Chrysanthemum-like 3D hierarchical magnetic γ-Fe2O3 and Fe3O4 superstructures: facile synthesis and application in adsorption of organic pollutants from water
Guoliang Li*a,
Jing Lanb and
Gang Lia
aState Key Laboratory of Environmental Aquatic Chemistry, Research Center for Eco-Environmental Sciences, Chinese Academy of Sciences, P.O. Box 2871, Beijing 100085, P. R. China. E-mail: glli@rcees.ac.cn; Tel: +86-10-62849339
bCollege of Chemistry and Pharmaceutical Sciences, Qingdao Agricultural University, Qingdao, 266109, P. R. China
First published on 28th November 2014
Abstract
Novel chrysanthemum-like 3D hierarchical magnetic γ-Fe2O3 and Fe3O4 superstructures self-assembled by nanobelts have been successfully synthesized through a simple one-pot solvothermal route without using structure-directing additives. The morphology and structure of the sample depend strongly on the reaction time. γ-Fe2O3 and Fe3O4 with superparamagnetic properties were obtained by thermal transformation of the precursor without changing the overall architectures. A possible formation mechanism of the chrysanthemum-like 3D hierarchical precursor was proposed. The BET surface area and the magnetic properties of the prepared γ-Fe2O3 and Fe3O4 were investigated. In addition, the adsorption capacity of the as-prepared magnetic iron oxide for organic pollutants was also determined by using 2,4-dichlorophenol and 2,4,6-trichlorophenol as probe pollutants.
1. Introduction
It has been recognized that properties and performances of nanomaterials are highly related to their morphologies, sizes, and structures.1 Therefore, developing and designing strategies for controlled preparation of nanomaterials with various morphologies, e.g. 0D (nanoparticles),2 1D (nanorods and nanowires),3 2D (nanorings and nanosheets),4 and 3D (hollow spheres),5 has aroused extensive attention. Particularly, constructing inorganic nanomaterials with 3D hierarchical architectures composed of nanoscale building units (e.g., nanobelts, nanoparticles, nanorods, etc.) have attracted considerable attention not only owing to their novel structural properties but also to their potential applications in a wide spectrum of fields.1a,6 To date, a number of methods have been developed to construct these structures, fabrication of which generally require additives, e.g. template materials or other structure-directing agents, in most cases.7 However, these additives need to be removed later, which might compromise the structural integrity and even cause secondary environmental pollution. In addition, additives involved synthesis procedures also usually experience disadvantages of high cost and troublesome synthetic procedures. Therefore, developing additives-free methods for preparation of those structures is more preferable due to their simplicity and adjustability.As one of the most important inorganic nanomaterials, metal oxide, e.g. maghemite (γ-Fe2O3) and magnetite (Fe3O4) have become a research hotspot due to their unique physicochemical characteristics (high magnetic saturation and nontoxicity) and extensive applications in adsorption, catalysis, magnetic resonance imaging (MRI), drug delivery, magnetic sensors and many other fields.8 Until now, magnetic iron oxides of various morphologies, e.g. nanoparticles,2a nanowires,3a,9 nanoflakes,10 nanorods,11 nanotubes,12 etc., have been fabricated through a variety of methods. What is more, a series of methods for synthesis of magnetic iron oxide of 3D architectures have also been developed.13 The majority of the developed methods usually lead to typical spheres-type structures. Wang et al. have reported the synthesis of iron oxides microspheres with poly-(vinylpyrrolidone) (PVP) as additive.13a The group of Biswas obtained oxides of iron in different crystalline forms through spray pyrolysis of iron precursors with hydrazine in furnace aerosol reactor.13d Also, unremitting efforts have been made to synthesize a kind of more interesting 3D hierarchical flower-like iron oxide structures. Zhang et al. introduced an ultrasound-assisted hydrothermal method for synthesis of 3D hierarchical flower-like Fe3O4 structures.13e Zhong's group prepared flower-like iron oxides with tetrabutylammonium bromide (TBAC) as template.13g Although these abovementioned methods for preparing 3D hierarchical iron oxides structures appear simple, they still involved surfactants additives or other technical assistance. To circumvent these problems, several groups developed additives-free methods, but the products either possess lower specific surface area (<40 m2 g−1) resulting low application efficiency or suffer redundant procedures,13b,c,f which does not favour large scale production and application. Therefore, from the practical point of view, developing facile methods for constructing 3D hierarchical magnetic iron oxide nanostructures with better application efficiency remains a challenge.
In this work, we report a facile route for the fabrication of chrysanthemum-like 3D hierarchical magnetic γ-Fe2O3 and Fe3O4 nanostructures through a simple solvothermal process followed by a calcination treatment of the obtained precursor. The obtained magnetic γ-Fe2O3 and Fe3O4 nanostructures were constructed by a number of 2D ultrathin nanobelts. A possible formation mechanism as investigated in detail was proposed. The magnetic and porous properties of the obtained product were also studied. In addition, the adsorption ability of the as-prepared magnetic iron oxides were also investigated by using chlorophenols as probe pollutants.
2. Experimental
2.1. Preparation of the precursor
The precursor was prepared through a solvothermal process. Typically, 0.405 g FeCl3·6H2O and 0.24 g urea were added to 70 mL ethylene glycol (EG) and magnetically stirred until completely dissolved. The mixture was transferred into a Teflon lined autoclave of 100 mL, which was then sealed, put into an electronic oven, and heated to 160 °C at a rate of 5 °C min−1 and maintained for 8 h. After the autoclave naturally cooled to room temperature, the green precipitate was collected by centrifugation, washed with absolute ethanol several times, and vaccum dried at 60 °C overnight. The obtained powder was kept in a desiccator for further use.2.2. Preparation of γ-Fe2O3 and Fe3O4
A portion of the as-prepared precursor was heated in air with a temperature ramp of 3 °C min−1 to 350 °C and held for 1 h. After the oven cooled to room temperature, the reddish brown γ-Fe2O3 product was obtained. Another portion of the as-prepared precursor was heated to 350 °C at a rate of 3 °C min−1 under protection of flowing nitrogen gas and maintained for 1 h, the black Fe3O4 was obtained.2.3. Characterization
Scanning electron microscopy (SEM) and energy dispersive X-ray analysis (EDAX) were performed on a Hitachi H7500N equipped with an energy dispersive X-ray spectroscope. Transmission electron microscopy images were taken by a Hitachi 2100 with an accelerating voltage of 200 kV. The crystal structure was determined by a PANalyticl X'Pert Pro X-ray diffractometer using Cu-Ka radiator. Fourier transform infrared (FTIR) spectroscopy was performed with a spectrometer (FTIR-7600) with the KBr pellet technique. Raman spectra was obtained on a Renishaw Invia Raman microscope. The TG/DSC was examined on a Netzsch STA449 instrument from room temperature to 800 °C with a heating rate of 10 °C min−1. The N2 adsorption–desorption isotherm was performed on a Micromeritics ASAP2000 V3.01 at 77 K after the samples were dehydrated and degassed at 120 °C for 2 h, the specific surface area was calculated using Brunauer–Emmett–Teller (BET) equation, pore diameters were calculated by Barret–Joyner–Halenda (BJH) equation. Magnetic property at room temperature was measured by a vibrating sample magnetometer (NIM-200C, National Institute of Metrology, China).2.4. Adsorption of chlorophenols
2,4-Dichlorophenol and 2,4,6-trichlorophenol were chosen as probe organic pollutants. The adsorption experiment was performed by mixing 20 mg γ-Fe2O3 and Fe3O4 with 150 mL of chlorophenols aqueous solutions (20 mg L−1) in capped glass bottles. The mixtures were shaken in a rotary shaker at the rate of 200 rpm under room temperature. The samples were pipetted at certain intervals (20 min) and the adsorbents were separated using a magnet. The concentration of phenols in the solution were determined through a reported method14 using an Agilent 1200 LC system consisting of a quaternary bump and a UV/vis detector set at 220 nm. The Langmuir equation was used to evaluate the adsorption isotherm, which is defined as: Qe = bqmCe/(1 + bCe), where Ce represents the equilibrium concentration of the probe pollutants (mg L−1); qe is the adsorption capacity at equilibrium (mg g−1); b and qm represent the adsorption constant and the monolayer adsorption capacity, respectively. The adsorption kinetics was simulated by pseudo-first-order kinetics equation, ln(C/C0) = kt, where C and C0 represent the concentration of the probe pollutants at times of t and 0 min; k and t refer to the adsorption rate constant and the adsorption time, respectively.3. Results and discussion
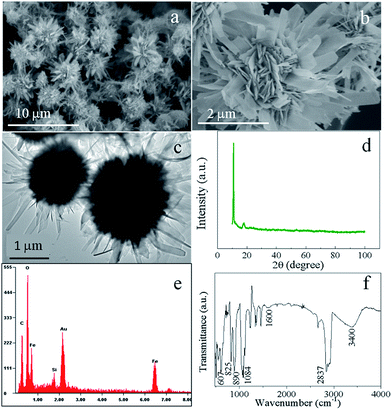
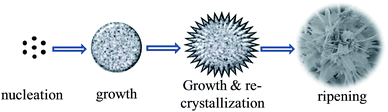
The morphology and structure of the precursor were examined by scanning electron microscopy (SEM) and transmission electron microscopy (TEM). Fig. 1a and b show the SEM images of the precursor, which clearly demonstrates that the sample appears hierarchical nanostructures self-assembled by nanobelts. The detailed morphology of the hierarchical nanostructure shown in Fig. 1b exhibits a chrysanthemum-like three dimensional hierarchical structure with diameters in the range of 2–4 μm. Also evidenced from Fig. 1b is that the microflower is constructed by nanobelts of several nanometers in thickness, which further can be evidenced by the TEM observations (Fig. 1c). The crystalline nature of the precursor obtained from X-ray powder diffraction (XRD, Fig. 1d) shows a similar pattern to a kind of ferrous alkoxide, e.g. Fe–EG,6b especially the strong peak around 2θ = 11 degrees. It is known that ethylene glycol is a strong reducing agent with a relatively high boiling point.15 During the solvothermal reaction, EG molecules lose protons forming diaions of –OCH2CH2O– and reduced Fe3+ to form Fe2+ cations. The formed –OCH2CH2O– then coordinated with Fe2+ ions forming ferrous alkoxide in green colour. EDAX result (Fig. 1e) clearly indicates the existence of C element in the raw sample. The FTIR spectrum was also carried out to further confirm the occurrence of ferrous alkoxide. As shown in Fig. 1f, the wide absorption band centered at around 3400 and 1600 cm−1 are OH stretching and HOH bending vibrational bands derived from the adsorbed water in the sample.16 The absorption bands located between 2500 and 3000 cm−1 are attributed to C–H stretching mode. Other characteristic peaks at 1084, 890, 825 and 607 cm−1 can be ascribed to C–C, C–O, –CH2– and Fe–O bands.17 The results clearly demonstrate the precursor is a kind of ferrous alkoxide as speculated. It is also noteworthy that the green precursor turned to reddish brown when dispersed in aqueous solvent, indicating the precursor can be easily oxidized to form ferric ions.To investigate the formation mechanism of the chrysanthemum-like 3D hierarchical nanostructure, SEM images of the product at different reaction time were taken. As shown in Fig. 2a, it is nanoparticles that were initially nucleated after 2 hours' solvothermal reaction. With the reaction time prolonged to 4 h, bigger spheres were formed at the expense of the initially formed nanoparticles. What is also worth noting is that the spheres are not of smooth surface, but with outshoots sprouting outward from the surface of the spheres forming urchin-like structures (Fig. 2b). Four more hours later, these outshoots grew larger forming nanobelt subunits, which constructed the chrysanthemum-like 3D hierarchical nanostructure (Fig. 2c). If further increased the reaction time to 16 h, with the growing of the nanobelts, the formed chrysanthemum collapsed (Fig. 2d). Based on those experimental results, a formation mechanism different from the generally popular two-stage “growth-assembly” growth mechanism involving the initial formation of primary 1D nanostructure and the subsequent self-assembly process,6a,18 was proposed here (Fig. 3). Similar to the commonly proposed mechanism, in the early stage of this preparation process, small nanoparticles were first nucleated at a fast rate under solvothermal condition. Then, different from the generally happened “aggregation”, Ostwald ripening phenomenon simultaneously functioned with increase of the solvothermal reaction. Those tiny nanoparticles grew to larger spheres at the expense of smaller ones of higher solubility to decrease the surface energy.19 Accompanying the formation of larger spheres, the particles on the outermost surface of the big sphere would continue to grow at a slow rate under low saturation conditions with bigger outshoots sprouting which served as starting seeds for further growth and recrystallization. At the same time, the smaller particles of higher surface energy near the surface can dissolve easily and diffuse outwards, acting as ingredient for growing of the outshoots. Therefore, with prolonging of the solvothermal reaction, these tiny outshoots undergone a ripening process growing larger forming nanobelts-constructed chrysanthemum-like 3D hierarchical structure. With the continuing growth of “petals” of the integrated chrysanthemum, the overall structure finally collapsed forming anomalous nanobelts and nanoplates.
 | ||
| Fig. 2 SEM images of the samples prepared for different solvothermal reaction time: 1 h (a), 4 h (b), 8 h (c), and 16 h (d). | ||
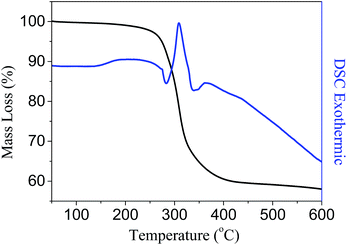
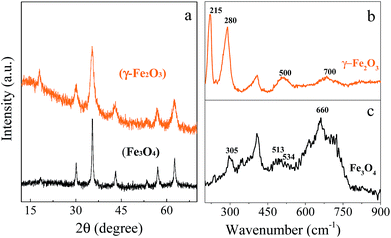
On the basis of the abovementioned experiment results, it is known the ferrous alkoxide precursor is unstable and can be easily oxidized. Therefore, a thermal transformation strategy was designed to prepare the chrysanthemum-like hierarchical nanostructures of γ-Fe2O3 and Fe3O4 with well-preserved morphological structure similar to that of the precursor. In order to determine the temperature for the thermal treatment of the precursor, the thermal gravimetric analysis (TG) and differential scanning calorimetric (DSC) were carried out. Fig. 4 shows a sharp mass loss of about 40% in weight and an obvious exothermic peak in the DSC curve around 308 °C, which indicates the precursor can be completely decomposed when the thermal temperature was above 308 °C. Therefore, we chose 350 °C to ensure a complete thermal transformation. When the precursor was heated in air to 350 °C and maintained for 1 h, the green precursor turned to reddish brown powder. X-ray diffraction (XRD) was carried out to investigate the crystal phase and crystallinity of the reddish brown product (Fig. 5a), which appears five main diffraction peaks around 2θ = 30.02, 35.35, 42.88, 56.89 and 62.57 degrees, all of which can be indexed to the (220), (311), (400), (511) and (440) planes of the γ-Fe2O3 (JPDS no. 39-1346). If the precursor was pyrolyzed at 350 °C for 1 h under protection of N2 atmosphere, the green precursor tuned to black. The XRD pattern of the black powder matches well with the reported data of Fe3O4 (JCPDS no. 85-1436). Due to the similarity of the XRD patterns of the γ-Fe2O3 and Fe3O4, Raman spectra were performed to further identify the difference. As shown in Fig. 5b, the peaks in the Raman spectrum of the γ-Fe2O3 around 500 and 700 cm−1 can be assigned as the T2g and A1g modes, respectively. While those peaks at 215 and 285 cm−1 are assigned to A1g and Eg mode of the α-Fe2O3, which can be ascribed to the degradation of γ-Fe2O3 to α-Fe2O3 induced by the laser irradiation.20 For the prepared Fe3O4 structures (Fig. 5c), the peaks at 305 and 534 cm−1 are assigned to the T2g mode, and the peaks at 513 and 660 cm−1 are characteristic peaks assigned to Eg and A1g mode, respectively.21
The overall morphology and size of the as-prepared iron oxide architectures after calcination treatment were studied by SEM, TEM and high-resolution TEM (HRTEM) to further investigate the morphology and architecture. As shown in Fig. 6a, the chrysanthemum-like morphology of the precursor well-preserved after thermal treatment in air. The nanobelt subunit building units also maintained integrated. TEM image further prove the maintenance of the overall morphology and structure of the precursor. HRSEM image (Fig. 6d) demonstrates that the nanobelts are further composed of well-crystallized nanoparticles and with mesopores of ∼4 nanometers distributed due to removal of organic species by calcination. Also shown in Fig. 6d is that the nanobelt appears polycrystalline nature and the particles have interplanar spacing of 0.296 nm, corresponding to the (220) lattice plane of γ-Fe2O3. The selected-area electron diffraction (SAED, inset picture in Fig. 6d) rings further indicate the polycrystalline nature of γ-Fe2O3. As illustrated in Fig. 6e and f, SEM and TEM images of Fe3O4 also proved the chrysanthemum-like 3D hierarchical structure maintained well after thermal treatment under protection of flowing N2 gas.
 | ||
| Fig. 6 SEM (a and b), TME (c) and HRTEM (d) images of the as-prepared γ-Fe2O3, SEM (e) and TEM (f) images of the as-prepared Fe3O4. Inset picture in Fig. 4c and d are the HRTEM and SAED pattern of the γ-Fe2O3. | ||
Fig. 7 shows the N2 adsorption–desorption isotherms and the pore size distributions of the as prepared γ-Fe2O3 and Fe3O4, which exhibits the typical type IV curves with H3 hysteresis loops according to the IUPAC classification, indicating occurrence of slit-like mesopores (2–50 nm) composed of sheet-like particles.22 The BET (Brunauer–Emmett–Teller) surface area and BJH (Barett–Joyner–Halenda) pore volume are 87.1 m2 g−1 and 0.165 cm3 g−1 for γ-Fe2O3, and 94.6 m2 g−1 and 0.177 cm3 g−1 for Fe3O4. Inset pictures in Fig. 6a and b indicate there are two types of pores distributed in the samples. One type is around 3.6 nm, which can be ascribed to the removal of organic moieties in the product by calcination treatment. The other type of around 12 nm could be the distance between the assembled nanobelts. Such hierarchical porous structure not only provides communicable channels for chemicals travelling from outer space into the inner voids of the material, but also supplies higher specific surface area for more pollutant molecules to be adsorbed, and thus bestow the prepared product great potentials in environmental field.
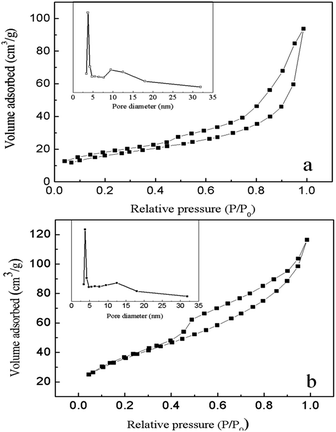 | ||
| Fig. 7 N2 adsorption–desorption isotherm and pore size distribution curves of the chrysanthemum-like γ-Fe2O3 (a) and Fe3O4 (b). | ||
The magnetic property of the chrysanthemum-like 3D hierarchical γ-Fe2O3 and Fe3O4 nanostructures were also investigated. Fig. 8 shows the magnetic hysteresis loops of the chrysanthemum-like γ-Fe2O3 and Fe3O4 measured at room temperature at the applied magnetic field from −20 to 20 kOe. Both samples exhibited a superparamagnetic characteristic at room temperature. The saturation magnetization of the chrysanthemum-like γ-Fe2O3 and Fe3O4 were 30.8 emu g−1 and 44.1 emu g−1 at a magnetic field of 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Oe, respectively, indicating a relatively strong magnetic response to the magnetic field. This property makes the products can be easily separated from aqueous solution under a magnetic field, and therefore endows them with great potential applications in environmental field.
000 Oe, respectively, indicating a relatively strong magnetic response to the magnetic field. This property makes the products can be easily separated from aqueous solution under a magnetic field, and therefore endows them with great potential applications in environmental field.
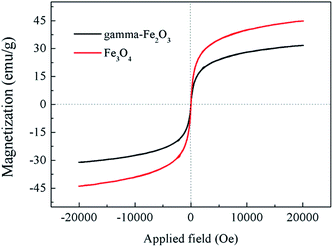 | ||
| Fig. 8 Magnetization curves measured at room temperature for the chrysanthemum-like 3D γ-Fe2O3 and Fe3O4 microflowers. | ||
The adsorption capacities of the as-prepared γ-Fe2O3 and Fe3O4 chrysanthemum-like hierarchical structures were investigated by using chlorophenols, e.g. 2,4-dichlorophenol (2,4-DCP) and 2,4,6-trichlorophenol (2,4,6-TCP) as probe organic pollutants. Fig. 9 shows the adsorption isotherm curves of the as-prepared γ-Fe2O3 and Fe3O4 for the probe pollutants, which fit well with the Langmuir adsorption model (r2 > 0.99) corresponding to complete monolayer adsorption. Also shown in Fig. 9 is that Fe3O4 nanostructures possess higher adsorption capacity for both the 2,4-DCP (81.9 mg g−1) and 2,4,6-TCP (107 mg g−1) than that of γ-Fe2O3 nanostructures (74.4 mg g−1 for 2,4-DCP and 83.7 mg g−1 for 2,4,6-TCP). The inset picture in Fig. 9 exhibits the adsorption kinetics of the prepared products for probe pollutants, which shows the plots fit well with the pseudo-first-order kinetics equation showing a linear pattern. The as-prepared Fe3O4 shows higher adsorption rate for both 2,4,6-TCP (0.0384 min−1) and 2,4-DCP (0.0159 min−1) than that of the γ-Fe2O3 (0.0228 min−1 for 2,4,6-TCP and 0.00813 min−1 for 2,4-DCP, respectively). This might be attributed to the higher BET surface area and pore volume, which supply more active sites not only on the surface but also in the inner voids of the adsorbent. Also, the porous structure supply communicable channels for pollutants travelling from the surface to inner voids of the mesoporous structure accelerating the mass transfer rate, and therefore results in higher adsorption rate of the Fe3O4 than γ-Fe2O3. It is also noteworthy that both the adsorbents have higher adsorption capacity for 2,4,6-TCP than 2,4-DCP. This can be ascribed to the physiochemical properties of the pollutants. It is known that more substituting group of Cl in the chlorophenol molecules lead to lower solubility than chlorophenols of fewer Cl substituting group. Therefore, 2,4,6-TCP have lower solubility than 2,4-DCP, which on the contrary results in higher adsorption.
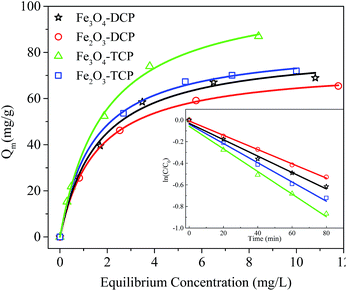 | ||
| Fig. 9 Adsorption isotherms and kinetics of the as-prepared γ-Fe2O3 and Fe3O4 for 2,4-dichlorophenol and 2,4,6-trichlorophenol. | ||
4. Conclusions
In summary, chrysanthemum-like 3D hierarchical γ-Fe2O3 and Fe3O4 nanostructures have been successfully fabricated by a facile one pot procedure in presence of urea and ethylene glycol. The unique structure was composed of nanobelt building units. Ostwald ripening phenomenon functioned in the early stage of the formation process, followed by re-growth driven by the inherent trend of minimizing system free energy. The as-prepared product also features mesoporous structure with high specific surface area and superparamagnetic property of high saturation magnetization. As-prepared magnetic iron oxide also shows higher adsorption capacity for chlorophenols. This study supplies a facile and reliable method for synthesizing 3D hierarchical magnetic γ-Fe2O3 and Fe3O4 materials that has potential applications in organic pollutants removal from aqueous system. They can also find their potential applications in many other fields like magnetic materials fabrication, gas sensors, etc.Acknowledgements
This work was jointly supported by the National Natural Science Foundation of China (no. 21207148), The National Key Technology R&D Program (no. 2012BAC07B02).Notes and references
- (a) T. Zhu, J. Li and Q. Wu, ACS Appl. Mater. Interfaces, 2011, 3, 3448 CrossRef CAS PubMed; (b) M. C. Orilall, N. M. Abrams, J. Lee, F. J. DiSalvo and U. Wiesner, J. Am. Chem. Soc., 2008, 130, 8882 CrossRef CAS PubMed; (c) J. Chen, L. N. Xu, W. Y. Li and X. L. Gou, Adv. Mater., 2005, 17, 582 CrossRef CAS; (d) H. T. Song, J. S. Choi, Y. M. Huh, S. Kim, Y. W. Jun, J. S. Suh and J. Cheon, J. Am. Chem. Soc., 2005, 127, 9992 CrossRef CAS PubMed; (e) X. M. Liu, X. G. Zhang and S. Y. Fu, Mater. Res. Bull., 2006, 41, 620 CrossRef CAS PubMed.
- (a) C. T. Yavuz, J. T. Mayo, W. W. Yu, A. Prakash, J. C. Falkner, S. Yean, L. Cong, H. J. Shipley, A. Kan, M. Tomson, D. Natelson and V. L. Colvin, Science, 2006, 314, 964 CrossRef PubMed; (b) L. Z. Zhang and J. C. Yu, Chem. Commun., 2003, 2078 RSC.
- (a) D. S. Xue, L. Y. Zhang, A. B. Gui and X. F. Xu, Appl. Phys. A: Mater. Sci. Process., 2005, 80, 439 CrossRef CAS; (b) Y. W. Jun, S. M. Lee, N. J. Kang and J. Cheon, J. Am. Chem. Soc., 2001, 123, 5150 CrossRef CAS.
- (a) W. Cheng, J. He, T. Yao, Z. Sun, Y. Jiang, Q. Liu, S. Jiang, F. Hu, Z. Xie, B. He, W. Yan and S. Wei, J. Am. Chem. Soc., 2014, 136, 10393 CrossRef CAS PubMed; (b) Q. Guo, S. J. Kim, M. Kar, W. N. Shafarman, R. W. Birkmire, E. A. Stach, R. Agrawal and H. W. Hillhouse, Nano Lett., 2008, 8, 2982 CrossRef CAS PubMed.
- (a) J.-S. Xu and Y.-J. Zhu, J. Colloid Interface Sci., 2012, 385, 58 CrossRef CAS PubMed; (b) B. Xu, B. Huang, H. Cheng, Z. Wang, X. Qin, X. Zhang and Y. Dai, Chem. Commun., 2012, 48, 6529 RSC; (c) G. L. Li, H. Y. Zhang, J. Lan, J. Li, Q. W. Chen, J. Y. Liu and G. B. Jiang, Dalton Trans., 2013, 42, 8541 RSC.
- (a) L. S. Zhong, J. S. Hu, H. P. Liang, A. M. Cao, W. G. Song and L. J. Wan, Adv. Mater., 2006, 18, 2426 CrossRef CAS; (b) S. W. Cao, Y. J. Zhu, M. Y. Ma, L. Li and L. Zhang, J. Phys. Chem. C, 2008, 112, 1851 CrossRef CAS.
- (a) T. Lan, Y. Liu, J. Dou, Z. Hong and M. Wei, J. Mater. Chem. A, 2014, 2, 1102 RSC; (b) L. R. Hu, Y. M. Ren, H. X. Yang and Q. Xu, ACS Appl. Mater. Interfaces, 2014, 6, 14644 CrossRef CAS PubMed; (c) L. Han, D. P. Yang and A. H. Liu, Biosens. Bioelectron., 2015, 63, 145 CrossRef CAS PubMed; (d) Y. Zhang, C. Hu, B. Feng, X. Wang and B. Wan, Appl. Surf. Sci., 2011, 257, 10679 CrossRef CAS PubMed; (e) W. Zhao, H. Chen, Y. Li, L. Li, M. Lang and J. Shi, Adv. Funct. Mater., 2008, 18, 2780 CrossRef CAS.
- (a) C. Corot, P. Robert, J. M. Idee and M. Port, Adv. Drug Delivery Rev., 2006, 58, 1471 CrossRef CAS PubMed; (b) D. A. Wheeler, G. Wang, Y. Ling, Y. Li and J. Z. Zhang, Energy Environ. Sci., 2012, 5, 6682 RSC; (c) P. Saharan, G. R. Chaudhary, S. K. Mehta and A. Umar, J. Nanosci. Nanotechnol., 2014, 14, 627 CrossRef CAS PubMed; (d) S. Laurent, D. Forge, M. Port, A. Roch, C. Robic, L. V. Elst and R. N. Muller, Chem. Rev., 2008, 108, 2064 CrossRef CAS PubMed; (e) S. N. Sun, C. Wei, Z. Z. Zhu, Y. L. Hou, S. S. Venkatraman and Z. C. Xu, Chin. Phys. B, 2014, 23, 19 Search PubMed; (f) M. B. Gawande, P. S. Branco and R. S. Varma, Chem. Soc. Rev., 2013, 42, 3371 RSC.
- U. Cvelbar, Z. Q. Chen, M. K. Sunkara and M. Mozetic, Small, 2008, 4, 1610 CrossRef CAS PubMed.
- Y. Hu, H. Qian, T. Mei, J. Guo and T. White, Mater. Lett., 2010, 64, 1095 CrossRef CAS PubMed.
- G. H. Jiang and J. S. Jiang, J. Inorg. Mater., 2005, 20, 1066 CAS.
- G. Gao, H. Wu, Y. Zhang, K. Wang, P. Huang, X. Zhang, S. Guo and D. Cui, J. Mater. Chem., 2011, 21, 12224 RSC.
- (a) D. Wang, C. Song, Y. Zhao and M. Yang, J. Phys. Chem. C, 2008, 112, 12710 CrossRef CAS; (b) P. Hu, L. Yu, A. Zuo, C. Guo and F. Yuan, J. Phys. Chem. C, 2009, 113, 900 CrossRef CAS; (c) L. S. Zhong, J. S. Hu, H. P. Liang, A. M. Cao, W. G. Song and L. J. Wan, Adv. Mater., 2006, 18, 2426 CrossRef CAS; (d) S. Basak, K. S. Rane and P. Biswas, Chem. Mater., 2008, 20, 4906 CrossRef CAS; (e) X. Y. Li, Z. J. Si, Y. Q. Lei, X. N. Li, J. K. Tang, S. Y. Song and H. J. Zhang, CrystEngComm, 2011, 13, 642 RSC; (f) L. Han, Y. Chen and Y. Wei, CrystEngComm, 2012, 14, 4692 RSC; (g) L. S. Zhong, J. S. Hu, H. P. Liang, A. M. Cao, W. G. Song and L. J. Wan, Adv. Mater., 2006, 18, 2426 CrossRef CAS; (h) Q. Zhang, X. W. Lu, L. Y. Chen, Y. X. Shi, T. Xu and M. L. Liu, Mater. Lett., 2013, 106, 447 CrossRef CAS PubMed.
- Y.-D. Feng, Z.-Q. Tan and J.-F. Liu, J. Sep. Sci., 2011, 34, 965 CrossRef CAS PubMed.
- (a) Q. Peng, Y. J. Dong and Y. D. Li, Angew. Chem., Int. Ed., 2003, 42, 3027 CrossRef CAS PubMed; (b) X. M. Sun and Y. D. Li, Angew. Chem., Int. Ed., 2004, 43, 597 CrossRef PubMed; (c) H. Deng, X. L. Li, Q. Peng, X. Wang, J. P. Chen and Y. D. Li, Angew. Chem., Int. Ed., 2005, 44, 2782 CrossRef CAS PubMed.
- M. L. Hair, J. Non-Cryst. Solids, 1975, 19, 299 CrossRef CAS.
- D. Larcher, G. Sudant, R. Patrice and J. M. Tarascon, Chem. Mater., 2003, 15, 3543 CrossRef CAS.
- S. W. Cao and Y. J. Zhu, J. Phys. Chem. C, 2008, 112, 12149 CAS.
- W. Ostwald, Z. Phys. Chem-Stoch. Ve, 1900, 34, 495 Search PubMed.
- J.-S. Xu and Y.-J. Zhu, ACS Appl. Mater. Interfaces, 2012, 4, 4752 CAS.
- X. Zhang, Y. Niu, X. Meng, Y. Li and J. Zhao, CrystEngComm, 2013, 15, 8166 RSC.
- (a) D. Y. Zhao, J. L. Feng, Q. S. Huo, N. Melosh, G. H. Fredrickson, B. F. Chmelka and G. D. Stucky, Science, 1998, 279, 548 CrossRef CAS; (b) M. Kruk and M. Jaroniec, Chem. Mater., 2001, 13, 3169 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2015 |