Preparation and characterization of SiO2/BiOX (X = Cl, Br, I) films with high visible-light activity
Fan Shen†
,
Li Zhou†,
Jiajia Shi,
Mingyang Xing* and
Jinlong Zhang*
Key Laboratory for Advanced Materials and Institute of Fine Chemicals, East China University of Science and Technology, 130 Meilong Road, Shanghai 200237, P. R. China. E-mail: mingyangxing@ecust.edu.cn; jlzhang@ecust.edu.cn
First published on 1st December 2014
Abstract
SiO2/BiOX (X = Cl, Br, I) thin films, which were well adhered to glass substrates with SiO2 as the intermediate layer, were prepared via a simple sol–gel method. The prepared films were characterized using X-ray diffraction (XRD), scanning electron microscopy (SEM), UV-visible diffuse reflectance spectroscopy (UV-vis DRS), X-ray photoelectron spectroscopy (XPS) and Raman. The results suggest that the films have a tetragonal PbFCl-type structure and crystallized well. These films showed high and stable photocatalytic activities for the degradation of RhB under visible light. This method is cheap and convenient, the size and morphology of the product are uniform, the production cycle is short, and the process is applicable to large-scale applications. SiO2/BiOX (X = Cl, Br, I) films might have promising applications in photocatalysis, photovoltaic film devices, nano-coating, pearlescent pigments and other fields.
1. Introduction
In recent years, n-type semiconductors have been widely used in the photodegradation of pollutants, such as TiO2,1–6 ZnO,7 CdS8,9 and so on. Among many semiconductor photocatalysts, TiO2 is one of the most effective photocatalysts, and many researchers have attempted to study it. However, only UV light can be absorbed by TiO2 because of its relatively broad band gap (Eg = 3.2 eV), which leads to low utilization of solar energy.10–12 Therefore, it is necessary to develop a photocatalyst that responds to visible light. Recently, various non-TiO2-based photocatalysts have been reported by a number of research groups, for example, oxides of vanadium family elements,13,14 bismuth compounds15–20 and some other visible-light photocatalysts. It is noteworthy that Bi compounds could easily form layered structures due to the special structure of Bi atoms, generating the visible light responsive Bi compounds. In a previous study, Bi is used as a dopant in oxide photocatalysts,21 but some later studies reported that Bi compounds showed high photocatalytic activities, such as Bi2O3,18 Bi2S3,19 BiOX (X = Cl, Br, I),16,17 BiW2O6,15 BiVO420 and some of the more complex Bi compounds. Among them, BiOX (X = Cl, Br, I) is an excellent visible-light-active photocatalyst with great chemical stability, which is worthy of further study.As a novel and efficient material, BiOX (X = Cl, Br, I) has attracted extensive attention because of its unique internal electronic structure, optical properties and photocatalytic activity. All BiOX (X = Cl, Br, I) compounds have a tetragonal PbFCl-type crystal structure, and the atomic arrangement consists of tetragonal [Bi2O2]2+ layers interleaved with double layers of halogen atoms, which interact mainly via Van der Waals forces. Thus, the binding force is so weak that the layered structure could easily dissociate along the [110] direction.17
Bismuth oxyhalides have promising applications as catalysts, ferroelectric materials and pigments. Bismuth oxyhalides exhibit a better performance than TiO2 (P25) in the photocatalytic degradation of organic pollutants. These photocatalysts could respond well to visible light. Hence, bismuth oxyhalides are becoming the hotshots in the field of photocatalysis. However, bismuth oxyhalide photocatalysts have also a lot of disadvantages, such as the very fine powder of their nanoparticles, which leads to the following limitations in large-scale applications. First, the separation of the photocatalyst from large volumes of reaction solution is very difficult.22 Second, agglomeration of the bismuth oxyhalides could make the particles of the catalyst grow,23 which might decrease its photocatalytic activity. Third, catalyst poisoning of the suspended phase could occur owing to the catalyst’s poor stability. Fourth, a shadowing effect always exists. Shadowing effect means that the light could be absorbed and scattered by the photocatalyst. Thus the light used in the photocatalytic reaction would be greatly reduced.24 Loading the bismuth oxyhalides on a solid substrate when preparing films with photocatalytic activity could overcome the above mentioned drawbacks. The preparation of nano-bismuth oxyhalide films could fix the photocatalyst particles and refine their size, and the photocatalyst would present a quantum size effect, small size effect, quantum confinement effect, surface and interface effects and others, making it possible to further improve the photocatalytic activity. Therefore, this research shows some theoretical as well as practical value.
Moreover, SiO2 films have been widely used in optics, microelectronics and other fields, because some researchers discovered that these films show nice transmittance under visible light and chemical stability.25 In this study, we used SiO2 as the substrate in combination with the BiOX films. The SiO2 substrate served as an isolation layer which could prevent the impurities in the glass support from affecting the photocatalytic activity of the BiOX films. In addition, the BiOX films could grow into different morphologies, which led to different photocatalytic activities.
2. Experimental
2.1 Pre-treatment of the glass substrate
A large number of silanol groups, existing on the surface of the glass, could make the glass bind to the coating. However, the untreated glass substrate would be polluted due to exposure to air for a long time. Hence, impurities might cover up the silanol groups and prevent the surface from forming films which might weaken the adhesive strength between the glass and the coating.26The glass substrates were treated using acid dipping, alcohol washing and ultrasonic cleaning. The method could be briefly described by the following process: 250 mL dilute hydrochloric acid was prepared in a jar, four glass substrates were placed in it and cleaned for 30 min using ultrasound. The glass substrates were taken out of the jar, washed with deionized water, immersed in ethanol and cleaned again for 30 min using ultrasound. Then, the glass substrates were washed with deionized water, followed by cleaning in deionized water for 30 min using ultrasound. Finally, the cleaned glass substrates were taken out of the water with tweezers, dried in a constant temperature and humidity oven, sealed and stored. The above solutions must be re-prepared every 2 months. During the process, rubber gloves must be worn, the glass substrates must be put face down using clean tweezers to avoid polluting the glass substrates.
2.2 Preparation of sols
2.3 Preparation of SiO2/BiOX composite films on the glass substrate
SiO2/BiOX composite films were loaded onto the glass substrates using a convenient dip-coating method. The device is simple and the film thickness can be controlled by adjusting the pulling speed, which was limited to 2–5 mm s−1. At room temperature (15–30 °C), the pre-cleaned glass substrates were placed into the SiO2 sol for 3 min, then, they were lifted at a speed of 3 mm s−1 to form the films, followed by calcination at 350 °C for 2 h. The obtained films were immersed in the respective BiOX (X = Cl, Br, I) precursor solution for 3 min, then, they were lifted at a speed of 3 mm s−1 to form the films. Finally, the given films were dried at 50 °C in an oven to generate the SiO2/BiOX composite films.2.4 Characterization
XRD analysis of the prepared films was carried out with a Rigaku D/max 2550 VB/PC apparatus using Cu Kα radiation (λ = 1.54056 Å), Ni as a filter, operated at 40 kV and 100 mA in the range of 10–80°. Scanning electron microscopy (SEM, JEOL USA, JSM-6360, voltage 15 kV) was used to observe the morphology of the films’ surface. The prepared films were cut into small pieces with a glass cutter, and gold was gilded on every small piece, which was fixed using double-sided adhesive. The characterization was carried out in a SEM test chamber. UV-vis absorption spectra were recorded with a scan UV-vis spectrophotometer (Varian Cary 500), while BaSO4 was used as a reference, and the measuring range was 400–800 nm. X-Ray photoelectron spectroscopy (XPS) was carried out using a Perkin-Elmer PHI 5000C ESCA System with Mg Kα radiation operated at 300 W.2.5 Photocatalysis test
The prepared the SiO2/BiOX (X = Cl, Br, I) films (effective size of the films is 25 × 33 mm) were vertically added into glass tubes containing 60 mL rhodamine B solution (10 mg L−1, RhB), and the distance between the glass tube and the light source was 25 cm. An iodine tungsten lamp was used as the light source, and the wavelength of the light through the filter was higher than 420 nm. The mixture was stirred for 30 min in the dark until absorption–desorption equilibrium was reached, and then put into the photocatalytic reactor. The surface of the films must be perpendicular to the light source. The illumination time was 240 min, and the analytical samples were taken from the mixture every 60 min. The concentration of the solution was analyzed by checking the absorbance at 553 nm with a UV-vis spectrophotometer (Shimadzu UV-2450).3. Results and discussion
3.1 XRD characterization
XRD characterization could be used to check the composition and structure of the samples. However, the execution could be difficult, because the thickness of the films was less than 400 nm. The peaks of the films might coincide with the peaks of the glass, and the peaks would be very low. In the analysis, the precursor solution was used to generate the powder for XRD to observe the crystal phase structures of the prepared films. The XRD patterns of the powders prepared using the BiOX (X = Cl, Br, I) precursor solutions (Fig. 2) show that the powders possess a high degree of crystallinity with the P4/nmm space groups and a tetragonal PbFCl-type structure.17The Sherrer equation could be used to figure out the particle size:27
 | (1) |
| Photocatalyst | Structure | Space group | Cell parameters/nm | Particle size/nm | |
|---|---|---|---|---|---|
| BiOCl | 06–0249 | Tetragonal | P4/nmm | a = b = 0.3894, c = 0.7369 | 34.1 |
| BiOBr | 09–0393 | Tetragonal | P4/nmm | a = b = 0.3924, c = 0.8101 | 27.2 |
| BiOI | 10–0445 | Tetragonal | P4/nmm | a = b = 0.3992, c = 0.9151 | 24.5 |
 | ||
| Fig. 3 XRD patterns of BiOX (X = Cl, Br, I) powders compared to their respective standard peaks: (a) BiOCl: 06–0249, (b) BiOBr: 09–0393, and (c) BiOI: 10–0445. | ||
3.2 SEM characterization
The SiO2/BiOX (X = Cl, Br, I) thin films were cut into small pieces with a glass cutter for SEM characterization, and the results are shown in Fig. 4. Fig. 4A shows the SEM image of a single layer of SiO2 film, revealing that the layer is dense with drying cracks. Fig. 4B shows the SEM image of the SiO2/BiOCl thin film, indicating that the BiOCl layer possesses nice uniformity and dispersion with a lamellar structure, and the thickness is about 50 nm. The sheet-like structure is consistent with the related literature.17 The SiO2/BiOBr thin film just exhibits a layered plate structure (Fig. 4C), and a fan-like structure can be observed from the SEM image of the SiO2/BiOI film (Fig. 4D). In general, the SiO2/BiOX (X = Cl, Br, I) thin films all possess different layered structures, because the crystals would grow along different directions due to the different halogen atoms. The various morphologies are expected to give different photocatalytic activities owing to the difference in light adsorption, transmission and distribution of surface electrons. | ||
| Fig. 4 SEM images of SiO2/BiOX (X = Cl, Br, I) samples: (A) SiO2, (B) SiO2/BiOCl, (C) SiO2/BiOBr, and (D) SiO2/BiOI thin films. | ||
3.3 XPS characterization of SiO2/BiOX (X = Cl, Br, I) films
XPS characterization could be used to analyze the valence bands of each element in the sample, thus, the separate peaks in the XPS spectra of Bi and X (X = Cl, Br, I) are shown in Fig. 5. As shown in Fig. 5a, the peaks with the binding energies of 165.1 and 159.8 eV are corresponding to Bi 4f5/2 and Bi 4f7/2 in BiOCl. The Cl 2p3/2 peak is at 198.8 eV (Fig. 5b). The data of the other elements are listed in Table 2, Bi 4f5/2, Bi 4f7/2 and the halogen atoms’ valence bands are consistent with the related literature,28–30 indicating that the samples prepared are BiOX (X = Cl, Br, I) crystals, in which all Bi is in the Bi+3 state and X in the X−1 state.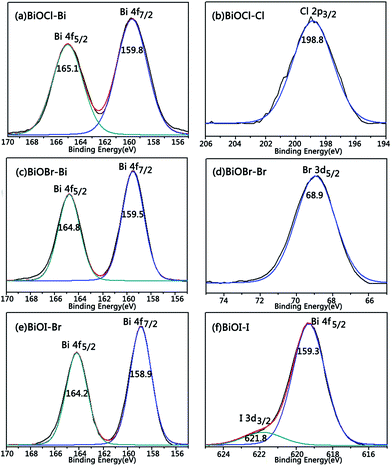 | ||
| Fig. 5 XPS spectra of the BiOX (X = Cl, Br, I) powder: (a) BiOCl–Bi; (b) BiOCl–Cl; (c) BiOBr–Bi; (d) BiOBr–Br; (e) BiOI–Bi; (f) BiOI–I. | ||
3.4 DRS characterization of the SiO2/BiOX (X = Cl, Br, I) film
UV-vis DRS was carried out to study the absorption of the SiO2/BiOX (X = Cl, Br, I) thin films, and the result is shown in Fig. 6. UV-vis DRS is often used to check the optical absorption properties of materials. From Fig. 6, with the increase in halogen atomic number, the light absorption range of the sample increased, and the corresponding band gap decreased. Especially BiOI presents strong absorption under visible light. The intrinsic absorption coefficient “α” of the semiconductors is a function of the wavelength of the incident light and the type of solid band transitions, and the relationship between α and the photon energy hν could be expressed as:31| αhν = A(hν − Eg)n/2 | (2) |
| ECB = X − EC + 0.5Eg | (3) |
 | ||
| Fig. 6 UV-vis diffuse reflectance spectra of BiOX (X = Cl, Br, I) powders: (a) BiOCl, (b) BiOBr, and (c) BiOI. | ||
3.5 Raman spectra of BiOCl
BiOCl was reported to have a tetragonal PbFCl-type structure consisting of [Bi2O2]2+ layers and double layers of Cl atoms.17 From the Raman spectrum (Fig. 7), the peak at 141 cm−1 can be assigned to the stretching vibration A1g of Bi–Cl, the peak at 197 cm−1 is attributed to the stretching vibration of Bi–Cl, and the peak at 396 cm−1 is caused by the vibration of Bi–O. The above results can further confirm that the sample is BiOCl.3.6 Photocatalytic activity of thin films
Fig. 8a shows the adsorbability of the prepared thin films for the degradation of RhB. After 0.5 h in the dark, the adsorbability showed a relatively stable trend. Fig. 8b shows that the concentration of RhB decreased more sharply under illumination conditions. SiO2/BiOBr exhibits the highest photocatalytic activity, and its degradation rate reached 98% after 3 h under the optimal conditions. The BiOX (X = Cl, Br, I) films are all superior to the SiO2/TiO2 film in their photocatalytic activity. The ranking order for the degradation activity towards RhB is: SiO2/BiOBr > SiO2/BiOI > SiO2/BiOCl.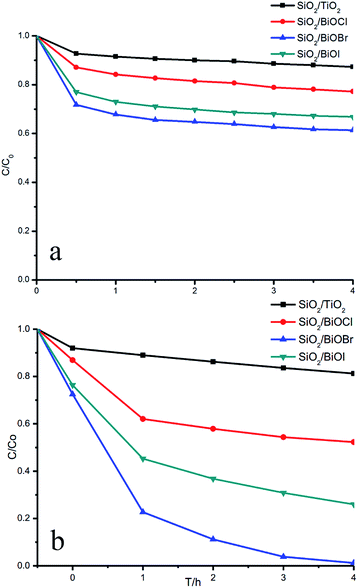 | ||
| Fig. 8 Adsorbability (a) and photocatalytic activity (b) of the different thin films for degradation of RhB. | ||
The results can be analyzed in two ways. On the one hand, the higher the valence band energy of the semiconductor, the stronger the oxidative ability.33 From Table 3, in the order BiOCl to BiOBr and BiOI, the valence band energy gradually decreases, so the ranking of the oxidative ability is: BiOCl > BiOBr > BiOI. On the other hand, the photocatalytic reaction necessitates the excitation of the semiconductor by absorbing photons.34 Thus, the visible light absorption capability could also affect the photocatalytic activity.35 From Table 3, with the halogen atomic number increasing, the band gap of BiOX decreases, showing that the visible light absorption capacity gradually increases. Thus the photocatalytic efficiency rises. The ranking of visible light absorption capacity is: BiOI > BiOBr > BiOCl. After comprehensive consideration of these two competing factors, the ranking order of degradation activity towards RhB of SiO2/BiOX (X = Cl, Br, I) under visible light is: SiO2/BiOBr > SiO2/BiOI > SiO2/BiOCl.
3.7 Mechanism of photocatalytic degradation
Under optical conditions, three possible mechanisms might explain the degradation of the dye on the surface of the semiconductor. In addition to the semiconductor photocatalytic mechanism, the dye-sensitized mechanism and dye-photolysis mechanism also probably exist.The dye-sensitized mechanism means that the dye is excited by light to form excited states, and the electrons in the excited states are transferred to the conduction band of the photocatalyst leading to electron injection.36 Then, the electrons and O2 in the water could combine to form ˙OOH/˙O2− groups, which have a high oxidative activity.37 As a result, the highly active group could degrade the dye. The dye-photolysis mechanism can be described as follows: the dye is excited by light to form the excited states,38 then, the electrons in the excited states could combine with O2 in the water to form highly active ˙O2− groups, which could degrade the dye. RhB is chemically stable, and a decrease in concentration could not be detected in the absence of catalyst. Thus, in this study the self-degradation mechanism could be ignored.
In general, deethylation is accompanied by a ring-opening reaction of the benzene rings during the photocatalytic degradation of RhB.39 During the photocatalytic process of the films, deethylation plays a dominant role, while the ring-opening reaction could be secondary.40 RhB (λmax = 553 nm) could generate Rhodamine (λmax = 498 nm) via deethylation in the photocatalytic reaction. During the photocatalytic reaction, deethylation is continually taking place. For de-ethyl RhB, the benzene rings are constantly attacked by ˙OH till complete degradation of the molecule. The blue-shift of the maximum absorption peak could correspond to the deethylation of RhB. With the deethylation step, the absorption peak was gradually blue-shifted from 553 to 552 nm, then to 510 nm, and finally to 498 nm, which was also observed visually with the colour gradually changing from red to yellow.
The photocatalytic activity of the semiconductors intrinsically depends on the electronic structures,41 because the photocatalytic activity is closely related to the conduction band (CB) and the valence band (VB), as well as to the mobility of the carriers. CB and VB could determine the oxidative ability and reductive ability of the catalyst, respectively, while the mobility of the carriers could determine the photocatalytic efficiency. For metal oxide photocatalysts, the VB consists of the 2p orbital of oxygen, but for bismuth-based semiconductors, the VB is a hybrid of the 2p orbital of oxygen and 6s orbital of Bi, and the CB is composed of the 6p orbital of Bi,42 which possesses a highly reductive activity.
Among the bismuth oxyhalides, BiOCl is an indirect wide band-gap semiconductor. The O2p and Cl3p orbitals occupy its VB,43 and the CB consists of the Bi6p orbital. From the DRS data, the band gap of BiOCl is 3.2 eV, so it should only have UV activity. However, the result of the photocatalytic degradation indicates that BiOCl also shows activity under visible light. We think that in this experiment, the photocatalytic degradation of RhB on BiOCl could be attributed to the dye-sensitized mechanism (Fig. 9). Meanwhile, during the photocatalytic degradation, with the gradual fading of the color of the solution, the photosensitization effect decreased, which would slow down the photocatalytic degradation (Fig. 8). For BiOBr and BiOI, their band gaps are 2.75 eV and 1.76 eV, respectively, so visible light can be absorbed. Thus, the photocatalytic degradation might include both photocatalysis and photosensitization. BiOBr had the highest photocatalytic activity with the appropriate CB and VB, as well as a nice response to visible light.
According to some literature, the visible light activity of SiO2/BiOX (X = Cl, Br, I) is attributed to the layered structure, the halogen atoms X exist between the [Bi2O2] layers.17 [Bi2O2], [X], [Bi2O2] would stack along the c axis to form special layered structures. The Bi atoms in this structure possess two states. One exhibits distorted [BiO4] polyhedrons, and another shows distorted [BiO4X4] polyhedrons. These layered structures with partially distorted polyhedrons are beneficial to the electron transfer and photocatalytic properties.
3.8 Recyclability of films
In order to investigate the stability of the photocatalytic films, the SiO2/BiOCl film was continuously used for 4 times (Fig. 10). The photocatalytic activity was only slightly decreased, indicating a firm adhesion between the prepared films and the substrates. The stable visible-light photocatalytic activity suggests the continuous usage of the catalysts in actual water degradation processes.4. Conclusions
SiO2/BiOX (X = Cl, Br, I) thin films with layered structures were prepared using a convenient sol–gel method. The films show a high and stable photocatalytic activity under visible-light irradiation. The SiO2/BiOX (X = Cl, Br, I) thin films have a tetragonal PbFCl-type structure, and the samples crystallized well and were stable. In addition, with increasing halogen atomic number, the particle size gradually decreased. The results of the DRS and photocatalytic activity tests indicated that the samples might respond well to visible light. The SiO2/BiOBr thin film showed the highest photocatalytic activity under visible light. Furthermore, the SiO2/BiOX (X = Cl, Br, I) thin films presented higher photocatalytic activity than the TiO2 film. The ranking of the degradation activity of SiO2/BiOX (X = Cl, Br, I) under visible light is: SiO2/BiOBr > SiO2/BiOI > SiO2/BiOCl. Two factors affect their photocatalytic activity: the energy band structure and the light absorption ability.Acknowledgements
This work has been supported by the National Nature Science Foundation of China (21237003, 21173077, 21377038, 21073060, 21203062), the National Basic Research Program of China (973 Program, 2013CB632403), the Research Fund for the Doctoral Program of Higher Education (20120074130001) and the Fundamental Research Funds for the Central Universities.Notes and references
- M. R. Hoffmann, S. T. Martin, W. Choi and D. W. Bahnemann, Chem. Rev., 1995, 95, 69–96 CrossRef CAS.
- M. Xing, J. Zhang and F. Chen, J. Phys. Chem. C, 2009, 113, 12848–12853 CAS.
- B. Qiu, M. Xing and J. Zhang, J. Am. Chem. Soc., 2014, 136, 5852–5855 CrossRef CAS PubMed.
- M. Xing, W. Fang, M. Nasir, Y. Ma, J. Zhang and M. Anpo, J. Catal., 2013, 297, 236–243 CrossRef CAS PubMed.
- M. Takeuchi, M. Matsuoka and M. Anpo, Res. Chem. Intermed., 2012, 38, 1261–1277 CrossRef CAS.
- N. Negishi and K. Takeuchi, Res. Chem. Intermed., 2003, 29, 861–879 CrossRef CAS PubMed.
- S. Chakrabarti and B. K. Dutta, J. Hazard. Mater., 2004, 112, 269–278 CrossRef CAS PubMed.
- Z. Gao, N. Liu, D. Wu, W. Tao, F. Xu and K. Jiang, Appl. Surf. Sci., 2012, 258, 2473–2478 CrossRef CAS PubMed.
- Y. Huo, X. Yang, J. Zhu and H. Li, Appl. Catal., B, 2011, 106, 69–75 CAS.
- R. Asahi, T. Morikawa, T. Ohwaki, K. Aoki and Y. Taga, Science, 2001, 293, 269–271 CrossRef CAS PubMed.
- J. Zhang, Y. Wu, M. Xing, S. A. K. Leghari and S. Sajjad, Energy Environ. Sci., 2010, 3, 715–726 CAS.
- M. Xing, J. Zhang and F. Chen, Appl. Catal., B, 2009, 89, 563–569 CrossRef CAS PubMed.
- R. Shi, Y. Wang, F. Zhou and Y. Zhu, J. Mater. Chem., 2011, 21, 6313–6320 RSC.
- H. Guo, J. Chen, W. Weng and S. Li, Appl. Surf. Sci., 2011, 257, 3920–3923 CrossRef CAS PubMed.
- H. Fu, C. Pan, W. Yao and Y. Zhu, J. Phys. Chem. C, 2005, 109, 22432–22439 CrossRef CAS PubMed.
- J. Henle, P. Simon, A. Frenzel, S. Scholz and S. Kaskel, Chem. Mater., 2007, 19, 366–373 CrossRef CAS.
- X. Zhang, Z. Ai, F. Jia and L. Zhang, J. Phys. Chem. C, 2008, 112, 747–753 CAS.
- L. Zhou, W. Wang, H. Xu, S. Sun and M. Shang, Chem.–Eur. J., 2009, 15, 1776–1782 CrossRef CAS PubMed.
- Y. Wang, J. Chen, P. Wang, L. Chen, Y.-B. Chen and L.-M. Wu, J. Phys. Chem. C, 2009, 113, 16009–16014 CAS.
- A. Kudo, K. Omori and H. Kato, J. Am. Chem. Soc., 1999, 121, 11459–11467 CrossRef CAS.
- K. Lv, H. Zuo, J. Sun, K. Deng, S. Liu, X. Li and D. Wang, J. Hazard. Mater., 2009, 161, 396–401 CrossRef CAS PubMed.
- L. Zhang, W. Wang, L. Zhou, M. Shang and S. Sun, Appl. Catal., B, 2009, 90, 458–462 CrossRef CAS PubMed.
- F. I. López-Salinas, G. A. Martínez-Castañón, J. R. Martínez-Mendoza and F. Ruiz, Mater. Lett., 2010, 64, 1555–1558 CrossRef PubMed.
- H. M. Coleman, C. P. Marquis, J. A. Scott, S. S. Chin and R. Amal, Chem. Eng. J., 2005, 113, 55–63 CrossRef CAS PubMed.
- Y. Kim, F. Zhao, M. Mitsuishi, A. Watanabe and T. Miyashita, J. Am. Chem. Soc., 2008, 130, 11848–11849 CrossRef CAS PubMed.
- P. C. Hidber, W. Helbig, E. Kim and G. M. Whitesides, Langmuir, 1996, 12, 1375–1380 CrossRef CAS.
- A. L. Patterson, Phys. Rev., 1939, 56, 978–982 CrossRef CAS.
- F. Dong, Y. Sun, M. Fu, Z. Wu and S. C. Lee, J. Hazard. Mater., 2012, 219–220, 26–34 CrossRef CAS PubMed.
- J. Xia, S. Yin, H. Li, H. Xu, L. Xu and Y. Xu, Dalton Trans., 2011, 40, 5249–5258 RSC.
- X. Zhang, L. Zhang, T. Xie and D. Wang, J. Phys. Chem. C, 2009, 113, 7371–7378 CAS.
- A. E. Morales, E. S. Mora and U. Pal, Rev. Mex. Fis. S, 2007, 53, 18 CAS.
- Y. Xu and M. A. Schoonen, Am. Mineral., 2000, 85, 543–556 CAS.
- D. Lawless, N. Serpone and D. Meisel, J. Phys. Chem., 1991, 95, 5166–5170 CrossRef CAS.
- H. Shi, J. Chen, G. Li, X. Nie, H. Zhao, P.-K. Wong and T. An, ACS Appl. Mater. Interfaces, 2013, 5, 6959–6967 CAS.
- A. Heller, Acc. Chem. Res., 1995, 28, 503–508 CrossRef CAS.
- R. Katoh, A. Furube, A. V. Barzykin, H. Arakawa and M. Tachiya, Coord. Chem. Rev., 2004, 248, 1195–1213 CrossRef CAS PubMed.
- H. Shi, G. Li, H. Sun, T. An, H. Zhao and P.-K. Wong, Appl. Catal., B, 2014, 158–159, 301–307 CrossRef CAS PubMed.
- Y. Xie and C. Yuan, Appl. Catal., B, 2003, 46, 251–259 CrossRef CAS.
- T. Wu, G. Liu, J. Zhao, H. Hidaka and N. Serpone, J. Phys. Chem. B, 1998, 102, 5845–5851 CrossRef CAS.
- J. Zhuang, W. Dai, Q. Tian, Z. Li, L. Xie, J. Wang, P. Liu, X. Shi and D. Wang, Langmuir, 2010, 26, 9686–9694 CrossRef CAS PubMed.
- M. Sathish, B. Viswanathan, R. P. Viswanath and C. S. Gopinath, Chem. Mater., 2005, 17, 6349–6353 CrossRef CAS.
- J. Tang, Z. Zou and J. Ye, Angew. Chem., Int. Ed., 2004, 43, 4463–4466 CrossRef CAS PubMed.
- C. Wang, C. Shao, Y. Liu and L. Zhang, Scr. Mater., 2008, 59, 332–335 CrossRef CAS PubMed.
Footnote |
| † Fan Shen and Li Zhou have contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2015 |





