (entries 1 and 6), electron-donating group such as p-chloroanisole (entry 7) and electron-neutral group such as chlorobenzene (entry 4) underwent smooth coupling with phenylboronic acid affording the desired biphenyls in good-excellent yields. Interestingly, our catalyst can also tolerate sterically bulky ortho-substituted chloride such as o-chloronitrobenzene (entry 3) and gave the desired product in reasonably good yields. No major differences in yields were noticed by replacing phenylboronic acid with p-tolylboronic acid (entry 10) and p-chloroboronic acid (entry 11).
| Entry | R–Cl | R′–B(OH)2 | Time (h) | Yieldb,c (%) | TONd |
|---|---|---|---|---|---|
| a Reaction conditions: 0.5 mmol aryl chloride, 0.75 mmol arylboronic acid, 1.5 mmol K2CO3, 6 mL DMF.b Isolated Yield.c Yields are average of two runs.d TON, product mole/Pd mole. | |||||
| 1 | 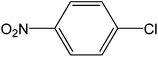 |
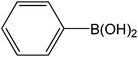 |
5 | 93 | 186 |
| 2 | 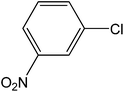 |
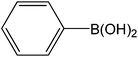
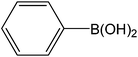 |
5 | 72 | 146 |
| 3 | 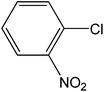 |
 |
5 | 66 | 132 |
| 4 | 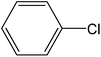 |
 |
5 | 95 | 190 |
| 5 |  |
 |
6 | 77 | 154 |
| 6 |  |
 |
6 | 85 | 170 |
| 7 | 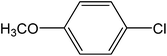 |
 |
5 | 86 | 172 |
| 8 | 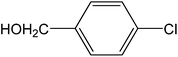 |
 |
5 | 88 | 176 |
| 9 |  |
 |
5 | 78 | 156 |
| 10 |  |
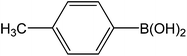 |
5 | 86 | 172 |
| 11 | 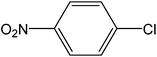 |
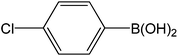 |
5 | 89 | 178 |
Reusability and heterogeneity test
Reusability is one of the most important properties of any heterogeneous catalyst to determine its sustainability and to check this property, the catalyst material in the model reaction between 4-bromoanisole and phenylboronic acid was separated from the reaction mixture by centrifugation and subsequent decantation, washed thoroughly with iPrOH–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), dried and reused for subsequent runs. The percentage conversions are diagrammatically represented in ESI: Fig. S2,† which shows that the catalyst could be reused at least for six times without comprising with its activity. In order to check the extent of Pd leaching, if any, into the solution we have performed ICP-AES analysis of the filtrate after 1st run, and only negligible amount (<1 ppm) of palladium was detected in the solution. To confirm this fact further, we have also performed the hot filtration test by using p-bromoanisole and phenylboronic acid as coupling partners. The reaction was stopped after 20 min. and the yield was determined to be about 30% (analyzed by GC). The solid catalyst was then separated from the solution by filtration under hot condition and the filtrate was then allowed to continue stirring to react for another 3 h. The product concentration in the filtrate was found to be almost constant (Fig. 5), indicating that no Pd species was leached into the solution and the catalyst was truly heterogeneous in nature. A slight change in surface morphology of the silica-based material before and after catalysis has been observed from the SEM pictures. The after catalysis material shows a little decrease in the silica particle size (Fig. 2c) however, this does not affect the reusability of the catalyst. The SEM-EDX (Fig. 2d) spectra and also the TEM image of the six times used catalyst clearly shows the presence of Pd on silica matrix which suggests that the catalyst could be used for further runs.
1), dried and reused for subsequent runs. The percentage conversions are diagrammatically represented in ESI: Fig. S2,† which shows that the catalyst could be reused at least for six times without comprising with its activity. In order to check the extent of Pd leaching, if any, into the solution we have performed ICP-AES analysis of the filtrate after 1st run, and only negligible amount (<1 ppm) of palladium was detected in the solution. To confirm this fact further, we have also performed the hot filtration test by using p-bromoanisole and phenylboronic acid as coupling partners. The reaction was stopped after 20 min. and the yield was determined to be about 30% (analyzed by GC). The solid catalyst was then separated from the solution by filtration under hot condition and the filtrate was then allowed to continue stirring to react for another 3 h. The product concentration in the filtrate was found to be almost constant (Fig. 5), indicating that no Pd species was leached into the solution and the catalyst was truly heterogeneous in nature. A slight change in surface morphology of the silica-based material before and after catalysis has been observed from the SEM pictures. The after catalysis material shows a little decrease in the silica particle size (Fig. 2c) however, this does not affect the reusability of the catalyst. The SEM-EDX (Fig. 2d) spectra and also the TEM image of the six times used catalyst clearly shows the presence of Pd on silica matrix which suggests that the catalyst could be used for further runs.
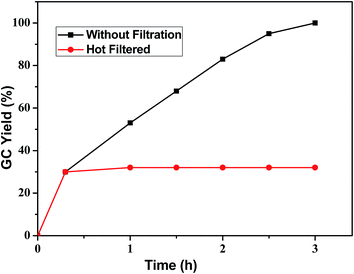 | ||
| Fig. 5 Hot filtration test for the coupling reaction between p-bromoanisole and phenylboronic acid catalyzed by PdNP@PPh2–SiO2. | ||
Conclusion
In summary, we have developed a silica-supported Pd nanocatalyst stabilized by an anchored phosphine without taking assistance of an external reductant or stabilizer. The supported catalyst exhibited excellent activity for the Suzuki–Miyaura cross-coupling reactions of aryl bromides and chlorides with a low catalyst loading (<0.5 mol%). The catalyst could be reused several times without compromising with its activity.Acknowledgements
The Council of Scientific and Industrial Research (CSIR), New Delhi, is gratefully acknowledged for financial support (Grant no. 02/0081/12). D. S. thanks the University Grants Commission (UGC), New Delhi for BSR-RFSMS fellowship. DST, New Delhi has also been acknowledged for providing Surface Area Analyzer to the Department under DST-FIST programme.References
- (a) K. C. Nicolaou, P. G. Bulger and D. Sarlah, Angew. Chem., Int. Ed., 2005, 44, 4442 CrossRef CAS PubMed; (b) X.-F. Wu, P. Anbarasan, H. Neumann and M. Beller, Angew. Chem., Int. Ed., 2010, 49, 9047 CrossRef CAS PubMed.
- Important reviews in Suzuki–Miyaura reaction: (a) N. Miyaura and A. Suzuki, Chem. Rev., 1995, 95, 2457 CrossRef CAS; (b) A. Fihri, M. Bouhrara, B. Nekoueishahraki, J.-M. Basset and V. Polshettiwar, Chem. Soc. Rev., 2011, 40, 5181 RSC; (c) A. J. J. Lennox and G. C. Lloyed-Jones, Chem. Soc. Rev., 2014, 43, 412 RSC.
- (a) A. F. Littke and G. C. Fu, Angew. Chem., Int. Ed., 1998, 37, 3387 CrossRef CAS; (b) J. P. Wolfe and S. L. Buchwald, Angew. Chem., Int. Ed., 1999, 38, 2413 CrossRef CAS; (c) A. F. Littke and G. C. Fu, Angew. Chem., Int. Ed., 2002, 41, 4176 CrossRef CAS; (d) L.-C. Liang, P.-S. Chien and M.-H. Huang, Organometallics, 2005, 24, 353 CrossRef CAS; (e) N. T. S. Phan, M. V. D. Sluys and C. W. Jones, Adv. Synth. Catal., 2006, 348, 609 CrossRef CAS and references there in; (f) Y.-Y. Peng, J. Liu, X. Lei and Z. Yin, Green Chem., 2010, 12, 1072 RSC; (g) S. C. To and F. Y. Kwong, Chem. Commun., 2011, 47, 5079 RSC; (h) W. Susanto, C.-Y. Chu, W. J. Ang, T.-C. Chou, L.-C. Lo and Y. Lam, Green Chem., 2012, 14, 77 RSC; (i) L. Monnereau, H. E. Moll, D. Semeril, D. Matt and L. Toupet, Eur. J. Inorg. Chem., 2014, 1364 CrossRef CAS; (j) F. Rajabi and W. R. Thiel, Adv. Synth. Catal., 2014, 356, 1873 CrossRef CAS.
- Important reviews in heterogeneous Suzuki–Miyaura reaction (a) L. Yin and J. Liebscher, Chem. Rev., 2007, 107, 133 CrossRef CAS PubMed; (b) V. Polshettiwar, C. Len and A. Fihri, Coord. Chem. Rev., 2009, 253, 2599 CrossRef CAS PubMed; (c) A. Molnar, Chem. Rev., 2011, 111, 2251 CrossRef CAS PubMed; (d) P. Zhou, H. Wang, J. Yang, J. Tang, D. Sun and W. Tang, RSC Adv., 2012, 2, 1759 RSC.
- (a) S. Pal and J. H. Clark, Green Chem., 2003, 5, 635 RSC; (b) L. Wang, A. Reis, A. Seifert, T. Philippi, S. Ernst, M. Jia and W. R. Thiel, Dalton Trans., 2009, 3315 RSC; (c) G. Jayamurugan, C. P. Umesh and N. Jayaraman, J. Mol. Catal. A: Chem., 2009, 307, 142 CrossRef CAS PubMed; (d) D.-H. Lee, J.-Y. Jung and M.-J. Jin, Green Chem., 2010, 12, 2024 RSC; (e) N. J. S. Costa, P. K. Kiyohara, A. L. Monteiro, Y. Coppel, K. Philippot and L. M. Rossi, J. Catal., 2010, 276, 382 CrossRef CAS PubMed; (f) N. Fukaya, M. Ueda, S.-Y. Onozawa, K. K. Bando, T. Miyaji, Y. Takagi, T. Sakakura and H. Yasuda, J. Mol. Catal. A: Chem., 2011, 58, 342 Search PubMed; (g) W. Chen, P. Li and L. Wang, Tetrahedron, 2011, 67, 318 CrossRef CAS PubMed; (h) M. Ghiaci, M. Zarghani, A. Khojastehnezhadb and F. Moeinpourc, RSC Adv., 2014, 4, 15496 RSC; (i) N. Fukaya, S. Onozawa, M. Udea, T. Miyaji, Y. Takagi, T. Sakakura and H. Yasuda, J. Mol. Catal. A: Chem., 2014, 385, 7 CrossRef CAS PubMed; (j) T. Iwai, R. Tanaka, T. Harada and M. Sawamura, Chem.–Eur. J., 2014, 20, 1057 CrossRef CAS PubMed.
- (a) M. Cai, J. Sha and Q. Xu, J. Mol. Catal. A: Chem., 2007, 268, 82 CrossRef CAS PubMed; (b) K. Dhara, K. Sarkar, D. Srimani, S. K. Saha, P. Chattopadhyay and A. Bhaumik, Dalton Trans., 2010, 39, 6395 RSC; (c) H. Zhao, J. Peng, R. Xiao and M. Cai, J. Mol. Catal. A: Chem., 2011, 337, 56 CrossRef CAS PubMed; (d) Q. Zhang, H. Su, J. Luo and Y. Wei, Tetrahedron, 2013, 69, 447 CrossRef CAS PubMed.
- (a) C. M. Crudden, M. Sateesh and R. Lewis, J. Am. Chem. Soc., 2005, 127, 10045 CrossRef CAS PubMed; (b) H. Yang, X. Han, Z. Ma, R. Wang, J. Liu and X. Ji, Green Chem., 2010, 12, 441 RSC; (c) Z. Zheng, H. Li, T. Liu and R. Cao, J. Catal., 2010, 270, 268 CrossRef CAS PubMed; (d) S. E. Hankari, A. E. Kadib, A. Finiels, A. Bouhaouss, J. J. E. Moreau, C. M. Crudden, D. Brunel and P. Hesemann, Chem.–Eur. J., 2011, 17, 8984 CrossRef PubMed; (e) R. Ghorbani-Vaghei, S. Hemmati and H. Veisi, J. Mol. Catal. A: Chem., 2014, 393, 240 CrossRef CAS PubMed.
- (a) S. S. Soomro, F. L. Ansari, K. Chatziapostolou and K. Kohler, J. Catal., 2010, 273, 138 CrossRef CAS PubMed; (b) S. S. Soomro, C. Roehlich and K. Kohler, Adv. Synth. Catal., 2011, 353, 767 CrossRef CAS; (c) A. Kumbhar, S. Jadhav, S. Kamble, G. Rashinkar and R. Salunkhe, Tetrahedron Lett., 2013, 54, 1331 CrossRef CAS PubMed.
- (a) A. Corma, H. Garcia and A. Leyva, Appl. Catal., A, 2002, 236, 179 CrossRef CAS; (b) F. Durap, M. Rakap, M. Aydemir and S. Ozkar, Appl. Catal., A, 2010, 382, 339 CrossRef CAS PubMed; (c) K. Okumura, T. Tomiyama, S. Okuda, H. Yoshida and M. Niwa, J. Catal., 2010, 273, 156 CrossRef CAS PubMed.
- (a) M. K. Richmond, S. L. Scott and H. Alper, J. Am. Chem. Soc., 2001, 123, 10521 CrossRef CAS PubMed; (b) C. Baleizao, A. Corma, H. Garcia and A. Leyva, Chem. Commun., 2003, 606 RSC.
- (a) C. Sarmah, D. Sahu and P. Das, Catal. Today, 2012, 198, 197 CrossRef CAS PubMed; (b) C. Sarmah, D. Sahu and P. Das, Catal. Commun., 2013, 41, 75 CrossRef CAS PubMed.
- (a) W. Han, C. Liu and Z.-L. Jin, Org. Lett., 2007, 9, 4005 CrossRef CAS PubMed; (b) M. Moreno-Manas, R. Pleixats and S. Villarroya, Organometallics, 2001, 20, 4524 CrossRef CAS; (c) C. Deraedt and D. Astruc, Acc. Chem. Res., 2014, 47, 494 CrossRef CAS PubMed.
- (a) P. Das, D. Sharma, A. K. Shil and A. Kumari, Tetrahedron Lett., 2011, 52, 1176 CrossRef CAS PubMed; (b) P. Dutta and A. Sarkar, Adv. Synth. Catal., 2011, 353, 2814 CrossRef CAS; (c) B. Basu and S. Paul, Appl. Organomet. Chem., 2013, 27, 588 CAS; (d) P. Veerakumar, M. Velayudham, K.-L. Lu and S. Rajagopal, Appl. Catal., A, 2013, 455, 247 CrossRef CAS PubMed; (e) M. Nandi and H. Uyama, RSC Adv., 2014, 4, 20847 RSC; (f) K. Karimi, M. Ghasemi and N. H. Naeini, Catal. Commun., 2013, 38, 10 CrossRef PubMed; (g) V. W. Faria, D. G. M. Oliveira, M. H. S. Kurz, F. F. Goncalves, C. W. Scheeren and G. R. Rosa, RSC Adv., 2014, 4, 13446 RSC.
- (a) N. J. S. Costa and L. M. Rossi, Nanoscale, 2012, 4, 5826 RSC; (b) B. J. Borah, S. J. Borah, K. Saikia and D. K. Dutta, Appl. Catal., A, 2014, 469, 350 CrossRef CAS PubMed.
- (a) S. U. Son, Y. Jang, K. Y. Yoon, E. Kang and T. Hyeon, Nano Lett., 2004, 4, 1147 CrossRef CAS; (b) M. Tamura and H. Fujihara, J. Am. Chem. Soc., 2003, 125, 15742 CrossRef CAS PubMed.
- (a) S. A. Buckler, L. Doll, F. K. Lind and M. Epstein, J. Org. Chem., 1962, 27, 794 CrossRef CAS; (b) J. A. Burns, J. C. Butler, J. Moran and G. M. Whitesides, J. Org. Chem., 1962, 56, 2648 CrossRef.
- (a) A. S. Roy, J. Mondal, B. Banerjee, P. Mondal, A. Bhaumik and S. M. Islam, Appl. Catal., A, 2014, 469, 320 CrossRef CAS PubMed; (b) P. P. Sarmah and D. K. Dutta, Green Chem., 2012, 14, 1086 RSC.
- K. S. W. Sing, D. H. Everett, R. A. W. Haul, L. Moscou, R. A. Pierotti, J. Rouquerol and T. Siemieniewska, Pure Appl. Chem., 1985, 57, 603 CrossRef CAS.
- N. Iranpoor, H. Firouzabadi, S. Motevalli and M. Talebi, J. Organomet. Chem., 2012, 708–709, 118 CrossRef CAS PubMed.
- (a) E. Bratt, O. Verho, M. J. Johansson and J.-E. Backvall, J. Org. Chem., 2014, 79, 3946 CrossRef CAS PubMed; (b) M. A. Düfert, K. L. Billingsley and S. L. Buchwald, J. Am. Chem. Soc., 2013, 135, 12877 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Textural data of BET studies, XRD patterns, catalyst optimization study, reusability diagram, 1H NMR and mass spectral data of cross-coupling products. See DOI: 10.1039/c4ra11186a |
| This journal is © The Royal Society of Chemistry 2015 |