Colorimetric “naked eye” detection of CN−, F−, CH3COO− and H2PO4− ions by highly nonplanar electron deficient perhaloporphyrins†
Nivedita Chaudhri and
Muniappan Sankar*
Department of Chemistry, Indian Institute of Technology Roorkee, Roorkee – 247667, India. E-mail: sankafcy@iitr.ac.in; Fax: +91-1332-27-3560; Tel: +91-1332-28-4753
First published on 21st November 2014
Abstract
Highly nonplanar perhaloporphyrin-based receptors have been synthesized and characterized by various spectroscopic techniques. Nonplanar and electron deficient nature was authenticated by electronic spectral, electrochemical redox and protonation–deprotonation studies. The colorimetric “naked eye” anion sensing ability of these sensors was probed by spectroscopic and electrochemical studies. These receptors were found to be highly selective and sensitive for the basic anions, such as CN−, F−, OAc− and H2PO4−, over the tested anions and were able to detect these anions even at nanomolar concentrations via anion induced deprotonation. These perhaloporphyrin receptors have been recovered from receptor–anion host–guest complexes formed during sensing events by acid treatment and were reused for the detection of basic anions without loss of their sensing ability.
Introduction
Porphyrinoids are a class of tetrapyrrole pigments that widely occur in nature.1a These pigments are highly coloured and exhibit varying degrees of π-conjugation leading to different nonplanar conformations of the porphyrin macrocycle, which are responsible for a variety of biological functions.1 Inspired by nature's fundamental biological processes involving enzyme–substrate or host–guest interactions, which are regulated by light, ions and small molecule concentrations, the design of effective anion receptors containing responsive functional groups as an integral part of a host macrocyclic framework has been an active area of research.2 Now-a-days the need for a suitable method of anion recognition, extraction and transportation is severely felt, and as a consequence, the field of anion supramolecular chemistry has grown rapidly over recent decades.3 In order to achieve an enhanced selectivity and sensitivity towards a particular anion, a fine tuning of binding sites is required, which is often difficult because of their wide range of geometries, larger size, high solvation energies and accessibility in a very narrow pH range as compared to their cationic counterparts.4 Cyanide is extremely toxic to living organisms; it binds strongly to iron in heme and the active site of cytochrome c, which completely stops the O2 transport in the blood and the mitochondrial electron-transport chain, thus inhibiting cellular respiration.5a The selective recognition of fluoride ions is of great importance for monitoring F− ion metabolism in nature, the analysis of drinking water and in the treatment of osteoporosis.5b,c Phosphate ions play a vital role in biological processes such as signalling, energy transduction, information storage and expression.6a The acetate ions are the most common building blocks in biosynthesis and a critical component of numerous metabolic processes. The rate of OAc− production and oxidation has been frequently used as an indicator of organic decomposition in marine sediments.6bCalixpyrroles,7 phlorins,8a corroles,8b,c sapphyrins,8d,e N-confused porphyrins,8f,g oxoporphyrinogens8h,i and porphyrins8j are excellent multifunctional candidates for a great variety of anion sensor applications via the NH groups of the pyrrole units. Several porphyrins bearing NH groups at the ortho-positions of the meso-aryl substituents have been utilized as effective anion sensors.9a,b Recently, porphyrin bearing NH groups at the para-positions of the meso-phenyl ring have been demonstrated for H2PO4− ion sensing.9c Many transformations and modifications have been carried out on porphyrin macrocycles for selective ion recognition.8j,9,10 The β-functionalisation of meso-tetraarylporphyrins is of great importance because the electronic properties of the porphyrin macrocycle can be altered by small changes in the substituents leading to larger steric and electronic effects on the porphyrin π-system1b,c,10a,b,11 than substituents at the meso-aryl positions. A few β- and meso-substituted porphyrins are known that utilize their protonated inner core as effective hydrogen bond makers for anion recognition.10 In most of the cases, planar porphyrins can recognize anions via peripheral functional groups,9a,b in which inner core NH groups are less effectively available for H-bond formation due to tautomerism.9c Recently, cyanide sensing through a chemodosimetric method by a new calix[4] pyrrole derivative,12a Pd-calixphyrin,12b and subphthalocyanine dye12c have been reported. Moreover, metalloporphyrin-based cyanide ion sensors through coordinative interactions are known.12d,e However, highly sensitive free base porphyrin-based chemosensors for CN− ions with very low detection limits (in ppb range) are not reported. In general, acidic receptors can recognize basic anions through deprotonation and it is quite common in organic hosts,13a–c whereas it is very difficult to deprotonate imino protons of planar porphyrins under ambient conditions.13d,e Therefore, herein, we tried to make highly nonplanar electron deficient perhaloporphyrins (Chart 1), which can be utilized as anion sensors through pyrrolinic NH deprotonation as a detection mechanism for the first time in porphyrin chemistry.
Results and discussion
Synthesis and characterization
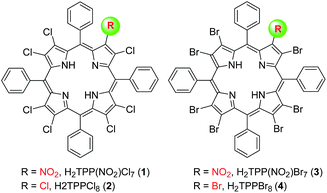
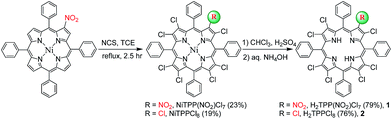
Herein, we report a straightforward synthetic route for the preparation of 1 and 2, which gives the direct substitution at β-pyrrole position of the macrocycle (Scheme 1). 3 and 4, having idiosyncratic nature, have also been synthesized using modified previously reported methods.14The representative UV-visible spectra of 1 and 3 are shown in toluene (Fig. S1, ESI†). The Table S1 in ESI† lists the electronic absorption spectral data of 1–4 in toluene at 298 K. 1 exhibited similar spectral features to 2 and showed red-shifted absorption in Soret (6 nm) and in the Qx(0,0) band (12 nm) relative to 2 possibly due to the electron withdrawing nature of the nitro group and increased nonplanarity by mixed susbtitution.15 Interestingly, 3 has shown red-shifted absorption in B (12 nm) and the Qx(0,0) band (17 nm) relative to 1 possibly due to increased nonplanarity produced by the larger size of bromo substituents. The B and Qx(0,0) bands of 1–4 showed an interesting trend in red-shift and aligns in the following order: 2 < 1 < 4 < 3. In 1H NMR of 1, the meso-o-phenyl protons showed an asymmetric multiplet (Fig. S2, ESI†) in contrast to 2, indicating the lower symmetry of the molecular structure. Fig. S3 in the ESI† shows the 1H NMR spectra of imino proton region of 1–4 in CDCl3 at 298 K. Interestingly, the downfield shift of NH or the acidity of imino-protons of 1–4 has shown the following order: 3 > 4 > 1 > 2, which readily reflects the extent of nonplanarity of the porphyrin core and electron withdrawing effect of the nitro substituent.16 The negative ion mode ESI mass spectrum of 1 is shown in Fig. S4 in ESI.†
The single crystals of 2 were obtained by vapour diffusion of CH3CN into a toluene solution of 2. The crystallographic data of 2·2CH3CN·2H2O is listed in Table S2 in the ESI.† The top and side ORTEP views of 2 are shown in Fig. S5 in the ESI.† The nonplanarity of the porphyrin macrocycle is induced by the steric repulsion among the peripheral substituents, which enforces the relief of the strain through bond lengths and angles. The selected average bond lengths and bond angles of 2 are listed in Table S3 in ESI,† which are in accordance with the reported studies.1b,c,15a,16 Notably, 2 has exhibited a severe nonplanar saddle-shape conformation (Fig. S5b in ESI†) with the displacement of the β-pyrrole carbons, (ΔCβ = ±1.21 Å) and 24 atoms core (Δ24 = 0.57 Å) from the porphyrin mean plane. This is further supported by the increment in Cβ–Cα–Cm angle (∼129°) with concomitant decrement in the N–Cα–Cm angle (∼124°) along with larger Cβ–Cβ bond length (1.345(11) Å) as compared to reported planar porphyrins.1c We have observed a higher ΔCβ for 2 than expected due to the strong hydrogen bonding interaction between solvate water molecules and the porphyrin core NH with a distance of 2.831 Å. It is known that 4 has a greater extent of nonplanarity (saddle-shape conformation) in comparison to 2 as evidenced from the single crystal X-ray structures.1b,c,15a,16
The ground state geometry optimisation of H2TPP(NO2)Cl7 (1) in gas phase was carried out by DFT calculations using B3LYP functional with a 6-311G(d,p) basis set. Fig. S6 in ESI† represents the fully optimised geometry of 1, which exhibits a severe nonplanar saddle-shape conformation as shown in Fig. S6b in ESI.† The selected average bond lengths and bond angles of 1 are listed in Table S4 in ESI.† The displacement of the β-pyrrole carbons, (ΔCβ = ±1.21 Å) and 24 atoms core (Δ24 = 0.52 Å) from the porphyrin mean plane of 1 authenticate the highly nonplanar conformation of the porphyrin core. This is further proven by the increment in the Cβ–Cα–Cm angle with concomitant decrement in the N–Cα–Cm angle along with a larger Cβ–Cβ bond length. The observed higher ΔCβ and Δ24 values of 1 in the gas phase are possibly due to enhanced nonplanarity provided by mixed β-substitution. The pictorial representation of frontier orbitals of 1 is shown in Fig. S7 in ESI.† The HOMO and HOMO−1 orbitals are found to be a2u and a1u, respectively, as expected for electron withdrawing β-substituted porphyrins. The results obtained from single crystal structure and DFT calculations are in good agreement with electronic spectral and NMR studies.
A wide variety of perhaloporphyrins have been examined in non-aqueous media.13e,17 Cyclic voltammograms and DPVs of 1–4 in CH2Cl2 containing TBAPF6 as supporting electrolyte are shown in Fig. S8 in ESI† and the redox data is listed in Table S5 in ESI.† Interestingly, the mixed substituted porphyrins (1 and 3) exhibited a ∼200 mV anodic shift in the reduction as compared to homo substituted porphyrins (2 and 4), whereas in oxidation only a 60 mV anodic shift was observed. This can be attributed to the strong electron withdrawing nature of the nitro substituent. Notably, the bromoporphyrins (3 and 4) exhibited a 90 mV cathodic shift in the oxidation potentials relative to their corresponding chloroporphyrins (1 and 2) is ascribed to enhanced nonplanarity provided by bulkier bromo groups.15,16
Protonation and deprotonation studies
To examine the effect of mixed substitution on nonplanarity, we have carried out protonation and deprotonation studies of 1–4 in toluene using trifluroacetic acid (TFA) and tetrabutylammonium hydroxide (TBAOH), respectively Fig. 1 shows the UV-visible spectral changes of 1 while increasing the conc. of TFA (20–60 μM) and TBAOH (0.07–11 μM). The protonation and deprotonation constants of 1–4 are calculated using Hill equation18 (Table 1). Fig. 1a represents the concomitant decrement in absorbance of 1 at 461 nm and rising of a new band at 490 nm upon increasing [TFA]. As protonation proceeds, the multiple Q bands are disappearing and a new single broad band rises at 752 nm accompanied with the red-shift of 22 nm in Qx(0,0) band. In all cases, we have obtained diprotonated porphyrin species, which is further confirmed by Hill plot having the slope value of ∼2 as shown in Fig. 1a (inset) and S9 in ESI.† 4 has shown the highest protonation constant (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) β2) as compared to all the other porphyrins.16b 3 and 4 have shown 15–18 times higher log
β2) as compared to all the other porphyrins.16b 3 and 4 have shown 15–18 times higher log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) β2 values as compared to 1 and 2. The protonation constants of 1–4 showed the following order: 4 > 3 > 2 > 1, which reveals the extent of basicity of the inner core nitrogens. The deprotonation of free base porphyrin is influenced by the electronic nature of the substituents, the extent of nonplanarity of the porphyrin core and the basicity of the base employed. Fig. 1b shows the concomitant decrement in the absorbance of 1 at 461 nm while increasing [TBAOH] and the new band rises at 507 nm. During deprotonation, a new band grows at 721 nm with the disappearance of multiple Q bands of 1. The Fig. 1b (inset) and S10 in ESI† show the Hill plot with the slope value of 2, indicating the formation of dianionic species in all cases. 3 showed highest deprotonation constant (log
β2 values as compared to 1 and 2. The protonation constants of 1–4 showed the following order: 4 > 3 > 2 > 1, which reveals the extent of basicity of the inner core nitrogens. The deprotonation of free base porphyrin is influenced by the electronic nature of the substituents, the extent of nonplanarity of the porphyrin core and the basicity of the base employed. Fig. 1b shows the concomitant decrement in the absorbance of 1 at 461 nm while increasing [TBAOH] and the new band rises at 507 nm. During deprotonation, a new band grows at 721 nm with the disappearance of multiple Q bands of 1. The Fig. 1b (inset) and S10 in ESI† show the Hill plot with the slope value of 2, indicating the formation of dianionic species in all cases. 3 showed highest deprotonation constant (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) β2 = 11.6), which is 7 fold greater than 1 and is ascribed to the increased nonplanarity of the porphyrin macrocycle. The log
β2 = 11.6), which is 7 fold greater than 1 and is ascribed to the increased nonplanarity of the porphyrin macrocycle. The log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) β2 values of 1–4 have shown the following order: 3 > 4 > 1 > 2, which suggests that porphyrins bearing mixed substitution with bulky bromo groups favour a high degree of deprotonation.
β2 values of 1–4 have shown the following order: 3 > 4 > 1 > 2, which suggests that porphyrins bearing mixed substitution with bulky bromo groups favour a high degree of deprotonation.
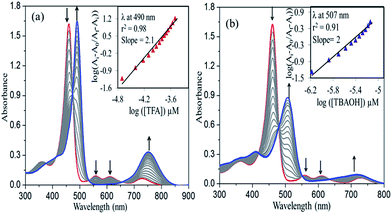 | ||
| Fig. 1 UV-visible spectral titrations of 1 with TFA (a) and TBAOH (b) in toluene at 298 K. Insets shows the corresponding Hill plots. | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) β2)a of 1–4 in toluene at 298 K
β2)a of 1–4 in toluene at 298 K
| Porphyrin | Protonation | Deprotonation | ||||
|---|---|---|---|---|---|---|
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) β2 β2 |
β2 | nb | log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) β2 β2 |
β2 | nb | |
a Within the error ±0.07 for log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) β2 and ±10% for β2.b n refers stoichiometry. β2 and ±10% for β2.b n refers stoichiometry. |
||||||
| 1 | 8.51 | 3.30 × 108 | 2.1 | 10.77 | 5.91 × 1010 | 2.0 |
| 2 | 9.21 | 1.90 × 109 | 2.0 | 10.21 | 1.65 × 1010 | 1.9 |
| 3 | 9.70 | 5.07 × 109 | 2.1 | 11.60 | 4.06 × 1011 | 2.1 |
| 4 | 10.53 | 3.43 × 1010 | 2.1 | 10.40 | 2.53 × 1010 | 2.0 |
Anion sensing
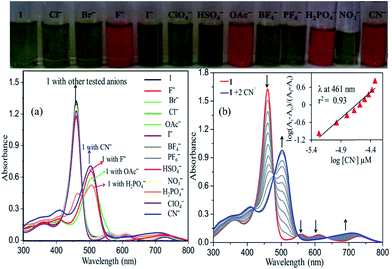
The anion recognition properties of 1–4 were studied in toluene with different anions such as CN−, F−, Cl−, Br−, I−, NO3−, HSO4−, PF6−, ClO4−, BF4−, CH3COO− and H2PO4− ions using UV-visible spectroscopy with the addition of the aliquot anion in the form of TBA salt. Among all, CN−, F−, CH3COO− and H2PO4− selectively interacted with 1–4 by showing a considerable red-shift (43–48 nm) in the UV-visible spectra, as shown in Fig. 2a and S11 in ESI,† whereas there were no shifts observed for the other anions. Interestingly, the green colour solution of 1 turned to dark pink upon addition of aliquots of CN−, F−, OAc− and H2PO4− solution, which enables the naked eye detection of these anions in solution (Fig. 2). The UV-visible spectra obtained for 1 with CN−, F−, OAc− and H2PO4− resemble the optical absorption spectrum of 12− obtained by the addition of TBAOH indicating the possibility of dianion formation during the addition of anions (Table S6 in ESI†).The UV-visible spectrophotometric titration of 1 with CN− ions is shown Fig. 2b. As we increased the concentration of CN− ions, the decrement in the absorbance of 1 was observed at 461 nm, 559 nm and 612 nm and the concomitant increment at 503 and 711 nm with multiple isosbestic points. The Hill plot (Fig. 2b inset) shows a straight line between log[CN−] and log(Ai − A0/Af − Ai) having slope value ∼2, which indicates 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (porphyrin-to-anion) stoichiometry. Moreover, the similar behaviour was observed for 2–4 with CN− ions as shown in Fig. S12 in ESI.† Furthermore, we have performed the UV-visible spectral titrations for 1–4 with F−, OAc− and H2PO4− anions and were found to have almost similar spectral features with variation in the association constants (Fig. S13 to S15 in ESI†). Table 2 lists the association constants obtained for CN−, F−, OAc− and H2PO4− ions with 1–4 in toluene at 298 K. Each of the system studied displays higher β2 values which are >108 M−2, establishing that these porphyrins (1–4) are capable of strongly interacting with 2 equiv. of CN−, F−, OAc− and H2PO4− ions.7–12,13e Interestingly, 1–4 exhibited considerably higher association constants (log
2 (porphyrin-to-anion) stoichiometry. Moreover, the similar behaviour was observed for 2–4 with CN− ions as shown in Fig. S12 in ESI.† Furthermore, we have performed the UV-visible spectral titrations for 1–4 with F−, OAc− and H2PO4− anions and were found to have almost similar spectral features with variation in the association constants (Fig. S13 to S15 in ESI†). Table 2 lists the association constants obtained for CN−, F−, OAc− and H2PO4− ions with 1–4 in toluene at 298 K. Each of the system studied displays higher β2 values which are >108 M−2, establishing that these porphyrins (1–4) are capable of strongly interacting with 2 equiv. of CN−, F−, OAc− and H2PO4− ions.7–12,13e Interestingly, 1–4 exhibited considerably higher association constants (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) β2 values ranges from 8.7 to 11.4) with anions such as CN−, F−, OAc− and H2PO4− ions as compared to other porphyrins known in the previous studies.7–12,13e Notably, the β2 values for 1 and 3 are ∼220 times higher than 2 and 4 for F− ions, which is ascribed to strong electron withdrawing nature of the nitro substituent and the effect of mixed substitution. With CN− ions, 3 and 4 showed two-to-six fold higher β2 values as compared 1 and 2. This is ascribed to enhanced nonplanarity of the macrocycle due to bulkier bromo groups relative to their chloro substituents.16 The general trend in β2 values for free base porphyrins with anions was found to be 3 > 1 > 4 > 2 (Table 2). Among the anions, the general trend in β2 values was found to be CN− > F− > OAc− > H2PO4−, which is slightly different from the expected trend (CN− > OAc− > F− > H2PO4−) according to their pKa values. The higher log
β2 values ranges from 8.7 to 11.4) with anions such as CN−, F−, OAc− and H2PO4− ions as compared to other porphyrins known in the previous studies.7–12,13e Notably, the β2 values for 1 and 3 are ∼220 times higher than 2 and 4 for F− ions, which is ascribed to strong electron withdrawing nature of the nitro substituent and the effect of mixed substitution. With CN− ions, 3 and 4 showed two-to-six fold higher β2 values as compared 1 and 2. This is ascribed to enhanced nonplanarity of the macrocycle due to bulkier bromo groups relative to their chloro substituents.16 The general trend in β2 values for free base porphyrins with anions was found to be 3 > 1 > 4 > 2 (Table 2). Among the anions, the general trend in β2 values was found to be CN− > F− > OAc− > H2PO4−, which is slightly different from the expected trend (CN− > OAc− > F− > H2PO4−) according to their pKa values. The higher log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) β2 values of F− ions are possibly due to the smaller ionic size (1.3 Å) and high electronegativity (4.1) of F− ions.
β2 values of F− ions are possibly due to the smaller ionic size (1.3 Å) and high electronegativity (4.1) of F− ions.
The detection limit (LOD) and quantification limit (LOQ) for CN−, F−, OAc− and H2PO4− ions were calculated in presence of 1–4 in toluene at 298 K (Table S7, ESI†). In all cases, the observed LOD (6–10 nM) and LOQ (18–31 nM) were found to be extremely low, which are in nanomolar scale. Hence these porphyrins 1–4 were highly sensitive to basic anions such as CN−, F−, OAc− and H2PO4− ions. 1–4 have shown positive cooperative binding as evidenced from the plot (Fig. 3 and S16 in ESI†), which indicates the interaction of first anion with N4 core distorts the macrocycle ring intern that favours second anion binding. The detection of anions further supported by the fluorescence quenching of 1–4 with increasing [F−] in toluene (Fig. S17, ESI†). We have carried out the 1H NMR studies for 1–4 in presence of F− ions and observed that the disappearance of N–H peak, whereas increasing [F−] indicates the formation of dianionic species (Fig. S18, ESI†).
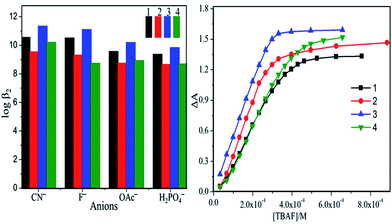 | ||
Fig. 3 Bar graph constructed log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) β2 vs. [anions] for 1–4 in toluene at 298 K (left). Sigmoidal curve for 1–4, ΔA vs. [TBAF] indicating positive cooperative behaviour (right). β2 vs. [anions] for 1–4 in toluene at 298 K (left). Sigmoidal curve for 1–4, ΔA vs. [TBAF] indicating positive cooperative behaviour (right). | ||
To probe further, we have carried out differential pulse voltammetric (DPV) studies of 1–4 with the excess addition of fluoride ions (Fig. S19, ESI†). The observed cathodic shift in oxidation (450–530 mV) and in reduction (230–360 mV) potentials after the addition of aliquots of F− also supports the formation of dianionic species13e (DPV titrations of 1–4 with F− ions, Fig. 4 and S20 in ESI†), whereas the opposite trend (anodic shift) was observed for the protonation of free base dodecaphenylporphyrins.10a,b The cation radicals of 1–4 formed after electrochemical oxidation are unstable and undergo disproportionation; hence, the peak currents for the anodic and cathodic processes are different.10a,b Moreover, we have carried out UV-visible spectral titrations for some planar porphyrins (H2TPP and H2TPP(Ph)4) and nonplanar porphyrins (H2TPPBr4 and H2TPP(Ph)8) with F− ions (Fig. S21 in ESI†) and found no spectral changes were observed even at a high conc. of anions. which clearly suggests that the highly nonplanar conformation with electron withdrawing substituents is necessary for anion recognition.
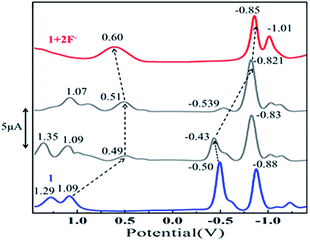 | ||
| Fig. 4 DPV traces of 1 while increasing concentration of F− ion in CH2Cl2 containing 0.1 M TBAPF6 at 298 K. | ||
It is known that the perhaloporphyrins, 2 and 4, hydrogen bonds with various polar solvents,19a including Lewis bases, and forms a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 host–guest complex19b with very low binding constants (0.2–16 M−1). In general, 1–4 with anions did not correspond to the 1
1 host–guest complex19b with very low binding constants (0.2–16 M−1). In general, 1–4 with anions did not correspond to the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 host–guest complex neither in the UV-visible spectral features (Fig. S22, ESI†) nor in binding constant values (very high log
1 host–guest complex neither in the UV-visible spectral features (Fig. S22, ESI†) nor in binding constant values (very high log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) β2 values ranges from 8.7 to 11.4) except for 3, which interacts with Cl− ions through hydrogen bonding (Fig. S23, ESI†) with 1
β2 values ranges from 8.7 to 11.4) except for 3, which interacts with Cl− ions through hydrogen bonding (Fig. S23, ESI†) with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry having a lower binding constant (log
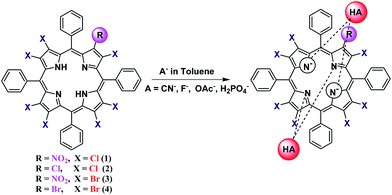
1 stoichiometry having a lower binding constant (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) β2 = 3.82). Based on our experimental evidence, we are representing the plausible mechanism of anion recognition by 1–4 in Fig. 5.
β2 = 3.82). Based on our experimental evidence, we are representing the plausible mechanism of anion recognition by 1–4 in Fig. 5.
Reversibility studies
Reversibility studies were carried out on these porphyrin–anion host–guest systems to acknowledge the range of their applicability.20 The reversibility test of these sensors with cyanide ions was studied. For example, 1·2CN− was prepared by adding 2 equivalents of cyanide ions to the solution of 1 in toluene, which led to the colour change from green to pink (Fig. 6). It was then treated with aliquots of 1 mM solution of TFA in toluene (Fig. S24a, ESI†). This led to a complete regeneration of 1, which can be visualized by colorimetric change from pink to green accompanied by UV-visible spectral changes as shown in Fig. 6. On this basis, we conclude that the formation and dissociation of 1·2CN− is a reversible process. To confirm the regeneration, the resulting mixture was washed with water and dried over anhydrous Na2SO4. Then, the recovered 1 was treated with 2 equivalents of CN− ions (Fig. S24b, ESI†), which exhibited similar binding constant as fresh solution of 1 with cyanide ions. The similar results were obtained for the receptors 2–4 with CN− ions and TFA solution as shown in Fig. S25–S27 in ESI.† Notably, the receptors 1–4 have exhibited similar results with other anions such as F−, CH3COO− and H2PO4− ions. These results clearly demonstrate that the receptors 1–4 can be recoverable and reusable for basic anion detection. | ||
| Fig. 6 (a) Colorimetric response of 1 for reversibility and reusability test with CN−; (b) reversibility test: treatment of the complex 1·2CN− with a solution of TFA. | ||
Conclusions
In conclusion, the perhaloporphyrins (1–4) were synthesised and exhibited colorimetric responses toward basic anions, such as CN−, F−, CH3COO− and H2PO4− ions, and were able to detect these anions in nanomolar concentration. The red-shifted electronic spectral features, the higher β2 values for deprotonation and anion recognition of 1–4 were interpreted in terms of enhanced nonplanarity and the electron withdrawing effect of NO2 and/or halo substituents. The large anodic shift in voltammetric studies and disappearance of 1H NMR signals for imino protons strongly supports the anion induced deprotonation. The spectroscopic studies and voltammetric titrations reveal that the formation of dianionic porphyrin species, which intern hydrogen bonded with protonated anions (HA). Our results clearly suggest that the highly nonplanar conformation with electron withdrawing substituents is necessary for anion recognition. The reversibility studies unambiguously demonstrated that these receptors 1–4 can be recoverable and reusable for basic anions detection without losing their sensing ability.These results reported herein will provide a new standpoint to develop recyclable electron deficient nonplanar porphyrin-based anion sensors.
Experimental section
Materials
Pyrrole, C6H5CHO, N-chlorosuccinimide, Cu(NO3)2·3H2O, Ni(OAc)2·4H2O, Na2S2O4, TFA, TBAOH and NaHCO3 were purchased from HiMedia, India, and used as received. All solvents employed in the present work were of analytical grade and distilled or dried before use. 1,1,2,2-Tetrachloroethane was purchased from Rankem and dried over molecular sieves (4 Å) before use. Silica gel (100–200 mesh) was purchased from Rankem and used as received. TBAPF6 was recrystallised twice with ethanol and dried at 50 °C under vacuum for 2 days. The tetrabutylammonium salts (TBAX, X = CN−, F−, Cl−, Br−, I−, HSO4−, BF4−, OAc−, H2PO4−, ClO4−, PF6− and NO3−) were purchased from Alfa Aesar and used as received. Dry CH2Cl2 for CV analysis was distilled thrice from CaH2 and the toluene (for UV-visible spectral studies) was dried and distilled from sodium–benzophenone mixture.Instrumentation and methods
Optical absorption spectra were recorded using an Agilent Cary 100 spectrophotometer using a pair of quartz cells of 3.5 ml volume and 10 mm path length and fluorescence spectra were recorded using a Hitachi F-4600 spectrofluorometer using a quartz cell of 10 mm path length. 1H NMR spectra were recorded using Bruker AVANCE 500 MHz and JEOL ECX 400 MHz spectrometers in CDCl3 and elemental analyses were performed using an Elementar vario EL III instrument. ESI mass spectra were recorded using a Bruker Daltanics microTOF mass spectrometer in negative ion mode using CH3CN as solvent. The X-ray quality single crystals of 2 were obtained by vapour diffusion of CH3CN into the toluene solution of 2. Single-crystal XRD data of 2 was collected on a Bruker Apex-II CCD diffractometer equipped with a liquid cryostat. The ground state geometry optimisation of 1 in the gas phase was carried out by DFT calculations using B3LYP functional with a 6-311G(d,p) basis set. Electrochemical measurements were carried out using CH instrument (CH 620E). A three electrode assembly was used consisted of a platinum working electrode, Ag/AgCl as a reference electrode and a Pt-wire as a counter electrode. The concentration of 1–4 was maintained at ∼1 mM. All measurements were performed in triple distilled CH2Cl2 containing 0.1 M TBAPF6 as supporting electrolyte, which was degassed by argon gas purging. Protonation, deprotonation and anion detection studies were carried out in distilled toluene at 298 K and the concentration of 1–4 were kept at ∼10–13 μM throughout the experiments, whereas the stock solution of anions were maintained from 0.003 to 0.05 M as per their need. The temperature inside the cell was 298 ± 0.2 K. The association constants (β2) and stoichiometry for anion binding were calculated using Hill equation.18 We calculated the limit of detection (LOD) and limit of quantification (LOQ) for the anions in toluene by 1–4 using the formulae LOD = 3.3(SD/S) and LOQ = 10(SD/S), where SD stands for the standard deviation of the blank and S stands for the slope of the regression line.19Synthetic procedures
NiTPP(NO2) was prepared by nitration of NiTPP using copper nitrate in acetic anhydride as reported in literature.21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) as eluent. The desired product was eluted as second fraction and the yield was found to be 23% (0.062 g, 0.064 mmol). NiTPPCl8 was collected as first fraction and the yield was found to be 19% (0.050 g, 0.053 mmol). The spectroscopic data of NiTPPCl8 was in accordance with reported study.22
1, v/v) as eluent. The desired product was eluted as second fraction and the yield was found to be 23% (0.062 g, 0.064 mmol). NiTPPCl8 was collected as first fraction and the yield was found to be 19% (0.050 g, 0.053 mmol). The spectroscopic data of NiTPPCl8 was in accordance with reported study.22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε) 448(5.12), 562(4.04), 604(3.80). 1H NMR in CDCl3 (500 MHZ): δH (ppm) 7.93–7.87 (m, 8H, meso-o-phenyl-H), 7.73–7.60 (m, 12H, meso-m,p-phenyl-H). ESI-MS (m/z): 987.82 [M·OCH3]− (calcd, 987.56). C44H20N5O2Cl7Ni: C, 55.19%; H, 2.11%; N, 7.31%. Found: C, 55.45%; H, 2.44%; N, 7.57%.
ε) 448(5.12), 562(4.04), 604(3.80). 1H NMR in CDCl3 (500 MHZ): δH (ppm) 7.93–7.87 (m, 8H, meso-o-phenyl-H), 7.73–7.60 (m, 12H, meso-m,p-phenyl-H). ESI-MS (m/z): 987.82 [M·OCH3]− (calcd, 987.56). C44H20N5O2Cl7Ni: C, 55.19%; H, 2.11%; N, 7.31%. Found: C, 55.45%; H, 2.44%; N, 7.57%.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε) 461(5.18), 562(3.88), 615(3.85), 732(3.78). 1H NMR in CDCl3 (500 MHZ): δH (ppm) 8.23–8.15 (m, 8H, meso-o-phenyl-H), 7.82–7.72 (m, 12H, meso-m,p-phenyl-H). ESI-MS (m/z): 900.58 [M]− (calcd, 900.86). C44H22N5O2Cl7: C, 58.66%; H, 2.46%; N, 7.77%. Found: C, 58.37%; H, 2.62%; N, 7.56%.
ε) 461(5.18), 562(3.88), 615(3.85), 732(3.78). 1H NMR in CDCl3 (500 MHZ): δH (ppm) 8.23–8.15 (m, 8H, meso-o-phenyl-H), 7.82–7.72 (m, 12H, meso-m,p-phenyl-H). ESI-MS (m/z): 900.58 [M]− (calcd, 900.86). C44H22N5O2Cl7: C, 58.66%; H, 2.46%; N, 7.77%. Found: C, 58.37%; H, 2.62%; N, 7.56%.The demetallation of NiTPPCl8 was carried out using concentrated H2SO4 as per the reported procedure23 and the spectroscopic data was in accordance with the proposed structure of 2.
Acknowledgements
We are grateful for the financial support provided by Council of Scientific and Industrial Research (01(2694)/12/EMR-II), Science and Engineering Research Board (SB/FT/CS-015/2012) and Board of Research in Nuclear Sciences (2012/37C/61BRNS/253). NC thanks Council of Scientific and Industrial Research (CSIR), India for the fellowship.Notes and references
- (a) L. R. Milgrom, The Colours of Life: An Introduction to the Chemistry of Porphyrins and Related Compounds, Oxford University Press, New York, 1997 Search PubMed; (b) J. A. Shelnutt, X.-Z. Song, J.-G. Ma, S.-L. Jia, W. Jentzen and C. J. Medforth, Chem. Soc. Rev., 1998, 27, 31 RSC; (c) M. O. Senge, The Porphyrin Handbook, ed. K. M. Kadish, K. M. Smith and R. Guilard, Academic Press, San Diego, 2000, vol. 1, p. 239 Search PubMed; (d) M. O. Senge, Chem. Commun., 2006, 243 RSC.
- (a) D. S. Kim and J. L. Sessler, Chem. Soc. Rev., 2014 10.1039/c4cs00157e; (b) V. V. Roznyatovskiy, C.-H. Lee and J. L. Sessler, Chem. Soc. Rev., 2013, 42, 1921 RSC; (c) P. D. Beer and P. A. Gale, Angew. Chem., Int. Ed., 2001, 40, 486 CrossRef CAS; (d) E. Matthews and P. D. Beer, Supramol. Chem., 2005, 17, 411 CrossRef.
- (a) A. Bianchi, K. Bowman-James and E. Garcia-Espana, Supra-molecular Chemistry of Anions, Wiley-VCH, New York, 1997 Search PubMed; (b) J. L. Sessler, P. A. Gale and W.-S. Cho, Anion Receptor Chemistry, RSC Publishing, Cambridge, UK, 2006 Search PubMed; (c) L. C. Gilday, N. G. White and P. D. Beer, Dalton Trans., 2012, 41, 7092 RSC.
- (a) P. D. Beer and P. A. Gale, Angew. Chem., 2001, 113, 502 CrossRef; (b) E. Matthews and P. D. Beer, Supramol. Chem., 2005, 17, 411 CrossRef.
- (a) B. Vennesland, E. E. Comm, C. J. Knownles, J. Westly and F. Wissing, Cyanide in Biology, Academic press, London, 1981 Search PubMed; (b) M. S. Frant and J. Ross, Science, 1966, 154, 1533 Search PubMed; (c) D. Briancon, Rev. Rheum., 1997, 64, 78 CAS.
- (a) R. L. P. Adams, J. T. Knowler and D. P. Leader, The Biochemistry of the Nucleic Acids, ed. Chapman and Hall, New York, 11th edn, 1992 Search PubMed; (b) X. F. Shang and X. F. Xu, BioSystems, 2009, 96, 165 CrossRef CAS PubMed.
- (a) C.-H. Lee, H. Miyaji, D.-W. Yoon and J. L. Sessler, Chem. Commun., 2008, 24 RSC; (b) S. K. Kim and J. L. Sessler, Acc. Chem. Res., 2014, 47, 2525 CrossRef CAS PubMed; (c) P. Anzenbacher, R. Nishiyabu and M. A. Palacois, Coord. Chem. Rev., 2006, 250, 2929 CrossRef CAS PubMed; (d) P. A. Gale and C.-H. Lee, Top. Heterocycl. Chem., 2010, 24, 39 CAS.
- See for selected examples: (a) A. J. Pistner, D. A. Lutterman, M. J. Ghidiu, Y.-Z. Ma and J. Rosenthal, J. Am. Chem. Soc., 2013, 135, 6601 CrossRef CAS PubMed; (b) C. I. M. Santos, E. Oliveira, J. F. B. Barata, M. A. F. Faustino, J. A. S. Cavaleiro, M. G. Neves and C. Lodeiro, J. Mater. Chem., 2012, 22, 13811 RSC; (c) P. Yadav and M. Sankar, Dalton Trans., 2014, 43, 14680 RSC; (d) J. L. Sessler and J. M. Davis, Acc. Chem. Res., 2001, 34, 989 CrossRef CAS PubMed; (e) D. G. Cho, Syntheses and studies of sapphyrins and anion receptors, Umi Dissertation Publishing, 2012 Search PubMed; (f) D. H. Won, M. Toganoh, H. Uno and H. Furuta, Dalton Trans., 2009, 6151 RSC; (g) Y. Xie, T. Marimoto and H. Furuta, Angew. Chem., Int. Ed., 2006, 45, 6907 CrossRef CAS PubMed; (h) F. D'Souza, N. K. Subbaiyan, Y. Xie, J. P. Hill, K. Ariga, K. Ohkubo and S. Fukuzumi, J. Am. Chem. Soc., 2009, 131, 16138 CrossRef PubMed; (i) J. P. Hill, A. L. Schumacher, F. D'Souza, J. Labuta, C. Redshaw, M. R. J. Elsegood, M. Aoyagi, T. Nakanishi and K. Ariga, Inorg. Chem., 2006, 45, 8288 CrossRef CAS PubMed; (j) M. Takeuci, T. Shioya and T. M. Swager, Angew. Chem., Int. Ed., 2001, 40, 3372 CrossRef.
- (a) D. P. Cormode, M. G. B. Drew, R. Jagessar and P. D. Beer, Dalton Trans., 2008, 6732 RSC; (b) P. D. Beer, D. P. Cormode and J. J. Davis, Chem. Commun., 2004, 414 RSC; (c) J. M. M. Rodrigues, A. S. Farinha, P. V. Muteto, S. M. Woranovicz-Barreira, F. A. A. Paz, M. G. Neves, J. A. S. Cavaleiro, A. C. Tome, M. T. Gomes, J. L. Sessler and J. P. C. Tome, Chem. Commun., 2014, 50, 1359 RSC.
- (a) R. Harada and T. Kojima, Chem. Commun., 2005, 716 RSC; (b) T. Honda, T. Kojima and S. Fukuzumi, Chem. Commun., 2009, 4994 RSC; (c) M. M. Kruk, A. S. Starukhina, N. Zh. Mamardashvilib, G. M. Mamardashvilib, Y. B. Ivanovab and O. V. Maltsevab, J. Porphyrins Phthalocyanines, 2009, 13, 1148 CrossRef CAS.
- (a) N. Agarwal and M. Ravikanth, Tetrahedron, 2004, 60, 4739 CrossRef CAS PubMed; (b) M. O. Senge, T. P. Forsyth, L. T. Nguyen and K. M. Smith, Angew. Chem., Int. Ed. Engl., 1994, 30, 2485 Search PubMed; (c) N. Agarwal, C.-H. Hung and M. Ravikanth, Tetrahedron, 2004, 60, 10671 CrossRef CAS PubMed.
- (a) S.-J. Hong, J. Yoo, S.-H. Kim, J. S. Kim, J. Yoon and C.-H. Lee, Chem. Commun., 2009, 189 RSC; (b) M. G. D. Holaday, G. Tarafdar, B. Adinarayana, M. L. P. Reddy and A. Srinivasan, Chem. Commun., 2014, 50, 10834 RSC; (c) E. Palomares, M. V. Martinez-Diaz, T. Torres and E. Coronado, Adv. Funct. Mater., 2006, 16, 1166 CrossRef CAS; (d) Y.-H. Kim and J.-I. Hong, Chem. Commun., 2002, 512 RSC; (e) L. D. Chen, X. U. Zou and P. Bühlmann, Anal. Chem., 2012, 84, 9192 CAS.
- (a) V. Amendola, D. Esteban-Gomez, L. Fabbrizzi and M. Licchelli, Acc. Chem. Res., 2006, 39, 343 CrossRef CAS PubMed; (b) A. Basu, S. K. Dey and G. Das, RSC Adv., 2013, 3, 6596 RSC; (c) Q. Wang, Y. Xie, Y. Ding, X. Li and W. Zhu, Chem. Commun., 2010, 46, 3669 RSC; (d) G. Lu, X. Zhang, X. Cal, Y. Fang, M. Zhu, W. Zhu, Z. Ou and K. M. Kadish, J. Porphyrins Phthalocyanines, 2013, 17, 941 CrossRef CAS; (e) Y. Fang, P. Bhyrappa, Z. Ou and K. M. Kadish, Chem.–Eur. J., 2014, 20, 524 CrossRef CAS PubMed.
- (a) P. Bhyrappa and V. Krishnan, Inorg. Chem., 1991, 30, 239 CrossRef CAS; (b) P. Bhyrappa and P. Bhavana, J. Chem. Soc., Perkin Trans. 2, 2001, 238 RSC.
- (a) M. O. Senge, V. Gerstung, K. Ruhlandt-Senge, S. Runge and I. Lehmann, J. Chem. Soc., Dalton Trans., 1998, 4187 RSC; (b) L. Jaquinod, R. G. Khoury, K. M. Shea and K. M. Smith, Tetrahedron, 1999, 5, 13151 CrossRef; (c) P. Bhyrappa, M. Sankar and B. Varghese, Inorg. Chem., 2006, 45, 4136 CrossRef CAS PubMed.
- (a) T. Wijesekera, A. Matsumoto, D. Dolphin and D. Lexa, Angew. Chem., Int. Ed. Engl., 1990, 29, 1028 CrossRef; (b) P. Bhyrappa and V. Krishnan, Chem. Lett., 1993, 869 CrossRef CAS.
- K. M. Kadish, G. Royal, E. V. Caemelbecke and L. Gueletti, The Porphyrin Handbook, ed. K. M. Kadish, K. M. Smith and R. Guilard, Academic Press, San Diego, 2000, vol. 9, p. 1 Search PubMed.
- (a) A. V. Hill, J. Physiol., 1910, 40, IV–VII Search PubMed; (b) A. Pfeil and J.-M. Lehn, J. Chem. Soc., Chem. Commun., 1992, 838 RSC.
- (a) P. Bhyrappa and P. Bhavana, Chem. Phys. Lett., 2001, 342, 39 CrossRef CAS; (b) P. Bhyrappa and P. Bhavana, Chem. Phys. Lett., 2002, 357, 108 CrossRef CAS.
- A. S. F. Farinha, M. J. F. Calvete, F. A. A. Paz, A. C. Tome, J. A. S. Cavaleiro, J. L. Sessler and J. P. C. Tome, Sens. Actuators, B, 2014, 201, 387 CrossRef CAS PubMed.
- V. M. Patel, J. A. Patel, S. S. Havele and S. R. Dhaneshwar, Int. J. ChemTech Res., 2010, 2, 756–761 CAS.
- A. Giraudeau, H. J. Callot and M. Gross, Inorg. Chem., 1979, 18, 201 CrossRef CAS.
- G. A. Spyroulias, A. P. Despotopoulos, C. P. Raptopoulou, A. Terzis, D. de Montauzon, R. Poilblanc and A. G. Coutsolelos, Inorg. Chem., 2002, 41, 2648 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: UV-visible absorption, fluorescence and 1H NMR spectra of 1–4, CV and DPV figures of 1–4, UV-visible titrations for protonation, deprotonation and anion sensing studies, evidences for the dianion formation through 1H NMR and electrochemical studies. CCDC 1033576. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4ra11368c |
| This journal is © The Royal Society of Chemistry 2015 |