A green technique to prepare uniform amine capped multi-walled carbon nanotubes to fabricate high strength, protein resistant polymer nanocomposites
Tanya Das*a,
Sunanda Royb,
Sun Tingb,
Liying Zhangb,
Yongmei Lib,
Chee Yoon Yue*a and
Xiao Hub
aSchool of Mechanical and Aerospace Engineering, Nanyang Technological University, 50 Nanyang Avenue, Singapore 639798. E-mail: tanyadas.iitmsc@gmail.com; mcyyue@ntu.edu.sg
bSchool of Materials Science and Engineering, Nanyang Technological University, Singapore 639798
First published on 5th January 2015
Abstract
An effort has been made to produce efficient amino functionalized carbon nanotubes (CNTs) without utilizing any hazardous chemicals, with an aim to use them in biomaterials as well as in advanced polymer nanocomposites. Because it is water soluble, biocompatible and rich in primary amines, allylamine (AA) was selected for surface functionalization. Grafting was performed by a novel approach, i.e. double UV-ozone induced grafting (DUVO) process, which is absolutely eco-friendly, fast, cost-effective and highly controllable. Optimized conditions have been identified to achieve the highest degree of grafting with uniform thickness. To explore their functionality, the PAA-g-MWCNTs were incorporated into Nylon 6 and PET matrices; remarkable increases in tensile strength (34% and 78%) and Young's modulus (44% and 30%) at only 0.3 wt% filler loading were noticed due to excellent dispersion and strong adhesion with the polymer matrix. Moreover, in vitro BSA and Fb protein adsorption tests showed that the nanocomposites containing PAA-g-MWCNTs possess significantly higher surface antifouling property as compared to the neat polymers. It was also interesting to note that the antifouling property of the composites increased with increasing polymer grafting density on the CNTs. These results clearly suggest that the DUVO-PAA-g-MWCNTs would be versatile novel filler materials in the field of advanced polymer nanocomposites as well as in biomaterials.
1. Introduction
Carbon nanotubes (CNTs) show extraordinarily high mechanical, thermal and electrical conductivity properties, and thus possess great potential in applications such as photovoltaic devices,1–3 superconductors,4 lithium batteries,5,6 supercapacitors,7,8 gas storage media,9 nanowires,10 and polymer reinforcement.11–13 Over the past two decades, numerous research articles have showed that despite the many advantageous properties of CNTs, the development of advanced polymer composites with as-grown CNTs is technologically very challenging. This may be due to their nonreactive surface properties and weak dispersion, preventing an efficient load transfer from the polymer matrix to the CNTs. Due to their poor dispersion and inefficient load transfer, the mechanical properties of polymer–CNT composites are often not as superior as anticipated. The functionalization of the sidewalls of CNTs has been found to be an effective way to prevent them from agglomerating, facilitating better dispersion and improving their compatibility with polymer matrices. There are two types of functionalization approaches categorized based on the noncovalent and covalent functionalization of CNTs. In the noncovalent functionalization process, CNTs are functionalized with aromatic compounds, surfactants, and polymers through π–π stacking or hydrophobic interactions.14,15 The advantage of this method is that it does not destroy the conjugation of the sidewalls of the CNTs, which enables them to retain their mechanical properties and improves their processability. However, due to the weak forces between the wrapped molecules, the efficiency of load transfer using this process can be low. On the other hand, covalent functionalization leads to the transformation of the carbon hybridization of the CNTs from sp2 C to sp3 C atoms, leaving holes in the structure, which could adversely affect their mechanical and electrical properties.16 Nevertheless, this functionalization of CNTs can improve their solubility as well as their dispersion in solvents or matrix polymers.17,18 The covalent attachment of chemical groups is commonly achieved by the oxidation of carbon nanotubes in concentrated HNO3, H2SO4, HCl and H2O2.19–21 The tips of CNTs have been proven to be more active than their sidewalls, and the treatment of CNTs with these acids could open the nanotube tips and facilitate the introduction of oxygen-containing groups at the opened ends. As a result, most of the acid oxidation reactions cause damage to the nanotubes as well as the sidewalls. Moreover, these harsh chemical treatments can break the CNTs into shorter tubes,22 which apparently affects the thermal conductivity of CNTs because this property is length dependent.23 Furthermore, these treatments are considered to be highly environmentally hazardous due to the involvement of large amounts of acids, which require huge volumes of water for purification.A particular attractive option is the radiation based modification of CNTs, which largely retains their structural integrity. Therefore, in the current study, we have demonstrated an environmentally safe process for the modification of CNTs. Modern global interest is focused on green chemistry techniques to modify surfaces under mild, eco-friendly conditions with low energy consumption to minimize environmental issues. In this particular context, UV-ozone treatment would be an appropriate choice for the functionalization of CNTs because it is fast, homogeneous, more controllable and precisely modifies only the surface properties, without affecting the bulk properties.24 Though plasma treatment process have recently shown great potential in the surface modification or surface grafting of CNTs,25–27 the UV-ozone treatment has some key advantages over it: (i) with the breakdown mechanism of O3 to O2, the process becomes more environmentally friendly, (ii) the surface modification can be carried out under atmospheric pressure with O3 and (iii) it is more cost-effective because no vacuum is required.28 To date, UV-ozone treatment has been basically employed for the surface hydrophilization of polymer surfaces.29,30 Though few recent studies have reported UV/O3 process for the surface treatment of CNTs,24,31,32 to the best of our knowledge, the utilization of this theory by us to graft polymers on CNTs is a new exploration in the area of surface functionalization of CNTs. Motivated by the aforementioned merits, in the present study, we demonstrate the surface functionalization of MWCNTs by two approaches, i.e., double UV-ozone induced grafting (DUVO) and UV-ozone induced UV-grafting (UVOUV) with an objective of identifying the most efficient and robust process. Allylamine was particularly chosen as a monomer for grafting because it is water soluble, rich in primary amine groups and has high reactivity towards carbon materials.33,34 In comparison with the common amine functionalization process of CNTs, which passes through a long chemical process (typically 4–6 days) through carboxylation and acylchlorination followed by amidation involving numerous hazardous chemicals,35,36 we believe that our process is more advantageous and promising based on the above-illustrated facts. In addition, to explore the versatile properties of the grafted MWCNTs in various fields, melt mixing with Nylon 6 and polyethylene terephthalate (PET) was conducted, and the mechanical properties of the composites were evaluated. Following this, the surface biocompatibilities of the nanocomposites were also investigated to establish their potentiality in the area of biomaterials. The mentioned matrices were chosen because they are widely used plastics in many engineering and daily applications.
2. Experimental methods
MWCNTs with an outer diameter of less than 8 nm and a length in the range of 10–30 μm (purity over 95%) were purchased from Cheap Tubes, USA. All the chemicals, including allylamine, benzophenone (BP, 98% purity), acetone, methanol, dimethylformamide (DMF), bovine serum albumin (BSA) and bovine fibrinogen proteins (Fb), were purchased from Sigma Aldrich. Nylon 6 and polyethylene terephthalate (PET) were provided from other collaborators. All the chemicals were used as received.2.1 UV-ozone treatment and preparation of polymer grafted MWCNTs
UV-ozone treatment of the MWCNTs was conducted using a UV/ozone surface processor equipment model Senlight PL16, Japan. A regulator was attached to the pump to manually control the oxygen flow into the chamber. Prior to the UV-ozone treatment, raw MWCNTs (∼1 g) were subjected to a 20 kHz ultrasonic processor for 20 min in DMF solution to separate the CNTs by disruption of their inherent aggregation. After vacuum drying (24 h), the raw MWCNTs were subjected to UV-ozone radiation treatment for 90 min. Then, the UV-ozone treated MWCNTs were divided into two parts: in the first part (I), ∼600 mg of activated MWCNTs were transferred into a round bottom flask containing an AA monomer solution (30 wt% in water) and then thoroughly mixed for 20 min under continuous nitrogen gas purging. The graft polymerization was carried out under UV irradiation for 6 and 10 h at room temperature, using a UV flood curing system (λ = 365 nm). The control samples were prepared in parallel using raw CNTs following the same process.In the second part of the experiment (II), the UV-ozone treated MWCNTs (300 mg) were introduced into another round bottom flask containing the same amount of monomer solution and thoroughly mixed for 5 minutes. Then, the CNTs were removed from the solution and dried at 40 °C for 2 h. The dried UV-ozone pretreated CNTs with a pre-adsorbed layer of AA monomer on their surfaces were placed again into the UV-ozone reaction chamber for graft copolymerization for up to 45 min. After graft polymerization, all the products were separately and repeatedly washed with deionized water, acetone and ethanol, followed by centrifugation to remove traces of unreacted residuals and homopolymers. The polymer grafted MWCNTs were then dried in a vacuum oven overnight and stored for further experiments. The samples that were polymerized under UV light for 6 and 10 h are denoted as 6-UVOUV-PAA-g-MWCNTs and 10-UVOUV-PAA-g-MWCNTs, respectively, and the sample grafted under double UV-ozone treatment process (II) is denoted as DUVO-PAA-g-MWCNTs. The details of the experiments are demonstrated in Fig. 1.
 | ||
| Fig. 1 Schematic representation of the synthesis of polymer grafted MWCNTs by DUVO and UVOUV processes. | ||
2.2 Preparation of polymer nanocomposites
The polymer composites were prepared by a simple solution blending and casting technique followed by melt mixing. Prior to use, the polymer pellets (Nylon 6 and PET) were vacuum dried at 70 °C for 12 h. In the case of the PET nanocomposites, the polymer pellets were first dissolved in 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP, ≥99%) solution, and then the PAA-g-MWCNTs were added to the solution and thoroughly mixed for several hours to ensure good dispersion. The Nylon 6 nanocomposites were similarly prepared in a formic acid solution. The films were obtained by solvent evaporation at 45 °C over a period of 2 days. These films were then annealed at 135 °C for 4 h under vacuum. Subsequently, the films were cut into small pieces, and then extruded above the polymer melting temperature (for Nylon 6 and PET, the processing temperatures were set at 250 and 270 °C, respectively, while the screw speed and mixing time were maintained the same, i.e., 100 rpm and 5 min, respectively). In each system, the nanocomposites were composed of only 0.2 and 0.3 wt% of grafted MWCNTs. For determining the tensile properties, the ASTM D638-V method was followed.2.3 Protein adsorption tests on nanocomposites
The protein-adsorption tests were carried out following our previously reported protocol.37 Bovine serum albumin (BSA) and fibrinogen (Fb) were chosen as model proteins for protein-adsorption measurements. Prior to adsorption, the neat polymer and nanocomposite films were hydrated in PBS solution for 4 h, and then incubated in 1 mg ml−1 of solution (2 h, 37 °C) of either protein. After rinsing several times with Milli-Q water to remove loosely bound protein, the films were then washed with 1% sodium dodecyl sulfate (SDS) to detach the adsorbed protein from the surface. A standard μBCA assay kit was used to estimate the protein content in the SDS washes, which was then quantified by measuring the absorption spectra at 562 nm (Tecan Infinite 200 series, Switzerland).2.4 Characterizations
The chemical compositions of the as-received, plasma modified and monomer grafted CNTs were determined using a Perkin-Elmer GX Fourier transform infrared spectroscope (FTIR) in the transmission mode. KBr pellets were prepared for the measurements.The elemental compositions of the raw and modified CNT surfaces were determined by X-ray photoelectron spectroscopy (XPS), using monochromatic Al Kα radiation as the photon source. The binding energies were referenced to the saturated hydrocarbon peak at 285 eV.
Raman spectroscopy was carried out using a Renishaw RM1000 laser spectrometer to investigate the structural changes of the MWCNTs.
Transmission electron microscopy (TEM) analysis was performed using a JEM-2010F electron microscope.
Field emission scanning electron microscopy (FESEM) was performed using a JEOL JSM-7600F scanning electron microscope.
3. Results and discussion
Fig. 1 illustrates the schematic representations of the plausible polymer grafted MWCNTs obtained by UVOUV process (I) and DUVO process (II). The reason for the UV-ozone pretreatment of CNTs before grafting is to generate polar peroxide groups on their chemically inert surfaces, which can later act as initiators during the polymerization process in the presence of monomers. Next, the graft polymerization reaction occurred through the cleavage of peroxide groups formed on the surfaces of the CNTs in the presence of strong UV-ozone irradiation (because the oxygen–oxygen chemical bond of peroxide is unstable and easily split into reactive radicals via homolytic cleavage). We believe that the DUVO grafting process has some advantages over the UVOUV grafting process, such as: (i) DUVO process requires only 45 min to accomplish the polymerization, whereas the UVOUV process takes several hours to complete. Some studies have even mentioned 24 h treatment in particular.38,39 (ii) Unlike the UVOUV grafting process, the DUVO process does not require any additional UV lamps, external gas cylinders or vacuum during polymerization; thus, (iii) it is more cost effective. In addition, this approach is considered to be a dry or solventless process, and therefore would be a very attractive process for practical and industrial applications.The FTIR spectra of the raw and all the polymer-coated MWCNTs are shown in Fig. 2. In the case of raw MWCNT, two small peaks for –OH (3600 cm−1) and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C (1606 cm−1) groups are observed. The appearance of a small –OH peak is probably the result of mild surface oxidation during the purification steps during manufacturing.28 As can be seen, after the UV-ozone treatment, the wavenumbers of the oxidized MWCNTs remained the same as those of the raw MWCNTs, but the chemical composition and peak intensities noticeably changed in the oxidized CNTs (see Fig. 1b). For all the poly-allylamine grafted MWCNTs (Fig. 2c–e), a strong absorption peak is observed in the range of 3100–3500 cm−1, which is attributed to the stretching vibrations and the deformation mode of the primary amine.40 Peaks in the range of 2800–3000 cm−1 are ascribed to the stretching vibration of the aliphatic C–H groups. The peak around 1580 cm−1 can be assigned to the bending modes of the primary amine groups. The appearance of another broad peak at 1164 cm−1 represents the C–N stretching vibrations.40 The presence of NH2 and C–N bands on the modified CNT samples confirms the attachment of primary amines due to the successful grafting of allylamine. It is also apparent that when the UV exposure time is increased, the intensities of the characteristic peaks for the amine group increases, indicating the increasing rate of grafting degree. However, when Fig. 2d and e are compared, the intensities and areas of the abovementioned peaks are found to be higher for the DUVO-PAA-g-MWCNT sample, which corroborates the conversion of more monomers to the resultant products.37,40
C (1606 cm−1) groups are observed. The appearance of a small –OH peak is probably the result of mild surface oxidation during the purification steps during manufacturing.28 As can be seen, after the UV-ozone treatment, the wavenumbers of the oxidized MWCNTs remained the same as those of the raw MWCNTs, but the chemical composition and peak intensities noticeably changed in the oxidized CNTs (see Fig. 1b). For all the poly-allylamine grafted MWCNTs (Fig. 2c–e), a strong absorption peak is observed in the range of 3100–3500 cm−1, which is attributed to the stretching vibrations and the deformation mode of the primary amine.40 Peaks in the range of 2800–3000 cm−1 are ascribed to the stretching vibration of the aliphatic C–H groups. The peak around 1580 cm−1 can be assigned to the bending modes of the primary amine groups. The appearance of another broad peak at 1164 cm−1 represents the C–N stretching vibrations.40 The presence of NH2 and C–N bands on the modified CNT samples confirms the attachment of primary amines due to the successful grafting of allylamine. It is also apparent that when the UV exposure time is increased, the intensities of the characteristic peaks for the amine group increases, indicating the increasing rate of grafting degree. However, when Fig. 2d and e are compared, the intensities and areas of the abovementioned peaks are found to be higher for the DUVO-PAA-g-MWCNT sample, which corroborates the conversion of more monomers to the resultant products.37,40
 | ||
| Fig. 2 FTIR spectra of the (a) raw MWCNTs, (b) UVO-MWCNTs, (c) 6-UVOUV-PAA-g-MWCNTs, (d) 10-UVOUV-PAA-g-MWCNTs and (e) DUVO-PAA-g-MWCNTs. | ||
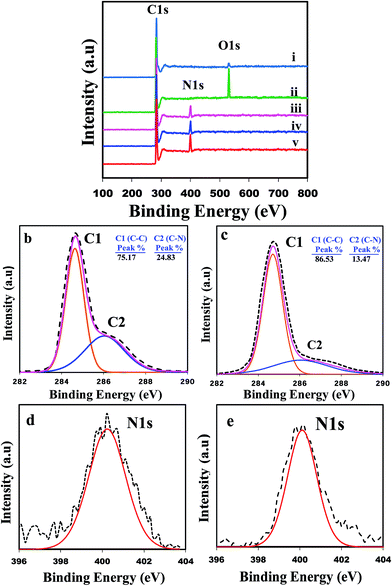
To confirm the abovementioned conclusion and investigate the chemical bonds involved in these modification processes in more detail, XPS measurements were further conducted. Fig. 3a compares the XPS survey spectra of the raw MWCNTs, UVO-MWCNTs (first oxidized), 6-, 10-UVOUV-PAA-g-MWCNTs and DUVO-PAA-g-MWCNT samples. The survey spectra show that raw MWCNT (Fig. 3a(i)) is composed of carbon and oxygen atoms, whereas the PAA-g-MWCNTs contains carbon and nitrogen atoms. Peaks located at binding energies (BE) of 284.8, 400.1 and 531.9 eV correspond to the C, N and O atoms, respectively.40 For raw MWCNTs, the small O1s peak again indicates the occurrence of very low levels of surface oxidation during the manufacturing process. The atomic composition data showed that the raw MWCNTs are comprised of 96.88% carbon and 3.12% oxygen, whereas the 6-UVOUV-PAA-g-MWCNTs and 10-UVOUV-PAA-g-MWCNTs contain 85.19% and 81.42% carbon and 14.81% and 18.58% nitrogen, respectively. It is also interesting to observe that for the DUVO-PAA-g-MWCNTs sample, the percentages of carbon (76.93%) and nitrogen (23.07%) atoms are considerably higher than that for the 10-UVOUV-PAA-g-MWCNTs sample. As a result of the highest amount of amine grafting, the intensity of the N peak for the DUVO-PAA-g-MWCNTs is also noticed to be the strongest amongst all the samples (see Fig. 3a(v)). To further confirm these facts, the high resolution C1s and N1s spectra for the DUVO-PAA-g-MWCNTs and 10-UVOUV-PAA-g-MWCNTs are presented (Fig. 3b–e). From the curve fitted high resolution C1s spectra (Fig. 3b and c), it can be clearly seen that though both the samples indicate two peaks at the same binding energy, i.e., C1 corresponding to the C–C bonds (sp3-hybridized carbon atoms, BE = 284.9 eV) and C2 corresponding to the C–N bonds generated from the amine linkages (–CH2–CH2–NH2, BE = 286.1 eV), the C2 peak for the DUVO-PAA-g-MWCNTs is significantly broader and larger than that of the 10-UVOUV-PAA-g-MWCNTs sample. Further comparison between the high resolution N1s peaks (Fig. 3f and g) clearly shows a larger N1s peak for the DUVO-PAA-g-MWCNTs sample, indicating again that the higher level of poly-allylamine grafting can be obtained using this particular process. Based on the abovementioned results, it can be understood that DUVO is a more feasible and effective process for the efficient grafting of MWCNTs.
As it is important to know the amount of free amino groups present on the surface of the grafted CNTs, the amino derivatization of CNTs with 4-(trifluoromethyl)-benzaldehyde (TFBA, C8H5F3O) was carried out. PAA-g-MWCNTs was soaked in TFBA solution for 4 h, and then washed several times with ethanol. The percentage of free amino groups was estimated with the help of F atom concentration by XPS measurement following the below-mentioned equation:
| %NH2 = [NH2/N] = [(F/3)/N] × 100. |
The F1s XPS spectra for the raw CNTs and amine modified CNTs are shown in Fig. 4. As can be seen, raw CNT does not have any F1s peak due to the lack of amino conjugation owing to the absence of amine groups on its surface. However, a sharp F1s peak was observed in the aminated CNT samples at the B.E. of 689.1 eV, assigned to the –CF3 functional group in TFBA molecules. The mechanism of conjugation is shown in Fig. 4. The percentages of amine groups were estimated to be ∼27% and 40% for the 10-UVOUV-PAA-g-MWCNTs and DUVO-PAA-g-MWCNTs samples, respectively.
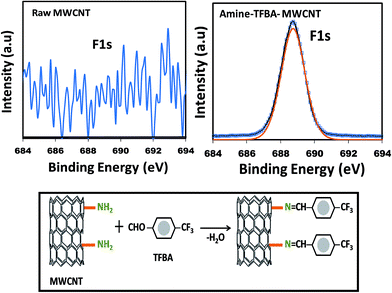 | ||
| Fig. 4 F1 XPS spectra of raw MWCNT and TFBA conjugated amine modified MWCNTs. The schematic represents the mechanism of the derivatization process. | ||
Subsequently, Raman spectra were acquired for all the samples because it is a very useful tool to determine the extent of structural disorder generated in the modified CNTs. Fig. 5a–c shows the Raman spectra of the raw and two polymer grafted MWCNTs. There are three distinct peaks in all the spectra. The peaks appearing at approximately 1351 cm−1 and 1576 cm−1 are denoted as the D and G bands, respectively. The D band is usually attributed to the presence of amorphous, disordered or sp3 hybridized carbons in the CNT, whereas the G band represents the tangential mode and is associated with the ordered sp2-hybridized carbon network.41 Another characteristic peak at the higher frequency side of 2677 cm−1 is the second-order overtone of the D-band, referred to as D*, and it is independent of the defect concentration. The ratio of the intensity of the D and G bands, (ID/IG), was calculated to determine the relative extent of structural defects in the MWCNTs due to the surface modification. The ID/IG ratios were found to be 0.21, 0.50 and 0.41 for the raw CNTs, DUVO-PAA-g-MWCNTs and 10-UVOUV-PAA-g-MWCNTs, respectively. Moreover, a slight shifting of the D band and G bands to higher wavenumbers (called blue shift)28 was noticed. The peak shifting associated with the increment of ID/IG ratio clearly demonstrates that the covalent grafting of poly-allylamine on the surface of the MWCNTs was successfully accomplished.
To estimate the relative amounts of polymer grafting achieved by the two different methods, TGA was performed under a nitrogen gas flow from room temperature to 800 °C. This information is very important in the case of polymer composites. This is because good polymer grafting could tremendously improve the miscibility and reinforcing ability of the CNTs into the polymer matrix. Fig. 6a shows the TGA curves of the raw and two differently polymer grafted MWCNTs. As can be seen, the TGA profile for raw CNT is very stable and does not exhibit any weight loss up to 650 °C, followed by a small loss, which corresponds to the decomposition of carbon materials (most likely disordered carbon). In contrast, the polymer grafted MWCNTs showed faster decomposition and less thermal stability. As known, a slight loss before 150 °C is most likely due to the desorption of water from the sample; the major weight loss in the temperature range from 270 to 500 °C corresponds to the decomposition of the polymer. Based on the TGA profiles, the amounts of grafted polymers in DUVO-PAA-g-MWCNTs and 10-UVOUV-PAA-g-MWCNTs were calculated to be ∼44% and 29%, respectively, confirming again the higher degree of polymer grafting achieved by the DUVO process.
Fig. 6b shows the dispersion stability of DUVO-PAA-g-MWCNTs and 10-UVOUV-PAA-g-MWCNTs in DMF over time. It can be clearly seen from the digital images that the DUVO-PAA-g-MWCNTs (Fig. 6b(i) and (ii)) provide a more stable and homogeneous solution than the 10-UVOUV-PAA-g-MWCNTs. Moreover, the DUVO-PAA-g-MWCNTs solution remained black even after 24 h, without noticeable sedimentation. This is certainly because of the higher amount of polymer (polar) grafting on the DUVO-MWCNTs, which facilitates their dispersion in DMF solvent via greater polar–polar interactions.
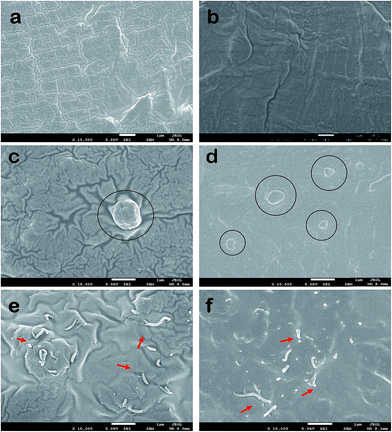
Fig. 7 shows the morphological comparison of the raw MWCNTs, DUVO-PAA-g-MWCNTs and 10-UVOUV-PAA-g-MWCNTs through FESEM and TEM images. From the micrographs, it is noticed that the surface of the raw CNTs is smooth and thin (Fig. 7a), whereas after polymer grafting, the tubes become thick and rough (Fig. 7b and c). It is also apparent from Fig. 7 that though both the process offers relatively uniform coating, the DUVO-PAA-g-MWCNTs were encapsulated by a much thicker layer of polymers owing to the higher degree of grafting (Fig. 7c and e), compared to the 10-UVOUV-PAA-g-MWCNTs (Fig. 7b and d). Despite this, the coating was found to be more uniform and the nanotubes thoroughly covered the DUVO-PAA-g-MWCNTs; this is believed to be the effect of double UVO treatment (because UV-ozone is a homogeneous treatment) and a faster grafting reaction. From the TEM images (Fig. 7d and e), the average diameter of the polymer coating in DUVO-PAA-g-MWCNTs and UVOUV-PAA-g-MWCNTs was found to be approximately 5–8 and 3–4 nm, respectively, again validating the facts explained above. All these evidences clearly demonstrate that double UV-ozone induced grafting is a versatile, controlled and promising technique for the effective grafting of MWCNTs. Moreover, this process is found to be more effective than several other grafting processes.42,43
Based on the best results found and also to explore their versatile potentiality, the DUVO-PAA-g-MWCNTs were incorporated into both Nylon 6 and PET matrices to fabricate various nanocomposites. Fig. 8a and b show the stress–strain curves for the Nylon 6 and PET composites, respectively. As can be seen, irrespective of the matrices, the addition of DUVO-PAA-g-MWCNTs tremendously increased both the tensile strength and Young's modulus of the composites. From both Fig. 8a and b, it can be clearly seen that the addition of raw MWCNTs (0.2 wt%) does not have any noticeable contribution on the mechanical properties (tensile strength), while the addition of the same amount, i.e. 0.2 wt% of DUVO-PAA-g-MWCNTs in Nylon 6 and PET resulted in increments of ∼13% and ∼40% in their mechanical properties, respectively, compared to the neat polymer. Further increasing the concentration of DUVO-PAA-g-MWCNTs to 0.3 wt% of the composites resulted in tremendous improvements in tensile strength as well as in Young's modulus. A minimum of five samples were tested for each set of composites, and the details of the results with standard deviation values are given in Table 1. The improvements in tensile strength and Young's modulus were found to be 34% and 44% for Nylon 6 and 78% and 30% for PET, respectively. These results are incomparably higher than many previous reports based on Nylon 6 and PET/CNT nanocomposites, even at higher CNT loadings.43–48 However, the elongation at break of the composites was found to be reduced compared to that of the pure polymer, indicating that the composites became brittle; moreover, their plastic deformation was decreased in comparison with the neat polymer, which is probably due to the confinement of the motion of the polymer chains as a result of strong adhesion between the modified MWCNTs and the polymer matrix. The abovementioned outstanding improvements in the mechanical properties using only 0.3 wt% of DUVO-PAA-g-MWCNTs in the composites clearly demonstrates the achievements of excellent wettability, efficient dispersion and strong interactions of the MWCNTs in the polymer matrix.
| Sample | Yield strength (MPa) | Increase in yield strength (%) | Young's modulus (MPa) | Increase in Young's modulus (%) |
|---|---|---|---|---|
| Nylon 6 | 50.1 (±3.7) | — | 435.4 (±16.8) | — |
| Nylon 6/0.2 wt% raw MWCNTs | 51.7 (±3.9) | 3 | 467.2 (±17.4) | 7 |
| Nylon 6/0.2 wt% DUVO-PAA-MWCNTs | 58.5 (±3.6) | 17 | 500.0 (±17.7) | 15 |
| Nylon 6/0.3 wt% DUVO-PAA MWCNTs | 67.3 (±4.0) | 34 | 625.3 (±17.6) | 44 |
| PET | 35.6 (±4.6) | — | 623.6 (±17.2) | — |
| PET/0.2 wt% raw MWCNTs | 37.6 (±4.6) | 6 | 700.5 (±18.1) | 12 |
| PET/0.2 wt% DUVO-PAA-MWCNTs | 50.0 (±4.6) | 40 | 802.4 (±17.6) | 29 |
| PET/0.3 wt% DUVO-PAA-MWCNTs | 63.4 (±4.6) | 78 | 808 (±18.4) | 30 |
To correlate with the abovementioned conclusions, the dispersion of CNTs in the polymer matrix was investigated through the cryo-fractured surfaces of the composites by FESEM analysis. Fig. 9a and b show the surface morphologies of neat Nylon 6 and PET polymers, whereas Fig. 9c–f show the surface morphologies of the Nylon 6 and PET nanocomposites. It can be clearly seen from Fig. 9e and f that irrespective of the polymer matrix, the modified MWCNTs (0.3 wt%) are well distributed throughout and also firmly embedded into the matrix. Moreover, from the fracture surfaces, it was observed that due to strong adhesion between the polymer–CNTs interfaces, at the time of fracture most of the CNTs were broken apart rather than pulled out from the matrix. This strong adhesion of the DUVO-PAA-g-MWCNTs to the polymers could be due to the hydrogen bonding interactions between the grafted poly-allylamine and the polar amide groups of Nylon 6 and the ester groups of PET, as well as high compatibility between themselves. This result could be the key factor contributing to the excellent mechanical properties of the abovementioned composites through the process of efficient load transfer from the polymer matrix to the modified MWCNTs. In contrary, extremely poor dispersion, large clumps and weak interfacial adhesion were observed for the raw MWCNT nanocomposites (see Fig. 9b and c). Thus, we certainly believe that the DUVO of poly-allylamine could be an excellent alternative choice for the effective amine functionalization of carbon nanotubes.
Because poly-allylamine is a known biocompatible polymer, in vitro protein adsorption experiments were carried out on both the neat polymers and their corresponding composites to demonstrate their biocompatibility. To date, poly-allylamine has been typically utilized on various polymeric substrates to enhance their surface wettability; however, we have probably used it for the first time to covalently functionalize the surface of carbon nanotubes for the fabrication of CNT reinforced bio-nanocomposites. We believe that these composite materials, and the CNTs themselves, would be very useful for many biomedical/orthopedic applications where antifouling properties as well as reinforced materials are important such as biosensors, where antibodies must be firmly immobilized on the transducer surface to improve the performance of the biosensors. This can only be achieved by the attachment of selective functional groups on the transducer surface, as well as in the antibodies; thus, surface modification is essential. It should also be noted that in most of the biosensors, moieties containing large amounts of amines are preferred because of their high reactivity towards biomolecules.49,50
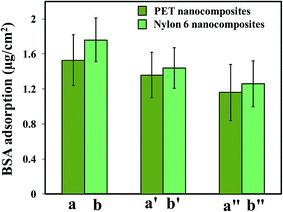
Fig. 10 shows the amount of adsorbed BSA and Fb proteins on the surfaces of the polymer nanocomposites. It is well known that the adsorption of protein on a substrate significantly depends on the surface characteristics of the substrate, such as hydrophilicity, roughness, charge, or chemistry. As can be seen from Fig. 10a and b, the adsorbed amount of BSA and Fb were noticeably reduced for both the nanocomposites compared to the neat polymers. The reductions in BSA and Fb protein adsorption were found to be ∼35% and 32% for the PET-nanocomposites, and 36% and 34% for the Nylon 6 nanocomposites, respectively as compared to the neat polymers.
 | ||
| Fig. 10 The bar diagrams illustrate the (a) BSA and (b) fibrinogen protein adsorption results of the DUVO-PAA-g-MWCNTs based nanocomposites (0.3 wt% CNTs samples were used). | ||
Fig. 11 demonstrates the time dependent surface stability of the composite films in protein medium. This is not only useful for determining the antifouling activity and surface stability of the films, but also a necessary step for continuous operation in a commercial environment. This investigation was carried out for a period of 8 days. Fig. 11a shows the kinetic study of protein adsorption, where the polymer films were incubated in 1 mg ml−1 protein solutions continuously for 8 days. It can be clearly seen that the nanocomposites strongly resisted protein adsorptions on their surfaces, and showed less change in antifouling activity with time compared to the neat polymers. Similarly, a strong decline in protein adsorption and stable antifouling activity were observed for the nanocomposites, even when the protein solutions were exchanged with freshly prepared (1 mg ml−1) solutions every two days (see Fig. 11b).
It may also be very interesting to determine the effect of the polymer grafting density of the CNTs on the antifouling behavior of the nanocomposites. To address this question, we preceded one step further and prepared three different nanocomposites comprised of 6- and 10-UVOUV-PAA-g-MWCNTs and DUVO-PAA-g-MWCNTs, which contained different degrees of polymer wrapping on their surfaces. Composites with 0.3 wt% CNTs loading were used. It is very interesting to see from Fig. 12 that the composites containing the CNTs with the highest degree of polymer grafting on their surfaces exhibited the lowest amount of protein adsorption compared to the two analogous specimens. The amount of protein adsorption decreases as the grafting degree increases. These results clearly indicate that the amount of biocompatible polymer wrapping on CNTs plays a significant role in altering the surface biocompatibility of such materials. This excellent antifouling property clearly indicates the promising biocompatible characteristics of the nanocomposites. Based on the abovementioned results, it can be certainly stated that the UV-ozone induced grafting process is indeed a promising technique and could be a new, alternative way to prepare uniform polymer enwrapped nanoparticles for the fabrication of advanced polymer nanocomposites and biocomposites.
4. Conclusions
A facile and novel strategy has been demonstrated to prepare effective amino-functionalized MWCNTs using UV-ozone induced UV-graft polymerization. Optimized conditions were identified to achieve the best polymer grafting. The XPS, FTIR, Raman, FESEM and TEM analysis results confirmed that poly-allylamine was successfully and more uniformly grafted on the DUVO-MWCNTs. Upon the incorporation of a very small amount of DUVO-PAA-g-MWCNTs as fillers into polymer matrices, namely, Nylon 6 and PET, the resulting nanocomposites showed tremendously high mechanical properties due to excellent dispersion, strong interfacial adhesion with the matrices and efficient load carrying capability. Moreover, in vitro protein adsorption tests demonstrated the good biocompatibility of the surface of the nanocomposites, which we believe would enable their use in various biomedical applications. The strategy established here is easily scalable, possibly applicable to other reactive monomers and offers a new way for the covalent functionalization of MWCNTs.Acknowledgements
This project was financially supported by the Singapore-MIT Alliance as well as A-Star, Singapore on the MIMO thematic program.Notes and references
- S. Ren, M. Bernardi, R. R. Lunt, V. Bulovic, J. C. Grossman and S. Gradečak, Nano Lett., 2011, 11, 5316–5321 CrossRef CAS PubMed.
- A. Bachtold, P. Hadley, T. Nakanishi and C. Dekker, Science, 2001, 294, 1317–1320 CrossRef CAS PubMed.
- H. Ago, K. Petritsch, M. S. P. Shaffer, A. H. Windle and R. H. Friend, Adv. Mater., 1999, 11, 1281–1285 CrossRef CAS.
- A. Y. Kasumov, R. Deblock, M. Kociak, B. Reulet, H. Bouchiat, I. I. Khodos, Y. B. Gorbatov, V. T. Volkov, C. Journet and M. Burghard, Science, 1999, 284, 1508–1511 CrossRef CAS.
- B. J. Landi, M. J. Ganter, C. D. Cress, R. A. DiLeo and R. P. Raffaelle, Energy Environ. Sci., 2009, 2, 638–654 CAS.
- S. W. Lee, N. Yabuuchi, B. M. Gallant, S. Chen, B. S. Kim, P. T. Hammond and Y. S. Horn, Nat. Nanotechnol., 2010, 5, 531–537 CrossRef CAS PubMed.
- W. Lu and R. Hartman, J. Phys. Chem. Lett., 2011, 2, 655–660 CrossRef CAS.
- V. Gupta and N. Miura, Electrochim. Acta, 2006, 52, 1721–1726 CrossRef CAS PubMed.
- W. H. Shin, H. M. Jeong, B. G. Kim, J. K. Kang and J. W. Choi, Nano Lett., 2012, 12, 2283 CrossRef CAS PubMed.
- A. M. Ajayan and S. Iijima, Nature, 1993, 361, 333–334 CrossRef.
- N. G. Sahoo, S. Rana, J. W. Cho, L. Li and S. H. Chan, Prog. Polym. Sci., 2010, 35, 837–867 CrossRef CAS PubMed.
- P. A. Song, L. H. Xu, Z. H. Guo, Y. Zhang and Z. P. Fang, J. Mater. Chem., 2008, 18, 5083–5091 RSC.
- P. M. Ajayan, O. Stephan, C. Colliex and D. Trauth, Science, 1994, 265, 1212–1214 CAS.
- G. S. Duesberg, M. Burghard, J. Muster and G. Philipp, Chem. Commun., 1998, 435–436 RSC.
- R. J. Chen, S. Bangsaruntip, K. A. Drouvalakis, N. W. S. Kam, M. Shim, Y. Li, W. Kim, P. J. Utz and H. Dai, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 4984–4989 CrossRef CAS PubMed.
- H. Park, J. Zhao and J. P. Lu, Nano Lett., 2006, 6, 916–919 CrossRef CAS PubMed.
- J. E. Riggs, Z. Guo, D. L. Carroll and Y. P. Sun, J. Am. Chem. Soc., 2000, 122, 5879–5880 CrossRef CAS.
- H. Tantang, J. Y. Ong, C. L. Loh, X. Dong, P. Chen, Y. Chen, X. Hu, L. P. Tan and L. J. Li, Carbon, 2009, 47, 1867–1870 CrossRef CAS PubMed.
- Y. Kanai, V. R. Khalap, P. G. Collins and J. C. Grossman, Phys. Rev. Lett., 2010, 104, 066401 CrossRef.
- V. Datsyuk, M. Kalyva, K. Papagelis, J. Parthenios, D. Tasis, A. Siokou, I. Kallitsis and C. Galiotis, Carbon, 2008, 46, 833–840 CrossRef CAS PubMed.
- Y. Li, C. Y. Yang and S. M. Chen, Int. J. Electrochem. Sci., 2011, 6, 4829–4842 CAS.
- F. Y. Meng, S. Ogata, D. S. Xu, Y. Shibutani and S. Q. Shi, Phys. Rev. B: Condens. Matter Mater. Phys., 2007, 75, 205403 CrossRef.
- I. D. Rosca and S. V. Hoa, Carbon, 2009, 47, 1958–1968 CrossRef CAS PubMed.
- M. L. Sham and J. K. Kim, Carbon, 2006, 44, 768–773 CrossRef CAS PubMed.
- B. Zhao, L. Zhang, X. Wang and J. Yang, Carbon, 2012, 50, 2710–2716 CrossRef CAS PubMed.
- L. Szetsen and W. Peng Jr, J. Phys. Chem. Solids, 2011, 72, 1101–1103 CrossRef PubMed.
- S. Roy, T. Das, C. Y. Yue and X. Hu, ACS Appl. Mater. Interfaces, 2014, 6, 664–670 CAS.
- K. Efimenko, W. E. Wallace and J. Genzer, J. Colloid Interface Sci., 2002, 254, 306–315 CrossRef CAS.
- L. G. Villa-Diaz, H. Nandivada, J. Ding, N. C Nogueira-de-Souza, P. H. Krebsbach, K. Sue O'Shea, J. Lahann and G. D. Smith, Nat. Biotechnol., 2010, 28, 581–583 CrossRef CAS PubMed.
- S. X. Liu, J. T. Kim and S. Kim, J. Food Sci., 2008, 73, E143–E150 CrossRef CAS PubMed.
- S. Kim, Y. I. Lee, D. H. Kim, K. J. Lee, B. S. Kim, M. Hussain and Y. H. Choa, Carbon, 2013, 51, 346–354 CrossRef CAS PubMed.
- E. Najafi, J. Y. Kim, S. H. Han and K. Shin, Colloids Surf., A, 2006, 284–285, 373–378 CrossRef PubMed.
- P. Li, H. Liu, J. Yang, D. Sun, Y. Chen, Y. Zhou, C. Cai and T. Lu, J. Mater. Chem. B, 2014, 2, 102–109 RSC.
- H. Liu, Y. Cui, P. Li, Y. Zhou, X. Zhu, Y. Tang, Y. Chen and T. Lu, Analyst, 2013, 138, 2647–2653 RSC.
- T. Ramanathan, F. T. Fisher, R. S. Ruoff and L. C. Brinson, Chem. Mater., 2005, 17, 1290–1295 CrossRef CAS.
- M. Moniruzzaman, J. Chattopadhyay, W. E. Billups and K. I. Winey, Nano Lett., 2007, 7, 1178–1185 CrossRef CAS PubMed.
- S. Roy, T. Das and C. Y. Yue, ACS Appl. Mater. Interfaces, 2013, 5, 5683–5689 CAS.
- Y. H. Yan, J. Cui, M. B. Chan-Park, X. Wang and Q. Y. Wu, Nanotechnology, 2007, 18, 115712 CrossRef.
- X. Ren, D. Shao, G. Zhao, G. Sheng, J. Hu, S. Yang and X. Wang, Plasma Processes Polym., 2011, 8, 589–598 CrossRef CAS.
- A. Abbas, C. Vivien, B. Bocquet, D. Guillochon and P. Supiot, Plasma Processes Polym., 2009, 6, 593–604 CrossRef CAS.
- C. Chen, B. Liang, D. Lu, A. Ogino, X. Wang and M. Nagatsu, Carbon, 2010, 48, 939–948 CrossRef CAS PubMed.
- P. Petrov, G. Georgiev, D. Momekova, G. Momekov and C. B. Tsvetanov, Polymer, 2010, 51, 2465–2471 CrossRef CAS PubMed.
- H. Xia, Q. Wang and G. Qiu, Chem. Mater., 2003, 15, 3879–3886 CrossRef CAS.
- Q. Zhou, Y. Hu, X. Sun and Y. Weng, Adv. Mater. Res., 2011, 287–290, 462–466 CAS.
- J. Y. Kim, H. J. Choi, C. S. Kang and S. H. Kim, Polym. Compos., 2010, 31, 858–869 CAS.
- N. G. Sahoo, H. K. F. Cheng, J. Cai, L. Li, S. H. Chan, J. Zhao and S. Yu, Mater. Chem. Phys., 2009, 117, 313–320 CrossRef CAS PubMed.
- C. Xu, Z. Jia, D. Wu, Q. Han and T. Meek, J. Electron. Mater., 2006, 35, 954–957 CrossRef CAS.
- G. Xin Chen, H. S. Kim, B. H. Park and J. S. Yoon, Polymer, 2006, 47, 4760–4767 CrossRef PubMed.
- F. Basarir, N. Cuong, W. K. Song and T. H. Yoon, Macromol. Symp., 2007, 249–250, 61–66 CrossRef.
- T. Sen and I. J. Bruce, Sci. Rep., 2012, 2, 564, DOI:10.1038/srep00564.
| This journal is © The Royal Society of Chemistry 2015 |