Calcium mitigates the stress caused by ZnSO4 as a sulphur fertilizer and enhances the sulforaphane formation of broccoli sprouts†
Abstract
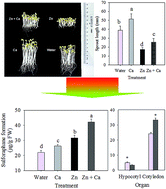
In order to improve the growth condition of broccoli sprouts under ZnSO4 application, exogenous CaCl2 was added in the cultural medium, and the growth profiles, key bioactive substances (glucosinolates, sulforaphane, ascorbic acid and phenolic compounds), antioxidant capacity, myrosinase activity and related genes expression of the broccoli sprouts were evaluated. Results showed that the stressful condition caused by ZnSO4 was effectively mitigated by CaCl2. Consequently, ascorbic acid and the total phenolics content as well as the antioxidant capacity of the broccoli sprouts decreased compared with that of the sole ZnSO4 treatment. However, sulforaphane formation increased because a higher glucoraphanin content, myrosinase activity and related gene expression was induced after the CaCl2 treatment. The glucoraphanin content and sulforaphane formation of the water-treated sprouts decreased steadily during germination. However, the sulforaphane formation of the sprouts treated with ZnSO4 plus CaCl2 increased after germinating for 2 days. These results suggest that CaCl2 could mitigate the stress caused by ZnSO4 and enhance the sulforaphane formation of broccoli sprouts.

 Please wait while we load your content...
Please wait while we load your content...