Competitive adsorption of water vapor with VOCs dichloroethane, ethyl acetate and benzene on MIL-101(Cr) in humid atmosphere†
Shikai Xian,
Ying Yu,
Jing Xiao,
Zhijuan Zhang,
Qibin Xia,
Haihui Wang and
Zhong Li*
School of Chemistry and Chemical Engineering & Guangdong Provincial Key Laboratory of Atmospheric Environment and Pollution Control, South China University of Technology, Guangzhou, 510640, China. E-mail: cezhli@scut.edu.cn
First published on 14th November 2014
Abstract
It is well-known that water vapor is omnipresent. It would inevitably have a negative influence on VOC adsorption on novel porous materials in actual situations. In this work, the competitive adsorption behavior of water vapor with three VOCs, 1,2-dichloroethane (DCE), ethyl acetate (EA) and benzene, on MIL-101 in a humid atmosphere was investigated by isotherm measurement, breakthrough experiments and TPD experiments. The results showed that adsorption capacities of MIL-101 for DCE, EA and benzene were individually up to 9.71, 5.79 and 3.76 mmol g−1, much higher than those of other conventional adsorbents. Breakthrough experiments indicated that the presence of water vapor in the feed stream resulted in a sharp decrease in the VOCs working capacities of MIL-101 due to competitive adsorption of water vapor on MIL-101 surfaces. The breakthrough times and the working capacities of these VOCs became smaller with an increase in the relative humidity. TPD experiments indicated that the desorption activation energies of water vapor, DCE, EA and benzene on MIL-101 were 72.9, 47.14, 41.9, and 38.16 kJ mol−1, respectively. The stronger interaction of water vapor with MIL-101 formed strong competitive adsorption with VOCs on MIL-101, resulting in the sharp decrease of the VOCs working capacities in a humid atmosphere.
1. Introduction
In recent decades, the release of anthropogenic toxic pollutants into the atmosphere has become a worldwide threat of growing concern. Air pollution is now recognized as a severe problem and has become increasingly serious both nationally and worldwide.1 Volatile organic compounds (VOCs) are the main pollutants in the ambient air released from chemical, petrochemical, and related industries. VOCs may cause short- and long-term adverse health risks for humans, such as headaches and eye, nose and throat irritation, dizziness, nausea, cancer, and even death even at very low concentrations. More and more countries and regions have proposed stringent legislation to impose stringent standards on VOC emissions from industries. Therefore, it is urgently necessary to develop safer and more efficient systems for the removal of VOCs from polluted air.There are many techniques available to abate the emission of VOCs, such as adsorption,2,3 catalytic oxidation,4 condensation,5 membrane separation6 and biological treatments. Among these, the adsorption method has been considered as one of the most cost-effective and environmentally friendly technologies for the removal of VOCs, especially at low concentration. Adsorbents play a key role in the adsorption technique. Up to now, much work has been conducted to investigate the adsorption of VOCs on some traditional absorbents, such as activated carbon (AC), silica gel, activated alumina and zeolites. Although activated carbon and zeolites were widely applied for adsorption of VOCs, their disadvantages of low capacity and difficulty in regeneration have limited their wider application.7
In recent years, a new class of porous materials assembled with metal ions and organic linkers, known as metal–organic frameworks (MOFs), has been rapidly developed as potential materials for VOC adsorption due to their ultrahigh surface area, sturdiness, open crystalline structure and adjustable chemical functionality.8,9 The presence of open metal sites (coordinatively unsaturated metal centers) or certain functionalizations on the pore surfaces of MOFs could enhance the adsorption selectivity/efficiency of MOFs towards certain toxic compounds via coordination bonds, acid–base/electrostatic interactions, pi-complex/H-bonding formation, etc.8 It is possible to take advantage of MOF materials in order to develop new technologies for environmental remediation purposes. Barea et al.8 reviewed performances of MOFs in environmental remediation processes, and their studies showed that some MOFs and modified MOFs exhibited remarkable adsorption capacities and good selectivities for organic molecules. Among these MOFs, MIL-101 (Matérial Institut Lavoisier, chromium-terephthalate-based solid) is considered as one of the most prominent representative MOFs, which is basically built up from a hybrid super-tetrahedral (ST) building unit, which is formed from rigid terephthalate ligands and trimeric chromium(III) octahedral clusters. It possesses a very large specific surface area with ordered micro/mesoporous zeotype architecture and high chemical and thermal stability.10 Therefore, MIL-101 is a promising candidate as an adsorbent for VOC capture applications. Zhao et al.11,12 measured the adsorption isotherms and kinetics of benzene and p-xylene on MIL-101, and reported that the maximum capacities of MIL-101 were 16.5 and 10.9 mmol g−1 at 288 K, respectively, much higher than activated carbons and zeolites. In addition, multiple cycle experiments of these VOCs adsorption–desorption on MIL-101 were carried out, and their results showed that the efficiency of VOC desorption can reach over 97%. Shi et al.13 reported that the adsorption capacity of MIL-101 for ethyl acetate is up to 10.5 mmol g−1 at 288 K and 54 mbar. Diffusion coefficients of ethyl acetate within MIL-101 are in the range of (1.617 to 2.264) × 10−10 cm 2 s−1 with a lower activation energy of 8.361 kJ mol−1. In addition, multiple cycle experiments of these VOCs adsorption–desorption on MIL-101 were carried out, and the results showed that desorption efficiency of VOCs can reach over 97%.11,13 Trung et al.14 measured the isotherms of C6–C9 on MIL-101, and then reported that its maximum capacities for C6, C7, C8 and C9 reached about 9.95, 8.82, 8.75 and 6.17 mmol g−1 respectively. Yang et al.15 reported that MIL-101 had higher adsorption capacities for selected VOCs than zeolite, activated carbon and other reported adsorbents, and thus it was suitable for the adsorptive removal of VOCs including polar acetone and nonpolar benzene, toluene, ethylbeznene, and xylenes. Huang et al.16 measured the isotherms of n-hexane, toluene, methanol, butanone, dichloromethane, and n-butylamine on MIL-101, and reported that MIL-101 had much higher affinity and adsorption capacity for VOCs than activated carbon, and its affinity to n-butylamine was the strongest among these VOCs. These studies above showed that the capacities of MIL-101 for VOCs were several times that of the ACs, showing a great application prospect in the fields of environmental remediation and protection. However, if MIL-101 is used as a novel adsorbent applied in actual cases, it will face the challenge of competitive adsorption of water vapor, which has not been studied yet.
It is well-known that water vapor is omnipresent. It often presents in various of polluted gases in the cases of actual situations, and would commonly have negative influence on VOC adsorption on adsorbents due to its strong competitive adsorption. The study of VOC adsorption on MIL-101 in the presence of water vapor has hardly been done so far. Competitive adsorption behaviour and the mechanism of water vapor and VOC adsorption on MIL-101(Cr) in a humid atmosphere has not been revealed yet. Therefore, it is necessary to investigate the effect of water vapor on VOC adsorption on MIL-101 and its competitive adsorption mechanism so that some new knowledge can be obtained and adsorption process of MIL-101 for practical application of VOCs removal can be optimized or designed rationally.
The purpose of this work is to investigate the competitive adsorption behavior of water vapor and three VOCs, EA, DCE and benzene, on MIL-101 with the help of fixed bed experiments. Adsorption isotherms of EA, DCE, benzene and water vapor were separately measured by using a gravimetric method. The breakthrough curves of EA, DCE and benzene were measured under the conditions of the absence and the presence of water vapor, and then compared. TPD experiments were conducted to estimate the interaction of water vapor and VOCs, EA, DCE and benzene, with the surfaces of MIL-101. The competitive adsorption mechanism of water vapor with EA, DCE and benzene on MIL-101 is discussed and reported here.
2. Experimental section
2.1. Materials
Chromium(III) nitrate nonahydrate (Cr(NO3)3·9H2O, >99.0%, Alfa), 1,4-benzenedicarboxylic acid (C8H6O4, >99.0%, Aldrich), hydrofluoric acid (HF, 48.0%, Merck), N,N-dimethylformamide (DMF, 99.5%, Mallinckrodt), ethanol (99.7%, Tianjin), NH4F (≥96.0%, Tianjin), and 1,2-dichloroethane (≥99.0%, Tianjin) were used as received from vendors without further purification.2.2. Synthesis of MIL-101 and characterization
The MIL-101 used in this work was synthesized by a hydrothermal method and the specifics are described in the ESI (S1†).The surface morphology and particle size of MIL-101 samples were observed by using a LEO 1530Vp scanning electron microscope (SEM) at an accelerating voltage of 5.0 kV after gold deposition. The synthesized MIL-101 was characterized by X-ray powder diffraction (XRD), which was performed on a Bruker D8 Advance X-ray diffractometer at 40 kV, 40 mA, with a scan speed of 2° min−1 and a step size of 0.02° in 2–25, using λCuKα radiation. Specific surface area and pore texture of the sample were measured using Micromeritics ASAP 2020.
2.3. Measurement of adsorption isotherms of DCE, EA and benzene vapor
Adsorption isotherms of DCE, EA and benzene vapor on a MIL-101 sample were measured by using a standard gravimetric technique (intelligent gravimetric analyzer, IGA-003, Hiden) at 298–318 K. This intelligent gravimetric analyzer (IGA-003, Hiden) is equipped with an ultra-sensitive balance of resolution 0.2 μg. Details of the measurement procedures can be available in the ESI (S2†). The equilibrium and instantaneous uptakes of DCE, EA and benzene on the sample can be calculated as follows:where MDCE (g mol−1) is the molecular weight of the VOC molecule; We (g) and Wt (g) are the amount of adsorbent (the MIL-101) at equilibrium and time t (s); Wa (g) is the initial weight of the sample (MIL-101); and Qe (mmol g−1) and Qt (mmol g−1) are the VOC amount adsorbed per gram of adsorbent at equilibrium and at time t (s), respectively ESI (S2†).
2.4. Measurement of adsorption isotherms of water vapor
The isotherm of water was measured on a Gravimetric water sorption analyzer (AQVADYNE DVS) equipped with a microbalance with an accuracy of 1 μg. Details of the measurement procedures are available in the ESI (S2†).2.5. Temperature programmed desorption experiments
Temperature programmed desorption (TPD) is an effective technique for surface analysis.11 In this work, TPD experiments were conducted to estimate the binding energy between an adsorbate and an adsorbent. Detailed descriptions of methods for the TPD experiment and estimation of desorption activation energy are given in the ESI (S3†).2.6. Determination of breakthrough curves under different relative humidities
Fixed bed adsorption experiments were conducted at 308 K to measure the breakthrough curves of DCE, EA and benzene on MIL-101 under conditions of relative humidities of 5%, 40%, and 80%, separately. The experimental setup is a flow-type fixed-bed adsorption system, which is shown in the ESI† (Fig. S1) and the detailed description of the method is given in the ESI (S4†).3. Results and discussion
3.1. Sample characteristics
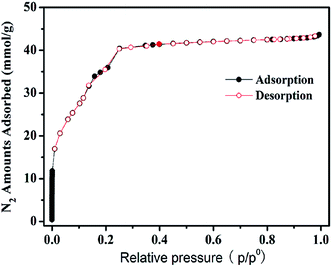
Fig. 1 shows the nitrogen adsorption and desorption isotherms of the MIL-101 sample. It can be seen that the N2 isotherm on MIL-101 was of typical type-V profile with secondary uptakes, which is characteristic of the presence of the two kinds of micropores.10 Textural properties of sample can be obtained from the isotherm by analyzing the nitrogen adsorption and desorption isotherms with the Micromeritics ASAP 2010 built in software. The BET specific surface area and total pore volume of the MIL-101 synthesized in this work were 3360 m2 g−1 and 1.75 cm3 g−1, respectively. In addition, the SEM image and XRD pattern of the sample are also obtained, which can be seen in ESI (S5, Fig. S2 and S3†).3.2. Isotherms of three VOCs on MIL-101
Fig. 2 presents the isotherms of DCE, EA, and benzene on MIL-101 at 308 K. It shows that the isotherms of DCE and EA were favorable ones, while the isotherm of benzene was an unfavorable isotherm. The isotherms of DCE and EA were higher than that of benzene, suggesting that the adsorption capacity of MIL-101 for DCE or EA was higher than that for benzene. The amounts adsorbed of DCE, EA and benzene on MIL-101 followed the order: DCE > EA > benzene. For example, at 8 mbar, the adsorption capacity of MIL-101 for DCE was up to 9.7 mmol g−1, that for DCE was about 5.5 mmol g−1, and that for DCE was about 3.3 mmol g−1. This difference in adsorption capacity may be ascribed to the different polarity of the three VOC molecules. DCE and EA are polar molecules whose dipole moments are 1.8 D and 1.78 D respectively. Benzene is non-polar molecule with dipole moments of zero. The different polarities of the three VOCs molecule result in different interactions between the VOC molecules and the framework of MIL-101 sample. As a result, the isotherms of DCE and EA were favorable ones due to their strong adsorption, while the isotherm of benzene was an unfavorable isotherm due to its weaker adsorption compared to DCE and EA. In addition, Table 1 gives the comparison between adsorption capacities of MIL-101 and some other porous materials for DCE, EA and benzene at similar conditions. It shows that the adsorption capacities of MIL-101 were much higher than those of the other materials. | ||
| Fig. 2 Adsorption isotherms of DCE, EA and benzene on the MIL-101 at low pressure and 308 K (points, experimental data; solid curves, fitted isotherm with Langmuir/Freundlich equation). | ||
| VOC | Material | Q (mmol g−1) | T (K) | Pressure (mbar) | Reference |
|---|---|---|---|---|---|
| DCE | MIL-101 | 9.7 | 308 | 8 | Present work |
| LC-1 | 3 | 303 | 8 | 17 | |
| HC-MWCNTs | 0.05 | 323 | 8 | 18 | |
| EA | MIL-101 | 5.5 | 308 | 8 | Present work |
| MCM-48 | 1 | 293 | 8 | 19 | |
| HDTMA clay | 0.4 | 308 | 8 | 20 | |
| Benzene | MIL-101 | 3.3 | 308 | 8 | Present work |
| MOF-5 | 0.8 | 303 | 8 | 21 | |
| Silicalite-1 | 1 | 295 | 8 | 22 | |
| H-ZSM-5 | 1.3 | 303 | 8 | 23 | |
| SBA-15 | 0.83 | 303 | 8 | 23 |
Langmuir and Freundlich equations were applied to fit the experimental isotherm data in order to describe the adsorption behavior of DCE, EA and benzene on MIL-101 sample, as shown in Fig. 2. The Langmuir equation seems to give a good fit to the experimental isotherm data of DCE and EA. However, the Langmuir equation was not a good fit for adsorption data of benzene, but the Freundlich equations can give a good fit.
The fitting parameters of Freundlich isotherm and Langmuir isotherm equations as well as their linear correlation coefficients (R2) are listed in the ESI (Table S1†). Examination of the data shows that the Langmuir and Freundlich equations were able to fit the experimental adsorption data well since their correlation coefficients R2 were up to 0.99.
3.3. Effects of water vapor on breakthrough curves of VOCs through the fixed bed of MIL-101
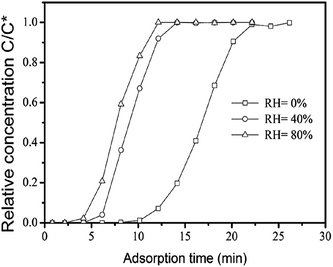
Fig. 3–5 show the adsorption breakthrough curves of DCE, EA, and benzene through a fixed bed packed with MIL-101 at different relative humidity. It was clearly visible that the relative humidity of the feed stream containing the VOC had a negative influence on the breakthrough curves of these VOCs in the packed bed. The breakthrough times of these VOCs sharply decreased with an increase in the humidity of the feed stream, implying that the working adsorption capacity of MIL-101 sharply decreased due to presence of water vapor in the feed stream. The higher relative humidity would make the breakthrough times of the VOCs become shorter. | ||
| Fig. 3 Effect of relative humidity on the breakthrough curves of EA through the fixed bed of the MIL-101 (T = 308 K, N2 flow rate = 70 ml min−1). | ||
 | ||
| Fig. 4 The effect of relative humidity on the breakthrough curves of DCE through the fixed bed of the MIL-101 (T = 308 K, N2 flow rate = 70 ml min−1). | ||
 | ||
| Fig. 5 Effect of relative humidity on the breakthrough curves of benzene through the fixed bed of the MIL-101 (T = 308 K, N2 flow rate = 70 ml min−1). | ||
Table 2 lists the breakthrough times and working adsorption capacities of EA, DCE and benzene on MIL-101 under conditions of different relative humidities. It shows that the breakthrough times and the working capacities of these VOCs became smaller with an increase in the relative humidity. When the relative humidity of the feed stream increased from 0% to 80%, the VOCs breakthrough times or the working adsorption capacities were decreased by about 45, 62 and 60.8% separately for DCE, EA, and benzene. This could be attributed to the competitive adsorption of water vapor on the surfaces of MIL-101. MIL-101 is an amphiphilic porous solid since its framework is composed of inorganic (metal cations and oxygen anions) and organic moieties (nonpolar carbon atoms and benzene ring).23,24 Thus it has polar sites due to the metal–oxygen clusters and very non-polar regions due to the presence of organic and mostly aromatic linker.23 In dry condition, the unsaturated Cr3+ sites of MIL-101 were also strong adsorption sites for the VOCs.11 In the case of the presence of water vapor in the adsorption system, H2O molecules preferentially adsorbed on the hydrophilic centers such as trivalent metal cations,25 and then if the concentration of water vapor became higher further additional water molecules are bound by hydrogen bridges to these water nucleation sites resulting in small water clusters.23,26 As a result, a part of the surface area of MIL-101 would be occupied by more H2O molecules, and thus less adsorption sites are available for adsorption of VOCs. In the fixed bed adsorption experiment, when the gaseous mixture containing VOCs and water vapor passed over the fixed bed of MIL-101, some water molecules preferentially adsorbed on the hydrophilic centers such as Cr3+ sites of MIL-101, and thus the surface active sites of MIL-101 for adsorption VOCs became less. As a consequence of that, the working adsorption capacity of the fixed bed of MIL-101 for VOCs would greatly decrease due to competitive adsorption of water molecules.
| Adsorbate | C0 (mol L−1) | RH (%) | Breakthrough time (min) | Working capacity (mmol g−1) |
|---|---|---|---|---|
| DCE | 5 × 10−5 | 5 | 16.3 | 2.282 |
| 40 | 10.7 | 1.498 | ||
| 80 | 8.9 | 1.246 | ||
| EA | 2 × 10−5 | 5 | 27.9 | 1.562 |
| 40 | 12.8 | 0.717 | ||
| 80 | 10.6 | 0.594 | ||
| BE | 10−5 | 5 | 11.4 | 0.319 |
| 40 | 6.24 | 0.175 | ||
| 80 | 4.46 | 0.125 |
Fig. 6a–c show a comparison of breakthrough curves of benzene at different temperatures in the presence and absence of water vapor. It is observed from Fig. 6 that breakthrough times of benzene in the presence and absence of water vapor decreased obviously as the temperature rose. This can be attributed to a decrease in adsorption capacity of MIL-101 for benzene due to the rising temperature, as shown in Fig. S6.† In addition, it was noticed that the breakthrough times in the presence of water vapor were always shorter than in the absence of water vapor at different temperatures. This means that although the rise of temperature could weaken adsorption of water vapor on MIL-101, there was still the negative effect of water vapor on benzene adsorption on MIL-101 in the range of temperatures studied.
 | ||
| Fig. 6 (a) The breakthrough curves of benzene on MIL-101 at 308 K. (b) The breakthrough curves of benzene on MIL-101 at 318 K. (c) The breakthrough curves of benzene on MIL-101 at 328 K. | ||
3.4. Isotherm of water vapor on MIL-101
Fig. 7 shows the adsorption isotherm of water vapor on MIL-101 at 308 K. It exhibits an S-shaped type of isotherm with a maximum water vapor uptake of 83 mmol g−1. The amount adsorbed of H2O vapor was low at a relative humidity below 30%, and then sharply increased when the relative humidity reached 40%. As the relative humidity increased, the amount adsorbed of water vapor water continued to rise.3.5. Desorption activation energies of the VOCs and H2O vapor on MIL-101
Fig. 8 shows a series of TPD curves of EA, DCE and benzene on MIL-101 at different heating rates. Each of these TPD curves exhibited one peak due to EA, DCE or benzene desorption, and its peak temperature (Tp) increased with an increase in heating rate.Knowing the values of Tp at the different heating rates employed (in Table 3), the desorption activation energy of EA, DCE or benzene can be estimated by using the Polanyi–Wigner equation (see ESI S2 and Fig. S4†). Calculations indicated that desorption activation energies of the three VOCs on MIL-101 were 47.14, 41.9, and 38.16 kJ mol−1, respectively, which followed the order: DCE > EA > benzene.
| VOC | Tp (K) | Ed (kJ mol−1) | R2 | ||||
|---|---|---|---|---|---|---|---|
| 4 K min−1 | 6 K min−1 | 8 K min−1 | 10 K min−1 | 12 K min−1 | |||
| EA | 357 | 365 | 373.5 | 378 | 382.5 | 41.9 | 0.997 |
| DCE | 361 | 368 | 374.5 | 379 | 385 | 47.14 | 0.995 |
| Benzene | 355 | 363 | 369.5 | 377.5 | 382.5 | 38.16 | 0.993 |
| H2O | 439 | 447 | 453 | 458 | 462 | 72.96 | 0.999 |
In similar manner, TPD experiments were also conducted for water vapor at different heating rates. Fig. 9 exhibits the TPD spectra of water vapor desorption from MIL-101 at different heating rates (Table 3). It is clearly visible that only one peak appeared in each of their TPD curves, and the peak temperature (Tp) increased with an increase in heating rate. In similar method to mentioned above, the desorption activation energy of water vapor on MIL-101 was estimated to be 72.9 kJ mol−1 (see ESI Fig. S5†), which was higher than that of the three VOCs. The higher desorption activation energy meant a stronger interaction between H2O molecules and the surface of MIL-101 compared to the three VOCs. This would make the presence of water vapor in the feed stream result in a sharp decrease in the VOCs working adsorption capacities of MIL-101 due to strong competitive adsorption of water vapor on the surfaces of MIL-101.
4. Conclusion
Adsorption behavior of 1,2-dichloroethane (DCE), ethyl acetate (EA), and benzene in a fixed bed of MIL-101 was investigated in the presence of water vapor. The results showed that the equilibrium amounts adsorbed of DCE, EA and benzene on MIL-101 followed the order: DCE > EA > benzene. The maximum adsorption capacities of MIL-101 for DCE, EA and benzene were separately up to 9.71, 5.79 and 3.76 mmol g−1, being much higher than those for some other adsorbents such as zeolites, activated carbons and clay. The presence of water vapor in the feed stream had a remarkable negative influence on the breakthrough behavior of VOCs in the fixed bed. The breakthrough times and the working capacities of these VOCs became smaller with an increase in the relative humidity. The isotherm of water vapor on MIL-101 at 308 K exhibited an S-shaped type of the isotherm, which can be expressed by the DSLF model. TPD experiments showed that the desorption activation energies of water vapor, DCE, EA and benzene on MIL-101 followed the order: water vapor > DCE > EA > benzene, and were 72.9, 47.14, 41.9, and 38.16 kJ mol−1, respectively. The higher desorption activation energy of water vapor suggested the interaction between water molecules and MIL-101 was stronger than that between DCE, EA, or benzene and MIL-101. The stronger interaction of water vapor with MIL-101 formed stronger competitive adsorption with VOCs on MIL-101. As a result, the existence of water vapor in the feed stream could lead to a sharp decrease in the working adsorption capacity of MIL-101 for these VOCs due to its strong competitive adsorption on the surfaces of MIL-101. Gaining a good understanding of water vapor impact on VOC adsorption on MOFs under a humid atmosphere would be helpful to design an effective adsorption process suitable for VOC capture in humid atmosphere or to arouse a study on novel adsorptive materials with resistance to competitive adsorption of water vapor. Therefore, the preparation of hydrophobic MOFs by surface modification to enhance their adsorption of VOCs for practical application would be worthy of investigation in the future.Acknowledgements
This work was supported by National Natural Science Foundation of China (no. 21436005 and no. 21376090), National Science Fund for Distinguished Young Scholars of China (no. 21225625), the Science Foundation of Guangzhou City, the Fundamental Research Funds for the Central Universities, and the State Key Lab of Subtropical Building Science (Grant C714004z).References
- X. B. Zhu, X. Gao, C. H. Zheng, Z. H. Wang, M. J. Ni and X. Tu, RSC Adv., 2014, 4, 37796–37805 RSC.
- S. J. Zhang, T. Shao, H. S. Kose and K. Tanju, Environ. Sci. Technol., 2010, 44(16), 6377–6383 CrossRef CAS PubMed.
- Y. S. Chen, Y. C. Hsu, C. C. Lin, C. Y. D. Tai and H. S. Liu, Environ. Sci. Technol., 2008, 42(7), 2631–2636 CrossRef CAS.
- J. C. Fang, X. Chen, Q. B. Xia, H. X. Xi and Z. Li, Chin. J. Chem. Eng., 2009, 17(5), 767–772 CrossRef CAS.
- H. Zaitan, D. Bianchi, O. Achak and T. Chafik, J. Hazard. Mater., 2008, 153(1–2), 852–859 CrossRef CAS PubMed.
- H. F. Zhen, S. M. J. Jang, W. K. Teo and K. Li, J. Appl. Polym. Sci., 2006, 99(5), 2497–2503 CrossRef CAS.
- N. Qi, W. S. Appel, M. D. LeVan and J. E. Finn, Ind. Eng. Chem. Res., 2006, 45(7), 2303–2314 CrossRef CAS.
- E. Barea, C. Montoro and J. A. R. Navarro, Chem. Soc. Rev., 2014, 43(16), 5419–5430 RSC.
- M. Eddaoudi, J. Kim, N. Rosi, D. Vodak, J. Wachter, M. O’Keeffe and O. M. Yaghi, Science, 2002, 295(5554), 469–472 CrossRef CAS PubMed.
- G. Férey, C. Mellot-Draznieks, C. Serre, F. Millange, J. Dutour, S. Surblé and I. Margiolaki, Science, 2005, 309(5743), 2040–2042 CrossRef PubMed.
- Z. X. Zhao, X. M. Li, S. S. Huang, Q. B. Xia and Z. Li, Ind. Eng. Chem. Res., 2011, 50(4), 2254–2261 CrossRef CAS.
- Z. Zhao, X. Li and Z. Li, Chem. Eng. J., 2011, 173(1), 150–157 CrossRef CAS PubMed.
- J. Shi, Z. Zhao, Q. Xia, Y. Li and Z. Li, J. Chem. Eng. Data, 2011, 56(8), 3419–3425 CrossRef CAS.
- T. K. Trung, N. A. Ramsahye, P. Trens, N. Tanchoux, C. Serre, F. Fajula and G. Férey, Microporous Mesoporous Mater., 2010, 134(1–3), 134–140 CrossRef CAS PubMed.
- K. Yang, Q. Sun, F. Xue and D. Lin, J. Hazard. Mater., 2011, 195, 124–131 CrossRef CAS PubMed.
- C.-Y. Huang, M. Song, Z.-Y. Gu, H.-F. Wang and X.-P. Yan, Environ. Sci. Technol., 2011, 45(10), 4490–4496 CrossRef CAS PubMed.
- C. Long, P. Liu, Y. Li, A. M. Li and Q. X. Zhang, Environ. Sci. Technol., 2011, 45(10), 4506–4512 CrossRef CAS PubMed.
- H. Sone, B. Fugetsu, T. Tsukada and M. Endo, Talanta, 2008, 74(5), 1265–1270 CrossRef CAS PubMed.
- S. Wu, J. Wang, G. Liu, Y. Yang and J. Lu, J. Membr. Sci., 2012, 390–391, 175–181 CrossRef CAS PubMed.
- A. M. Cavalcante, L. G. Torres and G. L. V. Coelho, Braz. J. Chem. Eng., 2005, 22, 75–82 CAS.
- W.-G. Shim, K.-J. Hwang, J.-T. Chung, Y.-S. Baek, S.-J. Yoo, S.-C. Kim, H. Moon and J.-W. Lee, Adv. Powder Technol., 2012, 23(5), 615–619 CrossRef CAS PubMed.
- L. Song, Z.-L. Sun, H.-Y. Ban, M. Dai and L. V. C. Rees, Phys. Chem. Chem. Phys., 2004, 6(19), 4722–4731 RSC.
- S. H. Jhung, J. H. Lee, J. W. Yoon, C. Serre, G. Ferey and J. S. Chang, Adv. Mater., 2007, 19(1), 121–124 CrossRef CAS.
- G. Akiyama, R. Matsuda, H. Sato, A. Hori, M. Takata and S. Kitagawa, Microporous Mesoporous Mater., 2012, 157, 89–93 CrossRef CAS PubMed.
- P. Küsgens, M. Rose, I. Senkovska, H. Fröde, A. Henschel, S. Siegle and S. Kaskel, Microporous Mesoporous Mater., 2009, 120(3), 325–330 CrossRef PubMed.
- Y.-K. Seo, J. W. Yoon, J. S. Lee, Y. K. Hwang, C.-H. Jun, J.-S. Chang, S. Wuttke, P. Bazin, A. Vimont, M. Daturi, S. Bourrelly, P. L. Llewellyn, P. Horcajada, C. Serre and G. Férey, Adv. Mater., 2012, 24(6), 806–810 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra10463c |
| This journal is © The Royal Society of Chemistry 2015 |