PEG based random copolymer micelles as drug carriers: the effect of hydrophobe content on drug solubilization and cytotoxicity†
Abstract
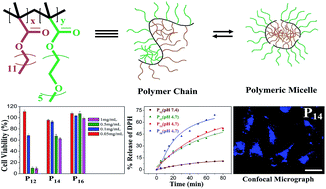
Polymeric micelles (PMs) of block copolymers have been extensively used as drug delivery vehicles in recent times. Polyethylene glycol (PEG) is being used for surface modification of these delivery systems, as PEG helps to protect from recognition by the reticuloendothelial system in the body. This work reports the synthesis and characterization of three new PEG-based random copolymers of poly(dodecyl methacrylate-co-polyethylene glycol methyl ether methacrylate), poly[DMAx-co-mPEGy] (where x : y = 1 : 2, 1 : 4, 1 : 6), with different hydrophobe (DMA) content. These copolymers were observed to exhibit good surface activity in aqueous solution. The micelle formation by these random amphiphilic copolymers in aqueous medium was studied using a steady-state fluorescence probe technique. The size and shape of the PMs were determined by use of light scattering and electron microscopic techniques, respectively. The aqueous solutions of all three copolymers exhibit a temperature-induced phase transition above a critical temperature (LCST) forming a stable emulsion. The LCST value of the micellar solution was observed to increase with an increase of mPEG content of the copolymer, but decrease with an increase of polymer concentration. The PMs were successfully used to solubilize the hydrophobic anticancer drug (S)-(+)-camptothecin (CPT); the solubilization capacity of the PMs was observed to increase with the decrease of hydrophobe content (or with the substantial increase of hydrophile content) in these copolymers. However, all three copolymers have the potential to protect the labile lactone ring of CPT from hydrolysis. Though all three copolymers showed a considerable release of the guest at acidic pH, the polymer having the maximum hydrophile content showed maximum guest release. Furthermore, the PMs of all three copolymers were observed to be easily permeable to the cancer cell membrane. In the permissible concentration regime, the polymers were found to be nontoxic and hemocompatible, but the cell viability as well as hemocompatibility was observed to deteriorate with the increase of DMA content in the copolymers.

 Please wait while we load your content...
Please wait while we load your content...