MoO2Cl2(DMF)2 catalyzed microwave assisted reductive cyclisation of nitroaromatics into dibenzodiazepines†
I. R. Siddiqui*,
Anushree Srivastava,
Archana Singh,
Shayna Shamim and
Pragati Rai
Laboratory of Green Synthesis, Department of Chemistry, University of Allahabad, Allahabad-211002, India. E-mail: dr.irsiddiqui@gmail.com
First published on 5th December 2014
Abstract
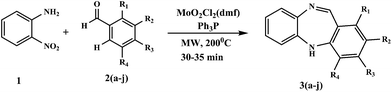
The paper describes a gentle and highly efficient protocol for the synthesis of dibenzodiazepines by reductive cyclisation of nitroaniline to dibenzodiazepines under microwave irradiation catalyzed by MoO2Cl2(DMF)2 involving Ph3P as reducing agent. This synergic approach results in the transformation of nitroaromatics into dibenzodiazepines in high yield and is applicable to the construction of a wide variety of dibenzo(di/ox)azepines and other structurally related heterocycles.
Introduction
Dibenzodiazepines have attracted considerable attention because of their efficient pharmacological activities.1 The benzodiazepine nucleus is a well studied traditional pharmacophoric scaffold that has emerged as the core structural unit of a variety of sedatives, hypnotic muscle-relaxants, atypical and potential antipsychotic,2 anti-dopaminergic and anti-muscarinic agents,3 compounds displaying H4 receptor affinity4 and RXR-antagonist activity drugs.5 Since the first reported synthesis of dibenzodiazepine derivatives by Schmutz in 1964–67 (clozapine and loxapine; Fig. 1) limited progress in their synthesis has been made.6 Existing routes to dibenzodiazepines generally rely on the preparation of amide7 or lactam8 intermediates and subsequent functionalization of the heterocyclic scaffold to introduce additional substituents (Scheme 1). Such transformations typically require harsh conditions and purification after each synthetic step, and are characterized by low atom economy and the use of protecting groups.9 | ||
| Scheme 1 Pairs of coupling partners traditionally used for synthesis of dibenzodiazepines. Reagents used: POCl3, PPA, SOCl2, RZnCl, Pd/C, SnCl2, peptide coupling reagents.14 Coupling of substrates with free NH2 groups leads to mixtures of products. | ||
Transition metal complexes with metal–oxygen multiple bonds are well known as catalysts for oxo-transfer oxidation reactions and particularly for reduction of organic functional groups.10 The catalyst MoO2Cl2(DMF)2 has advantages due to its straightforward synthesis (under air, using aqueous media), water solubility and possession of Cl ligands which are prone to hydrolysis and substitution by oxygen ligands.11 The redox ability of dioxomolybdenum reagents has been utilized in designing several useful organic transformations.12
Prompted by the above facts and to circumvent the above-mentioned drawbacks we report herein the novel, gentle and highly efficient synthesis of dibenzodiazepines by MoO2Cl2(DMF)2 catalyzed and microwave activated one-step reductive cyclisation of nitroaromatic compounds with Ph3P as reducing agent in excellent yield (75%) (Scheme 2).
The reduction of the dioxomolybdenum(VI) compounds by addition of phosphines like PPh3 leads to dinuclear m-oxo-bridged complexes. However, oxidation of the oxomolybdenum(V) complexes is restricted by the nature of the ligand and the oxidizing agents.13
Result and discussion
In our preliminary investigation encouraged by great progress towards the target molecule, we carried out a one pot reaction with the commercially available compounds 2-nitroaniline 1 and aromatic aldehyde 2a (see Table 6 below) as model reactants by conventional heating methods without any catalyst but no product formation was detected. However when a catalyst was used, product formation occurred in low yield (25–30%). Next we tried triphenylphosphine as reducing agent employed with the catalyst. Conversion of 1 and 2a into 3a was extremely sluggish and both the starting material and product were observed to decompose over several hours under reflux conditions. We envisaged that the efficiency of this modified synthesis could be improved significantly through the use of microwave irradiation methodology.In order to test the synthesis proposed in Scheme 2 and find the best reaction conditions, 2-nitroaniline 1 and aromatic aldehyde 2a in various solvents with and without catalyst were irradiated with microwaves but the product was only obtained in trace amounts. Our initial studies focused on examining the scope of the catalyst and application of various metal compounds. Commercially available molybdenum oxides such as MoO2, MoO3 and MoO2(acac)2 showed catalytic activities (40–50% yield, Table 1, entries 1–3). In contrast the catalytic activities of other metal compounds such as Na2MoO4 and ReO2 were very low (Table 1, entries 4 and 5). As far as catalytic activity is concerned MoO2Cl2(DMF)2 was superior to the others (Table 1, entry 6).
The use of H-donor solvents such as toluene and isopropanol allowed the formation of the desired product 3a. Good yields were obtained in both solvents although using isopropanol was less convenient because its low boiling point led to the microwave vial being exposed to high pressure. In order to increase the yield of the desired product further, we focused on the combination of MoO2Cl2(DMF)2 catalyst and a variety of phosphines, using any one of trimethyl, triethyl and triphenyl phosphine under microwave heating. The use of trimethyl and triethyl phosphines led to complex mixtures of 3a with very poor yields. Thus, a homogeneous solution of 1 and 2, Ph3P (2.4 eq.) and MoO2Cl2(DMF)2 (10 mol%) in toluene was heated at 200 °C using microwave heating for 30 min. With a view to expanding the scope and practical convenience of this dibenzodiazepine synthesis, a variety of solvents and phosphine combinations were studied (Table 2). Herein, we report the development of a new catalytic system based on Ph3P as reductant and MoO2Cl2(DMF)2 as the catalyst with toluene because of the poor microwave absorption properties of this solvent.
| Entry | Solvent | Phosphine | Temp. (°C) | Time (min) | Yieldb (%) |
|---|---|---|---|---|---|
| a Reaction conditions: nitroaniline (1 mmol), aromatic aldehyde (1 mmol), MoO2Cl2(DMF)2 catalyst and different solvents and phosphines.b Isolated yields. | |||||
| 1 | 1,4-Dioxane | PMe3 | 200 | 60 | 35 |
| 2 | 1,4-Dioxane | PEt3 | 200 | 65 | 44 |
| 3 | 1,4-Dioxane | PPh3 | 200 | 55 | 25 |
| 4 | Toluene | PMe3 | 200 | 40 | 65 |
| 5 | Toluene | PEt3 | 200 | 42 | 68 |
| 6 | Toluene | PPh3 | 200 | 30 | 75 |
| 7 | Isopropanol | PMe3 | 200 | 35 | 55 |
| 8 | Isopropanol | PEt3 | 200 | 47 | 62 |
| 9 | Isopropanol | PPh3 | 200 | 50 | 58 |
A temperature of 200 °C was chosen on the basis of previous MW reactions of nitroaniline. In order to optimize the reaction temperature the reaction of 1 and 2a was carried out by using MoO2Cl2(DMF)2 with Ph3P catalytic system at temperatures ranging from 160 °C to 210 °C with an increment of 10 °C each time. The results are displayed in Table 3.
We observed that the yield of product 3a was improved and the reaction time was shortened as the temperature was increased from 190–200 °C. The yield dropped when the temperature was further increased from 200 °C to 210 °C. Therefore, the most suitable reaction temperature was found to be 200 °C. Moreover screening of the time required for an excellent yield of product (entries 1 and 3, Table 4) showed that at 200 °C the reaction needed irradiation for 30 min to achieve complete conversion but that at longer times side reactions/degradative processes set in. This is derived from the fact that at irradiation times higher than 30 min (Table 4, entry 4 and 5), the yield fell off.
On a comparative point of view, regarding the methodology applied, the dibenzodiazepines were prepared in lower yields (40–45%) using thermal conditions than using microwave irradiation (70–80%). The results are summarised in Table 5. The results in Table 5 clearly show that the reaction was efficiently promoted by MW and the reaction time was strikingly shortened to 30–35 min from 12–15 h.
| Product | MP °C | Time | Yieldb | ||
|---|---|---|---|---|---|
| MW (min) | Thermal (h) | MW (%) | Thermal (%) | ||
| a Reaction conditions: nitroaniline (1 mmol), aromatic aldehydes (1 mmol), MoO2Cl2(DMF)2 (10 mol%), Ph3P (2.4 eq.) and toluene solvent.b Isolated yield. | |||||
| 3a | 138 | 31 | 12 | 72 | 42 |
| 3b | 144 | 30 | 14 | 74 | 44 |
| 3c | 152 | 30 | 13 | 73 | 40 |
| 3d | 128 | 32 | 12 | 75 | 42 |
| 3e | 120 | 31 | 14 | 78 | 45 |
| 3f | 158 | 33 | 13 | 80 | 43 |
| 3g | 148 | 34 | 15 | 76 | 44 |
| 3h | 136 | 32 | 12 | 75 | 42 |
| 3i | 132 | 35 | 13 | 77 | 41 |
| 3j | 146 | 34 | 15 | 78 | 40 |
This observation clearly indicates the existence of a non-thermal microwave effect. It is thus assumed that the microwave synergy plays a significant role in the reaction and it combines with the specific effect of the electromagnetic field of the microwaves in decreasing the activation energy of the reaction by stabilising the polar activated complex of the reaction, to provide excellent yields and reducing reaction time from hours to minutes.
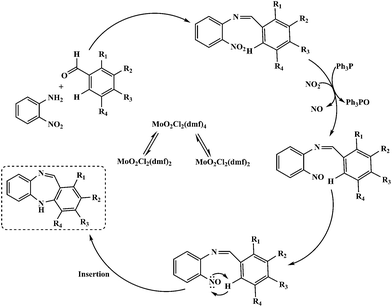
Mechanistically, microwave activated synthesis of dibenzodiazepines and reductive cyclization of nitroaromatics via intramolecular cyclization can be achieved by employing a catalytic system (Scheme 3). The structural assignments of the new compounds are based on chemical and spectroscopic evidence. In order to have accurate data for comparison to hand, we explored the scope of our proposed reaction where nitroaniline was reacted with a range of commercially available aromatic aldehydes under the optimised conditions. The results are listed in Table 6.
| Entry | Products | Substrate 2 | Time (min) | Yield (%) | MP (°C) | |||
|---|---|---|---|---|---|---|---|---|
| R1 | R2 | R3 | R4 | |||||
| a Reaction condition: nitroaniline (1 mmol), aromatic aldehydes (1 mmol), MoO2Cl2(DMF)2 (10 mol%), Ph3P (2.4 eq.) and toluene solvent at 200 °C in MW. | ||||||||
| 1 | 3a | H | H | H | H | 30 | 75 | 138 |
| 2 | 3b | H | H | H | Cl | 31 | 74 | 144 |
| 3 | 3c | H | H | H | NO2 | 30 | 73 | 152 |
| 4 | 3d | H | H | H | OCH3 | 32 | 75 | 128 |
| 5 | 3e | H | H | OCH3 | OCH3 | 31 | 78 | 120 |
| 6 | 3f | H | H | NO2 | Cl | 33 | 80 | 158 |
| 7 | 3g | H | H | NO2 | NO2 | 34 | 76 | 148 |
| 8 | 3h | H | H | H | CH3 | 32 | 75 | 136 |
| 9 | 3i | H | H | CH3 | CH3 | 35 | 77 | 132 |
| 10 | 3j | H | H | Cl | Cl | 34 | 78 | 146 |
Conclusion
The advantages of microwave heating to effect a wide variety of organic reactions are now well established.15 Key advantages of modern scientific microwave apparatus are the ability to control reaction conditions, precisely monitoring temperatures, pressure and reaction time. The microwave reactor “Monowave 300” (manufactured by Anton-paar Pvt. Ltd.) was used for irradiation reactions. The instrument uses a maximum of 850 W magnetron output power (2.45 GHz). The temperature was recorded using IR temperature sensor. The irradiation experiments were performed in temperature control mode. Care was taken to ensure efficient stirring in all experiments. In summary we devised a mild, convenient and efficient protocol for the synthesis of dibenzodiazepines from easily available and cost effective starting materials. The simple experimental procedure is convenient and environmentally benign.Experimental section
Materials
All chemicals were reagent grade, were purchased from Aldrich and Alfa Aesar and were used without purification. NMR spectra were recorded on a BRUKER AVANCE II-400FT Spectrometer (400 for 1H NMR, 100 MHz for 13C) using CDCl3 as solvent and TMS as an internal reference. Mass spectra were recorded on a JEOL SX-102 (FAB) mass spectrometer at 70 eV. Elemental analyses were carried out in a Coleman automatic carbon, hydrogen, oxygen, chlorine and nitrogen analyzer. All the reactions were monitored by TLC using 40 pre-coated sheets of silica gel G/UV-254 of 0.25 mm thickness (Merck 60F254). Melting points were determined by the open glass capillary method and were uncorrected.Method
Herein, for the microwave assisted synthesis of dibenzodiazepines 3(a–j), a mixture of 2-nitroaniline 1 (1 mmol), various aromatic aldehydes 2(a–j) (1 mmol), Ph3P (2.4 equivalents), 10% mol of MoO2Cl2(DMF)2 and toluene (3 ml) was irradiated with microwaves at 200 °C for 30–35 minutes. After completion of the reaction (indicated by TLC solvent system, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of ethyl acetate
1 ratio of ethyl acetate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane), the reaction mixture was filtered and allowed to stand for 1.5 hours to remove the solvent. The mixture was dissolved in H2O (100 ml) and the product was extracted with EtOAc (3 × 50 ml), washed with brine (3 × 20 ml) and dried over MgSO4. The solvent was removed by distillation under reduced pressure. The remaining residue was purified by flash column chromatography to obtain analytically pure 3(a–j). All products were characterized by comparison of their MP, 1H NMR spectra with those of authentic samples.4
hexane), the reaction mixture was filtered and allowed to stand for 1.5 hours to remove the solvent. The mixture was dissolved in H2O (100 ml) and the product was extracted with EtOAc (3 × 50 ml), washed with brine (3 × 20 ml) and dried over MgSO4. The solvent was removed by distillation under reduced pressure. The remaining residue was purified by flash column chromatography to obtain analytically pure 3(a–j). All products were characterized by comparison of their MP, 1H NMR spectra with those of authentic samples.4
Acknowledgements
We gratefully acknowledge the financial support from the University Grants Commission and CSIR. Authors are also thankful to SAIF, Punjab University Chandigarh for providing all the spectroscopic and analytical data.Notes and references
-
(a) D. E. Thurston and D. Subhas Bose, Chem. Rev., 1994, 94, 433 CrossRef CAS
; (b) G. Broggini, G. Molteni and G. Zecchi, Synthesis, 1995, 6, 647 CrossRef PubMed
; (c) G. Broggini, I. De Marchi, M. Martinelli, G. Paladino, T. Pilati and A. Terraneo, Synthesis, 2005, 13, 2246 CrossRef PubMed
; (d) B. Anwar, P. Grimsey, K. Hemming, M. Krajniewski and C. Loukou, Tetrahedron Lett., 2000, 41, 10107 CrossRef CAS
; (e) K. Hemming, C. S. Chambers, M. S. Hamasharif, H. João, M. N. Khan, N. Patel, R. Airley and S. Day, Tetrahedron, 2014, 70, 7306 CrossRef CAS PubMed
.
-
(a) S. Jafari, F. Fernandez Enright and X. Feng Huang, J. Neurochem., 2012, 120, 371 CrossRef CAS PubMed
; (b) J. F. F. LiBgeois, J. Bruhwyler, J. Damas, T. P. Nguyen, E. M. G. Chleide, M. G. A. Mercier, F. A. Register and J. E. Delarge, J. Med. Chem., 1993, 36, 2107 CrossRef
; (c) T. Kendrick, Br. J. Gen. Pract., 1999, 49, 745 CAS
.
- R. J. Miller and C. R. Hiley, Naunyn-Schmiedeberg’s Arch. Pharmacol., 1976, 292, 289 CrossRef CAS
.
- R. A. Smits, H. D. Lim, B. Stegink, R. A. Bakker, I. J. P. De Esch and R. Leurs, J. Med. Chem., 2006, 49, 4512 CrossRef CAS PubMed
.
-
(a) M. Kimura, M. Kishida, K. Konishi, H. Mitani, J. Sakaki and H. Uchiyama, US Patent, WO 2004089916 A1, PCT/EP2004/003806 and J. Sakaki, K. Konishi, M. Kishida, M. Kimura, H. Uchiyama and H. Mitani, US 20100173896 A1, US 12/724-977, 2010
; (b) L. Eyrolles, E. Kawachi, Y. Matsushima, O. Nakajima, H. Kagechika, Y. Hashimoto and K. Shudo, Med. Chem. Res., 1992, 2, 361 CAS
; (c) L. Eyralles, H. Kagechika, E. Kawachi, H. Fukasawa, T. Iijima, Y. Matsushima, Y. Hashimoto and K. Shudo, J. Med. Chem., 1994, 37, 1508 CrossRef
.
-
(a) F. Hunziker, F. Kunzle, J. Schmutz and O. Schindler, Helv. Chim. Acta, 1964, 47, 1163 CrossRef CAS
; (b) F. Hunziker, E. Fischer and J. Schmutz, Helv. Chim. Acta, 1967, 50, 1588 CrossRef CAS
; (c) J. Schmutz, F. Kunzle, F. Hunziker and R. Gauch, Helv. Chim. Acta, 1967, 50, 245 CrossRef CAS
.
-
(a) V. G. Noskov, M. N. Noskova, Y. L. Kruglyak, O. G. Strukov, A. P. Bezrukov and V. K. Kurochkin, Pharm. Chem. J., 1997, 31, 431 CrossRef
; (b) A. W. H. Wardrop, G. L. Sainsbury, J. M. Harrison and T. D. Inch, J. Chem. Soc., Perkin Trans. 1, 1976, 01, 1279 RSC
; (c) X. X. Xu, S. Guo, Q. Dang, J. Chen and X. Bai, J. Comb. Chem., 2007, 9, 773 CrossRef CAS PubMed
.
-
(a) R. A. Smits, H. D. Lim, B. Stegink, R. A. Bakker, I. J. de Esch and R. Leurs, J. Med. Chem., 2006, 49, 4512 CrossRef CAS PubMed
; (b) J. Su, H. Tang, B. A. McKittrick, D. A. Burnett, H. Zhang, A. Smith-Torhan, A. Fawzi and J. Lachowicz, Bioorg. Med. Chem. Lett., 2006, 16, 4548 CrossRef CAS PubMed
; (c) A. V. Joshua, S. K. Sharma, A. Strelkov, J. R. Scott, M. T. Martin-Iverson, D. N. Abrams, P. H. Silverstone and A. J. B. McEwan, Bioorg. Med. Chem. Lett., 2007, 17, 4066 CrossRef CAS PubMed
; (d) Y. Liao, B. J. Venhuis, N. Rodenhuis, W. Timmerman, H. Wikstrom, E. Meier, G. D. Bartoszyk, H. Bottcher, C. A. Seyfried and S. Sundell, J. Med. Chem., 1999, 42, 2235 CrossRef CAS PubMed
.
-
(a) X. L. Jiang, G. T. Lee, K. Prasad and O. Repic, Org. Process Res. Dev., 2008, 12, 1137 CrossRef CAS
; (b) M. Ebisawa, H. Umemiya, K. Ohta, H. Fukasawa, E. Kawachi, G. Christoffel, H. Gronemeyer, M. Tsuji, Y. Hashimoto, K. Shudo and H. Kagechika, Chem. Pharm. Bull., 1999, 47, 1778 CrossRef CAS
; (c) A. V. Joshua, S. K. Sharma, A. Strelkov, J. R. Scott, M. T. Martin-Iverson, D. N. Abrams, P. H. Silverstone and A. J. B. McEwan, Bioorg. Med. Chem. Lett., 2007, 17, 4066 CrossRef CAS PubMed
.
-
(a) W. A. Nugent and J. M. Mayer, Metal–Ligand Multiple Bonds, Wiley, New York, NY, 1988 Search PubMed
; (b) K. Tanaka, M. Honjo and T. Tanaka, Inorg. Chem., 1985, 24, 2662 CrossRef CAS
.
-
(a) K. G. Moloy, Inorg. Chem., 1988, 27, 677 CrossRef CAS
; (b) C. M. Tome, M. C. Oliveira, M. Pillinger, I. S. Goncalves and M. Abrantes, Dalton Trans., 2013, 3901 RSC
.
- M. R. Maddani and K. R. Prabhu, J. Indian Inst. Sci., 2010, 90, 287 CAS
.
-
(a) P. B. Huleatt, J. Lau, S. Chua, Y. L. Tan, H. A. Duong and C. L. L. Chai, Tetrahedron Lett., 2011, 52, 1339 CrossRef CAS PubMed
; (b) F. P. Cubillo, J. S. Scott and J. C. Walton, J. Org. Chem., 2009, 74, 4934 CrossRef PubMed
; (c) A. C. Fernandes and C. C. Ramao, Tetrahedron, 2006, 62, 9650 CrossRef CAS PubMed
.
-
(a) D. S. Surry and S. L. Buchwald, Angew. Chem., Int. Ed., 2008, 47, 6338 CrossRef CAS PubMed
; (b) D. S. Surry and S. L. Buchwald, Chem. Sci., 2011, 2, 27 RSC
; (c) J. F. Hartwig, Angew. Chem., Int. Ed., 1998, 37, 2046 CrossRef CAS
; (d) D. Maiti and S. L. Buchwald, J. Am. Chem. Soc., 2009, 131, 17423 CrossRef CAS PubMed
; (e) B. P. Fors, D. A. Watson, M. R. Biscoe and S. L. Buchwald, J. Am. Chem. Soc., 2008, 130, 13552 CrossRef CAS PubMed
; (f) J. F. Hartwig, Acc. Chem. Res., 2008, 41, 1534 CrossRef CAS PubMed
; (g) E. M. Beccalli, G. Broggini, G. Paladinoa and C. Zonia, Tetrahedron, 2005, 61, 61 CrossRef CAS PubMed
; (h) S. Fang, X. Niu, Z. Zhang, Y. Sun, X. Si, C. Shan, L. Wei, A. Xu, L. Feng and C. Ma, Org. Biomol. Chem., 2014, 12, 6895 RSC
.
-
(a) M. N. Joy, Y. D. Bodke, K. K. A. Khader, M. S. A. Padusha, A. M. Sajith and A. Muralidharan, RSC Adv., 2014, 4, 19766 RSC
; (b) A. Sharma, D. Vacchani and E. V. Eycken, Chem.–Eur. J., 2013, 19, 1158 CrossRef CAS PubMed
; (c) G. Broggini, V. Barbera, E. M. Beccalli, U. Chiacchio, A. Fasana, S. Galli and S. Gazzola, Adv. Synth. Catal., 2013, 355, 1640 CrossRef CAS
; (d) X. L. Yun, W. Y. Bi, J. H. Huang, Y. Liu, D. Z. Negrerie, Y. F. Du and K. Zhao, Tetrahedron Lett., 2012, 53, 5076 CrossRef CAS PubMed
; (e) L. Ciofi, A. Trabocchi, C. Lalli, G. Menchi and A. Guarna, Org. Biomol. Chem., 2012, 10, 2780 RSC
; (f) L. Basolo, A. Bernasconi, E. Borsini, G. Broggini and E. M. Beccalli, ChemSusChem, 2011, 4, 1637 CrossRef CAS PubMed
; (g) C. O. Kappe, Angew. Chem., Int. Ed., 2004, 43, 6250 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra11089g |
| This journal is © The Royal Society of Chemistry 2015 |