Photoluminescence, photocatalysis and Judd–Ofelt analysis of Eu3+-activated layered BiOCl phosphors†
Abstract
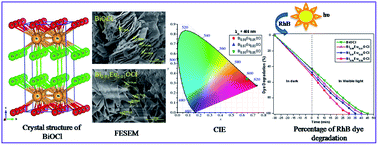
Eu3+-activated layered BiOCl phosphors were synthesized by the conventional solid-state method at relatively low temperature and shorter duration (400 °C for 1 h). All the samples were crystallized in the tetragonal structure with the space group P4/nmm (no. 129). Field emission scanning electron microscopy (FE-SEM) studies confirmed the plate-like morphology. Photoluminescence spectra exhibit characteristic luminescent 5D0 → 7FJ (J = 0–4) intra-4f shell Eu3+ ion transitions. The electric dipole transition located at 620 nm (5D0 → 7F2) was stronger than the magnetic dipole transition located at 594 nm (5D0 → 7F1). The evaluated Commission International de l'Eclairage (CIE) color coordinates of Eu3+-activated BiOCl phosphors were close to the commercial Y2O3:Eu3+ and Y2O2S:Eu3+ red phosphors. Intensity parameters (Ω2, Ω4) and various radiative properties such as transition probability (Atot), radiative lifetime (τrad), stimulated emission cross-section (σe), gain bandwidth (σe × Δλeff) and optical gain (σe × τrad) were calculated using the Judd–Ofelt theory. The experimental decay curves of the 5D0 level in Eu3+-activated BiOCl have a single exponential profile. In comparison with other Eu3+ doped materials, Eu3+-activated BiOCl phosphors have a long lifetime (τexp), low non-radiative relaxation rate (WNR), high quantum efficiency (η) and better optical gain (σe × τrad). The determined radiative properties revealed the usefulness of Eu3+-activated BiOCl in developing red lasers as well as optical display devices. Further, these samples showed efficient photocatalytic activity for the degradation of rhodamine B (RhB) dye under visible light irradiation. These photocatalysts are useful for the removal of toxic and non-biodegradable organic pollutants in water.

 Please wait while we load your content...
Please wait while we load your content...