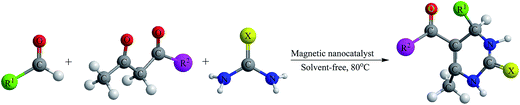Immobilization of phosphomolybdic acid nanoparticles on imidazole functionalized Fe3O4@SiO2: a novel and reusable nanocatalyst for one-pot synthesis of Biginelli-type 3,4-dihydro-pyrimidine-2-(1H)-ones/thiones under solvent-free conditions†
Jaber Javidi*a,
Mohsen Esmaeilpour*b and
Fatemeh Nowroozi Dodejiac
aDepartment of Pharmaceutics, School of Pharmacy, Shahid Beheshti University of Medical Sciences, Tehran, Iran
bChemistry Department, College of Science, Shiraz University, Shiraz, Iran
cStudents Research Committee, School of Pharmacy, Shahid Beheshti University of Medical Sciences, Tehran, Iran
First published on 21st November 2014
Abstract
This article introduces Fe3O4@SiO2-imid-H3PMo12O40 nanoparticles as heterogenic catalyst for the synthesis of 3,4-dihydropyrimidinones under solvent-free condition. This reaction proceeds through acetoacetate esters and aldehyde derivatives followed by cyclisation with a urea to the dihydropyrimidinone. Excellent yields of dihydropyrimidinones are obtained within a short reaction time. The suggested method offers several advantages such as short reaction times, high yields, easy purification, a cleaner reaction and ease of recovery and reusability of the catalyst by a magnetic field. Also, the aforementioned nanocatalyst can be easily recovered by an external magnetic field and reused for subsequent reactions at least 5 times without noticeable deterioration in catalytic activity. Particle size distribution, surface area, magnetic properties and percent of leaching of H3PMo12O40 (PMA) after any reaction were investigated by dynamic light scattering (DLS), nitrogen physisorption method (BET) and Inductively Coupled Plasma (ICP) analyzer.
Introduction
3,4-Dihydropyrimidin-2(1H)-ones are interesting compounds due to their biological importance and exhibit a wide range of pharmaceutical and therapeutic properties.1 These products are medicinally important as calcium channel blockers, anti-inflammatory agents, antihypertensive, anti-tumor, anti-inflammatory, adrenergic and neuropeptide antagonists.2 They are prepared by the Biginelli reaction. The Biginelli reaction consists of a three compound condensation of an aldehyde, α,β-ketoester and urea in the presence of an acid catalyst.3Recently, several procedures have been reported with Lewis acids and protic acids as catalysts for the synthesis of dihydropyrimidinone derivatives.4 Although most reported methods are worthwhile, many of them have one or more of the following drawbacks: tedious workup of the reaction mixture, difficulty in separation and recovery of the catalyst, expensive moisture sensitive reaction conditions, toxic metals, low yields and long reaction times.
Therefore, in spite of the large number of methods reported for this transformation, the development of more efficient, simple and milder protocols with suitable catalysts is needed.
Brønsted acids such as Keggin-type heteropolyacids (HPAs) have been used as efficient catalysts for a variety of organic reactions because of their superacidic and redox properties, low toxicity, ease of handling, low cost, high thermal stability, high proton mobility, water tolerance, stronger acids than homogeneous acid catalysts, development of clean technologies, recoverability and reusability.5 Although HPAs in their acidic form are versatile compounds, their main disadvantages are high solubility in polar solvents and low surface area (<10 m2 g−1). Therefore, in a homogeneous reaction the isolation of the products and the reuse of the catalyst after reaction become difficult.6 Therefore, in order to overcome this problem, these materials disperse on supports (such as silica, acidic ion-exchange resins, active carbon and etc.) which possess large surface area. The use of support allows the heteropolyacids to be dispersed over a large surface area and increases theirs catalytic activity.7
Nanoparticles have properties intermediate between those of bulk and single particles.8 They are expected to be suitable candidates for the design of highly active and selective catalysts. Nanostructured materials, a new branch of materials research, are attracting a great deal of attention because of their potential applications in areas such as optics,9 ceramics,10 electronics,11 catalysis,12 magnetic data storage,13 and nanocomposites. The unique properties and the improved performances of nanomaterials are determined by their sizes, surface structures and inter-particle interactions.
In previous work,14 we introduce a simple, repaid, inexpensive and one step method, solvothermal, for synthesis of H3PMo12O4 nanoparticles (PMAn) from H3PMo12O4 bulk particles (PMAb). Acidity of as-prepared nanoparticles was investigated by pyridine adsorption method. Results showed rising acidity by declining particle size of HPAs.
In recent years, nanomagnetic materials have emerged as viable alternatives to conventional heterogeneous supports.15 The magnetic separation technology offers many advantages over conventional filtration and other purification methods.
In family of nanomagnetic materials, magnetite (Fe3O4) exhibits excellent properties, so that makes it great potential applications in magnetic bio-separations,16 drug delivery,17 magnetic resonance imaging (MRI) contrast agents,18 hyperthermia treatment of cancer cells19 and catalysts.20
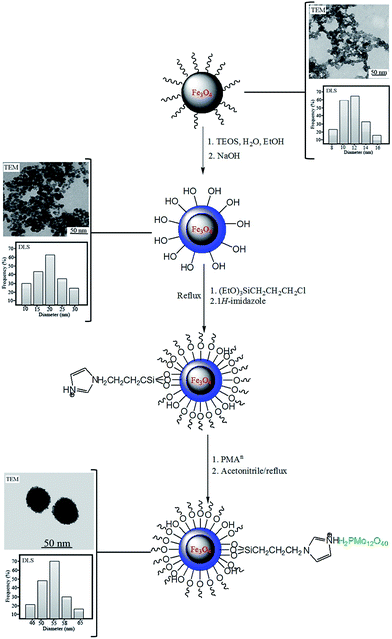
In our previous study,21 PMAn were supported on magnetic Fe3O4@SiO2-imid nanoparticles. Compared to other substrates (silica, acidic ion-exchange resins, active carbon and nano titania7b), Fe3O4@SiO2-imid nanoparticles have various advantages such as high loading capacity, low leaching and simple and efficient recovery procedure. Fig. 1 presents the procedure for the preparation of Fe3O4@SiO2-imid-PMAn stepwise. In this work, we tried to synthesize dihydropyrimidinones derivatives with using Fe3O4@SiO2-imid-PMAn and Fe3O4@SiO2-imid-PMAb catalysis.
Results and discussion
In our previous work,21 the Fe3O4, Fe3O4@SiO2 and Fe3O4@SiO2-imid-PMAn nano catalysts were characterized by various methods such as transmission electron microscopy (TEM), scanning electron microscopy (SEM), dynamic light scattering (DLS), Fourier transform infrared (FT-IR), vibrating sample magnetometer (VSM) and etc. As shown in Fig. 1 Fe3O4@SiO2-imid-PMAn nanoparticles have spherical shapes with approximately 50 nm diameters. The size distribution of these is centered at a value of 55 nm. The magnetic properties of Fe3O4, Fe3O4@SiO2, Fe3O4@SiO2-imid-PMAn nanoparticles were measured by VSM at room temperature. All the samples show a typical superparamagnetic behavior. Hysteresis phenomenon was not found and the magnetization and demagnetization curves were coincident. The saturation magnetization of samples (a–c) is 63.4, 39.7, 33.2 emu g−1, respectively.We wish to report that immobilization of phosphomolybdic acid on imidazole functionalized Fe3O4@SiO2 (Fe3O4@SiO2-imid-PMAn and Fe3O4@SiO2-imid-PMAb) catalyzed the Biginelli reaction of aromatic aldehydes, acetoacetate esters and urea to yielding the dihydropyrimidinones. In pursuit of developing a solvent-free methodology for the preparation of these biologically important compounds, we decided to explore the use of these magnetic nanocatalysts for the synthesis of various substituted 3,4-dihydropyrimidin-2(1H)-ones by Biginelli protocol under solvent-free condition (Scheme 1).
Initially the reaction between benzaldehyde (1 mmol), acetoacetate esters (1 mmol) and urea (1.5 mmol), as the model reaction was examined in the presence of various amount of the nanocatalysts and the results are presented in Table 1, entries 1–5. The best result was attributed to the reaction of benzaldehyde (1 mmol), ethyl acetoacetate (1 mmol) and urea (1.5 mmol) in presence of 0.03 g or 0.04 g of Fe3O4@SiO2-imid-PMAn or Fe3O4@SiO2-imid-PMAb nanocatalysts at 80 °C (Table 1, entry 4). The results show clearly that nanocatalysts are effective for this transformation and in the absence of it, the reaction did not take place even after higher reaction time (Table 1, entry 1).
| Entry | Solvent | Condition | Fe3O4@SiO2-imid-PMAn | Fe3O4@SiO2-imid-PMAb | ||||
|---|---|---|---|---|---|---|---|---|
| Amount of catalysts (g) | Time (min) | Yieldb (%) | Amount of catalysts (g) | Time (min) | Yieldb (%) | |||
| a Reaction condition: benzaldehyde (1 mmol), ethylacetoacetate (1 mmol), urea (1.5 mmol).b Isolated yield. | ||||||||
| 1 | Solvent-free | 80 °C | — | 60 | Trace | — | 90 | Trace |
| 2 | Solvent-free | 80 °C | 0.01 | 60 | 71 | 0.02 | 90 | 62 |
| 3 | Solvent-free | 80 °C | 0.02 | 30 | 92 | 0.03 | 90 | 81 |
| 4 | Solvent-free | 80 °C | 0.03 | 30 | 93 | 0.04 | 45 | 89 |
| 5 | Solvent-free | 80 °C | 0.04 | 30 | 92 | 0.05 | 45 | 89 |
| 6 | Solvent-free | 60 °C | 0.03 | 45 | 82 | 0.04 | 60 | 78 |
| 7 | Solvent-free | r.t | 0.03 | 60 | 21 | 0.04 | 90 | 17 |
| 8 | Toluene | Reflux | 0.03 | 60 | 83 | 0.04 | 90 | 73 |
| 9 | THF | Reflux | 0.03 | 60 | 80 | 0.04 | 90 | 75 |
| 10 | Ethanol | Reflux | 0.03 | 60 | 86 | 0.04 | 90 | 80 |
| 11 | H2O | Reflux | 0.03 | 60 | 38 | 0.04 | 90 | 26 |
| 12 | CH3CN | Reflux | 0.03 | 60 | 87 | 0.04 | 90 | 80 |
| 13 | CHCl3 | Reflux | 0.03 | 60 | 45 | 0.04 | 90 | 33 |
| 14 | CH2Cl2 | Reflux | 0.03 | 60 | 51 | 0.04 | 90 | 32 |
The effect of temperature was studied by carrying out the model reaction at different temperatures under solvent-free condition (room temperature, 60 °C and 80 °C) and the best results were obtained at 80 °C (Table 1, entries 4, 6 and 7). Then, the reactions were conducted in refluxing toluene, THF, EtOH, H2O, CH3CN, CH2Cl2 and CHCl3 as solvents and under solvent-less condition (Table 1, entries 4 and 8–14). When the reaction was performed in solvent-free, the progress of the reaction was higher in comparison with solvent conditions. Therefore, the solvent-free condition was used for the synthesis of dihydropyrimidinones derivatives.
In order to study the generality of the procedure, a series of aromatic aldehydes with the electron-donating and electron-withdrawing substituent were reacted with ethyl acetoacetate and urea under the optimized conditions. The results are presented in Table 2. Aromatic aldehydes containing both electron donating and electron withdrawing groups afforded high yields of the desired products.
| Entry | R1 | R2 | X | Fe3O4@SiO2-imid-PMAn | Fe3O4@SiO2-imid-PMAb | m.p. (°C) | |||
|---|---|---|---|---|---|---|---|---|---|
| Time (min) | Yieldb (%) | Time (min) | Yieldb (%) | Found | Reported (ref.) | ||||
| a Reaction condition aldehyde (1 mmol), β-diketoester (1 mmol), urea or thiourea (1.5 mmol), Fe3O4@SiO2-imid-PMAn (0.03 g) or and Fe3O4@SiO2-imid-PMAb (0.04 g), solvent-free, 80 °C.b Isolated yield. | |||||||||
| 1 | C6H5 | OEt | O | 30 | 93 | 45 | 89 | 205–206 | 206–208 (ref. 22) |
| 2 | 4-Br–C6H4 | OEt | O | 35 | 86 | 55 | 81 | 213–214 | 213–215 (ref. 23) |
| 3 | 2-Cl–C6H4 | OEt | O | 35 | 88 | 50 | 83 | 215–216 | 216–218 (ref. 24) |
| 4 | 4-Cl–C6H4 | OEt | O | 35 | 90 | 50 | 87 | 215–216 | 214–215 (ref. 25) |
| 5 | 2-NO2–C6H4 | OEt | O | 25 | 92 | 30 | 89 | 222–223 | 221 (ref. 26) |
| 6 | 3-NO2–C6H4 | OEt | O | 25 | 90 | 30 | 92 | 227–228 | 229–231 (ref. 27) |
| 7 | 4-NO2–C6H4 | OEt | O | 20 | 94 | 30 | 91 | 210 | 209–211 (ref. 25) |
| 8 | 4-Me–C6H4 | OEt | O | 35 | 91 | 50 | 88 | 203–204 | 205–206 (ref. 22) |
| 9 | 2-MeO–C6H4 | OEt | O | 40 | 91 | 60 | 85 | 262–263 | 262 (ref. 28) |
| 10 | 4-MeO–C6H4 | OEt | O | 40 | 90 | 60 | 82 | 200–201 | 201–203 (ref. 25) |
| 11 | 2-Furyl | OEt | O | 25 | 95 | 30 | 91 | 205–206 | 203–205 (ref. 29) |
| 12 | 2-Thiophene | OEt | O | 30 | 92 | 60 | 85 | 210–211 | 209–210 (ref. 30) |
| 13 | PhCH = CH | OEt | O | 40 | 85 | 60 | 80 | 223–224 | 225–227 (ref. 27) |
| 14 | C6H5 | OMe | O | 30 | 92 | 50 | 89 | 208–209 | 207–210 (ref. 25) |
| 15 | C6H5 | CH3 | O | 30 | 88 | 50 | 88 | 235–236 | 233–236 (ref. 31) |
| 16 | C6H5 | OEt | S | 30 | 91 | 45 | 84 | 211 | 208–210 (ref. 27) |
| 17 | 4-Cl–C6H4 | OEt | S | 25 | 90 | 45 | 88 | 179–180 | 180–182 (ref. 27) |
| 18 | 4-Me–C6H4 | OEt | S | 30 | 89 | 45 | 86 | 191–193 | 192–194 (ref. 27) |
| 19 | 4-MeO–C6H4 | OEt | S | 35 | 87 | 45 | 83 | 153 | 152–153 (ref. 32) |
Also heterocyclic aromatic compounds, such as furyl-2-carbaldehyde and thiophen-2-carbaldehyde produced the corresponding dihydropyrimidinones in excellent yields (Table 2, entries 11 and 12). Also, more importantly acid sensitive aldehydes such as 2-furfural, underwent condensation without formation of any side products (Table 2, entry 11).
The reaction of other 1,3-dicarbonyl compounds such as acetylacetone also run with benzaldehyde and urea in the presence of Fe3O4@SiO2-imid-PMAn and Fe3O4@SiO2-imid-PMAb nanoparticles under solvent free conditions and the corresponding dihydropyrimidinones were obtained in high yields (Table 2, entry 15). Also, the reaction of benzaldehyde and its 4-chloro, 4-methyl and 4-methoxy derivatives were treated with ethyl acetoacetate and thiourea in the presence of nanocatalysts under solvent-free at 80 °C and the related Biginelli products were obtained in high yields (Table 2, entry 16–19).
A comparison among Fe3O4@SiO2-imid-PMAn and Fe3O4@SiO2-imid-PMAb and the other catalysts, which were reported in the literature, in synthesis of 3,4-dihydropyrimidinones revealed advantages of these magnetic nanocatalysts over the most of them in term of higher yield and shorter reaction time (Table 3). According to these findings, Fe3O4@SiO2-imid-PMAn and Fe3O4@SiO2-imid-PMAb led to the best result and produced the maximum conversion in the shortest time (Table 3, entry 1, 2).
| Entry | Catalyst | Time | Yielda (%) | Literature reference |
|---|---|---|---|---|
| a Isolated yield. | ||||
| 1 | Fe3O4@SiO2-imid-PMAn | 30 min | 93 | This work |
| 2 | Fe3O4@SiO2-imid-PMAb | 45 min | 89 | This work |
| 3 | 12-Molybdophosphoric acid | 5 h | 80 | 24 |
| 4 | 12-Tungstophosphoric acid (PWA) | 6–7 h | 75 | 33 |
| 5 | Trichloroisocyanuric acid (TCCA) | 12 h | 94 | 34 |
| 6 | Silica sulfuric acid | 6 h | 91 | 35 |
| 7 | Sulfuric acid | 18 h | 71 | 3 |
| 8 | Montmorillonite KSF | 48 h | 82 | 28 |
| 9 | TiCl4–MgCl2·4CH3OH | 3 h | 90 | 36 |
| 10 | L-Pyrrolidine-2-carboxylicacid-4-hydrogen sulfate supported on silica gel | 6 h | 92 | 37 |
| 11 | Amberlyst-70 | 3 h | 81 | 38 |
| 12 | Sm(ClO4)3 | 9 | 72 | 39 |
| 13 | Chiral ligand/HCl | 6 day | 81 | 40 |
| 14 | NH4H2PO4 | 2 h | 85 | 41 |
| 15 | poly(4-vinylpyridine-co-divinylbenzene)–Cu(II) complex | 24 h | 70 | 42 |
Considering the general mechanistic pathway, the suggested mechanism for the formation of (3) using Fe3O4@SiO2-imid-PMAn or Fe3O4@SiO2-imid-PMAb is shown in Scheme 2. Protonation of the carbonyl group by Brønsted acid generates an electrophilic center on the carbonyl carbon atom, which is easily attacked by the urea to form minimum intermediate (1), which is the key rate-limiting step. Interception of this minimum intermediate (1) by β-dicarbonyl produces an open chain ureide (2), which subsequently cyclizes through dehydration process yielding compounds (3).
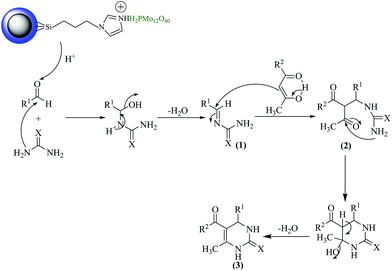 | ||
| Scheme 2 Proposed mechanism for synthesis of dihydropyrimidinones via Biginelli condensation protocol. | ||
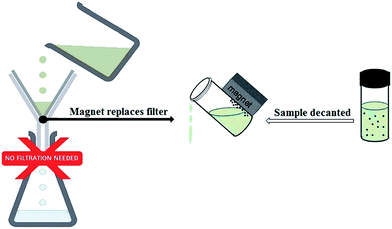
However, magnetically supported catalysts can be recovered with an external magnet due to the paramagnetic character of the support, resulting in remarkable catalyst recovery without the need for a filtration step (Scheme 3).
The activity of the recycle catalysts were also examined under the optimized conditions and the desired products were obtained in high yields after 4 runs without distinct deterioration in catalytic activity (Fig. 2).
The amounts of the nano heteropolyacid leaching for the reactions under the optimized conditions were detected. The molybdenum (Mo) amount in reaction medium after each reaction cycle was measured through ICP and details are provided in Table 4. The analysis of the reaction mixture by the ICP technique showed that the leaching of H3PMo12O40 was negligible. Additionally, the size and surface area of catalysts after each reaction cycle was investigated by DLS and nitrogen physisorption method (BET) respectively and results are provided in Table 4. As shown, the size of catalysts will be increased after each cycle. Generally, leaching of H3PMo12O40, increase of catalyst size and decrease of surface area led to a decrease in the yield of reaction.
Conclusion
In conclusion, we report here a high yielding one-pot synthesis of 3,4-dihydropyrimidin-2-(1H)-ones and thione analogs from readily available aldehydes, acetoacetate esters and urea or thiourea in presence of efficient magnetic nanocatalysts by Biginelli reactions under solvent-free conditions. The protocol offers several advantages such as easy preparation, heterogeneous nature and easy separation of the catalyst, short reaction times, easy isolation, very good yields and simple work up procedures. Also, in solvent-free reactions the purification process is simplified and the reaction is environmentally friendly. The nanocatalysts could be successfully recovered and recycled at least for five runs without noticeably decreasing in catalytic activity.Experimental
General methods
All starting materials were purchased from Fluka. The reactions were monitored by thin layer chromatography (TLC). 1H NMR, 13C NMR spectra were obtained at 250 MHz using a Bruker spectrometer in CDCl3 as the solvent and tetramethyl silane (TMS) as internal reference. Fourier transform infrared (FT-IR) spectra were obtained using a Shimadzu FT-IR 8300 spectrophotometer. Melting points were determined on a Mel-Temp apparatus. Magnetic characterization was carried out on a vibrating sample magnetometer (Meghnatis Daghigh Kavir Co., Iran) at room temperature, and dynamic light scattering (DLS) was recorded on a HORIBA-LB550 instrument. A transmission electron microscope (Philips EM208) with an accelerating voltage of 100 kV was used to examine morphology, and size of the nanoparticles. All compounds were identified by comparison of their spectral data and physical properties with those of the authentic sample and all yields refer to isolated products.General procedure
After completion of the reaction, ethyl acetate was added to the solidified mixture and the insoluble catalyst was separated by magnetic field. The filtrate was dried and organic medium was removed with a rotary evaporator under reduced pressure. Then, the resulting solid product was recrystallized from ethanol to give pure product.
Spectral data
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H), 6.38 (d, J = 15.8, 1H, H–C
C–H), 6.38 (d, J = 15.8, 1H, H–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 7.44–7.26 (m, 5H, Ar–H), 7.54 (s, 1H, NH), 9.15 (s, 1H, NH). 13C-NMR (62.9 MHz, DMSO-d6): δ = 14.18, 17.69, 51.82, 59.15, 97.70, 126.26, 127.51, 127.74, 128.04, 129.89, 136.16, 148.49, 152.57, 165.14. IR (KBr): 3241, 1704, 1650, cm−1.
CH), 7.44–7.26 (m, 5H, Ar–H), 7.54 (s, 1H, NH), 9.15 (s, 1H, NH). 13C-NMR (62.9 MHz, DMSO-d6): δ = 14.18, 17.69, 51.82, 59.15, 97.70, 126.26, 127.51, 127.74, 128.04, 129.89, 136.16, 148.49, 152.57, 165.14. IR (KBr): 3241, 1704, 1650, cm−1.Acknowledgements
Authors are grateful to the council of Iran National Science Foundation and University of Shiraz for their unending effort to provide financial support to undertake this work.References
- (a) A. K. Sharma, S. Jayakumar, M. S. Hundal and M. P. Mahajan, J. Chem. Soc., Perkin Trans. 1, 2002, 774 RSC; (b) S. Sasaki, N. Cho, Y. Nara, M. Harada, S. Endo and N. Suzuki, J. Med. Chem., 2003, 46, 113 CrossRef CAS PubMed; (c) S. Bartolini, A. Mai, M. Artico, N. Paesano, D. Rotili and C. Spadafora, J. Med. Chem., 2005, 48, 6776 CrossRef CAS PubMed; (d) A. R. Khosropour, I. Mohammadpoor-Baltork and H. Ghorbankhani, Catal. Commun., 2006, 7, 713 CrossRef CAS PubMed.
- (a) C. O. Kappe, Eur. J. Med. Chem., 2000, 35, 1043 CrossRef CAS; (b) C. O. Kappe, O. V. Shishkin, G. Uray and P. Verdino, Tetrahedron, 2000, 56, 1859 CrossRef CAS.
- D. Shobha, M. A. Chari, A. Mano, S. T. Selvan, K. Mukkanti and A. Vinu, Tetrahedron, 2009, 65, 10608 CrossRef CAS PubMed.
- (a) R. Ghosh, S. Maiti and A. Chakraborty, J. Mol. Catal. A: Chem., 2004, 217, 47 CrossRef CAS PubMed; (b) A. S. Paraskar, G. K. Dewker and A. Sudalai, Tetrahedron Lett., 2003, 44, 3305 CrossRef CAS; (c) S. Tu, F. Fang, S. L. Zhu, T. X. Zhang and Q. Zhuang, Synlett, 2004, 3, 537 CrossRef PubMed; (d) X. H. Chen, X. Y. Xu, H. Liu, L. F. Cun and L. Z. Gong, J. Am. Chem. Soc., 2006, 128, 14802 CrossRef CAS PubMed; (e) W. Y. Chen, S. Qin and J. R. Jin, Catal. Commun., 2007, 8, 123 CrossRef CAS PubMed; (f) M. M. Heravi, L. Ranjbar, F. Derikvand and B. Alimadadi, Mol. Diversity, 2008, 12, 191 CrossRef CAS PubMed.
- M. Esmaeilpour, J. Javidi, F. Dehghani and F. Nowroozi Dodeji, New J. Chem., 2014, 38, 5453 RSC.
- L. T. Aany Sofia, A. Krishnan, M. Sankar, N. K. Kala Raj, P. Manikandan, P. R. Rajamohanan and T. G. Ajithkumar, J. Phys. Chem. C, 2009, 113, 21114 Search PubMed.
- (a) Z. Zhang, F. Zhang, Q. Zhu, W. Zhao, B. Ma and Y. Ding, J. Colloid Interface Sci., 2011, 360, 189 CrossRef CAS PubMed; (b) A. Davood, K. Seyed-Mola and N. Razieh, J. Chem. Sci., 2014, 126, 95 CrossRef.
- M. Salavati-Niasari, J. Javidi and F. Davar, Ultrason. Sonochem., 2010, 17, 870 CrossRef CAS PubMed.
- K. Lance Kelly, E. Coronado, L. Lin Zhao and G. C. Schatz, J. Phys. Chem. B, 2003, 107, 668 CrossRef.
- D. Vollath, D. Vinga Szabó and J. Hauβelt, J. Eur. Ceram. Soc., 1997, 17, 1317 CrossRef CAS.
- W. Lu and C. M. Lieber, Nat. Mater., 2007, 6, 841 CrossRef CAS PubMed.
- M. Esmaeilpour, A. R. Sardarian and J. Javidi, Appl. Catal., A, 2012, 445–446, 359 CrossRef CAS PubMed.
- G. Reiss and A. Hütten, Nat. Mater., 2005, 4, 725 CrossRef CAS PubMed.
- J. Javidi, M. Esmaeilpour, Z. Rahiminezhad and F. Nowroozi Dodeji, J. Cluster Sci., 2014, 25, 1511 CrossRef CAS.
- (a) S. Shylesh, J. Schweizer, S. Demeshko, V. Schunemann, S. Ernst and W. R. Thiel, Adv. Synth. Catal., 2009, 351, 1789 CrossRef CAS; (b) E. Mohsen, J. Jaber, M. A. Mehdi and N. D. Fatemeh, J. Iran. Chem. Soc., 2014, 11, 499 CrossRef CAS.
- I. J. Bruce and T. Sen, Langmuir, 2005, 21, 7029 CrossRef CAS PubMed.
- (a) C. Alexiou, R. Jurgons, R. Schmid, A. Hilpert, C. Bergemann, F. Parak and H. Iro, J. Magn. Magn. Mater., 2005, 293, 389 CrossRef CAS PubMed; (b) J. L. Zhang, R. S. Srivastava and R. D. K. Misra, Langmuir, 2007, 23, 6342 CrossRef CAS PubMed.
- Y. M. Huh, E. S. Lee, J. H. Lee, Y. W. Jun, P. H. Kim, C. O. Yun, J. H. Kim and J. S. Suh, Adv. Mater., 2007, 19, 3109 CrossRef CAS.
- (a) M. F. Kircher, U. Mahmood, R. S. King, R. Weissleder and L. A. Josephson, Cancer Res., 2003, 63, 8122 CAS; (b) J. Jaber and E. Mohsen, Colloids Surf., B, 2013, 102, 265 CrossRef CAS PubMed; (c) H. Pardoe, P. R. Clark, T. G. Pierre, P. Moroz and S. K. A. Jones, Magn. Reson. Imaging, 2003, 21, 483 CrossRef CAS.
- (a) E. W. Wong, M. J. Bronikowski, M. E. Hoenk, R. S. Kowalczyk and B. D. Hunt, Chem. Mater., 2005, 17, 237 CrossRef CAS; (b) M. Esmaeilpour, J. Javidi, F. Nowroozi Dodeji and H. Hassannezhad, J. Iran. Chem. Soc., 2014, 11, 1703 CrossRef CAS PubMed.
- M. Esmaeilpour, J. Javidi and M. Zandi, Mater. Res. Bull., 2014, 55, 78 CrossRef CAS PubMed.
- D. S. Bose, L. Fatima and H. B. Mereyala, J. Org. Chem., 2003, 68, 587 CrossRef CAS PubMed.
- D. Russowsky, F. A. Lopes, V. S. S. Silva, K. F. S. Canto, M. G. Montes Doca and M. N. Godoi, J. Braz. Chem. Soc., 2004, 15, 165 CrossRef CAS PubMed.
- M. M. Heravi, K. Bakhtiari and F. F. Bamoharram, Catal. Commun., 2006, 7, 373 CrossRef CAS PubMed.
- E. H. Hu, D. R. Silder and U. H. Dolling, J. Org. Chem., 1998, 63, 3454 CrossRef CAS.
- A. Shaabani and A. Bazgir, Tetrahedron Lett., 2004, 45, 2575 CrossRef CAS PubMed.
- N. Y. Fu, Y. F. Yuan, Z. Cao, S. W. Wang, J. T. Wang and C. Peppe, Tetrahedron, 2002, 58, 4801 CrossRef CAS.
- F. Bigi, S. Carloni, B. Frullanti, R. Maggi and G. Sartori, Tetrahedron Lett., 1999, 40, 3465 CrossRef CAS.
- C. V. Reddy, M. Mahesh, P. V. K. Raju, T. R. Babu and V. V. N. Reddy, Tetrahedron Lett., 2002, 43, 2657 CrossRef CAS.
- G. Sabithia, G. S. K. K. Reddy, C. S. Reddy and J. S. Yadav, Synlett, 2003, 6, 858 CrossRef.
- M. Gohain, D. Prajapati and J. S. Sandhu, Synlett, 2004, 2, 235 Search PubMed.
- C. O. Kappe, J. Org. Chem., 1997, 62, 7201 CrossRef CAS PubMed.
- M. M. Heravi, F. Derikvand and F. Bamoharram, J. Mol. Catal. A: Chem., 2005, 242, 173 CrossRef CAS PubMed.
- M. A. Bigdeli, S. Jafari, Gh. H. Mahdavinia and H. Hazarkhani, Catal. Commun., 2007, 8, 1641 CrossRef CAS PubMed.
- P. Salehi, M. Dabiri, M. A. Zolfigol and M. A. B. Fard, Tetrahedron Lett., 2003, 44, 2889 CrossRef CAS.
- A. Kumar and R. A. Maurya, J. Mol. Catal. A: Chem., 2007, 272, 53 CrossRef CAS PubMed.
- A. Ghorbani-Choghamarani and P. Zamani, Chin. Chem. Lett., 2013, 24, 804 CrossRef CAS PubMed.
- S. H. Chandak, N. P. Lad and P. P. Upare, Catal. Lett., 2009, 131, 469 CrossRef.
- C. J. Liu and J. D. Wang, Molecules, 2010, 15, 2087 CrossRef CAS PubMed.
- D. Ding and C. G. Zhao, Eur. J. Org. Chem., 2010, 3802 CrossRef CAS PubMed.
- R. Tayebee, B. Maleki and M. Ghadamgahi, Chin. J. Catal., 2012, 33, 659 CrossRef CAS.
- R. V. Yarapathi, S. Kurva and S. Tammishetti, Catal. Commun., 2004, 5, 511 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra09929j |
| This journal is © The Royal Society of Chemistry 2015 |