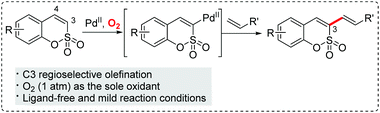
Regioselective palladium(II)-catalyzed aerobic oxidative Heck-type C3 alkenylation of sulfocoumarins†
Namhoon
Kim
ab,
Minsik
Min
ab and
Sungwoo
Hong
*ab
aDepartment of Chemistry, Korea Advanced Institute of Science and Technology, Daejeon, 305-701, Korea. E-mail: hongorg@kaist.ac.kr
bCenter for Catalytic Hydrocarbon Functionalization, Institute for Basic Science (IBS), Daejeon, 305-701, Korea
First published on 28th October 2015
Abstract
An efficient method for the direct C–H olefination of sulfocoumarins with a wide range of alkenes is developed. Moreover, O2 was successfully utilized as the sole oxidant for the oxidative Heck reaction. This approach enables the rapid generation of various 3-alkenylated sulfocoumarins.
Sulfocoumarins (1,2-benzoxathiine 2,2-dioxides) are bioisosteres of coumarins, where the carbonyl group in the coumarin ring is replaced by a sulfonyl group. Sulfocoumarin has been reported to be an important structural motif that inhibits the metalloenzyme, carbonic anhydrase.1 Consequently, the development of efficient methods for the rapid derivatization of sulfocoumarin has been the subject of intensive research.2 Despite significant synthetic efforts, an approach to functionalize this scaffold via C–H bond activation has not yet been reported.
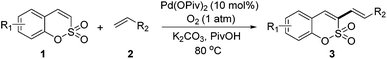
Since Fujiwara's discovery of the direct olefination of benzene,3 substantial progress has been achieved in the field of oxidative Heck reactions.4 Our group and Lee's group recently developed Pd(II)-catalyzed oxidative Heck reactions of coumarins and phosphacoumarins, respectively.5 These studies prompted us to explore the feasibility of an expeditious synthetic approach to install an olefin into sulfocoumarins. We speculated that the C3-palladated species could be accessed in a catalytic fashion because of the inherent nucleophilic characteristics of the 3-position of sulfocoumarins. Herein, we describe a regioselective Pd(II)-catalyzed C–H olefination of sulfocoumarins using 1 atm O2 as the only oxidant,6 which enables the construction of various 3-vinyl and 3-styryl sulfocoumarins (Scheme 1).
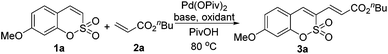
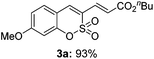
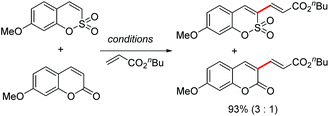
We studied the feasibility of the proposed strategy by investigating the possibility of C–H activation/olefination of sulfocoumarin (1a) with n-butyl acrylate (2a): the representative catalyst screening data for the conversion are listed in Table 1. The reaction utilized a Pd(OPiv)2 catalyst combined with Ag2CO3 to exclusively afford the C3-alkenylated product, presumably via an electrophilic palladation pathway (entry 1, 33%). Among the Pd species screened, Pd(OPiv)2 displayed the best catalytic efficiency. Both the base and the solvent were found to fundamentally affect the reaction efficiency; K2CO3 and pivalic acid were the optimal base and solvent, respectively. The properties of the oxidant were also critical for the reaction efficiency, and the use of Cu(OAc)2 dramatically improved the catalytic reactivity (entry 3). Based on the recent advances in palladium-catalyzed aerobic oxidation reactions, an intensive screen was conducted to promote the reactions using environmentally benign and economical oxidants.7 We therefore surveyed the capabilities of air and O2 as oxidizing agents. Indeed, we were pleased to observe that the C3-alkenylation process proceeded in air (entry 6, 76%) and the yields were higher than those obtained using the Pd(II)/Cu(II) catalytic system. When the reaction was subjected to treatment with 1 atm O2 as the only oxidant, an excellent product yield was obtained. Thus, this method presents an efficient and sustainable approach to the synthesis of various 3-vinylsulfocoumarin derivatives. Under the optimized reaction conditions, the C3 alkenylation of 1a in the presence of Pd(OPiv)2 (0.1 equiv.) and K2CO3 (3 equiv.) in pivalic acid at 80 °C afforded the product 3a in the highest yield (93%).
| Entry | Oxidant (equiv.) | Base (equiv.) | Yieldc (%) |
|---|---|---|---|
| a Reactions were conducted with sulfocoumarin, butyl acrylate (2.0 equiv.), Pd(OPiv)2 (0.2 equiv.), and base (3 equiv.) in PivOH at 80 °C for 8 h. b Butyl acrylate (1.1 equiv.), Pd(OPiv)2 (0.1 equiv.), and base (3 equiv.). c Yields were determined using 1H NMR analysis of the crude reaction mixture. d Isolation yield. Piv = pivaloyl, DDQ = 2,3-dichloro-5,6-dicyano-1,4-benzoquinone. | |||
| 1 | Ag2CO3 (3) | — | 33 |
| 2 | Oxone (1.2) | K2CO3 | 34 |
| 3 | Cu(OAc)2 (3) | K2CO3 | 61 |
| 4 | AgNO3 (3) | K2CO3 | 75 |
| 5 | DDQ (1) | K2CO3 | 14 |
| 6 | Air (1 atm) | K2CO3 | 76 |
| 7 | O2 (1 atm) | K2CO3 | 94 |
| 8 | N2 | K2CO3 | 15 |
| 9b | O2 (1 atm) | K2CO3 | 95 (93d) |
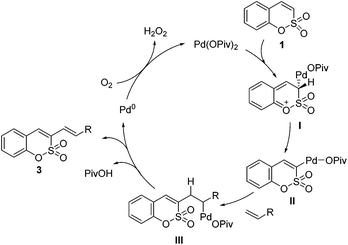
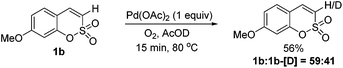
To gain some insight into the present alkenylation, a mechanistic analysis of the initial interaction of a Pd catalyst with sulfocoumarin 1b was performed using H/D exchange experiments.8 A significant level of deuterium incorporation (after 15 min, 41% D) was observed at the C3 position of sulfocoumarin (1b) when the reaction mixture was treated with AcOD as a deuterium source under the optimized conditions and in the absence of the alkene, as shown in Fig. 1 (see the ESI† for the full spectra).
 | (1) |
Based on our observations, a plausible catalytic mechanism for the alkenylation of sulfocoumarin involves the aforementioned electrophilic palladation pathway at the C3 position, followed by H-abstraction by the pivalate ligand to provide the intermediate II. In the presence of an alkene substrate, the C3-palladated species II inserts into the olefin, and the subsequent C–Pd β-hydride elimination of intermediate III provides the desired coupled product 3. Finally, the reoxidation of Pd(0) to Pd(II) by molecular oxygen completes the catalytic cycle.
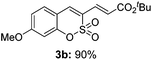
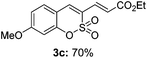
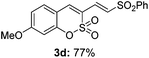
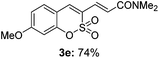
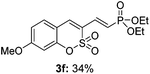
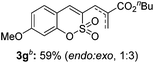
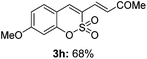
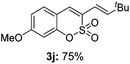
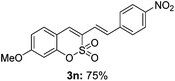
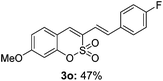
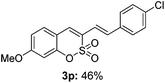
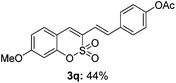
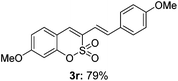
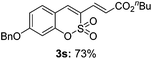
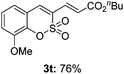
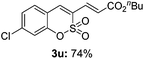
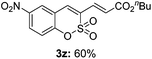
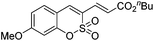
Both sulfocoumarin and alkene substrates were next investigated to extend the utility and generality of this methodology. In general, the present C3-alkenylation process was amenable to alkene substrates conjugated with a variety of functional groups from electron-donating to electron-withdrawing groups, as described in Table 2. For example, alkene substrates conjugated with ester (3a, 3b, and 3c), sulfonate (3d), amide (3e), or phosphonate (3f) groups smoothly coupled with 7-methoxy sulfocoumarin at the C3 position. When 2-methyl substituted methyl acrylate was employed as a substrate, a mixture of regioisomers 3g (endo![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exo, 1
exo, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
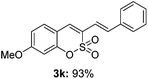
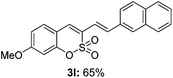
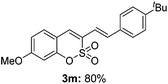
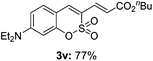
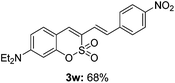
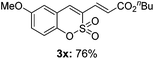
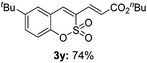
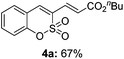
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) formed. The addition of the styryl group to the 3-position of the sulfocoumarin core was expected to induce a red-shift in the emission wavelength by extending the π-electron system.9 To our delight, various styrene substrates were compatible with the coupling reaction conditions, and modest to good yields of the desired products were obtained (3k–3q, 3w). For broad utility, the scope of the sulfocoumarin substrates was examined, and a broad range of functional groups (e.g., methoxy, benzoxy, chloro, diethylamino, alkyl, and nitro) on the sulfocoumarin core were compatible with the coupling conditions. Substitution with an electron-donating OMe group at the 7-position enhanced the reaction efficiency (3avs. 4a).
3) formed. The addition of the styryl group to the 3-position of the sulfocoumarin core was expected to induce a red-shift in the emission wavelength by extending the π-electron system.9 To our delight, various styrene substrates were compatible with the coupling reaction conditions, and modest to good yields of the desired products were obtained (3k–3q, 3w). For broad utility, the scope of the sulfocoumarin substrates was examined, and a broad range of functional groups (e.g., methoxy, benzoxy, chloro, diethylamino, alkyl, and nitro) on the sulfocoumarin core were compatible with the coupling conditions. Substitution with an electron-donating OMe group at the 7-position enhanced the reaction efficiency (3avs. 4a).
The C–H alkenylation of phosphacoumarin is less reactive compared to coumarin and sulfocoumarin counterparts, and the use of AgOAc as an oxidant was required to get comparable reactivity. When a competition experiment between sulfocoumarin and coumarin substrates was carried out under the optimized conditions, the sulfocoumarin substrate reacts preferentially over coumarin in the ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (eqn (2)).
1 (eqn (2)).
 | (2) |
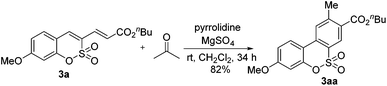
To showcase the applicability of the developed method, the present strategy was employed as a straightforward synthetic route to benzosulfocoumarin (eqn (3)). When 3-alkenylated sulfocoumarin 3a was treated with acetone and pyrrolidine in the presence of MgSO4, the desired benzosulfocoumarin (3aa) was produced in 82% yield via the inverse electron demand Diels–Alder reaction.10
 | (3) |
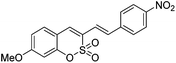
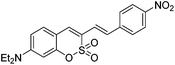
We next examined the photophysical and spectroscopic properties of 3-alkenylsulfocoumarins, and some of the new derivatives exhibited promising photonic luminescence (Table 3). The introduction of electron-donating NEt2 at position 7 on the sulfocoumarin core ring and the p-nitro group on styrene gave rise to notably red-shifted maxima and increased the value of ΦF. The comparison of the photophysical properties indicated that the coumarin core possesses higher photoluminescence efficiencies than those of sulfocoumarin and phosphacoumarin counterparts.
In summary, we developed a new protocol for effecting the direct C–H olefination of sulfocoumarins via a palladium-catalyzed oxidative Heck reaction using O2 as the sole oxidant. The reaction scope exhibits broad utility and functional group tolerance with complete regioselectivity. This simple and efficient approach offers convenient access to a variety of 3-vinyl and 3-styryl sulfocoumarin scaffolds, which are privileged structures in many biologically active compounds. In addition, our synthetic strategy led to the discovery of new sulfocoumarin-based derivatives that show promising photonic luminescence.
Acknowledgements
This research was supported financially by the Institute for Basic Science (IBS-R010-G1).Notes and references
- (a) A. Maresca, C. Temperini, H. Vu, N. B. Pham, S. A. Poulsen, A. Scozzafava, R. J. Quinn and C. T. Supuran, J. Am. Chem. Soc., 2009, 131, 3057 CrossRef CAS PubMed; (b) K. Tars, D. Vullo, A. Kazaks, J. Leitans, A. Lends, A. Grandane, R. Zlubovskis, A. Scozzafava and C. T. Supuran, J. Med. Chem., 2013, 56, 293 CrossRef CAS PubMed; (c) A. Grandane, S. Belyakov, P. Trapencieris and R. Zalubovskis, Tetrahedron Lett., 2012, 68, 5541 CrossRef CAS.
- (a) A. Grandane, M. Tanc, R. Zalubovskis and C. T. Supuran, Bioorg. Med. Chem., 2015, 23, 1430 CrossRef CAS PubMed; (b) M. Tanc, F. Carta, M. Bozdaq, A. Scozzafava and C. T. Supuran, Bioorg. Med. Chem., 2013, 21, 4502 CrossRef CAS PubMed; (c) A. Grandane, M. Tanc, R. Zalubovskis and C. T. Supuran, Bioorg. Med. Chem., 2014, 22, 1522 CrossRef CAS PubMed.
- (a) I. Moritani and Y. Fujiwara, Tetrahedron Lett., 1968, 35, 3863 Search PubMed; (b) Y. Fujiwara, I. Moritani, S. Danno, R. Asano and S. Teranishi, J. Am. Chem. Soc., 1969, 91, 7166 CrossRef CAS.
- For selected examples, see: (a) N. P. Grimster, C. Gauntlett, C. R. A. Godfrey and M. J. Gaunt, Angew. Chem., Int. Ed., 2005, 44, 3125 CrossRef CAS PubMed; (b) Y. Huang, F. Song, Z. Wang, P. Xi, N. Wu, Z. Wang, J. Lan and J. You, Chem. Commun., 2012, 48, 2864 RSC; (c) M. Miyasaka, K. Hirano, T. Satoh and M. Miura, J. Org. Chem., 2010, 75, 5421 CrossRef CAS PubMed; (d) D. Kim and S. Hong, Org. Lett., 2011, 13, 4466 CrossRef CAS PubMed; (e) M. Ye, G. Gao and J. Yu, J. Am. Chem. Soc., 2011, 133, 6964 CrossRef CAS PubMed; (f) J. Wu, X. Cui, L. Chen, G. Jiang and Y. Wu, J. Am. Chem. Soc., 2009, 131, 13888 CrossRef CAS PubMed; (g) Y. Yu, M. J. Niphakis and G. I. Georg, Org. Lett., 2011, 13, 5932 CrossRef CAS PubMed; (h) D. Cheng and T. Gallagher, Org. Lett., 2009, 11, 2639 CrossRef CAS PubMed; (i) L. Ming, C. Liu, W. Zhang, C. Zhou and A. Lei, Chem. Commun., 2014, 50, 1110 RSC; (j) Y. Moon, D. Kwon and S. Hong, Angew. Chem., Int. Ed., 2012, 51, 11333 CrossRef CAS PubMed.
- (a) M. Min, Y. Kim and S. Hong, Chem. Commun., 2013, 49, 196 RSC; (b) C. Kim, J. Son, B. Seo and P. Lee, Org. Lett., 2015, 17, 908 CrossRef CAS PubMed; (c) M. Min and S. Hong, Chem. Commun., 2012, 48, 9613 RSC.
- (a) E. M. Beck, N. P. Grimster, R. Hatley and M. J. Gaunt, J. Am. Chem. Soc., 2006, 128, 2528 CrossRef CAS PubMed; (b) Y. Xu, Y. Chok and T. P. Loh, Chem. Sci., 2011, 2, 1822 RSC; (c) C. Zheng and S. S. Stahl, Chem. Commun., 2015, 51, 12771 RSC; (d) J. Lv, Y. Liang, P. He, Z. Cai, J. Liu and F. Huang, RSC Adv., 2015, 5, 36171 RSC; (e) W. L. Chen, Y. R. Gao, S. I. Mao, Y. F. Zhang and Y. Q. Wang, Org. Lett., 2012, 14, 5920 CrossRef CAS PubMed; (f) Q. Huang, Q. Song, J. Cai, X. Zhang and S. Lin, Adv. Synth. Catal., 2013, 355, 1512 CrossRef CAS.
- For selected reviews, see: (a) A. N. Campbell and S. S. Stahl, Acc. Chem. Res., 2012, 45, 851 CrossRef CAS PubMed; (b) C. Liu, H. Zhang, W. Shi and A. Lei, Chem. Rev., 2011, 111, 1780 CrossRef CAS PubMed; (c) C. S. Yeung and V. M. Dong, Chem. Rev., 2011, 111, 1215 CrossRef CAS PubMed; (d) X. Chen, K. M. Engle, D.-H. Wang and J.-Q. Yu, Angew. Chem., Int. Ed., 2009, 48, 5094 CrossRef CAS PubMed; (e) S. S. Stahl, Angew. Chem., Int. Ed., 2004, 43, 3400 CrossRef CAS PubMed; (f) Y. Wu, J. Wnag, F. Mao and F. Y. Kwong, Chem. – Asian J., 2014, 9, 26 CrossRef CAS PubMed.
- (a) C. Hardacre, J. D. Holbrey and S. E. J. McMath, Chem. Commun., 2011, 367 Search PubMed; (b) H. Esaki, F. Aoki, M. Umehara, M. Kato, T. Maegawa, Y. Monguchi and H. Sajiki, Chem. – Eur. J., 2007, 13, 4052 CrossRef CAS PubMed.
- (a) G. Kim, K. Lee, H. Kwon and H. Kim, Org. Lett., 2011, 13, 2799 CrossRef CAS PubMed; (b) G. Signore, R. Nifosi, L. Albertazzi, B. Storti and R. Bizzarri, J. Am. Chem. Soc., 2010, 132, 1276 CrossRef CAS PubMed; (c) M. Min, B. Kim and S. Hong, Org. Biomol. Chem., 2012, 10, 2692 RSC.
- I. R. Pottle, P. R. Nandaluru, W. L. Benoit, D. O. Miller, L. N. Dawe and G. J. Bodwell, J. Org. Chem., 2011, 76, 9015 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5qo00294j |
| This journal is © the Partner Organisations 2015 |