Self-assembling nano mixed-brushes having co-continuous surface morphology by melt growing single crystals and comparison with solution patterned leopard-skin surface morphology†
S. Agbolaghiab,
M. Alizadeh-Osgoueiab,
S. Abbaspoorab and
F. Abbasi*ab
aInstitute of Polymeric Materials, Sahand University of Technology, Tabriz, Iran. E-mail: f.abbasi@sut.ac.ir
bFaculty of Polymer Engineering, Sahand University of Technology, Tabriz, Iran
First published on 19th November 2014
Abstract
Self-assembled mixed-brushes with co-continuous surface morphologies were developed from melts of poly(ethylene glycol)-b-polystyrene (PEG-b-PS) and poly(ethylene glycol)-b-poly(methyl methacrylate) (PEG-b-PMMA) diblock copolymers by a self-seeding technique. Some features of solution-grown matrix-dispersed mixed-brushes having controllable characteristics were briefly recalled and compared with the behavior of the corresponding melt-grown mixed-brushes. The observations implied that some major differences exist between the two growth systems. An obvious deduction made from atomic force microscopy (AFM) height images was that the patterned leopard-skin like surface morphology of the solution-induced mixed-brush single crystals changed to a co-continuous morphology in the melt state. Beside the alteration of the growth environment from solution to melt, this phenomenon was assigned to the dominant kinetic effect which replaced the thermodynamic effect that prevailed in the dilute solution systems. The ratio of PMMA- to PS-covered surface area on the substrate increased from 20/80 for the solution-grown mixed-brush single crystals to 50/50 for the melt-grown ones. Owing to an accelerated kinetic effect, the thickness of a melt-grown mixed-brush single crystal was significantly greater than that observed for a solution-grown mixed-brush single crystal of the same molecular weight. Similar trends, nevertheless, were observed for the thickness changes with molecular weight and crystallization temperature. The lateral sizes of the melt-grown single crystals were about 4-fold larger than those of the solution-grown single crystals (e.g., 24 vs. 6 μm). The thicknesses were also proven by the interface distribution function of small angle X-ray scattering analysis.
Introduction
Control of material properties is one of the main purposes of the science realm. Some features, such as mechanical properties, are prone to regulation by the bulk structure or the composition of the materials. Biological responses are mostly tuned by the surface chemistry of materials, however. Surface modification is widely employed to adjust surface characteristics. There are several methods for the successful alteration of surfaces, including mechanical methods, chemical coating and plasma etching.1–5Polymer brushes are self-assembled polymer chains with one end grafted to the substrate. The chains extend away from the substrate, giving a longer end to end distance as they are in the freely random coiled conformation.6 Successful modifications of polymer brushes, conducing adhesive, protein-repulsive, hydrophobic and hydrophilic surfaces, have been reported.7–9 To achieve a combination of properties in a single grafted layer, a polymer mixed-brush is used, where two polymer chains with different features are tethered on a common surface. Incorporating the second constituent significantly influences the brush’s structure. Mixed-brushes are prepared by grafting to,10–14 grafting from,15–23 a combination of both, and the single crystal growth of star block copolymers.24–31 Zhao applied atom transfer radical polymerization (ATRP) and then nitroxide-mediated radical polymerization (NMRP) to graft a polystyrene/poly(methyl methacrylate) (PS/PMMA) mixed-brush.24 Zhang et al. polymerized styrene and methyl methacrylate sequentially and obtained mixed-brushes on clay surfaces.32 A new generation of mixed-brushes has been developed by single crystal surface patterning.33
Recently, single crystals have been utilized in semiconductor microelectronics and solid-state science.34 PEO single crystals have also been adopted as a simplified ultrathin film system to probe the interfacial properties of different substrates.35 Our previous research contributed to the patterning of mixed-brushes and the epitaxial structures of solution-grown single crystals.33,36 In this work, we changed the growth system from a dilute solution to a melt. The features of melt-induced mixed-brushes drastically differed from those of the corresponding solution-grown samples. A lack of studies in this field inspired us to carry out experimental research in the melt state. A more detailed study led to a closer look at the mixed-brush single crystals developed from a polymer melt and their differences with dilute solution-grown samples,33 which appear major. The new results mainly concern the behavior of melt-grown mixed-brush single crystals and the data are compared to and complement previous work, which is briefly recalled.
Experimental
Diblock copolymers were synthesized by ATRP according to the literature.33,37 1H NMR spectroscopy on a Bruker (Avance DPX) spectrometer, GPC on a Waters 1515 (USA) gel permeation chromatography instrument, and differential scanning calorimetry (DSC) (Netzsch, F3 Maia) were adopted to analyze the characteristics of the block copolymers. The molecular characteristics of the PEG-b-PS and PEG-b-PMMA diblock copolymers are reported in Table S1.† In this work, we utilized the self-seeding approach to prepare mixed-brush single crystals from the melt state, which has been described elsewhere.38–41 The poly(ethylene glycol) (PEG)-b-PS and PEG-b-PMMA diblock copolymers were adopted in a 50/50 weight ratio. The homogeneous mixture of samples was put onto a cleaned silicon wafer under a high purity nitrogen stream, and heated to above the melting temperature (Tm = 65 °C), which was maintained for about 30 min to obtain a homogeneous bulk layer. Afterward, it was switched to the primary crystallization temperature (−10 °C) for 5–6 h. Then, it was transferred to the self-seeding temperature (Ts = 41 °C), and maintained for 30 min. The sample was then moved to a desired secondary crystallization temperature (Tc), which was maintained for 2–3 days. The characteristics of the single crystals were investigated by DSC (Table S1†), AFM, a Nanoscope IIIA in the tapping mode, a transmission electron microscopy (TEM) microscope (EM 208 Philips) equipped with a selected area electron diffraction (SAED) facility, and a Bruker-AXS Nanostar SAXS with a count rate of 1000 counts per s per channel and a spatial resolution of 400–500 μm.Results and discussion
In the past few decades, it was thought that melt-grown crystals were rarely single layer, once-folded, and ideal;42–44 but our conducted experiments in this work elucidated that even in the melt state, intervention of the polymer brushes can suppress the increased thickness, multilayer structures, and screw dislocation reported in previous studies.45 Similar to solution-grown systems,46,47 the population of ideal single crystals with grafted brushes on the surface was high in comparison to that in homopolymer single crystals (without any tethered chains on the surface of the substrate). It is worth stating that based on the content of the chains dispersed homogeneously on the silicon wafer, there existed some limitations in the melt growth systems. More details are reported in the ESI.†Co-continuous versus matrix-dispersed surface morphologies
In the melt growth systems, the PMMA patches are connected together as if they were a unified phase region, whereas the PS-covered phase regions are homogeneous and continuous. A thermodynamic effect is reflected in the matrix-dispersed surface morphology of the solution-grown mixed-brush single crystals.33 In the melt growth systems, in addition to a thermodynamic effect, a kinetic effect has an important influence on the surface morphologies. At the beginning of the growth process from the melt, the diblock copolymers of PEG-b-PS and PEG-b-PMMA are employed with the weight ratio of 50/50. Upon complete mixing of the diblock copolymer chains the growth commenced. The possibility of the presence of each type of crystallizable chain to attach to the growing seeds is to an extent the same; because the concentration of crystallizable chains and, consequently, the growth rate is high. However, despite the fact that in the solution growth systems the weight ratio was 50/50 as well, due to a low concentration and, consequently, the opportunity of thermodynamic selectivity, the chain attachments were based on their priorities.33In the melt state, the portion of PMMA chains was enhanced compared to in the solution state in amyl acetate at the same molecular weight and crystallization temperature. One of the reasons behind developing such morphologies could be ascribed to the fact that the PEG-b-PMMA chains with a more compact conformation (due to more attraction to the PEG block) would diffuse and reach the growth region more easily compared to the more extended chains (PEG-b-PS).
Disregarding the interaction with the substrate, PMMA and PS brushes have similar radii of gyration (Rg) for similar molecular weights.48,49 By drawing a comparison between the melt and solution states, we reached some conceivable results. For a PEG5000-b-PMMA8700/PEG5000-b-PS14800 mixed-brush single crystal from the melt at Tc = 30 °C (Fig. 1(a)), though the gyration radius of the PS chains is higher than the PMMA ones, the thickness of the substrate covered by PMMA brushes (even with a significantly lower molecular weight than the PS ones) is less than the PS-covered substrate thickness (9.08 nm vs. 10.21 nm). It declares that similar to solution-grown systems,37 in the melt-grown mixed-brush single crystal system the attractive interaction of the PMMA brushes with the substrate surface49 has led to a considerably higher osmotic pressure (the pressure exerted by a tethered chain on the surface of a single crystal substrate causing its required surface area to be expanded) for them in comparison with the PS brushes. When the length of the extended chain of PEG5000 (∼27 nm)50 is divided by the crystalline substrate thickness of the corresponding single crystal, the fold numbers can be calculated roughly. On the basis of the effective length, the fold numbers of PMMA- and PS-covered substrates are 3 and 2.6, respectively. The PEG5000-b-PMMA8700/PEG5000-b-PS10000 mixed-brush single crystal at Tc = 30 °C is another sample, in which the osmotic pressure of the PS chains is reduced with decreasing MPSn and, subsequently for a lower required coverage surface area (the fold number of PS-covered substrate changed from 2.6 to 2.3); but the matrix-dispersed morphology did not appear (Fig. 1(b)). The next system to compare is the PEG5000-b-PMMA8700/PEG5000-b-PS4600 mixed-brush single crystal at Tc = 30 °C. For the higher Rg of the PMMA chains and their attractive interaction with the substrate, the demanded surface area and, consequently, the osmotic pressure are higher for the PMMA brushes compared to the PS brushes. Simply, in previous mixed-brush single crystals (PEG5000-b-PMMA8700/PEG5000-b-PS14800 and PEG5000-b-PMMA8700/PEG5000-b-PS10000), one of the parameters (greater Rg) was to the benefit of the PS brushes and the other one (attractive interaction with the substrate) was to the advantage of the PMMA brushes; but here (PEG5000-b-PMMA8700/PEG5000-b-PS4600), both hegemonies are with PMMA tethered chains. By comparing with former samples, here, the osmotic pressure of the PS chains has decreased (the fold number of the PS-covered substrate reached 2.1) and, consequently, the difference between the osmotic pressures of the PS and PMMA brushes is elevated. So, the chance for matrix-dispersed surface morphology has increased. But regarding the AFM height images in Fig. 1(c), the surfaces do not yet have matrix-dispersed morphology.
In the upcoming example, again the osmotic pressure of the PMMA chains has increased and the difference between the osmotic pressures of PS and PMMA is higher than the previous example (PEG5000-b-PMMA8700/PEG5000-b-PS4600). This sample is a PEG5000-b-PMMA17100/PEG5000-b-PS4600 single crystal at Tc = 30 °C, in which the substrate thicknesses in the PS- and PMMA-covered phase regions are 12.79 and 8.02 nm, respectively. In these two samples with a constant osmotic pressure of PS (for constant MPSn), the osmotic pressure of the PMMA chains rises due to increasing MPMMAn and increasing of the required coverage surface area. For single crystals of PEG5000-b-PMMA8700/PEG5000-b-PS4600 (Fig. 1(c)) and PEG5000-b-PMMA17100/PEG5000-b-PS4600 (Fig. 1(d)) the morphological behaviors were similar to each other. In fact, in PEG5000-b-PMMA17100/PEG5000-b-PS4600 mixed-brush single crystals, the chance of getting a matrix-dispersed surface morphology has been increased again; because the difference between the osmotic pressures of the two kinds of brushes (PS and PMMA) has been enhanced. The fold numbers of the PS- and PMMA-covered substrates are 2.1 and 3.4, respectively. Despite the fact that the osmotic pressures of the PMMA and PS brushes are the highest and the lowest among all of the grown single crystals, respectively, and respecting the extension of the tethered chains, there is no trace of a matrix-dispersed surface morphology (Fig. 1(d)). Therefore, in melt growth systems there exists another presiding parameter, which is the kinetic effect.
The mentioned examples indicate that the surface morphologies of the melt-grown mixed-brush single crystals are completely distinct from those grown from the solution state (i.e., co-continuous vs. leopard-skin). In the solution environment, amyl acetate (at 23–30 °C) is a very good solvent for the PS chains and a partially poor solvent for the PMMA chains.51,52 On the contrary, in the melt state both chains present in the theta condition.53,54 The theta condition for PS (compared to the very good condition in the solution system) causes the PS chains to take a packed conformation in comparison with the very good solvent environment and thereby their hindrance is reduced against the PMMA chains. On the contrary, PMMA chains contain a higher extended conformation. Hence, their hindrance gets increased against the PS chains. On the other hand, PMMA brushes attract the substrate, and this could in turn not allow them to be extended. Here, from a conformational perspective, the osmotic pressure of the PS chains is decreasing whereas that of the PMMA chains is increasing to an extent compared to the solution state. Therefore, in addition to conformation, the osmotic pressures of the contributing chains (PS and PMMA) approach each other. It may be thought that the differences between the extension of the tethered chains as well as the osmotic pressure of PS and PMMA brushes have reduced due to being in the same kind of growth condition (theta condition). So, there is no possibility of getting a matrix-dispersed surface morphology. In the solution-grown systems there were some samples in which the substrate height variances were very low. For instance, for the PEG5000-b-PMMA17100/PEG5000-b-PS14800 mixed-brush single crystal at Tc = 23 °C, the substrate thicknesses of the PS- and PMMA-covered phase regions were equal to 4.0 and 2.7 nm, respectively. In this sample, the substrate height variance was 1.3 nm (48% of lower thickness and 32.5% of higher thickness). For the PEG5000-b-PMMA17100/PEG5000-b-PS4600 single crystal grown from the melt state (the highest osmotic pressure difference between the PMMA and PS brushes) at Tc = 23 °C (10.95 nm for the thickness of the PS-covered substrate and 6.91 nm for that of the PMMA-covered substrate) the substrate height variance is 4.04 nm (∼58.5% of lower thickness and ∼37 of higher thickness). Indeed, the difference in osmotic pressure in PEG5000-b-PMMA17100/PEG5000-b-PS4600 at Tc = 23 °C grown from the melt is high compared to that of PEG5000-b-PMMA17100/PEG5000-b-PS14800 at Tc = 23 °C grown from the dilute solution. Even though the osmotic pressure of the PMMA brushes is higher, they are not allowed to be dispersed in the PS-matrix. Beside the osmotic pressure, the extension of the tethered chains (PS and PMMA) is another parameter which could have an effect on the surface morphology. In the theta condition of the melt state the conformations of the PS and PMMA chains approach each other. So, their tendency to attract the opposite type of chain increases. This could in turn conduce co-continuous surface morphologies.
In addition, in the theta solution system at Tc = 23 °C, the decrease of the substrate thickness from the homo-PEG5000 (10.33 nm) to the PEG5000-b-PS4600 (6.50 nm) single crystal with the lowest osmotic pressure was 37%. This reduction is due to the entropic effect of the tethered chains on the substrate. However, in the melt system at the same crystallization temperature and for the same samples the decrease of the substrate thickness was 18.5% (from 13.51 nm to 11.00 nm). Due to a higher thickness of the pristine single crystal (homo-PEG5000), and without considering the kinetic effect, this decrease must have been larger; because for the same decrease in the substrate thickness the tethered chains will have a more enlarged compactness in the vicinity of each other in comparison with the corresponding solution-grown sample. So, to suppress this higher compactness of the amorphous brushes (to have a lower entropy enhancement by the compactness of the brushes), the substrate thickness has to undergo a greater decrease. Actually, here, the kinetic effect does not let the crystalline chains have more fold numbers. Therefore, the substrate thickness and, consequently, the amorphous brush thickness and the total thickness are more enlarged than those from the corresponding solution growth environment. Likewise, at Tc = 23 °C for the theta solution-grown single crystals of homo-PEG5000 and PEG5000-b-PMMA17100 (having the highest osmotic pressure), the substrate thickness variance was 74% (from 10.33 nm to 2.70 nm). In the respective melt-grown single crystals at the same crystallization temperature, the substrate thickness variance was 49.5% (from 13.51 nm to 6.83 nm), however. Here, the mentioned kinetic effect satisfies the condition as well. The kinetic effect contributes to the high concentration of crystallizable chains, the accessibility of the polymer chains in the growth system and, consequently, a high growth rate in the polymer melt state.
Surface morphologies developed from the PEG-b-PS and PEG-b-PMMA chains in the bulk state are randomly distributed. This phenomenon can also result from the kinetic effect, otherwise, like solution-grown mixed-brush single crystals having regular leopard-skin surfaces, it must possess a common surface morphology. It is interesting that the total, substrate and brush thicknesses in the PS- and PMMA-covered phase regions, for a given molecular weight, in all parts of a sample and even in different samples with distinct surface morphologies are consistent with each other. The AFM height profiles proved our claims. This phenomenon resembled the solution systems.
Characteristics and trends
On the basis of the Hoffman–Lauritzen theory,55 the crystal thickness is thermodynamically determined by the surface energy, γe. The dCRYST of a homopolymer single crystal is proportional to the reciprocal undercooling, 1/ΔT, and follows a linear relationship with 1/ΔT (eqn (1)):51,55–57
 | (1) |
In the homopolymer case, γe is defined by the fold-surface free energy, γc; however, in diblock copolymer single crystals, an additional term, γt, or the free-energy contribution from the tethered chains, must be brought into consideration (i.e., γe = γc + γt). γt is related to the tethering density and the conformation of the tethered amorphous chains. Up to now, it was thought that the amorphous blocks can affect the crystallization through changing the free energy. Here, we introduce another effective parameter, i.e., the kinetic effect.
In this work, the brushes fabricated from the melt-grown single crystals are embedded in higher regimes than those from the solution-grown mixed-brush single crystals.33 More details based on the reduced tethering densities (![[small sigma, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e10d.gif) )51,58,59 are reported in the ESI.† In highly compacted regimes, the effect of the tethered chains is more dominant on the substrate thickness. In our growth systems, despite the fact that the melt fabricated brushes are in more compact regimes, the respective substrate thicknesses are thicker. Hence, the considerable increase in substrate thickness could not be due to the dominant effect of enthalpy to entropy, which has been an acceptable theory to determine the substrate thickness in single crystals to date. This theory states that thermodynamically, the final crystal morphology of the PEO diblock copolymers reflects the balance between an enthalpic driving force to minimize the fold-surface energy and the entropic term resulting from the stretching of the amorphous blocks.60 In the melt state, the concentration is higher and, subsequently, the growth rate is higher. So, the crystallizable chains do not have enough time to form more folds. Therefore, they construct substrates with larger thicknesses. In conclusion, in melt systems the kinetic effect prevails the growth condition. It was demonstrated that extremely high regimes for tethered chains on the substrate do not allow the respective single crystals to develop.46
)51,58,59 are reported in the ESI.† In highly compacted regimes, the effect of the tethered chains is more dominant on the substrate thickness. In our growth systems, despite the fact that the melt fabricated brushes are in more compact regimes, the respective substrate thicknesses are thicker. Hence, the considerable increase in substrate thickness could not be due to the dominant effect of enthalpy to entropy, which has been an acceptable theory to determine the substrate thickness in single crystals to date. This theory states that thermodynamically, the final crystal morphology of the PEO diblock copolymers reflects the balance between an enthalpic driving force to minimize the fold-surface energy and the entropic term resulting from the stretching of the amorphous blocks.60 In the melt state, the concentration is higher and, subsequently, the growth rate is higher. So, the crystallizable chains do not have enough time to form more folds. Therefore, they construct substrates with larger thicknesses. In conclusion, in melt systems the kinetic effect prevails the growth condition. It was demonstrated that extremely high regimes for tethered chains on the substrate do not allow the respective single crystals to develop.46
By comparing the theta solvent and the melt state, similar results were obtained. For instance, at Tc = 23 °C for the PS4600-covered phase regions, the ![[small sigma, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e10d.gif) values were 11.37 and 7.33 in the melt state and theta solvent, respectively.
values were 11.37 and 7.33 in the melt state and theta solvent, respectively.
Although in the solution and melt growth systems of the mixed-brush single crystals the thicknesses are completely different (i.e., the total and substrate thicknesses are greater in the melt state) the trends of thickness change with crystallization temperature and molecular weight are similar for both systems. When a crystallizable chain attaches to a growing seed in the melt state and tends to fold, the presence of other chains in its vicinity (in a big population) does not allow it to have thermodynamically dictated folds. Hence, lower folding results in a more enlarged substrate thickness. Subsequently, when the substrate has lower folding the grafted amorphous chains are closer to each other on the surface of the single crystal, and this would in turn increase the height of the brushes. Fig. 2(a) and (b) display the increasing trends of total and substrate thicknesses vs. the crystallization temperature, respectively. In the melt state, the slopes of the graphs of total and substrate thicknesses vs. the crystallization temperature are steeper for both the PS- and PMMA-covered regions. These steeper slopes might indicate an accelerated kinetic effect in the melt-grown single crystals. In detail, in the melt state the thicknesses of the substrates are greater; so, the compactness of the tethered chains is higher, i.e., the brushes are in higher regimes compared to in the solution state. Hence, by increasing the crystallization temperature, the escalating of the substrate thickness will be more hampered in comparison to the solution state. But the graphs of Fig. 2(b) depict that the situation has happened in reverse, and the slopes of the substrate thickness as well as the overall height vs. the crystallization temperature variations are steeper for the melt-grown samples. The kinetic effect causes the slope of the thickness variances vs. the crystallization temperature to be steeper than in the solution systems through partial frustration of the tethered amorphous chains.
Although the radius of gyration (Rg) of the PS brushes with Mn = 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 g mol−1 is drastically higher than the Rg of the PMMA brushes with Mn = 8700 g mol−1, at Tc = 23 °C the thickness of the PMMA-covered regions was lower than the PS-covered regions (3.5 nm vs. 4.0 nm).33 On the basis of this fact we concluded that due to the attractive interaction of the PMMA brushes with the substrate, their osmotic pressure was higher and, consequently the respective substrate thickness was lower (even for the lowest molecular weight PMMA brushes).
800 g mol−1 is drastically higher than the Rg of the PMMA brushes with Mn = 8700 g mol−1, at Tc = 23 °C the thickness of the PMMA-covered regions was lower than the PS-covered regions (3.5 nm vs. 4.0 nm).33 On the basis of this fact we concluded that due to the attractive interaction of the PMMA brushes with the substrate, their osmotic pressure was higher and, consequently the respective substrate thickness was lower (even for the lowest molecular weight PMMA brushes).
The total thickness of the grown single crystals is obtainable from the AFM height profiles. The crystalline substrate thickness (dCRYST) can be determined from eqn (2).51,61
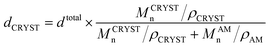 | (2) |
Resembling the solution-grown systems, in the melt-grown mixed-brush single crystals the features of each phase region affected only its own thickness. More details with elucidating examples are provided in the ESI.†
The thermodynamic parameters including the solvent type, molecular weight, interaction with the substrate, and temperature exist in both the dilute solution and the melt state. In the solution growth systems the thickness is mainly affected by the osmotic pressure of the grafted chains, which is determined by their interaction with the solvent and respective substrate. The domain sizes of the PMMA-patches were also completely controllable in the PS-matrix phase. Here, we discuss some detailed influences of the kinetic effect in the melt condition based on the graphs of Fig. 3(a)–(d).
By altering the growth condition from solution to melt, the substrate thickness enhancement for both the PS and PMMA brushes is the same (Fig. 3(a) and (b)). For PEG5000-b-PMMA13100/PEG5000-b-PS4600 at Tc = 23 °C, dPEG for the PMMA-covered regions changes from 3.00 nm in solution to 7.16 nm in the melt (Fig. 3(a)). Likewise, dPEG for the PS-covered regions changes from 6.8 nm in solution to 10.96 nm in the melt, and for both substrates the height difference is 4.16 nm (Fig. 3(b)). The PMMA brushes get an expanded conformation moving from the poor solvent to the theta state. This causes the brushes to exert a higher osmotic pressure on the substrate. On the other hand, the PS brushes are transferred from a very good solvent to a melt theta condition. So, they should get a more compact conformation. In the mentioned sample (PEG5000-b-PMMA13100/PEG5000-b-PS4600), MPMMAn is greater than MPSn and the PMMA brushes have attractive interactions with the PEG substrate.33 All of these effects would not allow the PS- and PMMA-covered substrates to heighten thickness at the same level. So, the PMMA-covered phase regions in the structure of the mixed-brush single crystals benefit more from the kinetic effect to increase their substrate thickness. Simply, the chains with higher hindrance to impede increase of the substrate thickness in solution state share a greater contribution in kinetic dominant growth condition and when the growth condition changes from solution to melt, they significantly increase the thickness of respective substrate in comparison to the chains with lower hindrance. Regardless of the substrate effect, although in the solution state the PMMA and PS brushes were in partially poor and very good solvents, respectively, the height of the PMMA chains was more enlarged than the PS chains. Here, in the melt state, the PMMA chains are in the theta condition and have a more expanded conformation. This in turn increases the height variance of the two kinds of brushes. On the other hand, the growth condition of the PS chains is changed from very good to theta. So, they have a smaller conformation. This also enhances the respective amorphous height variance. Actually, when the thicknesses of the PS- and PMMA-covered substrates increase at the same level, the PMMA brushes are more heightened in the melt state. The amorphous height variance of −3.83 nm for the melt in comparison with −0.4 nm for the solution proves this fact. In addition to the effect of the brushes’ conformation, the highly escalated PMMA-covered substrate compared to the PS-covered substrate from solution to melt results in more compressed brushes. In fact, the kinetic effect has highly suppressed the PMMA chain thermodynamics in comparison to the PS chains. For these reasons, the amorphous height variance of the PS and PMMA brushes is greater in the melt state (= −3.83 nm) than in the solution state (= −0.4 nm).
The higher hindrance caused by increasing MPSn and the hindrance induced by the kinetic effect are two reasons for the steeper slope of the amorphous height variance in the melt state. In detail, although the conformation of the PS chains is more compact in comparison to in a good solvent, the PS chains are in higher regimes in the melt state. This could be attributed to the greater substrate thickness of the melt-grown single crystals. Hence, in the melt, by increasing MPSn, the PS brushes exert a higher force against each other (in comparison to the corresponding sample grown from solution) and, consequently, are more extended. Therefore, the growth of the PS brushes height to that of the PMMA chains is more accelerated (i.e., the graph is steeper Fig. 3(b)). Another reason for the steeper amorphous height variance vs. MPSn graph is that when MPSn increases from 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 to 14
000 to 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 g mol−1, once again the hindrance of the PS brushes is enhanced. So, here the kinetic effect further affects the substrate and, consequently, the PS chains are more extended (in comparison to the corresponding variances from 4600 to 10
800 g mol−1, once again the hindrance of the PS brushes is enhanced. So, here the kinetic effect further affects the substrate and, consequently, the PS chains are more extended (in comparison to the corresponding variances from 4600 to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1).
000 g mol−1).
Now, we draw a comparison between the slopes of the substrate height variances between the solution and melt states. In the melt state, although the PS brushes are in a more compressed condition, due to the kinetic effect they do not have the opportunity to exert their thermodynamically dictated osmotic pressure. So, they are not capable of forcing the PS-covered substrate to approach the PMMA-covered substrate compared to the PS brushes with lower molecular weights. In conclusion, due to the dominant kinetic effect in the melt-grown condition, with increasing MPSn the variance between the substrate thicknesses of the PS- and PMMA-covered regions in the melt state is lower than in solution, and the slope of the respective graph for the melt is less than that of the solution (Fig. 3(b)).
Finally, the slope of the total height variance in the melt is steeper than that in the solution because the slope of the brush height variances is bigger than the slope of the decrease of the substrate thicknesses.
In Fig. 3(c), for PEG5000-b-PMMA13100/PEG5000-b-PS10000 the pristine thickness of the PS brushes (= 6.4 nm) is more enlarged than that of the PMMA brushes (= 4.6 nm) in solution. So, when the single crystal is grown from the melt, the kinetic effect only neutralizes the attractive interactions of the PMMA chains with the substrate as well as their more expanded conformation (from solution to melt), while for PEG5000-b-PMMA13100/PEG5000-b-PS4600 (Fig. 3(b), with a negative amorphous height variance, which means that the height of the PMMA chains is greater than that of the PS ones) the kinetic effect also reciprocates the higher height of the PMMA brushes. Indeed, due to the higher pristine height of the PS chains than the PMMA ones in the solution state, the kinetic effect neutralizes this hindrance. Therefore, for the PEG5000-b-PMMA13100/PEG5000-b-PS10000 mixed-brushes in comparison to PEG5000-b-PMMA13100/PEG5000-b-PS4600, the substrate of the PS-covered area has a higher elevation compared to the PMMA-covered regions in moving from solution to melt. This causes a greater substrate thickness variance for the PEG5000-b-PMMA13100/PEG5000-b-PS10000 sample in both the solution and melt states. The graphs of substrate thickness variances are parallel for solution and melt conditions (Fig. 3(d)). When MPSn is 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1, by increasing MPMMAn from 13
000 g mol−1, by increasing MPMMAn from 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 to 17
100 to 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 g mol−1, first, due to the more enlarged pristine thickness of the PMMA-covered substrate (for the kinetic effect) and second, for the more expanded conformation of the PMMA chains (from the poor to the theta condition), the PMMA brushes are embedded in higher regimes. So, the respective hindrance is greater and, consequently, the kinetic effect has more effect and the substrate thickness is much more elevated than before. In fact, if the kinetic effect did not intervene, through passing from 13
100 g mol−1, first, due to the more enlarged pristine thickness of the PMMA-covered substrate (for the kinetic effect) and second, for the more expanded conformation of the PMMA chains (from the poor to the theta condition), the PMMA brushes are embedded in higher regimes. So, the respective hindrance is greater and, consequently, the kinetic effect has more effect and the substrate thickness is much more elevated than before. In fact, if the kinetic effect did not intervene, through passing from 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 to 17
100 to 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 g mol−1, the substrate thickness would have highly decreased and the substrate thickness variance would be more increased. Anyway, in the melt the kinetic effect has highly neutralized the higher hindrance of the PMMA brushes having MPMMAn = 17
100 g mol−1, the substrate thickness would have highly decreased and the substrate thickness variance would be more increased. Anyway, in the melt the kinetic effect has highly neutralized the higher hindrance of the PMMA brushes having MPMMAn = 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 g mol−1, and consequently, has further elevated the substrate.
100 g mol−1, and consequently, has further elevated the substrate.
In Fig. 3(d) the PS brushes are always thicker than the PMMA ones but in the melt sometimes the PMMA brushes overtake them. Here, the hindrance of the PMMA chains is greater. So, the kinetic effect affects them more and keeps the substrate thickness of the PMMA-covered regions higher than that of the PS chains. As a result, the PMMA chains get more compressed. On the other hand, the PMMA chains are transferred from a poor to theta condition and the chains are larger whereas the PS chains are moved from a very good to theta condition. Therefore, the PMMA brushes have a better opportunity to overtake the PS ones height-wise in the melt state.
Eventually, the reason for the steeper graphs for the PS and PMMA brushes height variance in the melt state when MPSn is 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1 and MPMMAn changes from 13
000 g mol−1 and MPMMAn changes from 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 to 17
100 to 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 g mol−1 could be attributed to some features. First, the PMMA chain condition is altered and, subsequently, they are more stretched. Second, due to the kinetic effect the pristine thickness of the PEG substrate in the melt is larger than that in the solution. Therefore, the brushes exert a higher force to each other and, consequently, are highly heightened. Third, in the melt state the kinetic effect prevents the substrates from having their thermodynamic based thickness, and thereby, the substrates have larger thicknesses. So, the brushes are more compressed together and, consequently, have more elevation. This could cause the PMMA brushes to approach the height of the PS ones with higher acceleration (i.e., bigger slope) in comparison to the similar situation in the solution state.
100 g mol−1 could be attributed to some features. First, the PMMA chain condition is altered and, subsequently, they are more stretched. Second, due to the kinetic effect the pristine thickness of the PEG substrate in the melt is larger than that in the solution. Therefore, the brushes exert a higher force to each other and, consequently, are highly heightened. Third, in the melt state the kinetic effect prevents the substrates from having their thermodynamic based thickness, and thereby, the substrates have larger thicknesses. So, the brushes are more compressed together and, consequently, have more elevation. This could cause the PMMA brushes to approach the height of the PS ones with higher acceleration (i.e., bigger slope) in comparison to the similar situation in the solution state.
The ratio of the PMMA-covered to PS-covered surface areas on the substrate surface of mixed-brush single crystals was determined by Image J software for AFM. The ratio of PMMA to PS brushes was 20/80. In the polymer melt-grown corresponding mixed-brush single crystals this ratio was 50/50. The graphs (a) and (b) in Fig. 4 depict these ratios. As explained previously, the extension of the tethered PS and PMMA chains could be an effective parameter on the surface morphology. In the theta condition of the melt state the conformations of the PS and PMMA chains approach each other and, consequently, the system’s tendency to attract the opposite type of chain increases. Hence, this could in turn lead to co-continuous surface morphologies with a higher portion of PMMA brushes.
Another significant difference between the crystals grown in solution and from the melt is the orientation of the other four growth faces. Contrary to the single crystals grown from a dilute solution, in which the overall lateral habits were square or truncated square with (120), (110) and (020) prisms,33 in the melt state the general shapes are hexagonal. Here in the melt-grown mixed-brush single crystals from the PEG-b-PS and PEG-b-PMMA polymer chains, when the (140) fronts are predominant the lateral habit is square (Fig. 5) as well, whereas when the (100) growth faces appear in the electron diffraction (ED) patterns the overall shape turns to hexagonal habit (Fig. 5).
In some rare cases, in some parts of the melt-grown single crystal surface, like in solution, the matrix-dispersed morphology has been developed. In these samples, the PMMA-covered phases are dispersed in the PS-matrix (Fig. 5). In the hexagonal shaped, solution-grown crystals, these faces are parallel to the (120) planes,39 whereas in the melt-grown crystals they correspond (and are often tangential) to the (140) planes.64 In the melt-grown crystals the pair of truncating prism faces are parallel to the (100) planes instead of being normal to them, as in the solution-grown crystals.39
Some details of the micro-structure of the two-phase system can be analyzed using the interface distribution function (IDF) developed by Ruland.65 The IDF (g1(r)) (eqn (3)) provides a series of distance distributions with alternating signs.
 | (3) |
Fig. 5 and 6 depict the analytical features of PEG5000-b-PMMA17100/PEG5000-b-PS4600 mixed-brush single crystals in the frame of height image and height profile of the AFM and SAXS graphs.
In the theta solvent the chains take a shape and conformation as if they are present in their bulk state. The slope of the total thickness vs. MPSn for the PEG-b-PS single crystals grown from the melt is considerably higher than that of those grown from the theta solvent. The total and amorphous brush thicknesses of the single crystal substrate in the melt state are also significantly higher than the corresponding thicknesses in the theta solvent. In the melt state the polymer chains are more available, and this could in turn cause an accelerated kinetic effect. However, in the dilute solution growth condition the chains are far away from each other. This space is enough to allow the solvent and the interaction with the substrate effects to be more effective. Fig. 7(a) and (b) draw comparison between the characteristics of the PS-covered phase regions in the theta solvent and the melt state vs. MPSn and the crystallization temperature, respectively.
 | ||
| Fig. 7 Comparison between the thicknesses of the single crystals grown from the theta solvent and the polymer melt state at Tc = 23 °C vs. MPSn (a); crystallization temperature (b). | ||
Regarding the lateral sizes of the melt- and solution-grown mixed-brush single crystals, the melt-grown single crystals were 4 times larger than the solution ones (24.04 vs. 6.03 μm). In the melt system, due to the higher concentration of crystallizable chains, for the same growth time a bigger number of chains can attach to the growing seeds, and this leads to a greater lateral size.
Conclusions
Contrary to previous solution-grown mixed-brush single crystals having patterned leopard-skin like surface morphology, the melt-grown ones had co-continuous morphology. The portion of the PMMA/PS brushes in the solution-grown mixed-brush single crystals (= 20/80) reached 50/50 in the polymer melt-grown corresponding mixed-brush single crystals. In the theta condition of the melt state the conformations of the PS and PMMA chains approach each other and, consequently, the system’s tendency to attract chains of the opposite type increases. This could result in co-continuous surface morphologies with a higher ratio of PMMA brushes. Although for a higher concentration and, consequently, a higher growth rate, the thicknesses were considerably higher than the corresponding thicknesses in the dilute solution, the trends of thickness change with molecular weight and crystallization temperature were the same. The steeper slopes proved the accelerated kinetic effect in the melt-grown single crystals. In brief, the co-continuous morphologies of the mixed-brush single crystals and the conspicuously larger thicknesses in the melt state were ascribed to the dominant kinetic effect, instead of the thermodynamic effect which was dominant in the dilute solution growth systems.Acknowledgements
The authors are indebted to the Iran National Science Foundation.Notes and references
- J. Lahann, S. Mitragotri, T.-N. Tran, H. Kaido, J. Sundaram, I. S. Choi, S. Hoffer, G. A. Somorjal and R. Langer, Science, 2003, 299, 371 CrossRef CAS PubMed.
- D. Manos and D. Flamm, Plasma Etching: An Introduction, Academic Press, Inc., New York, 1989 Search PubMed.
- T. P. Russel, Science, 2002, 297, 964 CrossRef PubMed.
- V. Pan, R. Wesley, R. Lvginbuhl, D. Denton and B. Ratner, Biomacromolecules, 2001, 2, 32 CrossRef PubMed.
- Y. Nagasaki and K. Kataoka, Trends Polym. Sci., 1996, 4, 59 CAS.
- S. T. Milner, Science, 1991, 251, 905 CrossRef CAS PubMed.
- M. Motornov, S. Minko, M. Nitschke, K. Grundke and M. Stamm, Polym. Mater. Sci. Eng., 2003, 88, 264 CAS.
- B. Zhao and W. J. Brittain, Prog. Polym. Sci., 2000, 25, 677 CrossRef CAS.
- P. Gong, T. Wu, J. Genzer and I. Szleifer, Macromolecules, 2007, 40, 8765 CrossRef CAS.
- V. Koutsos, E. M. van der Vegte and G. Hadziioannou, Macromolecules, 1999, 32, 1233 CrossRef CAS.
- L. Ionov, B. Zdyrko, A. Sidorenko, S. Minko, V. Klep, I. Luzinov and M. Stamm, Macromol. Rapid Commun., 2004, 25, 260 CrossRef.
- P. A. Johnson, M. A. Gaspar and R. Levicky, J. Am. Chem. Soc., 2004, 126, 9910 CrossRef CAS PubMed.
- H. Nakashima, K. Furukawa, K. Ajito, Y. Kashimura and K. Torimitsu, Langmuir, 2005, 21, 511 CrossRef CAS PubMed.
- L. S. Penn, H. Huang, M. D. Sindkhedkar, S. E. Rankin, K. Chittenden, R. P. Quirk, R. T. Mathers and Y. Lee, Macromolecules, 2002, 35, 7054 CrossRef CAS.
- O. Prucker and J. Rühe, Macromolecules, 1998, 31, 592 CrossRef CAS.
- R. Jordan, A. Ulman, J. F. Kang, M. H. Rafailovich and J. Sokolov, J. Am. Chem. Soc., 1999, 121, 1016 CrossRef CAS.
- A. Vidal, A. Guyot and J. P. Kennedy, Polym. Bull., 1980, 2, 315 CrossRef CAS.
- B. de Boer, H. K. Simon, M. P. L. Werts, E. W. van der Vegte and G. Hadziioannou, Macromolecules, 2000, 33, 49 CrossRef.
- M. W. Weimer, H. Chen, E. P. Giannelis and D. Y. Sogah, J. Am. Chem. Soc., 1999, 121, 1615 CrossRef CAS.
- M. Ejaz, S. Yamamoto, K. Ohno, Y. Tsujii and T. Fukuda, Macromolecules, 1998, 31, 5934 CrossRef CAS.
- K. Matyjaszewski, P. J. Miller, N. Shukla, B. Immaraporn, A. Gelman, B. B. Luokala, T. M. Siclovan, G. Lickelbick, T. Vallant, H. Hoffmann and T. Pakula, Macromolecules, 1999, 32, 8716 CrossRef CAS.
- M. Weck, J. J. Jackiw, R. R. Rossi, P. S. Weiss and R. H. Grubbs, J. Am. Chem. Soc., 1999, 121, 4088 CrossRef CAS.
- W. R. Hertler, D. Y. Sogah and F. P. Boettcher, Macromolecules, 1990, 23, 1264 CrossRef CAS.
- B. Zhao, Polymer, 2003, 44, 4079 CrossRef CAS.
- S. Minko, S. Patil, V. Datsyuk, F. Simon, K.-J. Eichhorn, M. Motornov, D. Usov, I. Tokarev and M. Stamm, Langmuir, 2002, 18, 289 CrossRef CAS.
- S. Minko, D. Usov, E. Goreshnik and M. Stamm, Macromol. Rapid Commun., 2001, 22, 206 CrossRef CAS.
- S. Minko, M. Müller, D. Usov, A. Scholl, C. Froeck and M. Stamm, Phys. Rev. Lett., 2002, 88, 035502 CrossRef CAS.
- B. Zhao and W. J. Brittain, Macromolecules, 2000, 33, 8813 CrossRef CAS.
- S. Minko, M. Müller, M. Motornov, M. Nitschke, K. Grundke and M. Stamm, J. Am. Chem. Soc., 2003, 125, 3896 CrossRef CAS PubMed.
- M. Lemieux, S. Minko, D. Usov, M. Stamm and V. V. Tsukruk, Langmuir, 2003, 19, 6126 CrossRef CAS.
- J. Wang, S. Kara, T. E. Long and T. C. Ward, J. Polym. Sci., Part A: Polym. Chem., 2000, 38, 3742 CrossRef CAS.
- J. Zhang, Y. Yang, C. Zhao and H. Zhao, J. Polym. Sci., Part A: Polym. Chem., 2007, 45, 5329 CrossRef CAS.
- S. Abbaspoor, F. Abbasi and S. Agbolaghi, RSC Adv., 2014, 4, 17071 RSC.
- H. Minemawari, T. Yamada, H. Matsui, J. Tsutsumi, S. Haas, R. Chiba, R. Kumai and T. Hasegawa, Nature, 2011, 475, 364 CrossRef CAS PubMed.
- X. Jiang, X. Liu, Q. Liao, X. Wang, D. D. Yan, H. Huo, L. Li and J. J. Zhou, Soft Matter, 2014, 10, 3238 RSC.
- S. Agbolaghi, F. Abbasi and S. Abbaspoor, Colloid Polym. Sci., 2014, 292, 1375 CAS.
- S. Abbaspoor, F. Abbasi and S. Agbolaghi, J. Polym. Res., 2014, 21(493), 1 CAS.
- B. Lotz and A. J. Kovacs, Colloid Polym. Sci., 1966, 209(2), 97 CAS.
- B. Lotz, A. J. Kovacs, G. A. Bassett and A. Keller, Colloid Polym. Sci., 1966, 209, 115 CAS.
- G. Vidotto, D. Lévy and A. J. Kovacs, Colloid Polym. Sci., 1969, 230, 1 Search PubMed.
- D. J. Blundell, A. Keller and A. J. Kovacs, J. Polym. Sci., Part B: Polym. Phys., 1966, 4, 481 CrossRef CAS.
- S. Z. D. Cheng, H. S. But and B. Wunderlich, Polymer, 1988, 29, 579 CrossRef CAS.
- S. Z. D. Cheng, J. Chen and J. J. Janimak, Polymer, 1990, 31, 1018 CrossRef CAS.
- G. Vidotto, D. Lévy and A. J. Kovacs, Kolloid Z. Z. Polym., 1969, 230, 289 CAS.
- P. H. Geil, Polymer Single Crystals, Interscience, New York, London, 1963 Search PubMed.
- S. Agbolaghi, F. Abbasi and S. Abbaspoor, Polym. Bull., 2014, 71, 3177 CrossRef CAS PubMed.
- S. Agbolaghi, F. Abbasi and K. Jalili, J. Polym. Res., 2014, 21(380), 1 CAS.
- Y. Chen, dissertation, University of Akron, 2005.
- R. M. Van Horn, PhD dissertation, University of Akron, 2009.
- V. Cauda, C. H. Argyo and T. H. Bein, J. Mater. Chem., 2010, 20, 8693 RSC.
- J. X. Zheng, H. Xiong, W. Y. Chen, K. Lee, R. M. Van Horn, R. P. Quirk, B. Lotz, E. L. Thomas, A.-C. Shi and S. Z. D. Cheng, Macromolecules, 2006, 39, 641 CrossRef CAS.
- J. Brandup and E. H. Immergut, Polymer Handbook, Wiley, New York, 1975 Search PubMed.
- M. Rubinstein, Polymer physics, Oxford University Press, 2003 Search PubMed.
- L. H. Sperling, Introduction to physical polymer science, John Wiley, 2006 Search PubMed.
- J. D. Hoffman and J. I. Lauritzen, J. Res. Natl. Bur. Stand., Sect. A, 1961, 65, 297 CrossRef.
- B. Wunderlich, Macromolecular Physics, Academic Press, New York, 1980 Search PubMed.
- J. I. Lauritzen and J. D. Hoffman, J. Res. Natl. Bur. Stand., Sect. A, 1960, 64, 73 CrossRef.
- L. J. Fetters, N. Hadjichristidis, J. S. Lindner and J. W. Mays, J. Phys. Chem., 1994, 23, 619 CAS.
- M. S. Kent, Macromol. Rapid Commun., 2000, 21, 243 CrossRef CAS.
- E. A. DiMarzio, C. M. Guttman and J. D. Hoffman, Macromolecules, 1980, 13, 1194 CrossRef CAS.
- W. Y. Chen, J. X. Zheng, S. Z. D. Cheng, C. Y. Li, P. Huang, L. Zhu, H. Xiong, Q. Ge, Y. Guo, R. P. Quirk, B. Lotz, L. Deng, C. Wu and E. L. Thomas, Phys. Rev. Lett., 2004, 93, 028301 CrossRef.
- A. Keller and A. O’Connor, Disc. Faraday Soc., 1958, 25, 114 RSC.
- B. Wunderlich, Macromolecular Physics: Crystal Structure, Morphology, Defects, Academic Press, New York, 1973, vol. 1 Search PubMed.
- A. J. Kovacs and A. Gonthier, Colloid Polym. Sci., 1972, 250, 530 CAS.
- W. Ruland, Colloid Polym. Sci., 1977, 255, 417 CAS.
- N. Stribeck and W. Ruland, J. Appl. Crystallogr., 1978, 11, 535 CrossRef CAS.
- H. Hamie, PhD dissertation, University of Haute Alsace, 2010.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra09311a |
| This journal is © The Royal Society of Chemistry 2015 |






