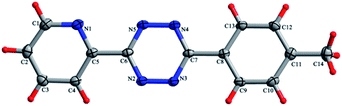
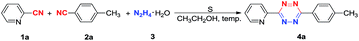Novel 3,6-unsymmetrically disubstituted-1,2,4,5-tetrazines: S-induced one-pot synthesis, properties and theoretical study†
Chen Li‡
a,
Haixia Ge‡a,
Bing Yinab,
Mengyao Shea,
Ping Liu*a,
Xiangdong Lia and
Jianli Li*a
aCollege of Chemistry & Materials Science, Northwest University, Xi'an 710069, P. R. China. E-mail: lijianli@nwu.edu.cn; liuping@nwu.edu.cn
bCollege of Chemistry, Beijing Normal University, Beijing 100875, P. R. China
First published on 14th January 2015
Abstract
18 unprecedented tetrazines unsymmetrically substituted at C3 and C6 by an aromatic heterocycle have been successfully prepared by the S-induced reaction of aromatic nitriles with hydrazine hydrate under thermal conditions. The spectral property investigation suggests that compounds 4d, 4e, 4f, 4p, 4q, 4r, and 4v can display intense fluorescence in the visible region, and their fluorescent properties are affected by the substituents both in tetrazine and in phenyl rings. Moreover, the electrochemical behaviors of these synthesized tetrazines are demonstrated to be fully reversible. Furthermore, density functional theory (DFT) calculations for these compounds were performed to investigate their optimized structures, Fukui function and reactivity or selectivity in the inverse electron demand Diels–Alder reaction.
Introduction
Since 1,2,4,5-tetrazine was first reported by Hantzsch and Lehmann in 1900,1 studies of 1,2,4,5-tetrazine and its 3,6-disubstituted derivatives have received considerable attention due to their definitely unique skeleton and chemical/physical properties, and special application potential in the fields of industry, agriculture and medicine.2,3The 1,2,4,5-tetrazine ring system, which consists of an electron-deficient aromatic heterocycle with four nitrogens, is known for its high colour, biological activity and electrical property. And this system can be applied to biocompatible labelling agents,4 sensors,5 on/off fluorescence switching,6 live cell imaging,7 and components in photovoltaic cells.8 Additionally, the 1,2,4,5-tetrazines typically have high positive enthalpies of formation and high densities, which are suitable for the development of energetic materials with desirable properties, such as explosive, propellant and pyrotechnics.9,10
As the good electron acceptors, 1,2,4,5-tetrazine derivatives can be used as 4-π components in inverse-type Diels–Alder reactions,11 and provide an access to many heterocycles12 and natural products.13 Furthermore, 1,2,4,5-tetrazine derivatives exhibit various biological activities such as antibacterial, antiviral and antitumor properties,2,14,15 therefore these compounds can served as pesticides, herbicides and anti-inflammatory, antimalarial drugs (Fig. 1).16–19
Among many reported 1,2,4,5-tetrazine derivatives,20 the 3,6-symmetrically disubstituted ones are common due to their synthetic accessibility.1b,13,21 In contrast, the reported 3,6-unsymmetrically disubstituted-1,2,4,5-tetrazines are limited, and especially their substituents at C3 and C6 positions are usually the alkyl and some specific substituent groups (methylthio, methoxycarbonyl and methylsulfinyl).11a,21,22 Furthermore, 3,6-unsymmetrically disubstituted-1,2,4,5-tetrazines with the aromatic heterocyclic substituents at C3 and C6 have rarely been reported. In addition, it is known that the substituents have an important effect on biological activities and chemical/physical properties of compounds. For 3,6-unsymmetrically disubstituted-1,2,4,5-tetrazines, the aromatic heterocyclic substituents at C3 and C6 will have the more significant effect on their spectral and electrochemical properties. Therefore, such 1,2,4,5-tetrazine derivatives may be served as potential materials with special properties. Based on this aim, we have devoted our recent efforts to the design and synthesis of new 3,6-unsymmetrically disubstituted-1,2,4,5-tetrazines, and the investigation of their application potential.
Herein, we detailed S-induced one-pot synthesis method for target compounds, and 18 novel 3,6-unsymmetrically disubstituted-1,2,4,5-tetrazines were first reported (Scheme 1). Moreover, the spectral and electrochemical properties23 of obtained compounds were examined. And a systematic theoretical investigation based on the density functional theory (DFT)24 calculation were carried out. Amazingly, these 1,2,4,5-tetrazines with the aromatic heterocyclic substituents at C3 and C6 positions not only can serve as special materials having both optical and electrochemical features, but also show the good reactivity or selectivity in the inverse-type Diels–Alder reaction.
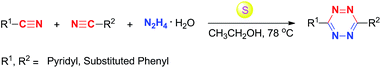 | ||
| Scheme 1 Preparation of 3,6-unsymmetrically disubstituted-1,2,4,5-tetrazines from nitriles and hydrazine hydrate. | ||
Results and discussion
Synthesis
As shown in Table 1, with 2-cyano-pyridine 1a (1.0 equivalent), 4-methylbenzonitrile 2a (1.0 equivalent) and hydrazine hydrate 3 (10 equivalents), the optimum reaction conditions for the synthesis of the corresponding 3-(pyridin-2-yl)-6-(p-tolyl)-1,2,4,5-tetrazine 4a by an intermolecular cyclization were evaluated. We investigated the influences of reaction temperature, amount of sulfur and reaction time on the yield of product. Firstly, the CH3CH2OH was chosen as solvent. It is noteworthy that ethanol is not only low cost and less toxic, but also more efficient than other solvents. Then, different temperatures were examined (entries 10, 13, 14) and 78 °C was found to be the optimal temperature (entry 10). So the reaction temperature was the boiling point of ethanol, 78 °C. Moreover, the amount of sulfur was also investigated. At 78 °C, the reaction of 1.0 equivalent 1a and 2a, 10.0 equivalents 3 with 0.1 equivalent sulfur resulted in only 27% of product 4a (Table 1, entry 3). When using 1.0 equivalent sulfur, the yield was improved to 34% (Table 1, entry 4). When 4.0 equivalent sulfur were used, the yield of 4a was increased to 72% (Table 1, entry 6). These results reveal that 4.0 equivalents sulfur are suitable for this reaction. The reaction time was also tested. When the reaction time was increased to 12 h, the product 4a was generated in 80% yield (Table 1, entry 10). Thus, the optimal condition involved the following parameters: ethanol as a solvent, reaction temperature at 78 °C, the amount of sulfur as inducer, and the reaction time.| Entry | T (°C) | Sulfur (equiv.) | t [h] | Yieldb (%) |
|---|---|---|---|---|
| a Reaction condition: 1a, 2a (each 1.0 equivalent, 0.5 mmol), hydrazine hydrate 3 (10.0 equivalents, 5.0 mmol), ethanol solution (3.0 mL), sulfur (4.0 equivalents, 2.0 mmol) at 78 °C.b Isolated product yields. | ||||
| 1 | rt | 0.05 | 9 | 17 |
| 2 | 78 | 0.05 | 9 | 20 |
| 3 | 78 | 0.1 | 9 | 27 |
| 4 | 78 | 1 | 9 | 34 |
| 5 | 78 | 2 | 9 | 59 |
| 6 | 78 | 4 | 9 | 72 |
| 7 | 78 | 4 | 5 | 55 |
| 8 | 78 | 4 | 7 | 63 |
| 9 | 78 | 4 | 10 | 76 |
| 10 | 78 | 4 | 12 | 80 |
| 11 | 78 | 4 | 14 | 81 |
| 12 | 78 | 0 | 12 | 0 |
| 13 | 58 | 4 | 12 | 64 |
| 14 | 68 | 4 | 12 | 73 |
| 15 | 88 | 4 | 12 | 79 |
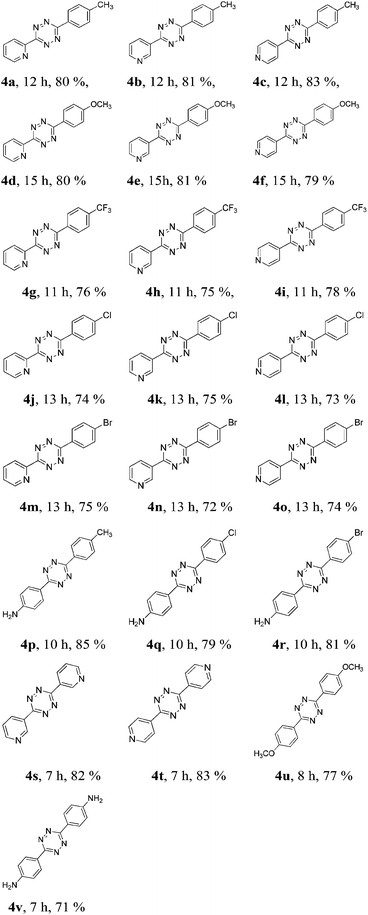
Under the optimized condition, additional aromatic nitriles were examined to expand the substrate scope, and the results are summarized in Table 2. To our delight, the reaction of various nitriles, such as cyanopyridine and substituted benzonitrile, with 3 underwent smoothly in excellent yields.
On the basis of the related literature,3a a tentative mechanism for the S-induced reaction between nitriles and hydrazine hydrate, which synthesizes 3,6-unsymmetrically disubstituted-1,2,4,5-tetrazine derivatives via addition–elimination–rearrangement, was proposed in Scheme 2. To inducing the reaction, the S is involved in the reaction process by reacting with hydrazine hydrate to form NH2NHSH.3a The possible structure of NH2NHSH was calculated at DFT level using double-ζ basis set (6-31G** for H element, 6-31+G* for S, N elements). Then, the NH2NHSH interacts with nitrile to produce the key intermediate [1], and this result is supported by the Fukui function f(r)− calculation. The f(r)− calculation result shows that the nitrogen atom connecting with SH of the NH2NHSH should be the first choice for the nitrile electrophilic attack since its value of condensed Fukui function (0.144) is larger than those of other atoms. The 3D representation of Fukui function f(r)− of NH2NHSH and the condensed f(r)− values of N atoms of NH2NHSH are shown in Scheme 2. The intermediate [1] further reacts with the other nitrile to generate [2]. Then, the [2] might be activated by eliminating of hydrogen sulfide to form intermediate [3]. Subsequently, the intermediate [3] converts to the [4] by electrocyclic rearrangement. Finally, the intermediate [4] converts to the target product by air oxidation. In the process of reaction, symmetrical products are also generated, but their yields are very low. The result may be arise from the fact that the concentration of R1-CN is much lower than that of R2-CN after the intermediate [1] is produced. Then [1] more easily reacts with the nitrile with a higher concentration to form the unsymmetrical product. Thus the yield of the unsymmetrical product is much higher than that of the symmetrical product. Moreover, with a kind of nitrile as the substrate, the major product is the symmetrical tetrazines.
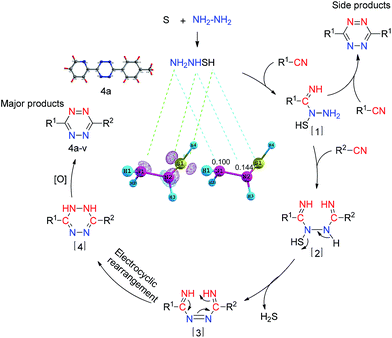 | ||
| Scheme 2 A tentative S-induced mechanism for synthesis of 3,6-unsymmetrically disubstituted-1,2,4,5-tetrazine derivatives. | ||
UV-vis spectroscopy
The UV-vis absorbance spectra of eight tetrazines 4d, 4e, 4f, 4p, 4q, 4r, 4u, 4v in dichloromethane lie in the ultraviolet region, and their absorption maxima are in the range of 270–346 nm (Fig. 2). Furthermore, these compounds all have an absorption maximum in the region of 317–346 nm, which belongs to the electron n–π* transition absorption band. For compounds 4v, a shoulder peak can be observed around 300 nm. The maximum absorption wavelengths (n–π* transition) of these compounds show a certain variation regularity, which is 4d < 4e < 4f < 4u, and 4p < 4q < 4r < 4v. The result reveals that the conjugated degree increase can cause a red shift.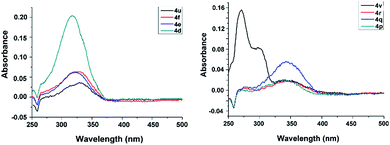 | ||
| Fig. 2 UV-vis absorbance spectra of the compounds 4d, 4e, 4f, 4p, 4q, 4r, 4u, and 4v in dichloromethane (c = 1 × 10−6 mol L−1). | ||
Fluorescence
Among all the compounds, we noticed that only 4d, 4e, 4f, 4p, 4q, 4r, 4u, and 4v can display fluorescence, and their fluorescence was shown in the visible region with the emission wavelengths lying in the range of 405–443 nm. And the fluorescence spectra and data of these compounds in dichloromethane are shown in Fig. 3 and Table 3. Moreover, the fluorescence quantum yields (Φu) of eight tetrazines were determined using optically matching solutions of quinine sulfate (Φf = 0.55 in 0.05 mol L−1 H2SO4) as standards. The quantum yields were calculated according to the related ref. 25. It is noted that these compounds with a fluorescence have the common structural character, which is the existence of an phenyl substituent (4-XC6H4, X = OCH3, NH2) at the C3 position.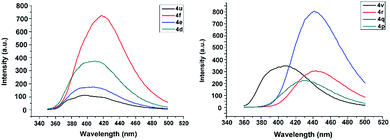 | ||
| Fig. 3 Fluorescence spectra of the compounds 4d, 4e, 4f, 4p, 4q, 4r, 4u, and 4v in dichloromethane (c = 1 × 10−6 mol L−1). | ||
| Entry | λexc, max | λemi, maxa | Φub |
|---|---|---|---|
| a The fluorescence spectra of compounds 4d, 4e, 4f, 4p, 4q, 4r, 4u, and 4v in dichloromethane (c = 1 × 10−6 mol L−1). The excitation slit width is 1.5 nm, the emission slit width is 3 nm.b Fluorescence quantum yield was determined by using quinine sulfate as a standard with Φ = 0.55 in 0.05 mol L−1 H2SO4. | |||
| 4d | 335 | 407 | 0.54 |
| 4e | 335 | 405 | 0.35 |
| 4f | 335 | 418 | 0.67 |
| 4u | 335 | 396 | 0.10 |
| 4p | 344 | 430 | 0.22 |
| 4q | 344 | 441 | 0.58 |
| 4r | 344 | 443 | 0.37 |
| 4v | 344 | 408 | 0.43 |
According to the phenyl substituent at C3, the eight compounds can be divided into two series: (1) 4u, 4d, 4e, 4f (–OCH3 series) and (2) 4v, 4p, 4q, 4r (–NH2 series). Comparison of fluorescence data of the 4u (4-CH3OC6H4) and 4v (4-NH2C6H4) show that the fluorescent wavelength of the latter is longer, since the amino group possesses stronger electron-donating ability resulting in bigger π-conjugation. Then, we compared the fluorescence data of –OCH3 series 4u, 4d, 4e, 4f, and it is found that the fluorescent wavelengths of 4d, 4e and 4f are longer than that of 4u. Interestingly, this same situation exists for –NH2 series compounds 4v, 4p, 4q and 4r. Furthermore, the observed change trends of fluorescent wavelengths of two series of compounds are 4f > 4d > 4e > 4u and 4r > 4q > 4p > 4v, respectively. This result could be explained by the intramolecular charge transfer (ICT). For 4d, 4e, 4f and 4p, 4q, 4r, the whole molecule could form ICT from strong electron-donating groups (4-XC6H4, X = OCH3, NH2) in C3 position to electron-withdrawing group (strong electron-withdrawing group –C5H5N (pyridyl) or weaker electron-withdrawing groups (4-XC6H4, X = CH3 Cl, Br)) in the C6 position, and thus the degree of π-conjugation is increased and the fluorescent wavelengths of these compounds are longer than those of 4u and 4v, respectively. The above results reveal that the substituents in tetrazine and in phenyl rings both have the important effects on the fluorescence of these compounds. More importantly, the fluorescence of these compounds are in the visible range, suggesting their good application potential as the photoelectricity functional material and in photocatalyst technology.
Electrochemistry
The synthesized tetrazines unsymmetrically substituted at C3 and C6 by the aromatic heterocycle are novel and interesting compounds, which are different from those reported ones (without substituents at C3 and C6 or substituted at C3 and C6 by alkyl, specific substituent group methylthio, methoxycarbonyl, etc.) in terms of structure and properties. Especially, the synthesized tetrazines are poor solubility and remain stable in KOH environment, and this result is verified by experiment test and structure analysis. For 4a–v, we have performed the electrochemical investigation,26 which is totally different from the ones published in many closely related reports. Herein, the electrochemical test was carried out in the beaker using three-electrode system and CHI660B electrochemical workstation at room temperature. The foam nickel previously prepared was used as the working electrode. A platinum electrode and a saturated calomel electrode (SCE) were used as the counter electrode and the reference electrode, respectively. The typical CV curves for the obtained samples in 2 M KOH electrolyte versus SCE at scan rate of 20 mV s−1 were well defined and fully reversible (Fig. 4).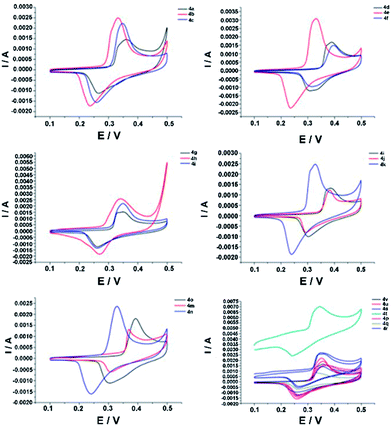 | ||
| Fig. 4 Cyclic voltammetry (CV) curves for compounds 4a–v, the obtained samples in 2 M KOH electrolyte versus SCE at scan rate of 20 mV s−1, the voltage window was ranged from 0.0–0.5 V. | ||
Moreover, it is found that all the investigated tetrazines can exhibit very high reduction potentials, and the electron-rich or -poor group in the heterocyclic substituent can cause a slightly shift of the redox potential. For the reversible system, the equation as follows:
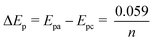 | (1a) |
| E1/2 = (Epa + Epc)/2 | (1b) |
| Entry | Epa | Epc | ΔEp | E1/2 | n |
|---|---|---|---|---|---|
| a Epa represents the oxidation potential; Epc represents the reduction potential; ΔEp is the potential difference between Epa and Epc; E1/2 is the half-wave potential; n is the number of electron transferred. | |||||
| 4a | 0.357 | 0.265 | 0.092 | 0.311 | 0.64 |
| 4b | 0.332 | 0.236 | 0.097 | 0.284 | 0.61 |
| 4c | 0.347 | 0.258 | 0.089 | 0.303 | 0.66 |
| 4d | 0.389 | 0.309 | 0.080 | 0.349 | 0.74 |
| 4e | 0.330 | 0.235 | 0.094 | 0.283 | 0.63 |
| 4f | 0.396 | 0.314 | 0.082 | 0.355 | 0.72 |
| 4g | 0.352 | 0.261 | 0.091 | 0.307 | 0.65 |
| 4h | 0.340 | 0.270 | 0.070 | 0.305 | 0.84 |
| 4i | 0.349 | 0.260 | 0.089 | 0.305 | 0.66 |
| 4j | 0.383 | 0.294 | 0.089 | 0.316 | 0.66 |
| 4k | 0.328 | 0.240 | 0.088 | 0.284 | 0.67 |
| 4l | 0.384 | 0.302 | 0.082 | 0.343 | 0.72 |
| 4m | 0.369 | 0.307 | 0.062 | 0.338 | 0.95 |
| 4n | 0.327 | 0.240 | 0.087 | 0.284 | 0.68 |
| 4o | 0.393 | 0.304 | 0.089 | 0.349 | 0.66 |
| 4p | 0.370 | 0.260 | 0.110 | 0.315 | 0.54 |
| 4q | 0.327 | 0.263 | 0.064 | 0.295 | 0.92 |
| 4r | 0.355 | 0.260 | 0.095 | 0.306 | 0.62 |
| 4s | 0.343 | 0.243 | 0.100 | 0.293 | 0.59 |
| 4t | 0.350 | 0.265 | 0.085 | 0.308 | 0.69 |
| 4u | 0.352 | 0.261 | 0.091 | 0.307 | 0.65 |
| 4v | 0.355 | 0.263 | 0.092 | 0.309 | 0.64 |
Theoretical study
All the calculations were carried out with the Gaussian 09 program package27 using the hybrid density functional theory (B3LYP) method. Basis sets of double-ζ quality (6-31G** for C, H atoms, 6-31+G* for other atoms) were used for the geometry optimization. The optimized structures were confirmed to be local minimum due to the non-existence of imaginary frequency. Fukui function based on optimized structures has been calculated to determine the site reactivity and site selectivity.We systematically performed calculations for all the synthesized tetrazine derivatives 4a–v. The geometry optimization of 4a was based on its X-ray crystal structure (Fig. 5), and the others were based on the molecular structures drawn by Chem3D and Gaussview.
To investigate the site reactivity concerning nucleophilic attack or site selectivity, the calculation of Fukui function f(r)+ for synthesized tetrazines was carried out.28 The equation of Fukui function f(r)+ as follows:
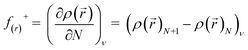 | (2a) |
| f(r)+ = qxN+1 − qxN | (2b) |
For 4a, the 3D representation of the Fukui function and the condensed Fukui function of atoms are shown in Fig. 6 (4b–v, see in the ESI†). The 3D representation of f(r)+ clearly demonstrates that the region around the N atoms of the 1,2,4,5-tetrazine skeleton possess higher reactivity than other parts. Therefore the 1,2,4,5-tetrazine skeleton as the electron-deficient unit may has the stronger electrophilic ability, and can be used as the 4-π components in the inverse-type Diels–Alder reaction. Furthermore, the f(r)+ calculation results also indicate the regioselectivity in the inverse-type Diels–Alder reaction.
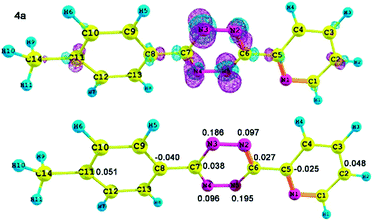 | ||
| Fig. 6 3D representation of the Fukui function f(r)+ (positive in red color and negative in green color) and the condensed Fukui function f(r)+ of related atoms of 4a. | ||
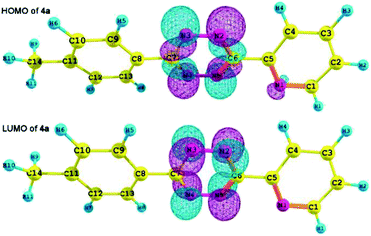
The frontier orbitals HOMO and LUMO for all the synthesized tetrazine derivatives have been also calculated. The 3D representations of HOMO and LUMO orbitals of 4a are shown in Fig. 7 (the shapes of HOMO and LUMO orbitals of 4b–v are shown in the ESI†). The energies of HOMO, LUMO, and the LUMO–HOMO gap for 4a–v are depicted in Table 5.
| Entry | EHOMO | ELUMO | ΔEL–H |
|---|---|---|---|
| a EHOMO, ELUMO, ΔEL–H represent the energies of HOMO, LUMO, and the LUMO–HOMO gap, respectively. | |||
| 4a | −6.265 | −2.746 | 3.52 |
| 4b | −6.455 | −2.980 | 3.48 |
| 4c | −6.569 | −3.063 | 3.51 |
| 4d | −6.208 | −2.691 | 3.52 |
| 4e | −6.299 | −2.926 | 3.37 |
| 4f | −6.406 | −3.004 | 3.40 |
| 4g | −6.643 | −3.131 | 3.51 |
| 4h | −6.832 | −3.356 | 3.48 |
| 4i | −6.957 | −3.445 | 3.51 |
| 4j | −6.491 | −2.978 | 3.51 |
| 4k | −6.681 | −3.205 | 3.48 |
| 4l | −6.800 | −3.290 | 3.51 |
| 4m | −6.501 | −2.988 | 3.51 |
| 4n | −6.691 | −3.214 | 3.48 |
| 4o | −6.810 | −3.299 | 3.51 |
| 4p | −5.717 | −2.545 | 3.17 |
| 4q | −5.876 | −2.752 | 3.12 |
| 4r | −5.882 | −2.762 | 3.12 |
| 4s | −6.723 | −3.271 | 3.45 |
| 4t | −6.973 | −3.453 | 3.52 |
| 4u | −5.881 | −2.618 | 3.26 |
| 4v | −5.435 | −2.404 | 3.03 |
 |
−6.324 | −2.832 | 3.49 |
 |
−8.154 | −4.519 | 3.64 |
 |
−6.630 | −3.095 | 3.54 |
 |
−6.337 | −2.803 | 3.53 |
The inverse-type Diels–Alder reactions between 1,2,4,5-tetrazines and electron-rich dienes depends on the LUMOdiene–HOMOdienophile gap.29 Thus for these 1,2,4,5-tetrazine derivatives as the electron-deficient dienophile, elevating their HOMO energies can facilitate the reaction. We compared the energies of HOMO, LUMO, and the LUMO–HOMO gap for 4a–v, the result reveals that using NH2, CH3O, and CH3 as substituent can increase the HOMO energies in comparison to the CN and CF3, that is to say, the electron-donating substituents will increase the HOMO energies of these compounds. Moreover, we investigated the frontier molecular orbitals (FMOs) of previously reported 3,6-disubstituted-1,2,4,5-tetrazines, which have the good reactivity in the inverse-type Diels–Alder reaction.11d,11f,34 The HOMO energies of our tetrazine derivatives are comparable to those of the reported tetrazine derivatives, and some of them even have the higher HOMO energies than the reported ones (Table 5). Therefore, almost all of the compounds (4a–v) have the good potential of participating in the inverse-type Diels–Alder reaction.
Conclusions
In conclusion, tetrazines unsymmetrically substituted at C3 and C6 positions by aromatic heterocyclic groups have been systematacially synthesized, and 18 novel 3,6-unsymmetrically disubstituted-1,2,4,5-tetrazines have been reported for the first time. The spectral and electrochemical properties of these synthesized tetrazines have also been investigated. These synthesized tetrazine derivatives display the intense fluorescence and fully reversible electrochemical behaviors, and the result indicate the good potential of these compounds in photonic applications. And it is found that the substituents both in tetrazine and in phenyl rings have the important effects on their spectral and electrochemical properties. In addition, the DFT calculations suggest that these synthesized tetrazines, as the electron-deficient dienophile, can exert the good reaction reactivity or selectivity in the inverse electron demand Diels–Alder reaction. The above results indicates that these 3,6-unsymmetrically disubstituted-1,2,4,5-tetrazine derivatives have the large potential to be the good lead compounds that can be used in many fields. Moreover, the further investigation of these tetrazine derivatives is warranted for developing their applications.Experimental
General remarks
1H NMR (400 MHz), 13C NMR (100 MHz) spectra were recorded on 400 MHz spectrometers. Chemical shifts for 1H NMR spectra are reported in parts per million downfield from TMS, chemical shifts for 13C NMR spectra are reported in ppm relative to internal chloroform. Infrared spectra (IR) were recorded with KBr pellets. Silica gel (200–300 mesh) was used for column chromatography. High-resolution mass spectra were obtained using ESI. The UV-vis absorption spectra, fluorescence spectra were recorded in dichloromethane as solvent on a UV-2520 spectrophotometer, RF-5301 PC fluorescence spectrophotometer, respectively. The single-crystal structure of 4a was determined by X-ray crystallography (ESI†).General procedure for the S-induced the reaction of nitriles and hydrazine hydrate
To a round-bottom flask, two kinds of nitriles (each 0.5 mmol), sulfur (64 mg, 2.0 mmol) were added to an ethanol solution (3.0 mL). Then hydrazine hydrate (250 mg, 5.0 mmol) was added in drops into the solution. The mixture was stirred overnight at the required reaction temperature. The reaction was monitored by TLC. After completion, the mixture was cooled, dichloromethane (8 mL) and water (5 mL) were added, and the sulfur was separated by the filtration. After the extraction with dichloromethane, the separated organic layer was dried over CaCl2, and concentrated under reduced pressure. The crude residue was purified by flash chromatography on silica gel using petroleum ether/ethyl acetate as eluent to afford the desired product.3-(Pyridin-2-yl)-6-(p-tolyl)-1,2,4,5-tetrazine (4a)
Silica gel chromatography (petroleum ether–ethyl acetate = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, Rf = 0.26); purple solid (99.6 mg, 80% yield); mp: 178–181 °C; 1H NMR (400 MHz, CDCl3) δ 8.97 (d, J = 3.4 Hz, 1H), 8.70 (d, J = 7.9 Hz, 1H), 8.60 (d, J = 8.0 Hz, 2H), 8.00 (t, J = 7.1 Hz, 1H), 7.62–7.53 (m, 1H), 7.44 (d, J = 7.9 Hz, 2H), 2.50 (s, 3H) ppm; 13C NMR (100 MHz, CDCl3) δ 164.6, 163.5, 151.1, 150.6, 144.1, 137.7, 130.4, 129.0, 128.6, 126.5, 124.0, 22.0 ppm; IR (KBr) νmax/cm−1 1605, 1580, 1393, 1245, 1177, 1116, 919, 812; HRMS (ESI) m/z: [M + Na]+ calcd for C14H11N5 + Na+ 250.1093; found: 250.1093.
1, Rf = 0.26); purple solid (99.6 mg, 80% yield); mp: 178–181 °C; 1H NMR (400 MHz, CDCl3) δ 8.97 (d, J = 3.4 Hz, 1H), 8.70 (d, J = 7.9 Hz, 1H), 8.60 (d, J = 8.0 Hz, 2H), 8.00 (t, J = 7.1 Hz, 1H), 7.62–7.53 (m, 1H), 7.44 (d, J = 7.9 Hz, 2H), 2.50 (s, 3H) ppm; 13C NMR (100 MHz, CDCl3) δ 164.6, 163.5, 151.1, 150.6, 144.1, 137.7, 130.4, 129.0, 128.6, 126.5, 124.0, 22.0 ppm; IR (KBr) νmax/cm−1 1605, 1580, 1393, 1245, 1177, 1116, 919, 812; HRMS (ESI) m/z: [M + Na]+ calcd for C14H11N5 + Na+ 250.1093; found: 250.1093.
3-(Pyridin-3-yl)-6-(p-tolyl)-1,2,4,5-tetrazine (4b)
Silica gel chromatography (petroleum ether–ethyl acetate = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, Rf = 0.40); purple solid (100.9 mg, 81%); mp: 197–199 °C; 1H NMR (400 MHz, CDCl3) δ 9.85 (s, 1H), 8.88 (dd, J = 6.6, 5.3 Hz, 2H), 8.54 (dd, J = 8.2, 5.3 Hz, 2H), 7.77 (d, J = 8.5 Hz, 1H), 7.55–7.59 (m, 1H), 7.43 (d, J = 8.0 Hz, 1H), 2.49 (s, 3H) ppm; 13C NMR (100 MHz, CDCl3) δ 164.6, 162.9, 153.3, 149.4, 144.2, 135.1, 130.4, 128.9, 128.4, 128.2, 124.2, 22.0 ppm; IR (KBr) νmax/cm−1 2361, 1604, 1583, 1393, 1178, 1107, 1017, 917, 849, 817, 593; HRMS (ESI) m/z: [M + Na]+ calcd for C14H11N5 + Na+ 250.1093; found: 250.1093.
1, Rf = 0.40); purple solid (100.9 mg, 81%); mp: 197–199 °C; 1H NMR (400 MHz, CDCl3) δ 9.85 (s, 1H), 8.88 (dd, J = 6.6, 5.3 Hz, 2H), 8.54 (dd, J = 8.2, 5.3 Hz, 2H), 7.77 (d, J = 8.5 Hz, 1H), 7.55–7.59 (m, 1H), 7.43 (d, J = 8.0 Hz, 1H), 2.49 (s, 3H) ppm; 13C NMR (100 MHz, CDCl3) δ 164.6, 162.9, 153.3, 149.4, 144.2, 135.1, 130.4, 128.9, 128.4, 128.2, 124.2, 22.0 ppm; IR (KBr) νmax/cm−1 2361, 1604, 1583, 1393, 1178, 1107, 1017, 917, 849, 817, 593; HRMS (ESI) m/z: [M + Na]+ calcd for C14H11N5 + Na+ 250.1093; found: 250.1093.
3-(Pyridin-4-yl)-6-(p-tolyl)-1,2,4,5-tetrazine (4c)
Silica gel chromatography (petroleum ether–ethyl acetate = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, Rf = 0.39); purple solid (103.4 mg, 83%), mp: 231–233 °C; 1H NMR (400 MHz, CDCl3) δ 8.92 (d, J = 5.9 Hz, 2H), 8.56 (d, J = 8.2 Hz, 2H), 8.47 (d, J = 6.0 Hz, 2H), 7.43 (d, J = 8.1 Hz, 2H), 2.50 (s, 3H) ppm; 13C NMR (100 MHz, CDCl3) δ 164.6, 162.6, 151.0, 144.2, 139.2, 130.2, 128.4, 128.3, 121.0, 21.7 ppm; IR(KBr) νmax/cm−1 2914, 1604, 1595, 1393, 1212, 1179, 1054, 921, 828, 598; HRMS (ESI) m/z: [M + Na]+ calcd for C14H11N5 + Na+ 250.1093; found: 250.1093.
1, Rf = 0.39); purple solid (103.4 mg, 83%), mp: 231–233 °C; 1H NMR (400 MHz, CDCl3) δ 8.92 (d, J = 5.9 Hz, 2H), 8.56 (d, J = 8.2 Hz, 2H), 8.47 (d, J = 6.0 Hz, 2H), 7.43 (d, J = 8.1 Hz, 2H), 2.50 (s, 3H) ppm; 13C NMR (100 MHz, CDCl3) δ 164.6, 162.6, 151.0, 144.2, 139.2, 130.2, 128.4, 128.3, 121.0, 21.7 ppm; IR(KBr) νmax/cm−1 2914, 1604, 1595, 1393, 1212, 1179, 1054, 921, 828, 598; HRMS (ESI) m/z: [M + Na]+ calcd for C14H11N5 + Na+ 250.1093; found: 250.1093.
3-(4-Methoxyphenyl)-6-(pyridin-2-yl)-1,2,4,5-tetrazine (4d)
Silica gel chromatography (petroleum ether–ethyl acetate = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, Rf = 0.18); white solid (106.0 mg, 80%); mp: >270 °C; 1H NMR (400 MHz, CDCl3) δ 8.70 (d, J = 4.4 Hz, 1H), 8.41 (d, J = 8.0 Hz, 1H), 7.99 (d, J = 8.5 Hz, 2H), 7.88 (t, J = 7.7 Hz, 1H), 7.40 (dd, J = 12.4, 6.4 Hz, 1H), 7.02 (d, J = 8.4 Hz, 2H), 3.89 (s, 3H) ppm; 13C NMR (100 MHz, CDCl3) δ 172.2, 150.3, 150.1, 149.7, 137.4, 129.9, 125.7, 121.3, 121.2, 114.9, 100.3, 55.8 ppm; IR(KBr) νmax/cm−1 1606, 1517, 1437, 1408, 1307, 1249, 1173, 1115, 1029, 985, 828, 601; HRMS (ESI) m/z: [M + Na]+ calcd for C14H11N5O + Na+ 288.0861; found: 288.0827.
1, Rf = 0.18); white solid (106.0 mg, 80%); mp: >270 °C; 1H NMR (400 MHz, CDCl3) δ 8.70 (d, J = 4.4 Hz, 1H), 8.41 (d, J = 8.0 Hz, 1H), 7.99 (d, J = 8.5 Hz, 2H), 7.88 (t, J = 7.7 Hz, 1H), 7.40 (dd, J = 12.4, 6.4 Hz, 1H), 7.02 (d, J = 8.4 Hz, 2H), 3.89 (s, 3H) ppm; 13C NMR (100 MHz, CDCl3) δ 172.2, 150.3, 150.1, 149.7, 137.4, 129.9, 125.7, 121.3, 121.2, 114.9, 100.3, 55.8 ppm; IR(KBr) νmax/cm−1 1606, 1517, 1437, 1408, 1307, 1249, 1173, 1115, 1029, 985, 828, 601; HRMS (ESI) m/z: [M + Na]+ calcd for C14H11N5O + Na+ 288.0861; found: 288.0827.
3-(4-Methoxyphenyl)-6-(pyridin-3-yl)-1,2,4,5-tetrazine (4e)
Silica gel chromatography (petroleum ether–ethyl acetate = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, Rf = 0.23); white solid (107.4 mg, 81%); mp: 158–159 °C; 1H NMR (400 MHz, CDCl3) δ 9.17 (s, 1H), 8.73 (d, J = 4.2 Hz, 1H), 8.37 (d, J = 7.9 Hz, 1H), 7.97 (d, J = 8.7 Hz, 2H), 7.46 (dd, J = 7.8, 4.9 Hz, 1H), 7.02 (d, J = 8.7 Hz, 2H), 3.89 (s, 3H) ppm; 13C NMR (100 MHz, CDCl3) δ 168.4, 163.7, 162.0, 151.4, 148.6, 134.5, 129.5, 126.5, 123.8, 122.2, 114.5, 55.3 ppm; IR (KBr) νmax/cm−1 1607, 1571, 1403, 1250, 1178, 1026, 835, 705.
1, Rf = 0.23); white solid (107.4 mg, 81%); mp: 158–159 °C; 1H NMR (400 MHz, CDCl3) δ 9.17 (s, 1H), 8.73 (d, J = 4.2 Hz, 1H), 8.37 (d, J = 7.9 Hz, 1H), 7.97 (d, J = 8.7 Hz, 2H), 7.46 (dd, J = 7.8, 4.9 Hz, 1H), 7.02 (d, J = 8.7 Hz, 2H), 3.89 (s, 3H) ppm; 13C NMR (100 MHz, CDCl3) δ 168.4, 163.7, 162.0, 151.4, 148.6, 134.5, 129.5, 126.5, 123.8, 122.2, 114.5, 55.3 ppm; IR (KBr) νmax/cm−1 1607, 1571, 1403, 1250, 1178, 1026, 835, 705.
3-(4-Methoxyphenyl)-6-(pyridin-4-yl)-1,2,4,5-tetrazine (4f)
Silica gel chromatography (petroleum ether–ethyl acetate = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, Rf = 0.22); white solid (104.7 mg, 79%); mp: 164–165 °C; 1H NMR (400 MHz, CDCl3) δ 8.77 (d, J = 5.0 Hz, 2H), 7.97 (d, J = 8.6 Hz, 2H), 7.86 (d, J = 5.0 Hz, 2H), 7.02 (d, J = 8.6 Hz, 2H), 3.89 (s, 3H) ppm; 13C NMR (100 MHz, CDCl3) δ 169.1, 164.6, 162.1, 150.6, 137.1, 129.5, 122.1, 121.3, 114.5, 55.4 ppm; IR(KBr) νmax/cm−1 1598, 1400, 1313, 1179, 824, 698; HRMS (ESI) m/z: [M + H]+ calcd for C14H11N5O + H+ 266.1042; found: 266.1049.
1, Rf = 0.22); white solid (104.7 mg, 79%); mp: 164–165 °C; 1H NMR (400 MHz, CDCl3) δ 8.77 (d, J = 5.0 Hz, 2H), 7.97 (d, J = 8.6 Hz, 2H), 7.86 (d, J = 5.0 Hz, 2H), 7.02 (d, J = 8.6 Hz, 2H), 3.89 (s, 3H) ppm; 13C NMR (100 MHz, CDCl3) δ 169.1, 164.6, 162.1, 150.6, 137.1, 129.5, 122.1, 121.3, 114.5, 55.4 ppm; IR(KBr) νmax/cm−1 1598, 1400, 1313, 1179, 824, 698; HRMS (ESI) m/z: [M + H]+ calcd for C14H11N5O + H+ 266.1042; found: 266.1049.
3-(Pyridin-2-yl)-6-(4-(trifluoromethyl)phenyl)-1,2,4,5-tetrazine (4g)
Silica gel chromatography (petroleum ether–ethyl acetate = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, Rf = 0.25); purple solid (115.2 mg, 76%); 1H NMR (400 MHz, CDCl3) δ 9.00 (d, J = 4.2 Hz, 1H), 8.84 (d, J = 8.0 Hz, 2H), 8.73 (d, J = 7.8 Hz, 1H), 8.03 (t, J = 7.7 Hz, 1H), 7.90 (d, J = 8.0 Hz, 2H), 7.60 (t, 1H) ppm; 13C NMR (100 MHz, CDCl3) δ 163.6, 163.4, 150.9, 149.8, 137.4, 134.7, 134.5, 128.5, 126.5, 126.2, 126.1, 124.1 ppm; IR(KBr) νmax/cm−1 1581, 1398, 1327, 1156, 1113, 1067, 910, 825; HRMS (ESI) m/z: [M + H]+ calcd for C14H8F3N5 + H+ 304.0810; found: 304.0809.
1, Rf = 0.25); purple solid (115.2 mg, 76%); 1H NMR (400 MHz, CDCl3) δ 9.00 (d, J = 4.2 Hz, 1H), 8.84 (d, J = 8.0 Hz, 2H), 8.73 (d, J = 7.8 Hz, 1H), 8.03 (t, J = 7.7 Hz, 1H), 7.90 (d, J = 8.0 Hz, 2H), 7.60 (t, 1H) ppm; 13C NMR (100 MHz, CDCl3) δ 163.6, 163.4, 150.9, 149.8, 137.4, 134.7, 134.5, 128.5, 126.5, 126.2, 126.1, 124.1 ppm; IR(KBr) νmax/cm−1 1581, 1398, 1327, 1156, 1113, 1067, 910, 825; HRMS (ESI) m/z: [M + H]+ calcd for C14H8F3N5 + H+ 304.0810; found: 304.0809.
3-(Pyridin-3-yl)-6-(4-(trifluoromethyl)phenyl)-1,2,4,5-tetrazine (4h)
Silica gel chromatography (petroleum ether–ethyl acetate = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, Rf = 0.29); purple solid (113.7 mg, 75%); 1H NMR (400 MHz, CDCl3) δ 9.89 (s, J = 1.6 Hz, 1H), 8.93 (dq, J = 4.8, 1.6 Hz, 2H), 8.81 (d, J = 8.2 Hz, 2H), 7.91 (d, J = 8.3 Hz, 2H), 7.60 (dd, J = 7.9, 4.9 Hz, 1H) ppm; 13C NMR (100 MHz, CDCl3) δ 187.6, 163.5, 163.2, 153.5, 149.4, 135.2, 134.7, 128.4, 127.5, 126.3, 126.3, 124.0 ppm; IR(KBr) νmax/cm−1 1587, 1517, 1394, 1321, 1193, 1162, 1114, 1067, 909, 863, 830; HRMS (ESI) m/z: [M + H]+ calcd for C14H8F3N5 + H+ 304.0810; found: 304.0817.
1, Rf = 0.29); purple solid (113.7 mg, 75%); 1H NMR (400 MHz, CDCl3) δ 9.89 (s, J = 1.6 Hz, 1H), 8.93 (dq, J = 4.8, 1.6 Hz, 2H), 8.81 (d, J = 8.2 Hz, 2H), 7.91 (d, J = 8.3 Hz, 2H), 7.60 (dd, J = 7.9, 4.9 Hz, 1H) ppm; 13C NMR (100 MHz, CDCl3) δ 187.6, 163.5, 163.2, 153.5, 149.4, 135.2, 134.7, 128.4, 127.5, 126.3, 126.3, 124.0 ppm; IR(KBr) νmax/cm−1 1587, 1517, 1394, 1321, 1193, 1162, 1114, 1067, 909, 863, 830; HRMS (ESI) m/z: [M + H]+ calcd for C14H8F3N5 + H+ 304.0810; found: 304.0817.
3-(Pyridin-4-yl)-6-(4-(trifluoromethyl)phenyl)-1,2,4,5-tetrazine (4i)
Silica gel chromatography (petroleum ether–ethyl acetate = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, Rf = 0.28); purple solid (118.2 mg, 78%); 1H NMR (400 MHz, CDCl3) δ 8.96 (d, J = 4.5 Hz, 2H), 8.83 (d, J = 8.1 Hz, 2H), 8.51 (d, J = 4.5 Hz, 2H), 7.92 (d, J = 8.2 Hz, 2H) ppm; 13C NMR (100 MHz, CDCl3) δ 163.8, 163.2, 151.2, 138.8, 134.8, 134.5, 128.6, 126.4, 126.4, 121.3 ppm; IR(KBr) νmax/cm−1 1633, 1400, 1325, 1170, 1132, 1067, 1108, 918, 841; HRMS (ESI) m/z: [M + H]+ calcd for C14H8F3N5 + H+ 304.0810; found: 304.0805.
1, Rf = 0.28); purple solid (118.2 mg, 78%); 1H NMR (400 MHz, CDCl3) δ 8.96 (d, J = 4.5 Hz, 2H), 8.83 (d, J = 8.1 Hz, 2H), 8.51 (d, J = 4.5 Hz, 2H), 7.92 (d, J = 8.2 Hz, 2H) ppm; 13C NMR (100 MHz, CDCl3) δ 163.8, 163.2, 151.2, 138.8, 134.8, 134.5, 128.6, 126.4, 126.4, 121.3 ppm; IR(KBr) νmax/cm−1 1633, 1400, 1325, 1170, 1132, 1067, 1108, 918, 841; HRMS (ESI) m/z: [M + H]+ calcd for C14H8F3N5 + H+ 304.0810; found: 304.0805.
3-(4-Chlorophenyl)-6-(pyridin-2-yl)-1,2,4,5-tetrazine (4j)
Silica gel chromatography (petroleum ether–ethyl acetate = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, Rf = 0.27); purple solid (99.5 mg, 74%); mp: 218–219 °C; 1H NMR (400 MHz, CDCl3) δ 8.98 (d, J = 4.6 Hz, 1H), 8.70 (d, J = 7.9 Hz, 1H), 8.66 (d, J = 8.5 Hz, 2H), 8.01 (t, J = 7.7 Hz, 1H), 7.66–7.55 (m, 3H) ppm; 13C NMR (100 MHz, CDCl3) δ 164.0, 163.7, 151.2, 150.4, 139.9, 137.7, 130.2, 130.0, 129.9, 126.7, 124.2 ppm; IR(KBr) νmax/cm−1 2361, 1633, 1588, 1394, 1118, 1085, 913, 813; HRMS (ESI) m/z: [M + H]+ calcd for C13H8ClN5 + H+ 270.0546; found: 270.0544.
1, Rf = 0.27); purple solid (99.5 mg, 74%); mp: 218–219 °C; 1H NMR (400 MHz, CDCl3) δ 8.98 (d, J = 4.6 Hz, 1H), 8.70 (d, J = 7.9 Hz, 1H), 8.66 (d, J = 8.5 Hz, 2H), 8.01 (t, J = 7.7 Hz, 1H), 7.66–7.55 (m, 3H) ppm; 13C NMR (100 MHz, CDCl3) δ 164.0, 163.7, 151.2, 150.4, 139.9, 137.7, 130.2, 130.0, 129.9, 126.7, 124.2 ppm; IR(KBr) νmax/cm−1 2361, 1633, 1588, 1394, 1118, 1085, 913, 813; HRMS (ESI) m/z: [M + H]+ calcd for C13H8ClN5 + H+ 270.0546; found: 270.0544.
3-(4-Chlorophenyl)-6-(pyridin-3-yl)-1,2,4,5-tetrazine (4k)
Silica gel chromatography (petroleum ether–ethyl acetate = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, Rf = 0.28); purple solid (100.9 mg, 75%); mp: 217–219 °C; 1H NMR (400 MHz, CDCl3) δ 9.86 (s, 1H), 8.90 (t, J = 6.8 Hz, 2H), 8.63 (d, J = 8.5 Hz, 2H), 7.57–7.63 (m, 3H) ppm; 13C NMR (100 MHz, CDCl3) δ 163.9, 163.1, 153.5, 149.5, 139.9, 135.3, 130.1, 130.0, 129.6, 127.9, 124.2 ppm; IR(KBr) νmax/cm−1 1638, 1589, 1407, 1172, 1098, 912, 820; HRMS (ESI) m/z: [M + H]+ calcd for C13H8ClN5 + H+ 270.0546; found: 270.0547.
1, Rf = 0.28); purple solid (100.9 mg, 75%); mp: 217–219 °C; 1H NMR (400 MHz, CDCl3) δ 9.86 (s, 1H), 8.90 (t, J = 6.8 Hz, 2H), 8.63 (d, J = 8.5 Hz, 2H), 7.57–7.63 (m, 3H) ppm; 13C NMR (100 MHz, CDCl3) δ 163.9, 163.1, 153.5, 149.5, 139.9, 135.3, 130.1, 130.0, 129.6, 127.9, 124.2 ppm; IR(KBr) νmax/cm−1 1638, 1589, 1407, 1172, 1098, 912, 820; HRMS (ESI) m/z: [M + H]+ calcd for C13H8ClN5 + H+ 270.0546; found: 270.0547.
3-(4-Chlorophenyl)-6-(pyridin-4-yl)-1,2,4,5-tetrazine (4l)
Silica gel chromatography (petroleum ether–ethyl acetate = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, Rf = 0.28); purple solid (98.2 mg, 73%); mp: 242–243 °C; 1H NMR (400 MHz, CDCl3) δ 8.94 (d, J = 4.5 Hz, 2H), 8.64 (d, J = 8.0 Hz, 2H), 8.49 (d, J = 4.5 Hz, 2H), 7.63 (d, J = 8.1 Hz, 2H) ppm; 13C NMR (100 MHz, CDCl3) δ 164.49, 163.36, 151.60, 140.41, 139.41, 130.28, 130.00, 121.58, 100.38 ppm; IR(KBr) νmax/cm−1 1635, 1592, 1400, 1170, 1091, 1007, 915, 830; HRMS (ESI) m/z: [M + H]+ calcd for C13H8ClN5 + H+ 270.0546; found: 270.0544.
1, Rf = 0.28); purple solid (98.2 mg, 73%); mp: 242–243 °C; 1H NMR (400 MHz, CDCl3) δ 8.94 (d, J = 4.5 Hz, 2H), 8.64 (d, J = 8.0 Hz, 2H), 8.49 (d, J = 4.5 Hz, 2H), 7.63 (d, J = 8.1 Hz, 2H) ppm; 13C NMR (100 MHz, CDCl3) δ 164.49, 163.36, 151.60, 140.41, 139.41, 130.28, 130.00, 121.58, 100.38 ppm; IR(KBr) νmax/cm−1 1635, 1592, 1400, 1170, 1091, 1007, 915, 830; HRMS (ESI) m/z: [M + H]+ calcd for C13H8ClN5 + H+ 270.0546; found: 270.0544.
3-(4-Bromophenyl)-6-(pyridin-2-yl)-1,2,4,5-tetrazine (4m)
Silica gel chromatography (petroleum ether–ethyl acetate = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, Rf = 0.28); purple solid (117.4 mg, 75%); mp: 191–193 °C; 1H NMR (400 MHz, CDCl3) δ 8.98 (d, J = 4.3 Hz, 1H), 8.70 (d, J = 7.9 Hz, 1H), 8.57 (d, J = 8.3 Hz, 2H), 8.01 (t, J = 7.7 Hz, 1H), 7.77 (d, J = 8.3 Hz, 2H), 7.58 (dd, J = 7.4, 4.8 Hz, 1H) ppm; 13C NMR (100 MHz, CDCl3) δ 164.1, 163.7, 151.2, 150.3, 137.7, 132.9, 130.6, 129.9, 128.5, 126.7, 124.2 ppm; IR(KBr) νmax/cm−1 1584, 1393, 1116, 1066, 913, 862, 588; HRMS (ESI) m/z: [M + H]+ calcd for C13H8BrN5 + H+ 314.0041; found: 314.0029.
1, Rf = 0.28); purple solid (117.4 mg, 75%); mp: 191–193 °C; 1H NMR (400 MHz, CDCl3) δ 8.98 (d, J = 4.3 Hz, 1H), 8.70 (d, J = 7.9 Hz, 1H), 8.57 (d, J = 8.3 Hz, 2H), 8.01 (t, J = 7.7 Hz, 1H), 7.77 (d, J = 8.3 Hz, 2H), 7.58 (dd, J = 7.4, 4.8 Hz, 1H) ppm; 13C NMR (100 MHz, CDCl3) δ 164.1, 163.7, 151.2, 150.3, 137.7, 132.9, 130.6, 129.9, 128.5, 126.7, 124.2 ppm; IR(KBr) νmax/cm−1 1584, 1393, 1116, 1066, 913, 862, 588; HRMS (ESI) m/z: [M + H]+ calcd for C13H8BrN5 + H+ 314.0041; found: 314.0029.
3-(4-Bromophenyl)-6-(pyridin-3-yl)-1,2,4,5-tetrazine (4n)
Silica gel chromatography (petroleum ether–ethyl acetate = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, Rf = 0.27); purple solid (112.7 mg, 72%); mp: 229–231 °C; 1H NMR (400 MHz, CDCl3) δ 9.87 (s, 1H), 8.89–8.92 (m, 2H), 8.55 (d, J = 8.6 Hz, 2H), 7.79 (d, J = 8.6 Hz, 2H), 7.58 (dd, J = 7.9, 4.9 Hz, 1H) ppm; 13C NMR (100 MHz, CDCl3) δ 164.0, 163.1, 153.4, 149.4, 135.1, 132.8, 130.4, 129.6, 128.4, 127.8, 124.1 ppm; IR(KBr) νmax/cm−1 1635, 1588, 1400, 1109, 914, 820, 590; HRMS (ESI) m/z: [M + H]+ calcd for C13H8BrN5 + H+ 314.0041; found: 314.0036.
1, Rf = 0.27); purple solid (112.7 mg, 72%); mp: 229–231 °C; 1H NMR (400 MHz, CDCl3) δ 9.87 (s, 1H), 8.89–8.92 (m, 2H), 8.55 (d, J = 8.6 Hz, 2H), 7.79 (d, J = 8.6 Hz, 2H), 7.58 (dd, J = 7.9, 4.9 Hz, 1H) ppm; 13C NMR (100 MHz, CDCl3) δ 164.0, 163.1, 153.4, 149.4, 135.1, 132.8, 130.4, 129.6, 128.4, 127.8, 124.1 ppm; IR(KBr) νmax/cm−1 1635, 1588, 1400, 1109, 914, 820, 590; HRMS (ESI) m/z: [M + H]+ calcd for C13H8BrN5 + H+ 314.0041; found: 314.0036.
3-(4-Bromophenyl)-6-(pyridin-4-yl)-1,2,4,5-tetrazine (4o)
Silica gel chromatography (petroleum ether–ethyl acetate = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, Rf = 0.28); purple solid (115.8 mg, 74%); mp: 244–247 °C; 1H NMR (400 MHz, CDCl3) δ 8.95 (d, J = 5.8 Hz, 2H), 8.57 (d, J = 8.6 Hz, 2H), 8.50 (d, J = 5.9 Hz, 2H), 7.80 (d, J = 8.6 Hz, 2H) ppm; 13C NMR (100 MHz, CDCl3) δ 164.3, 163.0, 151.2, 139.0, 132.9, 130.2, 129.7, 128.7, 121.2 ppm; IR(KBr) νmax/cm−1 1638, 1584, 1396, 1173, 1106, 1005, 920, 831, 593; HRMS (ESI) m/z: [M + H]+ calcd for C13H8BrN5 + H+ 314.004; found: 314.0048.
1, Rf = 0.28); purple solid (115.8 mg, 74%); mp: 244–247 °C; 1H NMR (400 MHz, CDCl3) δ 8.95 (d, J = 5.8 Hz, 2H), 8.57 (d, J = 8.6 Hz, 2H), 8.50 (d, J = 5.9 Hz, 2H), 7.80 (d, J = 8.6 Hz, 2H) ppm; 13C NMR (100 MHz, CDCl3) δ 164.3, 163.0, 151.2, 139.0, 132.9, 130.2, 129.7, 128.7, 121.2 ppm; IR(KBr) νmax/cm−1 1638, 1584, 1396, 1173, 1106, 1005, 920, 831, 593; HRMS (ESI) m/z: [M + H]+ calcd for C13H8BrN5 + H+ 314.004; found: 314.0048.
4-(6-(p-Tolyl)-1,2,4,5-tetrazin-3-yl)aniline (4p)
Silica gel chromatography (petroleum ether–ethyl acetate = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, Rf = 0.32); yellow solid (110.5 mg, 85%); mp: 180–181 °C; 1H NMR (400 MHz, CDCl3) δ 7.88 (d, J = 7.8 Hz, 2H), 7.81 (d, J = 8.1 Hz, 2H), 7.28 (d, J = 12.7 Hz, 2H), 6.74 (d, J = 8.1 Hz, 2H), 4.01 (s, 2H), 2.42 (s, 3H) ppm; 13C NMR (100 MHz, CDCl3) δ 168.4, 166.9, 149.4, 141.4, 130.0, 129.6, 127.9, 120.5, 115.1, 21.7 ppm; IR(KBr) νmax/cm−1 3130, 1600, 1516, 1401, 1261, 1098, 1019, 825.
1, Rf = 0.32); yellow solid (110.5 mg, 85%); mp: 180–181 °C; 1H NMR (400 MHz, CDCl3) δ 7.88 (d, J = 7.8 Hz, 2H), 7.81 (d, J = 8.1 Hz, 2H), 7.28 (d, J = 12.7 Hz, 2H), 6.74 (d, J = 8.1 Hz, 2H), 4.01 (s, 2H), 2.42 (s, 3H) ppm; 13C NMR (100 MHz, CDCl3) δ 168.4, 166.9, 149.4, 141.4, 130.0, 129.6, 127.9, 120.5, 115.1, 21.7 ppm; IR(KBr) νmax/cm−1 3130, 1600, 1516, 1401, 1261, 1098, 1019, 825.
4-(6-(4-Chlorophenyl)-1,2,4,5-tetrazin-3-yl)aniline (4q)
Silica gel chromatography (petroleum ether–ethyl acetate = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, Rf = 0.31); yellow solid (111.8 mg, 79%); mp: 192–194 °C; 1H NMR (400 MHz, CDCl3) δ 7.93 (d, J = 8.5 Hz, 2H), 7.81 (d, J = 8.5 Hz, 2H), 7.46 (d, J = 8.5 Hz, 2H), 6.74 (d, J = 8.5 Hz, 2H), 4.04 (s, 2H) ppm; 13C NMR (100 MHz, CDCl3) δ 168.7, 165.2, 149.3, 136.7, 129.5, 129.3, 128.9, 128.8, 120.0, 114.8 ppm; IR(KBr) νmax/cm−1 3133, 1629, 1400, 1181, 1089, 821.
1, Rf = 0.31); yellow solid (111.8 mg, 79%); mp: 192–194 °C; 1H NMR (400 MHz, CDCl3) δ 7.93 (d, J = 8.5 Hz, 2H), 7.81 (d, J = 8.5 Hz, 2H), 7.46 (d, J = 8.5 Hz, 2H), 6.74 (d, J = 8.5 Hz, 2H), 4.04 (s, 2H) ppm; 13C NMR (100 MHz, CDCl3) δ 168.7, 165.2, 149.3, 136.7, 129.5, 129.3, 128.9, 128.8, 120.0, 114.8 ppm; IR(KBr) νmax/cm−1 3133, 1629, 1400, 1181, 1089, 821.
4-(6-(4-Bromophenyl)-1,2,4,5-tetrazin-3-yl)aniline (4r)
Silica gel chromatography (petroleum ether–ethyl acetate = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, Rf = 0.34); yellow solid (132.4 mg, 81%); mp: 236–238 °C; 1H NMR (400 MHz, CDCl3) δ 7.86 (d, J = 8.2 Hz, 2H), 7.81 (d, J = 8.3 Hz, 2H), 7.62 (d, J = 8.0 Hz, 2H), 6.74 (d, J = 8.1 Hz, 2H), 4.04 (s, 2H) ppm; 13C NMR (100 MHz, DMSO) δ 174.0, 169.0, 157.3, 137.5, 134.3, 134.3, 134.2, 129.3, 121.4, 118.8 ppm; IR(KBr) νmax/cm−1 3131, 1627, 1521, 1401, 1178, 1129, 986, 823.
1, Rf = 0.34); yellow solid (132.4 mg, 81%); mp: 236–238 °C; 1H NMR (400 MHz, CDCl3) δ 7.86 (d, J = 8.2 Hz, 2H), 7.81 (d, J = 8.3 Hz, 2H), 7.62 (d, J = 8.0 Hz, 2H), 6.74 (d, J = 8.1 Hz, 2H), 4.04 (s, 2H) ppm; 13C NMR (100 MHz, DMSO) δ 174.0, 169.0, 157.3, 137.5, 134.3, 134.3, 134.2, 129.3, 121.4, 118.8 ppm; IR(KBr) νmax/cm−1 3131, 1627, 1521, 1401, 1178, 1129, 986, 823.
3,6-Di(pyridin-3-yl)-1,2,4,5-tetrazine (4s)30
Silica gel chromatography (methanol–dichloromethane = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, Rf = 0.40); purple solid (96.8 mg, 82%); mp: 203–204 °C; 1H NMR (400 MHz, CDCl3) δ 9.86 (s, 2H), 8.90 (t, J = 6.6 Hz, 4H), 7.57 (dd, J = 7.8, 5.0 Hz, 2H) ppm; 13C NMR (100 MHz, CDCl3) δ 163.6, 153.7, 149.6, 135.4, 127.8, 124.3 ppm; IR(KBr) νmax/cm−1 1584, 1387, 1126, 1016, 917, 821, 704, 598.
20, Rf = 0.40); purple solid (96.8 mg, 82%); mp: 203–204 °C; 1H NMR (400 MHz, CDCl3) δ 9.86 (s, 2H), 8.90 (t, J = 6.6 Hz, 4H), 7.57 (dd, J = 7.8, 5.0 Hz, 2H) ppm; 13C NMR (100 MHz, CDCl3) δ 163.6, 153.7, 149.6, 135.4, 127.8, 124.3 ppm; IR(KBr) νmax/cm−1 1584, 1387, 1126, 1016, 917, 821, 704, 598.
3,6-Di(pyridin-4-yl)-1,2,4,5-tetrazine (4t)31
Silica gel chromatography (methanol–dichloromethane = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, Rf = 0.40); purple solid (98.0 mg, 83%); mp: >200 °C; 1H NMR (400 MHz, CDCl3) δ 8.97 (d, J = 4.9 Hz, 4H), 8.53 (d, J = 4.9 Hz, 4H) ppm; 13C NMR (100 MHz, CDCl3) δ 163.7, 151.3, 138.6, 121.4 ppm; IR(KBr) νmax/cm−1 2361, 1588, 1557, 1411, 1389, 1261, 1110, 1053, 921, 831, 715, 600.
20, Rf = 0.40); purple solid (98.0 mg, 83%); mp: >200 °C; 1H NMR (400 MHz, CDCl3) δ 8.97 (d, J = 4.9 Hz, 4H), 8.53 (d, J = 4.9 Hz, 4H) ppm; 13C NMR (100 MHz, CDCl3) δ 163.7, 151.3, 138.6, 121.4 ppm; IR(KBr) νmax/cm−1 2361, 1588, 1557, 1411, 1389, 1261, 1110, 1053, 921, 831, 715, 600.
3,6-Bis(4-methoxyphenyl)-1,2,4,5-tetrazine (4u)32
Silica gel chromatography (petroleum ether–ethyl acetate = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, Rf = 0.27); white solid (113.2 mg, 77%); mp: 170–171 °C; 1H NMR (400 MHz, CDCl3) δ 7.93 (d, J = 8.8 Hz, 4H), 7.00 (d, J = 8.8 Hz, 4H), 3.88 (s, 6H) ppm; 13C NMR (100 MHz, CDCl3) δ 167.0, 161.7, 129.3, 122.9, 114.4, 55.4 ppm; IR(KBr) νmax/cm−1 2361, 1608, 1401, 1250, 1173, 987, 828.
1, Rf = 0.27); white solid (113.2 mg, 77%); mp: 170–171 °C; 1H NMR (400 MHz, CDCl3) δ 7.93 (d, J = 8.8 Hz, 4H), 7.00 (d, J = 8.8 Hz, 4H), 3.88 (s, 6H) ppm; 13C NMR (100 MHz, CDCl3) δ 167.0, 161.7, 129.3, 122.9, 114.4, 55.4 ppm; IR(KBr) νmax/cm−1 2361, 1608, 1401, 1250, 1173, 987, 828.
4,4′-(1,2,4,5-Tetrazine-3,6-diyl)dianiline (4v)33
Silica gel chromatography (petroleum ether–ethyl acetate = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, Rf = 0.20); yellow solid (93.8 mg, 71%); mp: >280 °C; 1H NMR (400 MHz, DMSO) δ 7.62 (d, J = 7.4 Hz, 4H), 6.66 (d, J = 7.5 Hz, 4H), 5.82 (s, 4H) ppm; 13C NMR (100 MHz, DMSO) δ 171.2, 156.7, 133.9, 122.1, 118.8 ppm; IR(KBr) νmax/cm−1 3126, 1627, 1903, 1400, 1307, 1178, 831, 776.
1, Rf = 0.20); yellow solid (93.8 mg, 71%); mp: >280 °C; 1H NMR (400 MHz, DMSO) δ 7.62 (d, J = 7.4 Hz, 4H), 6.66 (d, J = 7.5 Hz, 4H), 5.82 (s, 4H) ppm; 13C NMR (100 MHz, DMSO) δ 171.2, 156.7, 133.9, 122.1, 118.8 ppm; IR(KBr) νmax/cm−1 3126, 1627, 1903, 1400, 1307, 1178, 831, 776.
General procedure for preparation of solid electrode loaded test samples for electrochemical testing
The synthesized powder sample was mixed with acetylene black and caking agent polyvinylidene fluoride (PVDF) in certain proportion, and a suitable amount of anhydrous CH3CH2OH as demulsifier was added. And the mixture was grinded into a paste in the agate mortar, then it was besmeared on the nickel foam sheet several times (1 cm × 1.5 cm). At last, the nickel foam sheet was dried at 60 °C for 24 h and compressed with a compression stress of 10 MPa.Acknowledgements
The project was supported by the National Natural Science Foundation of China (NSFC 21272184; 20972124; 21143010; 21103137), the Shaanxi Provincial Natural Science Fund Project (no. 2012JQ2007), the Northwest University Science Foundation for Postgraduate Students (no. YZZ13042), the Xi'an City Science and Technology Project [no. CXY1429(6); CXY1434(7)], and the Chinese National Innovation Experiment Program for University Students (no. 201210697011).References
- (a) A. Hantzsch and M. Lehmann, Ber. Dtsch. Chem. Ges., 1900, 33, 3668 CrossRef CAS; (b) J. Sauer, D. K. Heldmann, J. Hetzenegger, J. Krauthan, H. Sichert and J. Schuster, Eur. J. Org. Chem., 1998, 2885 CrossRef CAS.
- S. Jaiswal, P. C. Varma, L. O'Neill, B. Duffy and P. McHale, Mater. Sci. Eng., C, 2013, 33, 1925 CrossRef CAS PubMed.
- (a) P. Audebert, S. Sadki, F. Miomandre, G. Clavier, M. C. Vernières, M. Saoud and P. Hapiot, New J. Chem., 2004, 28, 387 RSC; (b) P. Audebert, F. Miomandre, G. Clavier, M. Vernières, S. Badré and R. Méallet-Renault, Chem.–Eur. J., 2005, 11, 5667 CrossRef CAS PubMed; (c) C. Dumas-Verdes, F. Miomandre, E. Lépicier, O. Galangau, T. Vu, G. Clavier, R. Méallet-Renault and P. Audebert, Eur. J. Org. Chem., 2010, 2525 CrossRef CAS; (d) N. K. Devaraj, S. Hilderbrand, R. Upadhyay, R. Mazitschek and R. Weissleder, Angew. Chem., 2010, 122, 293 CrossRef; (e) Z. Li, J. Ding, N. Song, X. Du, J. Zhou, J. Lu and Y. Tao, Chem. Mater., 2011, 23, 1977 CrossRef CAS; (f) Q. Zhou, P. Audebert, G. Clavier, R. Méallet-Renault, F. Miomandre, Z. Shaukat, T. Vu and J. Tang, J. Phys. Chem. C, 2011, 115, 21899 CrossRef CAS.
- (a) N. K. Devaraj, R. Weissleder and S. A. Hilderbrand, Bioconjugate Chem., 2008, 19, 2297–2299 CrossRef CAS PubMed; (b) M. L. Blackman, M. Royzen and J. M. Fox, J. Am. Chem. Soc., 2008, 130, 13518 CrossRef CAS PubMed; (c) D. S. Liu, A. Tangpeerachaikul, R. Selvaraj, M. T. Taylor, J. M. Fox and A. Y. Ting, J. Am. Chem. Soc., 2012, 134, 792 CrossRef CAS PubMed; (d) J. Lub, W. Ten Hoeve, R. Rossin, S. M. Van Den Boschg and M. S. Robillard, WO patent, 2012049624, 2012.
- Y. Zhao, Y. Li, Z. Qin, R. Jiang, H. Liu and Y. Li, Dalton Trans., 2012, 41, 13338 RSC.
- (a) G. Clavier and P. Audebert, Chem. Rev., 2010, 110, 3299 CrossRef CAS PubMed; (b) J. Malinge, C. Allain, A. Brosseau and P. Audebert, Angew. Chem., 2012, 124, 8662 CrossRef; (c) C. Quinton, V. Alain-Rizzo, C. Dumas-Verdes, G. Clavier, F. Miomandre and P. Audebert, Eur. J. Org. Chem., 2012, 1394 CrossRef CAS.
- (a) N. K. Devaraj, S. Hilderbrand, R. Upadhyay, R. Mazitschek and R. Weissleder, Angew. Chem., 2010, 122, 2931 CrossRef; (b) C. M. Cole, J. Yang, J. Šečkutė and N. K. Devaraj, ChemBioChem, 2013, 14, 205 CrossRef CAS PubMed.
- Z. Li, J. Ding, N. Song, J. Lu and Y. Tao, J. Am. Chem. Soc., 2010, 132, 13160 CrossRef CAS PubMed.
- (a) D. E. Chavez and M. A. Hiskey, J. Energ. Mater., 1999, 17, 357 CrossRef CAS; (b) P. F. Pagoria, G. S. Lee, A. R. Mitchell and R. D. Schmidt, Thermochim. Acta, 2002, 384, 187 CrossRef CAS; (c) D. E. Chavez, M. A. Hiskey and R. D. Gilardi, Org. Lett., 2004, 6, 2889 CrossRef CAS PubMed; (d) T. Wei, W. Zhu, X. Zhang, Y. F. Li and H. Xiao, J. Phys. Chem. A, 2009, 113, 9404 CrossRef CAS PubMed; (e) A. Saikia, R. Sivabalan, B. G. Polke, G. M. Gore, A. Singh, A. Subhananda Rao and A. K. Sikder, J. Hazard. Mater., 2009, 170, 306 CrossRef CAS PubMed.
- (a) D. E. Chavez, M. A. Hiskey and R. D. Gilardi, Angew. Chem., 2000, 112, 1861 CrossRef; (b) D. E. Chavez, S. K. Hanson, J. M. Veauthier and D. A. Parrish, Angew. Chem., 2013, 125, 7014 CrossRef.
- (a) S. M. Sakya, K. K. Groskopf and D. L. Boger, Tetrahedron Lett., 1997, 38, 3805 CrossRef CAS; (b) D. L. Boger, R. P. Schaum and R. M. Garbaccio, J. Org. Chem., 1998, 63, 6329 CrossRef CAS PubMed; (c) A. Hamasaki, R. Ducray and D. L. Boger, J. Org. Chem., 2006, 71, 185 CrossRef CAS PubMed; (d) H. Xie, L. Zu, H. R. Oueis, H. Li, J. Wang and W. Wang, Org. Lett., 2008, 10, 1923 CrossRef CAS PubMed; (e) R. Pipkorn, W. Waldeck, B. Didinger, M. Koch, G. Mueller, M. Wiessler and K. Braun, J. Pept. Sci., 2009, 15, 235 CrossRef CAS PubMed; (f) J. Schoch, M. Wiessler and A. Jäschke, J. Am. Chem. Soc., 2010, 132, 8846 CrossRef CAS PubMed; (g) W. Chen, D. Wang, C. Dai, D. Hamelberg and B. Wang, Chem. Commun., 2012, 48, 1736 RSC.
- (a) J. F. Geldard and F. Lions, J. Org. Chem., 1965, 30, 318 CrossRef CAS; (b) D. L. Boger and S. M. Sakya, J. Org. Chem., 1988, 53, 1415 CrossRef CAS; (c) D. L. Boger and M. Zhang, J. Am. Chem. Soc., 1991, 113, 4230 CrossRef CAS; (d) R. E. Sammelson, M. M. Olmstead, M. J. Haddadin and M. J. Kurth, J. Org. Chem., 2000, 65, 9265 CrossRef CAS; (e) J. C. Gonzales, T. Dedola, L. Santana, E. Uriarte, M. Begala, D. Copez and G. Podda, J. Heterocycl. Chem., 2000, 37, 907 CrossRef; (f) J. Sauer, P. Bäuerlein, W. Ebenbeck, C. Gousetis, H. Sichert, T. Troll, F. Utz and U. Wallfahrer, Eur. J. Org. Chem., 2001, 2629 CrossRef CAS; (g) J. Sauer, G. R. Pabst, U. Holland, H. Kim and S. Loebbecke, Eur. J. Org. Chem., 2001, 697 CrossRef CAS; (h) J. Sauer, P. Bäuerlein, W. Ebenbeck, J. Schuster, I. Sellner, H. Sichert and H. Stimmelmayr, Eur. J. Org. Chem., 2002, 791 CrossRef CAS; (i) L. I. Robins, R. D. Carpenter, J. C. Fettinger, M. J. Haddadin, D. S. Tinti and M. J. Kurth, J. Org. Chem., 2006, 71, 2480 CrossRef CAS PubMed; (j) Y. H. Gong, P. Audebert, J. Tang, F. Miomandre, G. Clavier, S. Badré and J. Marrot, J. Electroanal. Chem., 2006, 592, 147 CrossRef CAS PubMed; (k) M. J. Haddadin and E. H. Ghazvini Zadeh, Tetrahedron Lett., 2010, 51, 1654 CrossRef CAS PubMed.
- (a) N. Saracoglu, Tetrahedron, 2007, 63, 4199 CrossRef CAS PubMed; (b) D. L. Boger, C. W. Boyce, M. A. Labroli, C. A. Sehon and Q. Jin, J. Am. Chem. Soc., 1999, 121, 54 CrossRef CAS; (c) D. L. Boger, D. R. Soenen, C. W. Boyce, M. P. Hedrick and Q. Jin, J. Org. Chem., 2000, 65, 2479 CrossRef CAS; (d) D. L. Boger and S. E. Wolkenberg, J. Org. Chem., 2000, 65, 9120 CrossRef CAS PubMed; (e) D. L. Boger and J. Hong, J. Am. Chem. Soc., 2001, 123, 8515 CrossRef CAS PubMed; (f) Z. Wan, G. H. C. Woo and J. K. Snyder, Tetrahedron, 2001, 57, 5497 CrossRef CAS.
- A. Borkowski, M. Szala and D. Wolicka, Chem. Ecol., 2011, 27, 57 CrossRef CAS.
- W. Hu, G. Rao and Y. Sun, Bioorg. Med. Chem. Lett., 2004, 14, 1177 CrossRef CAS PubMed.
- (a) J. Hajimichael, S. Botar, E. Bleicher, L. Pap, I. Szekely and K. Marmarosi, US pat., 5455237, 1995; (b) J. Hajimichael, D. Botár, E. Bleicher, D. Pap, D. Székely, N. K. K. Mármarosi and J. Ori, EP patent, 0635499, 1999.
- K. H. Pilgram, L. E. Wittsell, R. D. Skiles, A. L. James and J. W. Dawson, J. Agric. Food Chem., 1977, 25, 888 CrossRef CAS.
- S. A. Lang Jr, B. D. Johnson, E. Cohen, A. E. Sloboda and E. Greenblatt, J. Med. Chem., 1976, 19, 1404 CrossRef CAS.
- D. Nhu, S. Duffy, V. M. Avery, A. Hughes and J. B. Baell, Bioorg. Med. Chem. Lett., 2010, 20, 4496 CrossRef CAS PubMed.
- (a) J. Yang, M. R. Karver, W. Li, S. Sahu and N. K. Devaraj, Angew. Chem., Int. Ed., 2012, 51, 5222 CrossRef CAS PubMed; (b) W. W. Zajac Jr, J. F. Siuda, S. M. J. Nolan and T. M. Santosusso, J. Org. Chem., 1971, 36, 3539 CrossRef; (c) N. O. Abdel, M. A. Kira and M. N. Tolba, Tetrahedron Lett., 1968, 9, 3871 CrossRef; (d) C. L. Lim, S. H. Pyo, T. Y. Kim, E. S. Yim and B. H. Han, Bull. Korean Chem. Soc., 1995, 16, 374 CAS; (e) M. D. Coburn, G. A. Buntain, B. W. Harris, M. A. Hiskey, K. Y. Lee and D. G. Ott, J. Heterocycl. Chem., 1991, 28, 2049 CrossRef CAS.
- K. Pilgram and R. D. Skiles, J. Org. Chem., 1976, 41, 3392 CrossRef CAS.
- W. Hu and F. Xu, J. Heterocycl. Chem., 2008, 45, 1745 CrossRef CAS.
- P. Audebert, F. Miomandre, G. Clavier, M. C. Vernières, S. Badré and R. Méallet-Renault, Chem.–Eur. J., 2005, 11, 5667 CrossRef CAS PubMed.
- (a) Z. Bayat, S. Daneshnia and S. J. Mahdizadeh, Phys. E, 2011, 43, 1569 CrossRef CAS PubMed; (b) M. Moral, G. García, A. Peñas, A. Garzón, J. M. Granadino-Roldán, M. Melguizo and M. Fernández-Gómez, Chem. Phys., 2012, 408, 17 CrossRef CAS PubMed; (c) M. Plugge, V. Alain-Rizzo, P. Audebert and A. M. Brouwer, J. Photochem. Photobiol., A, 2012, 234, 12 CrossRef CAS PubMed.
- (a) X. Li, G. Zhang, H. Ma, D. Zhang, J. Li and D. Zhu, J. Am. Chem. Soc., 2004, 126, 11543 CrossRef CAS PubMed; (b) J. R. Lakowicz, in Principles of Fluorescence Spectroscopy, Kluwer Academic/Plenum, New York, 2nd edn, 1999 Search PubMed.
- M. L. Li, W. W. Xu, W. R. Wang, Y. P. Liu, B. Cui and X. H. Guo, J. Power Sources, 2014, 248, 465 CrossRef CAS PubMed.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery Jr, J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, in Gaussian 09, Revision A.02, Gaussian, Inc., Wallingford CT, 2009 Search PubMed.
- (a) D. Qi, L. Zhang, L. Wan, Y. Zhang, Y. Bian and J. Jiang, Phys. Chem. Chem. Phys., 2011, 13, 13277 RSC; (b) D. Qi, L. Zhang, L. Zhao, X. Cai and J. Jiang, ChemPhysChem, 2012, 13, 2046 CrossRef CAS PubMed.
- J. Balcar, G. Chrisam, F. X. Huber and J. Sauer, Tetrahedron Lett., 1983, 24, 1481 CrossRef CAS.
- J. Spanget-Larsen, E. W. Thulstrup and J. Waluk, Chem. Phys., 2000, 254, 135 CrossRef CAS.
- J. Li, Y. Peng, H. W. Liang, Y. Yu, B. J. Xin, G. H. Li, Z. Shi and S. H. Feng, Eur. J. Inorg. Chem., 2011, 2712 CrossRef.
- M. Pluggea, V. Alain-Rizzob, P. Audebertb and A. M. Brouwera, J. Photochem. Photobiol., A, 2012, 234, 12 CrossRef PubMed.
- A. R. Katritzky, J. Soloducho and S. Belyakov, ARKIVOC, 2000, 1, 37 CrossRef CAS.
- R. A. Caboni and R. V. Lindsey, Jr, J. Am. Chem. Soc., 1959, 81, 4342 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 973196. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4ra10808f |
| ‡ The authors Chen Li and Haixia Ge contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2015 |