Tuned magnetism–luminescence bifunctionality simultaneously assembled into flexible Janus nanofiber
Fei Bi,
Xiangting Dong*,
Jinxian Wang and
Guixia Liu
Key Laboratory of Applied Chemistry and Nanotechnology at Universities of Jilin Province, Changchun University of Science and Technology, Changchun 130022, China. E-mail: dongxiangting888@163.com; Fax: +86 0431 85383815; Tel: +86 0431 85582574
First published on 5th January 2015
Abstract
A new structure of [CoFe2O4/PVP]//[YAG:5%Eu3+/PVP] magnetic–luminescent bifunctional Janus nanofibers has been successfully fabricated via electrospinning technology using a homemade parallel spinneret. Electrospinning-derived YAG:5%Eu3+ luminescent nanofibers and CoFe2O4 magnetic nanofibers are incorporated into polyvinyl pyrrolidone (PVP) matrix and electrospun into Janus nanofibers with CoFe2O4 magnetic nanofibers/PVP as one strand nanofiber and YAG:5%Eu3+ luminescent nanofibers/PVP as another strand nanofiber. [CoFe2O4/PVP]//[YAG:5%Eu3+/PVP] magnetic–luminescent bifunctional Janus nanofibers possess superior magnetic and luminescent properties due to their special nanostructure, and the luminescent characteristics and saturation magnetizations of the Janus nanofibers can be tuned by adding various amounts of YAG:5%Eu3+ luminescent nanofibers and CoFe2O4 magnetic nanofibers. Compared with CoFe2O4/YAG:5%Eu3+/PVP composite nanofibers, the magnetic–luminescent bifunctional Janus nanofibers provide higher performances due to the isolation of YAG:5%Eu3+ luminescent nanofibers from CoFe2O4 magnetic nanofibers. More importantly, the design conception and construction technology are of universal significance to fabricate other bifunctional Janus nanofibers.
1 Introduction
Nowadays, the development of the magnetic–luminescent bifunctional nanomaterials has attracted particular interest because of their wide applications in biological systems such as diagnostic analysis and controlled drug release.1–5 Most of the magnetic–luminescent nanomaterials are core–shell structures. In general, organic dyes and quantum dots (QDs) have been used as the luminescence shell of the core–shell structured magnetic–luminescent nanomaterials.6–9 However, the photobleaching and quenching properties of organic dyes and the toxicity of QDs have seriously limited their applications. Compared with organic dyes and QDs, lanthanide-doped nanomaterials have begun to attract attention due to their excellent luminescence properties. Among these luminescent materials, Eu3+-activated Y3Al5O12 (YAG) is an important phosphor with a variety of applications in many luminescent and optical devices due to its excellent performance.10–13 As a type of magnetic material, cobalt ferrite (CoFe2O4) has received considerable attention because of its moderate saturation magnetization, high coercivity and excellent physical and chemical stability, as well as because of its potential applications in electronic devices, drug delivery technology, magnetic resonance imaging, and information storage.14–16Presently, researchers are mainly focused on the preparation, properties and applications of magnetic–luminescent bifunctional nanoparticles. To obtain new morphologies of magnetic–luminescent nanomaterials, the fabrication of one-dimensional (1D) magnetic–luminescent nanomaterials is an urgent subject of study.
Electrospinning is a simple and versatile technique to process polymers and related materials into one-dimensional structural fibers with controllable compositions, diameters, and porosities for a variety of applications. This method not only has attracted extensive academic investigations, but it is also been applied in many areas.17,18 To date, various magnetic–luminescent bifunctional 1D nanomaterials have been prepared via electrospinning.19–21 From these studies, it has been proved that the existence of dark-colored magnetic nanomaterials greatly decreases the luminescence of rare earth compounds if magnetic nanomaterials are directly blended with the rare earth luminescent compounds.22–26 Therefore, if the strong luminescence of the magnetic–luminescent bifunctional nanomaterials is to be achieved, rare earth compounds must be effectively isolated from magnetic nanomaterials to avoid their direct contact. In the procedure of seeking a way to ultimately reduce the impact of magnetic nanomaterials on the luminescent properties of the magnetic–luminescent bifunctional nanofibers, we were inspired by the reports about the Janus particles.27–29 Janus particles have two distinguished surfaces/chemistries on their two sides. Pierre-Gilles de Gennes, a winner of the Nobel Prize in Physics, introduced the Janus particles to the scientific community. These Janus-type morphologies allow the control of composition and surface anisotropy, providing additional degrees of freedom in the design of composite materials. Adopting the unique feature of the asymmetry dual-sided Janus structure, Janus nanofibers can successfully help to realize the effective separation of magnetic nanomaterials from rare earth luminescent compounds, and it is expected that the Janus nanofibers simultaneously exhibit both excellent magnetic and luminescent properties.
In this study, we designed and fabricated magnetic–luminescent bifunctional [CoFe2O4/PVP]//[YAG:5%Eu3+/PVP] Janus nanofibers via electrospinning using a homemade spinneret. This Janus nanostructure can successfully realize the effective separation of CoFe2O4 nanofibers from YAG:5%Eu3+ nanofibers. The structure, morphology, luminescence characteristics and magnetic properties of the [CoFe2O4/PVP]//[YAG:5%Eu3+/PVP] Janus nanofibers were investigated in detail, and some meaningful results were obtained.
2 Experimental section
2.1 Chemicals
Polyvinyl pyrrolidone (PVP, Mw = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300
300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) and N,N-dimethylformamide (DMF) were purchased from Tianjin Tiantai Fine Chemical Reagents Co., Ltd. HNO3 was bought from Beijing Chemical Company. Y2O3 (99.99%), Eu2O3 (99.99%), Al(NO3)3·9H2O, Fe(NO3)3·9H2O and Co(NO3)2·6H2O were bought from Sinopharm Chemical Reagent Co., Ltd. All chemicals were of analytical grade and directly used as received without further purification. Y(NO3)3·6H2O and Eu(NO3)3·6H2O were prepared by dissolving Y2O3 and Eu2O3 in dilute nitric acid, followed by crystallizing from the solution through evaporating the excess water and HNO3 by heating.
000) and N,N-dimethylformamide (DMF) were purchased from Tianjin Tiantai Fine Chemical Reagents Co., Ltd. HNO3 was bought from Beijing Chemical Company. Y2O3 (99.99%), Eu2O3 (99.99%), Al(NO3)3·9H2O, Fe(NO3)3·9H2O and Co(NO3)2·6H2O were bought from Sinopharm Chemical Reagent Co., Ltd. All chemicals were of analytical grade and directly used as received without further purification. Y(NO3)3·6H2O and Eu(NO3)3·6H2O were prepared by dissolving Y2O3 and Eu2O3 in dilute nitric acid, followed by crystallizing from the solution through evaporating the excess water and HNO3 by heating.
2.2 Preparation of CoFe2O4 nanofibers and YAG:5%Eu3+ nanofibers
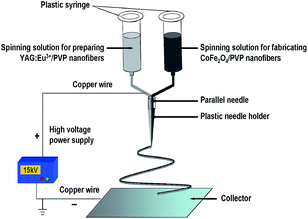
A traditional single-spinneret electrospinning instrument was used to prepare CoFe2O4 nanofibers (named S1) and YAG:5%Eu3+ nanofibers (named S2). In a typical procedure of preparing spinning solution for fabricating CoFe2O4 nanofibers, 1 mmol of Fe(NO3)3·9H2O, 0.5 mmol of Co(NO3)2·6H2O and 2.2 g of PVP were dissolved in 15.8 g of DMF with continuous stirring. The spinning solution for preparing YAG:5%Eu3+ nanofibers was prepared as follows: 0.95 mmol of Y(NO3)3·6H2O, 0.05 mmol of Eu(NO3)3·6H2O, 1.38 g of Al(NO3)3·9H2O and 2.4 g of PVP were added in 15.6 g of DMF to form a uniform solution with vigorous stirring. The spinning solutions were stirred for 4 h to form homogeneous mixture solutions for next-step electrospinning. Then, the spinning solutions were respectively injected into a traditional single-spinneret electrospinning setup, [Fe(NO3)3 + Co(NO3)2]/PVP composite nanofibers and [Y(NO3)3 + Al(NO3)3 + Eu(NO3)3]/PVP composite nanofibers were respectively prepared by electrospinning. The electrospinning parameters were as follows: the distance between the spinneret (a plastic needle) and collector was fixed at 18–20 cm and high voltage power supply was maintained at 12–15 kV. The room temperature was 20–24 °C and the relative humidity was 60%–70%. YAG:5%Eu3+ nanofibers and CoFe2O4 nanofibers could be obtained when the relevant composite nanofibers were annealed in air at 900 °C for 8 h and 700 °C for 4 h with a heating rate of 1 °C min−1, respectively.2.3 Fabrication of [CoFe2O4/PVP]//[YAG:5%Eu3+/PVP] Janus nanofibers and CoFe2O4/YAG:5%Eu3+/PVP composite nanofibers
Two different types of spinning solutions were prepared to fabricate Janus nanofibers. The spinning solution for preparing the strand CoFe2O4/PVP fiber of the Janus nanofibers was developed as follows: CoFe2O4 magnetic nanofibers (S1) were ultrasonically dispersed in DMF for 20 min at room temperature, then a certain amount of PVP was added into the abovementioned mixture with stirring for 12 h, and the final mixture was denoted as spinning solution A. In the preparation of spinning solution for fabricating the strand YAG:5%Eu3+/PVP fiber of the Janus nanofibers, YAG:5%Eu3+ luminescent nanofibers (S2) were added into DMF, followed by ultrasonic dispersion for 20 min. Then, a certain amount of PVP was dissolved in the abovementioned solution with stirring for 12 h. A mixed solution of YAG:5%Eu3+ nanofibers, PVP and DMF was prepared as the spinning solution B. The dosages of these materials are shown in Table 1.| Samples | Spinning solutions | PVP/g | DMF/g | CoFe2O4 nanofibers (S1)/g | YAG:5%Eu3+ nanofibers (S2)/g |
|---|---|---|---|---|---|
| Sa1 | A1 (CoFe2O4/PVP = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
0.300 | 2.007 | 0.300 | |
B1 (YAG:5%Eu3+/PVP = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
0.300 | 2.200 | 0.300 | ||
| Sa2 | A2 (CoFe2O4/PVP = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) 3) |
0.900 | 6.023 | 0.300 | |
B1 (YAG:5%Eu3+/PVP = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
0.300 | 2.200 | 0.300 | ||
| Sa3 | A3 (CoFe2O4/PVP = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) 5) |
1.500 | 10.035 | 0.300 | |
B1 (YAG:5%Eu3+/PVP = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
0.300 | 2.200 | 0.300 | ||
| Sa4 | A1 (CoFe2O4/PVP = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
0.300 | 2.007 | 0.300 | |
B2 (YAG:5%Eu3+/PVP = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
0.600 | 4.400 | 0.300 | ||
| Sa5 | A1 (CoFe2O4/PVP = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
0.300 | 2.007 | 0.300 | |
B3 (YAG:5%Eu3+/PVP = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) 3) |
0.900 | 6.600 | 0.300 | ||
| Sa6 | A1 (CoFe2O4/PVP = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
0.300 | 2.007 | 0.300 | |
B4 (YAG:5%Eu3+/PVP = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) 5) |
1.500 | 11.000 | 0.300 | ||
| Sb1 | Mixing A1 and B1 | 0.600 | 4.207 | 0.300 | 0.300 |
[CoFe2O4/PVP]//[YAG:5%Eu3+/PVP] Janus nanofibers were prepared using an electrospinning setup with a homemade parallel spinneret, as indicated in Fig. 1. The two types of spinning solutions were respectively loaded into each syringe, and the spinneret was vertically settled. A piece of flat iron net used as a collector was placed about 18 cm away from the tip of the plastic nozzle to collect the Janus nanofibers. A positive direct current (DC) voltage of 15 kV was applied between the spinneret and the collector. The electrospinning process was carried out at an ambient temperature of 22–24 °C and a relative air humidity of 60%–70%.
In addition, CoFe2O4/YAG:5%Eu3+/PVP composite nanofibers (named Sb1, as shown in Table 1), as a contrast sample, were also prepared to study the superiority of the structure of Janus nanofibers. CoFe2O4/YAG:5%Eu3+/PVP composite nanofibers were fabricated by mixing the spinning solution A1 (CoFe2O4/PVP = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and the spinning solution B1 (YAG:5%Eu3+/PVP = 1
1) and the spinning solution B1 (YAG:5%Eu3+/PVP = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) together using a traditional single-spinneret electrospinning setup, and the spinning parameters were the same as those in the fabrication of the Janus nanofibers.
1) together using a traditional single-spinneret electrospinning setup, and the spinning parameters were the same as those in the fabrication of the Janus nanofibers.
3 Characterization
The samples were identified by an X-ray powder diffractometer (XRD, Bruker D8 FOCUS) with Cu Kα radiation, and the operation voltage and current were kept at 40 kV and 20 mA, respectively. The morphology and internal structure of samples were observed by a field emission scanning electron microscope (SEM, XL-30) and a transmission electron microscope (TEM, JEM-2010), respectively. The luminescent properties of the samples were investigated by a Hitachi fluorescence spectrophotometer F-7000. The magnetic performance of the samples was measured by a vibrating sample magnetometer (VSM, MPMS SQUID XL). The ultraviolet-visible diffuse reflectance spectrum of the samples was determined by a UV-1240 ultraviolet-visible spectrophotometer. All the measurements were performed at room temperature.4 Results and discussion
4.1 Crystal structure
The phase compositions of [CoFe2O4/PVP]//[YAG:5%Eu3+/PVP] Janus nanofibers (Sa1) and CoFe2O4/YAG:5%Eu3+/PVP composite nanofibers (Sb1) were characterized using XRD analysis, as shown in Fig. 2. It can be seen that XRD patterns of [CoFe2O4/PVP]//[YAG:5%Eu3+/PVP] Janus nanofibers and CoFe2O4/YAG:5%Eu3+/PVP composite nanofibers are conformed to the cubic phase with a primitive structure of YAG (PDF#33-0040) and the cubic spinel structure of CoFe2O4 (PDF#22-1086), and the diffraction peak of the amorphous PVP (2θ = 22.2°) also can be observed, indicating that [CoFe2O4/PVP]//[YAG:5%Eu3+/PVP] Janus nanofibers and CoFe2O4/YAG:5%Eu3+/PVP composite nanofibers contain crystalline YAG:7%Tb3+, CoFe2O4 and amorphous PVP.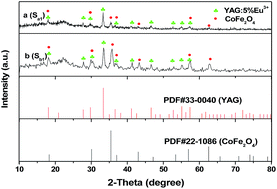 | ||
| Fig. 2 XRD patterns of [CoFe2O4/PVP]//[YAG:5%Eu3+/PVP] Janus nanofibers (Sa1) (a) and CoFe2O4/YAG:5%Eu3+/PVP composite nanofibers (Sb1) (b) with PDF standard cards of YAG and CoFe2O4. | ||
4.2 Morphology
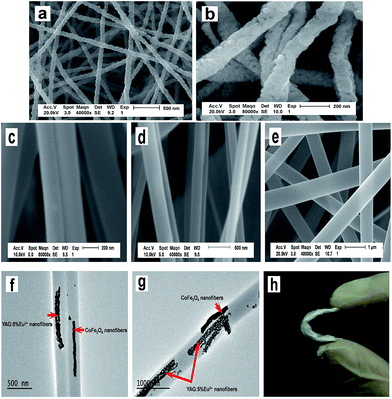
The morphologies of the as-prepared CoFe2O4 nanofibers (S1) and YAG:5%Eu3+ nanofibers (S2) were observed by means of SEM, as presented in Fig. 3a and b. CoFe2O4 nanofibers and YAG:5%Eu3+ nanofibers have coarse surfaces, the size distribution of the as-prepared nanofibers are almost uniform, and the diameters of the CoFe2O4 nanofibers and YAG:5%Eu3+ nanofibers are 81.43 ± 9.2 nm and 126.84 ± 16.9 nm at the confidence level of 95%, respectively, as demonstrated in Fig. 4a and b. The morphologies and structures of [CoFe2O4/PVP]//[YAG:5%Eu3+/PVP] Janus nanofibers (Sa1) and CoFe2O4/YAG:5%Eu3+/PVP composite nanofibers (Sb1) were characterized by the combination of SEM and TEM analyses. As seen from Fig. 3c–e, the surface of the Janus nanofibers and composite nanofibers is smooth, and each [CoFe2O4/PVP]//[YAG:5%Eu3+/PVP] Janus nanofiber consists of two nanofibers assembled side-by-side. A TEM image of [CoFe2O4/PVP]//[YAG:5%Eu3+/PVP] Janus nanofibers is presented in Fig. 3f. One strand nanofiber of the Janus nanofiber is composed of CoFe2O4 magnetic nanofibers and PVP, and the other one consists of YAG:5%Eu3+ luminescent nanofibers and PVP. The mean diameter of an individual nanofiber of the Janus nanofibers (Sa1) is ca. 283.06 ± 36.7 nm at the confidence level of 95%, as revealed in Fig. 4c. It can be observed from Fig. 3g that CoFe2O4 magnetic nanofibers and YAG:5%Eu3+ luminescent nanofibers are dispersed in the CoFe2O4/YAG:5%Eu3+/PVP composite nanofibers. The diameter of the CoFe2O4/YAG:5%Eu3+/PVP composite nanofibers (Sb1) is 632.98 ± 33.6 nm at the confidence level of 95%, as revealed in Fig. 4d. From the abovementioned SEM and TEM analyses, we can confirm that the [CoFe2O4/PVP]//[YAG:5%Eu3+/PVP] Janus nanofibers have been successfully fabricated. As seen in Fig. 3h, the Janus nanofibers are flexible nanofibers.4.3 Luminescent properties of [CoFe2O4/PVP]//[YAG:5%Eu3+/PVP] Janus nanofibers
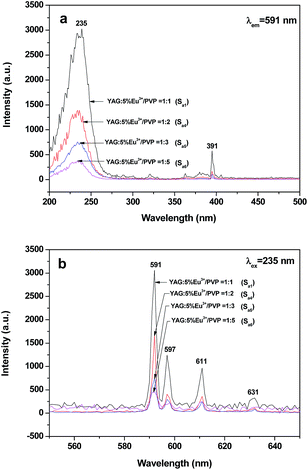
To investigate the impact of the mass ratio of YAG:5%Eu3+ luminescent nanofibers to PVP on the luminescent performance, a series of [CoFe2O4/PVP]//[YAG:5%Eu3+/PVP] Janus nanofibers (Sa1, Sa4, Sa5 and Sa6) were fabricated. To perform this study, the mass ratio of CoFe2O4 nanofibers to PVP was fixed at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and the mass ratio of YAG:5%Eu3+ nanofibers to PVP was varied from 1
1 and the mass ratio of YAG:5%Eu3+ nanofibers to PVP was varied from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 1
1 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5. It can be observed from Fig. 5a that the excitation spectra (monitored by 591 nm) of the samples show a predominant excitation band (200–300 nm) centered at 235 nm, which is assigned to the charge transfer from the 2p orbital of O2− ions to the 4f orbital of Eu3+ ions, while the sharp excitation peak at 391 nm is due to the 7F0 → 5L6 transition of Eu3+ ions. As shown in Fig. 5b, characteristic emission peaks of Eu3+ are observed under the excitation of 235 nm ultraviolet light and ascribed to the energy levels transitions of 5D0 → 7F1 (591 nm), 5D0 → 7F1 (597 nm), 5D0 → 7F2 (611 nm) and 5D0 → 7F2 (631 nm) of Eu3+ ions. The 5D0 → 7F1 energy level transition at 591 nm is the predominant emission peak. It is found from Fig. 5 that the peaks have the same spectral shape when the amount of luminescent substance increases, whereas the intensities of the excitation and emission peaks are strengthened, indicating that the luminescent intensity of the Janus nanofibers can be tuned by adjusting the amount of luminescent material.
5. It can be observed from Fig. 5a that the excitation spectra (monitored by 591 nm) of the samples show a predominant excitation band (200–300 nm) centered at 235 nm, which is assigned to the charge transfer from the 2p orbital of O2− ions to the 4f orbital of Eu3+ ions, while the sharp excitation peak at 391 nm is due to the 7F0 → 5L6 transition of Eu3+ ions. As shown in Fig. 5b, characteristic emission peaks of Eu3+ are observed under the excitation of 235 nm ultraviolet light and ascribed to the energy levels transitions of 5D0 → 7F1 (591 nm), 5D0 → 7F1 (597 nm), 5D0 → 7F2 (611 nm) and 5D0 → 7F2 (631 nm) of Eu3+ ions. The 5D0 → 7F1 energy level transition at 591 nm is the predominant emission peak. It is found from Fig. 5 that the peaks have the same spectral shape when the amount of luminescent substance increases, whereas the intensities of the excitation and emission peaks are strengthened, indicating that the luminescent intensity of the Janus nanofibers can be tuned by adjusting the amount of luminescent material.
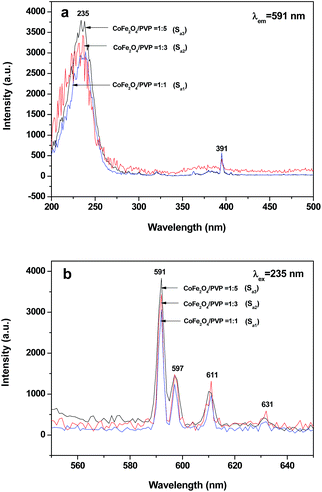
The excitation spectra (monitored at 591 nm) and emission spectra (excited by 235 nm) of [CoFe2O4/PVP]//[YAG:5%Eu3+/PVP] Janus nanofibers (Sa1, Sa2 and Sa3) containing different amounts of CoFe2O4 magnetic nanofibers are indicated in Fig. 6. To perform this investigation, the mass ratio of YAG:5%Eu3+ nanofibers to PVP was fixed at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and the mass ratio of CoFe2O4 nanofibers to PVP was varied from 1
1 and the mass ratio of CoFe2O4 nanofibers to PVP was varied from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 1
1 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5. As seen from Fig. 6, the excitation and emission intensity of [CoFe2O4/PVP]//[YAG:5%Eu3+/PVP] Janus nanofibers decreased with the increasing amount of CoFe2O4 nanofibers introduced into the Janus nanofibers. This phenomenon may result from the light absorbance of the dark-colored CoFe2O4 nanofibers. From the ultraviolet-visible diffuse reflectance spectrum of CoFe2O4 nanofibers (S1) illustrated in Fig. 7, it is observed that CoFe2O4 nanofibers can absorb light at ultraviolet wavelengths (<400 nm) much more strongly than in the visible range (400–700 nm). Both the exciting light (235 nm) and emitting light (591–631 nm) can be absorbed by dark-colored CoFe2O4. Thus, the exciting light and emitting light are absorbed by the CoFe2O4 nanofibers, resulting in the decrease in the intensity of excitation and emission peaks. Furthermore, more the CoFe2O4 nanofibers are introduced into the Janus nanofibers, more the decrease in the intensity of excitation and emission peaks occurs.
5. As seen from Fig. 6, the excitation and emission intensity of [CoFe2O4/PVP]//[YAG:5%Eu3+/PVP] Janus nanofibers decreased with the increasing amount of CoFe2O4 nanofibers introduced into the Janus nanofibers. This phenomenon may result from the light absorbance of the dark-colored CoFe2O4 nanofibers. From the ultraviolet-visible diffuse reflectance spectrum of CoFe2O4 nanofibers (S1) illustrated in Fig. 7, it is observed that CoFe2O4 nanofibers can absorb light at ultraviolet wavelengths (<400 nm) much more strongly than in the visible range (400–700 nm). Both the exciting light (235 nm) and emitting light (591–631 nm) can be absorbed by dark-colored CoFe2O4. Thus, the exciting light and emitting light are absorbed by the CoFe2O4 nanofibers, resulting in the decrease in the intensity of excitation and emission peaks. Furthermore, more the CoFe2O4 nanofibers are introduced into the Janus nanofibers, more the decrease in the intensity of excitation and emission peaks occurs.
To illustrate the advantages of the nanostructure of the magnetic–luminescent bifunctional Janus nanofibers, CoFe2O4/YAG:5%Eu3+/PVP composite nanofibers (Sb1), as a contrast sample, were also fabricated by mixing the spinning solution A1 (CoFe2O4/PVP = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and the spinning solution B1 (YAG:5%Eu3+/PVP = 1
1) and the spinning solution B1 (YAG:5%Eu3+/PVP = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) together, followed by electrospinning via the traditional single-spinneret electrospinning setup. From the contrast between the Janus nanofibers (Sa1) (CoFe2O4/PVP = 1
1) together, followed by electrospinning via the traditional single-spinneret electrospinning setup. From the contrast between the Janus nanofibers (Sa1) (CoFe2O4/PVP = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, YAG:5%Eu3+/PVP = 1
1, YAG:5%Eu3+/PVP = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and CoFe2O4/YAG:5%Eu3+/PVP composite nanofibers (Sb1), which have the same components, as shown in Fig. 8, one can see that the emission intensity of the Janus nanofibers is considerably stronger than that of CoFe2O4/YAG:5%Eu3+/PVP composite nanofibers. This result can be attributed to the isolation of YAG:5%Eu3+ from CoFe2O4. As illustrated in Fig. 9, YAG:5%Eu3+ nanofibers and CoFe2O4 nanofibers are promiscuously dispersed in the CoFe2O4/YAG:5%Eu3+/PVP composite nanofiber. The exciting light in the composite nanofiber has to pass through CoFe2O4 nanofibers to reach and excite YAG:5%Eu3+ nanofibers. In this process, a large part of the exciting light is absorbed by CoFe2O4 nanofibers, and thus the exciting light is considerably weakened before it reaches the YAG:5%Eu3+ nanofibers. Similarly, the emitting light emitted by YAG:5%Eu3+ nanofibers also has to pass through CoFe2O4 nanofibers and is absorbed by them. Consequently, both the exciting and emitting light are severely weakened. For [CoFe2O4/PVP]//[YAG:5%Eu3+/PVP] Janus nanofibers, YAG:5%Eu3+ nanofibers and CoFe2O4 nanofibers are separated in their own strand, so that the exciting light and emitting light in the YAG:5%Eu3+ nanofibers strand will be little affected by CoFe2O4 nanofibers. The overall effect is that the [CoFe2O4/PVP]//[YAG:5%Eu3+/PVP] Janus nanofibers possess much higher luminescent performance than the CoFe2O4/YAG:5%Eu3+/PVP composite nanofibers.
1) and CoFe2O4/YAG:5%Eu3+/PVP composite nanofibers (Sb1), which have the same components, as shown in Fig. 8, one can see that the emission intensity of the Janus nanofibers is considerably stronger than that of CoFe2O4/YAG:5%Eu3+/PVP composite nanofibers. This result can be attributed to the isolation of YAG:5%Eu3+ from CoFe2O4. As illustrated in Fig. 9, YAG:5%Eu3+ nanofibers and CoFe2O4 nanofibers are promiscuously dispersed in the CoFe2O4/YAG:5%Eu3+/PVP composite nanofiber. The exciting light in the composite nanofiber has to pass through CoFe2O4 nanofibers to reach and excite YAG:5%Eu3+ nanofibers. In this process, a large part of the exciting light is absorbed by CoFe2O4 nanofibers, and thus the exciting light is considerably weakened before it reaches the YAG:5%Eu3+ nanofibers. Similarly, the emitting light emitted by YAG:5%Eu3+ nanofibers also has to pass through CoFe2O4 nanofibers and is absorbed by them. Consequently, both the exciting and emitting light are severely weakened. For [CoFe2O4/PVP]//[YAG:5%Eu3+/PVP] Janus nanofibers, YAG:5%Eu3+ nanofibers and CoFe2O4 nanofibers are separated in their own strand, so that the exciting light and emitting light in the YAG:5%Eu3+ nanofibers strand will be little affected by CoFe2O4 nanofibers. The overall effect is that the [CoFe2O4/PVP]//[YAG:5%Eu3+/PVP] Janus nanofibers possess much higher luminescent performance than the CoFe2O4/YAG:5%Eu3+/PVP composite nanofibers.
 | ||
| Fig. 8 Emission spectra of [CoFe2O4/PVP]//[YAG:5%Eu3+/PVP] Janus nanofibers (Sa1) and CoFe2O4/YAG:5%Eu3+/PVP composite nanofibers (Sb1). | ||
 | ||
| Fig. 9 Schematic diagram of the situation of the exciting light and emitting light in CoFe2O4/YAG:5%Eu3+/PVP composite nanofibers and [CoFe2O4/PVP]//[YAG:5%Eu3+/PVP] Janus nanofibers. | ||
4.4 Magnetic properties of [CoFe2O4/PVP]//[YAG:5%Eu3+/PVP] Janus nanofibers
The typical hysteresis loops for CoFe2O4 nanofibers (S1), [CoFe2O4/PVP]//[YAG:5%Eu3+/PVP] Janus nanofibers (Sa1, Sa2 and Sa3) containing different mass ratios of CoFe2O4 nanofibers to PVP and CoFe2O4/YAG:5%Eu3+/PVP composite nanofibers (Sb1) are shown in Fig. 10, and the saturation magnetizations of the samples are summarized in Table 2. As seen from Fig. 10, the saturation magnetization of the CoFe2O4 nanofibers is 41.34 emu g−1, which is similar to the data reported by previous studies14–16. The saturation magnetization of [CoFe2O4/PVP]//[YAG:5%Eu3+/PVP] Janus nanofibers containing different mass ratios of CoFe2O4 nanofibers to PVP are 20.32 emu g−1, 6.73 emu g−1 and 3.12 emu g−1, as revealed in Fig. 10 and Table 2. It is known that the saturation magnetization of a magnetic composite material depends on the mass percentage of the magnetic substance in the magnetic composite material.21–23 It is found that the saturation magnetization of the [CoFe2O4/PVP]//[YAG:5%Eu3+/PVP] Janus nanofibers is increased with the amount of CoFe2O4 magnetic nanofibers introduced into the CoFe2O4/PVP strand, implying that the magnetism of the Janus nanofibers can be tunable by adjusting the amount of CoFe2O4 magnetic nanofibers. The saturation magnetization of the CoFe2O4/YAG:5%Eu3+/PVP composite nanofibers is 20.53 emu g−1, which is close to that of the Janus nanofibers marked c (20.32 emu g−1) in Fig. 10. Combining luminescence with magnetism analysis, it is found that when the Janus nanofibers have a close magnetic property to the CoFe2O4/YAG:5%Eu3+/PVP composite nanofibers, the luminescent intensity of the Janus nanofibers is considerably higher than that of the composite nanofibers, demonstrating that the novel Janus nanofibers have better magnetic–luminescent performance than the composite nanofibers.| Samples | Saturation magnetization (Ms)/(emu g−1) |
|---|---|
| CoFe2O4 nanofibers (S1) | 41.34 |
CoFe2O4/YAG:5%Eu3+/PVP composite nanofibers (Sb1) (CoFe2O4/PVP = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
20.53 |
[CoFe2O4/PVP]//[YAG:5%Eu3+/PVP] Janus nanofibers (Sa1) (CoFe2O4/PVP = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
20.32 |
[CoFe2O4/PVP]//[YAG:5%Eu3+/PVP] Janus nanofibers (Sa2) (CoFe2O4/PVP = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) 3) |
6.73 |
[CoFe2O4/PVP]//[YAG:5%Eu3+/PVP] Janus nanofibers (Sa3) (CoFe2O4/PVP = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) 5) |
3.12 |
5 Conclusions
In summary, magnetic–luminescent bifunctional [CoFe2O4/PVP]//[YAG:5%Eu3+/PVP] Janus nanofibers have been successfully synthesized by electrospinning technology using a homemade parallel spinneret. One strand nanofiber of the Janus nanofiber is composed of CoFe2O4 magnetic nanofibers and PVP, and the other one consists of YAG:5%Eu3+ luminescent nanofibers and PVP. The average diameter of each strand of the Janus nanofiber is ca. 283.06 ± 36.7 nm. It is very gratifying to see that the magnetic–luminescent bifunctional Janus nanofibers simultaneously possess both excellent luminescent performance and magnetic properties. Furthermore, the luminescent intensity and magnetism of the Janus nanofibers can be tuned via adjusting the content of luminescent and magnetic compounds. In addition, the design conception and preparation method of the Janus nanofibers are of universal significance to fabricate other one-dimensional multifunctional nanostructures. The new high-performance [CoFe2O4/PVP]//[YAG:5%Eu3+/PVP] magnetic–luminescent bifunctional Janus nanofibers have potential applications in the fields of medical diagnostics, drug target delivery, optical imaging, anti-counterfeiting technology and future nanomechanics.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (NSFC 50972020, 51072026), Specialized Research Fund for the Doctoral Program of Higher Education (20102216110002, 20112216120003), the Science and Technology Development Planning Project of Jilin Province (Grant nos 20130101001JC, 20070402), the Science and Technology Research Project of the Education Department of Jilin Province during the eleventh five-year plan period (Under grant no 2010JYT01), Key Research Project of Science and Technology of Ministry of Education of China (Grant no 207026).Notes and references
- W. Wang, Z. Y. Li, X. R. Xu, B. Dong, H. N. Zhang, Z. J. Wang, C. Wang, R. H. Baughman and S. L. Fang, Small, 2011, 7, 597 CrossRef CAS PubMed.
- W. Wang, M. Zou and K. Z. Chen, Chem. Commun., 2010, 46, 5100 RSC.
- J. Feng, S. Y. Song, R. P. Deng, W. Q. Fan and H. J. Zhang, Langmuir, 2010, 26, 3596 CrossRef CAS PubMed.
- H. Y. Chen, D. C. Colvin, B. Qi, T. Moore, J. He, O. T. Mefford, F. Alexis, J. C. Gore and J. N. Anker, J. Mater. Chem., 2012, 22, 12802 RSC.
- P. Lu, J. L. Zhang, Y. L. Liu, D. H. Sun, G. X. Liu, G. Y. Hong and J. Z. Ni, Talanta, 2010, 83, 450 CrossRef PubMed.
- H. G. Wang, L. Sun, Y. P. Li, X. L. Fei, M. D. Sun, C. Q. Zhang, Y. X. Li and Q. B. Yang, Langmuir, 2011, 27, 11609 CrossRef CAS PubMed.
- P. Sun, H. Y. Zhang, C. Liu, J. Fang, M. Wang, J. Chen, J. P. Zhang, C. B. Mao and S. K. Xu, Langmuir, 2010, 26, 1278 CrossRef CAS PubMed.
- F. Grasset, F. Dorson, Y. Molard, S. Cordier, V. Demange, C. Perrin, V. Marchi-Artzner and H. Haneda, Chem. Commun., 2008, 39, 4729 RSC.
- L. N. Chen, J. Wang, W. T. Li and H. Y. Han, Chem. Commun., 2012, 48, 4971 RSC.
- X. M. Li, X. Xing, W. J. Yang, W. L. Li and L. F. Kong, Semiconductor Optoelectronics, 2010, 31, 71 CAS.
- R. Lopez, E. A. Aguilar and J. Zarate-Medina, J. Eur. Ceram. Soc., 2010, 30, 73 CrossRef CAS PubMed.
- P. F. S. Pereira, M. G. Matos, L. R. Ávila, E. C. O. Nassor, A. Cestari, K. J. Ciuffi, P. S. Calefi and E. J. Nassar, J. Lumin., 2010, 130, 488 CrossRef CAS PubMed.
- H. K. Yang and J. H. Jeong, J. Phys. Chem. C, 2010, 114, 226 CAS.
- J. C. Fu, J. L. Zhang, Y. Peng, J. G. Zhao, G. G. Tan, N. J. Mellors, E. Q. Xie and W. H. Han, Nanoscale, 2012, 4, 3932 RSC.
- C. Fernandes, C. Pereira, M. P. F. García, A. M. Pereira, A. Guedes, R. F. Pacheco, A. Ibarra, M. R. Ibarra, J. P. Araújo and C. Freire, J. Mater. Chem. C, 2014, 2, 5818 RSC.
- Q. Cao, Z. W. Liu and R. C. Che, New J. Chem., 2014, 38, 3193 RSC.
- H. Wang, Y. Li, L. Sun, Y. Li, W. Wang, S. Wang, S. Xu and Q. Yang, J. Colloid Interface Sci., 2010, 350, 396 CrossRef CAS PubMed.
- Z. Y. Hou, C. X. Li, P. G. Ma, G. G. Li, Z. Y. Cheng, C. Peng, D. M. Yang, P. P. Yang and J. Lin, Adv. Funct. Mater., 2011, 21, 2356 CrossRef CAS.
- J. B. Mu, C. L. Shao, Z. C. Guo, Z. Y. Zhang, M. Y. Zhang, P. Zhang, B. Chen and Y. C. Liu, ACS Appl. Mater. Interfaces, 2011, 3, 590 CAS.
- J. Song, M. L. Chen, M. B. Olesen, C. X. Wang, R. Havelund, Q. Li, E. Q. Xie, R. Yang, P. Bøggild, C. Wang, F. Besenbacher and M. D. Dong, Nanoscale, 2011, 3, 4966 RSC.
- N. Lv, Q. L. Ma, X. T. Dong, J. X. Wang, W. S. Yu and G. X. Liu, Chem. Eng. J., 2014, 243, 500 CrossRef CAS PubMed.
- S. L. Chen, H. Q. Hou, F. Harnisch, S. A. Patil and A. A. Carmona-Martinez, Energy Environ. Sci., 2011, 4, 1417 CAS.
- N. Lv, Q. L. Ma, X. T. Dong, J. X. Wang, W. S. Yu and G. X. Liu, ChemPlusChem, 2014, 79, 690 CrossRef CAS.
- Q. L. Ma, W. S. Yu, X. T. Dong, J. X. Wang and G. X. Liu, Nanoscale, 2014, 6, 2945 RSC.
- Q. L. Ma, J. X. Wang, X. T. Dong, W. S. Yu and G. X. Liu, ChemPlusChem, 2014, 79, 290 CrossRef CAS.
- X. Xi, J. X. Wang, X. T. Dong, Q. L. Ma, W. S. Yu and G. X. Liu, Chem. Eng. J., 2014, 254, 259 CrossRef CAS PubMed.
- T. Nisisako, T. Torii, T. Takahashi and Y. Takizawa, Adv. Mater., 2006, 18, 1152 CrossRef CAS.
- H. R. Kyung, C. M. David and L. Joerg, Nat. Mater., 2005, 4, 759 CrossRef PubMed.
- S. Isaac, Y. Xin, J. C. Emily, M. M. Nicholas, T. M. Gerald, K. Olga and C. B. Anna, ACS Nano, 2013, 7, 1224 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2015 |