The anti-cancer activity of green tea, coffee and cocoa extracts on human cervical adenocarcinoma HeLa cells depends on both pro-oxidant and anti-proliferative activities of polyphenols†
Abstract
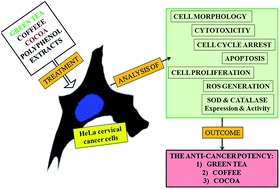
It has been shown before that dietary polyphenols possess cancer chemopreventive effects. As cervical cancer is the second leading genital malignancy in women after breast cancer, the anti-cervical cancer effects of polyphenol extracts of commonly used beverages (green tea, coffee and cocoa) were tested and compared in HeLa cells. All the extracts induced apoptosis of HeLa cells, but green tea was the most potent. However, as opposed to green tea which induced a strong anti-proliferative response in HeLa cells, coffee and cocoa extracts promoted the proliferation of surviving cells. After short-term exposure, green tea and coffee extracts, but not cocoa, induced the formation of intracellular reactive oxygen species. Only the green tea extract increased the production of superoxide anion radicals and decreased reduced glutathione levels. Gene expression of Cu/Zn and Mn-superoxide dismutase or catalase was unaltered in cells treated with extracts, but green tea partially inhibited catalase activity. The cytotoxic activity of green tea and coffee extracts was partially inhibited by vitamin C. The in vitro anti-cervical cancer potency of tested polyphenol extracts is related to their pro-oxidant and anti-proliferative activities and are exhibited in the following order: green tea > coffee > cocoa, with only green tea showing both pro-oxidative and anti-proliferative action.

 Please wait while we load your content...
Please wait while we load your content...