Delivery of SiC-based nanoparticles into live cells driven by cell-penetrating peptides SAP and SAP-E†
Abstract
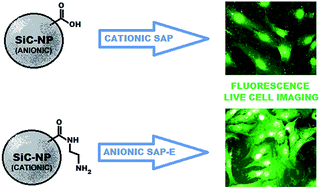
The delivery of SiC-based nanoparticles (SiC-NPs) into living eukaryotic cells is facilitated in the presence of cell-penetrating peptides, both cationic (SAP) and anionic (SAP-E). The SiC-NP surface functional group modification combined with rational CPP selection introduces an additional mode of delivery control.

 Please wait while we load your content...
Please wait while we load your content...