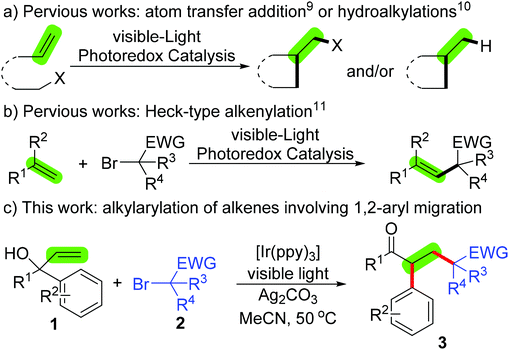
Alkylation/1,2-aryl migration of α-aryl allylic alcohols with α-carbonyl alkyl bromides using visible-light photoredox catalysis†
Yang
Li
a,
Bang
Liu
a,
Xuan-Hui
Ouyang
a,
Ren-Jie
Song
*a and
Jin-Heng
Li
*ab
aState Key Laboratory of Chemo/Biosensing and Chemometrics, College of Chemistry and Chemical Engineering, Hunan University, Changsha 410082, China. E-mail: srj0731@hnu.edu.cn; jhli@hnu.edu.cn
bState Key Laboratory of Applied Organic Chemistry Lanzhou University, Lanzhou 730000, China
First published on 1st September 2015
Abstract
A novel visible-light-induced alkene difunctionalization strategy is described for the synthesis of 1,5-dicarbonyl compounds from two reaction partners, α-aryl allylic alcohols and α-carbonyl alkyl bromides. This method is successful by sequential alkylation of an alkene C–C double bond and intramolecular 1,2-aryl migration, and shows a broad substrate scope, excellent functional group tolerance and good selectivity.
Introduction
Alkenes represent an important class of chemical feedstock with broad utility in organic synthesis for the construction of more complex molecular entities. Thus, methods for the efficient functionalization of alkenes at the C–C double bond positions are of intense interest.1,2 Particularly attractive are the alkene difunctionalization transformations wherein two functional groups are introduced across alkenes in a catalytic and selectivity-controlled manner.2–7 Despite remarkable progress in the alkene difunctionalization field, the dicarbofunctionalization of alkenes involving the simultaneous incorporation of an arene group and an alkyl group has been more limited3–7 and mostly restricted to the requirement of a stoichiometric amount of oxidants (often hypervalent iodine reagents or peroxides) and the synthesis of oxindoles and related heterocycles.3–5,7 Liu's group had first reported the alkylarylation of activated alkenes with aryl C(sp2)–H bonds and alkyl C(sp3)–H bonds adjacent to a CN group in the presence of Pd(II) catalysts and hypervalent iodine reagents to assemble CN-containing oxindoles.3 Later, our group illustrated an oxidative alkylarylation of activated alkenes with aryl C(sp2)–H bonds and alkyl C(sp3)–H bonds adjacent to a heteroatom (O, S or N atom) for the synthesis of functionalized 3-(2-oxoethyl)indolin-2-ones using the Fe catalyst/peroxide system.4 The group of Liu,5a the group of Cheng5b and our group5c have independently described that similar transformations could be achieved using hypervalent iodine reagents or peroxides as the alkyl resources. The group of Xu/Ji developed a metal-free peroxide-mediated alkylarylation of alkenes (α,α-diaryl allylic alcohols) with alkyl C(sp3)–H bonds adjacent to an oxygen atom through intramolecular 1,2-aryl migration.6 A Pd-catalyzed oxidative Heck-type insertion strategy for the alkylarylation of activated alkenes with aryl C(sp2)–H bonds and α-C(sp3)-Br bonds in α-carbonyl alkyl bromides, which has very recently been reported by our group, appears to be a good alternative; however, such a process is limited to an activated system, thus only enabling intramolecular aryl C(sp2)–H functionalization to access oxindoles and related heterocycles.7 Thus, further discovery of a new mild strategy for general alkylarylation of alkenes leading to diverse complex molecules is highly desirable.Recently, visible-light photoredox catalysis has proven to be a powerful and environmentally benign methodology for the construction of various C–C bonds in synthesis.8–11 In the field, alkene functionalization initiated by the in situ generation of alkyl radicals from alkyl halides through atom transfer radical addition (alkylation–halogenation),9 hydroalkylation (Scheme 1a)10 and alkenylation (Scheme 1b)11 have been well explored. Herein, we report a novel visible-light photoredox catalysis strategy for the alkylarylation of α-aryl allylic alcohols with α-carbonyl alkyl bromides through alkylation/1,2-aryl migration (Scheme 1c).12 This visible-light photoredox catalysis is applicable to a wide range of α-carbonyl alkyl bromides, including primary-, secondary- and tertiary-α-bromoalkyl esters, ketones and amides, and even the more challenging 2-bromo-2,2-difluoroacetate.
Results and discussion
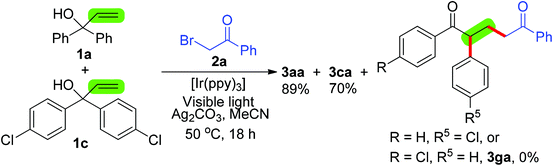
We started optimization studies by investigating the reaction between 1,1-diphenylprop-2-en-1-ol (1a) and 2-bromoacetophenone (2a), a primary alkyl bromide, using the visible-light photoredox catalysis strategy (Table 1). The results demonstrated that among the three photocatalysts [Ir(ppy)3] was more efficient than [Ru(bpy)3Cl2] and eosin (entries 1–3). In the presence of 2 mol% [Ir(ppy)3], 1.2 equiv. Ag2CO3 and 36 W compact fluorescent light, the desired alkylation/1,2-phenyl migration product 3aa was formed from substrate 1a and alkyl bromide 2a in 90% yield (entry 1). Notably, a photocatalyst was necessary for successful alkylation/1,2-aryl migration, as its absence resulted in no detectable product 3aa (entry 4). The amount of [Ir(ppy)3] was found to affect the reaction, as 2 mol% [Ir(ppy)3] was preferred (entry 1 versus entries 4 and 5). However, the yield of 3aa decreased to 55% using 5 W blue LED light instead of 36 W compact fluorescent light (entry 1 versus entry 6). In addition, the reaction did not take place without additional visible light (entry 7). It should be noted that the reaction could occur without bases, albeit with a lower yield (23% yield, entry 8). Extensive screening of the effect of bases revealed that adding a base, such as Ag2CO3, Ag2O, AgOAc, Na2CO3 and NaOH, improved the reaction, and using 1.2 equiv. Ag2CO3 was the best choice (entry 1 versus entries 8–14). After varying reaction temperatures and solvents, we found that this reaction in MeCN at 50 °C gave the best results (entry 1 versus entries 15–18). We were delighted to find that a reaction on a 1 gram scale of substrate 1a was successfully performed in good yield (entry 19).| Entry | [M] (mol%) | Base (equiv.) | Solvent | T (°C) | Yield (%) |
|---|---|---|---|---|---|
| a Reaction conditions: 1a (0.2 mmol), 2a (0.4 mmol), [M], base and solvent (2 mL) with 36 W compact fluorescent light under an argon atmosphere for 16 h. b 5 W blue LED light instead of 36 W compact fluorescent light. c Without additional light. d 1a (1 g, 4.76 mmol) for 72 h. | |||||
| 1 | [Ir(ppy)3] (2) | Ag2CO3 (1.2) | MeCN | 50 | 90 |
| 2 | [Ru(bpy)3Cl2] (2) | Ag2CO3 (1.2) | MeCN | 50 | 8 |
| 3 | Eosin Y (2) | Ag2CO3 (1.2) | MeCN | 50 | 5 |
| 4 | — | Ag2CO3 (1.2) | MeCN | 50 | 0 |
| 5 | [Ir(ppy)3] (5) | Ag2CO3 (1.2) | MeCN | 50 | 91 |
| 6b | [Ir(ppy)3] (2) | Ag2CO3 (1.2) | MeCN | 50 | 55 |
| 7c | [Ir(ppy)3] (2) | Ag2CO3 (1.2) | MeCN | 50 | 0 |
| 8 | [Ir(ppy)3] (2) | — | MeCN | 50 | 23 |
| 9 | [Ir(ppy)3] (2) | Ag2CO3 (2) | MeCN | 50 | 90 |
| 10 | [Ir(ppy)3] (2) | Ag2CO3 (1) | MeCN | 50 | 85 |
| 11 | [Ir(ppy)3] (2) | Ag2O (1.2) | MeCN | 50 | 52 |
| 12 | [Ir(ppy)3] (2) | AgOAc (1.2) | MeCN | 50 | 62 |
| 13 | [Ir(ppy)3] (2) | Na2CO3 (1.2) | MeCN | 50 | 75 |
| 14 | [Ir(ppy)3] (2) | NaOH (1.2) | MeCN | 50 | 35 |
| 15 | [Ir(ppy)3] (2) | Ag2CO3 (1.2) | MeCN | rt | 60 |
| 16 | [Ir(ppy)3] (2) | Ag2CO3 (1.2) | MeCN | 80 | 45 |
| 17 | [Ir(ppy)3] (2) | Ag2CO3 (1.2) | toluene | 50 | 50 |
| 18 | [Ir(ppy)3] (2) | Ag2CO3 (1.2) | DMF | 50 | 13 |
| 19d | [Ir(ppy)3] (2) | Ag2CO3 (1.2) | MeCN | 50 | 92 |
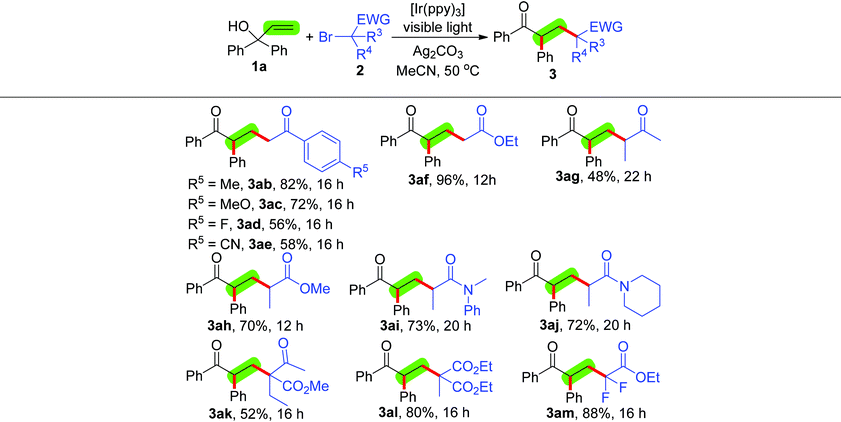
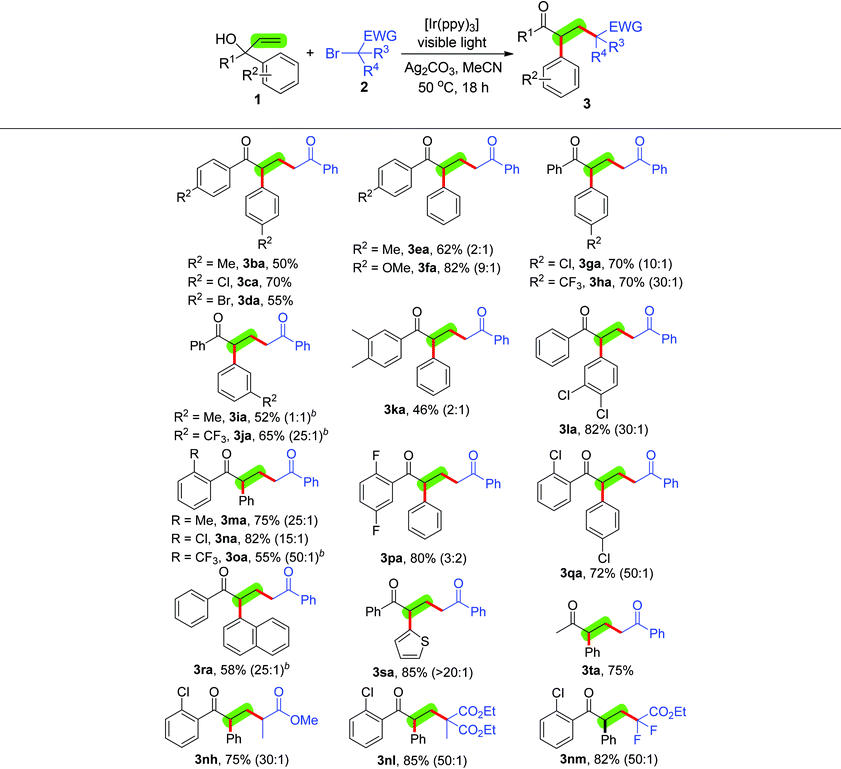
Having established the optimal reaction conditions, we turned our attention to investigate the scope of this alkylation/1,2-aryl migration protocol with respect to α-aryl allylic alcohols 1 and α-carbonyl alkyl bromides 2 (Tables 2 and 3). First, we explore this new transformation by using different α-carbonyl alkyl bromides 2b–m, including primary-, secondary- and tertiary-α-bromoalkyl ketones, esters and amides, and 2-bromo-2,2-difluoroacetate, to react with 1,1-diphenylprop-2-en-1-ol (1a), [Ir(ppy)3], Ag2CO3 and 36 W compact fluorescent light (Table 2). The optimal conditions proved to be compatible with both primary-α-bromoalkyl ketones 2b–e and ester 2f, giving the corresponding alkylation/1,2-aryl migration products 3ab–af in moderate to excellent yields. In addition, in ketones 2b–e the electronic properties of the substituted aryl groups affected the reaction, and their reactive order is as follows: electron-rich > electron-deficient. A number of secondary-α-bromoalkyl ketones 2g, esters 2h and amides 2i–j also worked well and led to the desired products 3ag–aj in moderate to good yields, although ketone 2g had the least reactivity. The alkylation/1,2-aryl migration of 1,1-diphenylprop-2-en-1-ol (1a) with tertiary-α-bromoalkyl esters 2k and 2l successfully afforded 3ak and 3al in high yields with excellent levels of regioselective control. Interestingly, 2-bromo-2,2-difluoroacetate (2m) also had high reactivity and delivered a two fluoro atom-containing product 3am in 88% yield.
| a Reaction conditions: 1a (0.2 mmol), 2 (0.4 mmol), [Ir(ppy)3] (2 mol%), Ag2CO3 (1.2 equiv.) and MeCN (2 mL) with 36 W compact fluorescent light at 50 °C under an argon atmosphere. |
|---|
|
| a Reaction conditions: 1 (0.2 mmol), 2 (0.4 mmol), [Ir(ppy)3] (2 mol%), Ag2CO3 (1.2 equiv.) and MeCN (2 mL) with 36 W compact fluorescent light at 50 °C under an argon atmosphere for 18 h. The ratio of product 3/its isomer is given in parenthesis determined by GC-MS or 1H NMR analysis of the crude product. b For 24 h. |
|---|
|
We next set out to apply the optimal conditions to the alkylation/1,2-aryl migration of various α-aryl allylic alcohols 1 with 2-bromoacetophenone (2a), methyl 2-bromopropanoate (2h), diethyl 2-bromo-2-methylmalonate (2l) or 2-bromo-2,2-difluoroacetate (2m) (Table 3). In the presence of 2-bromoacetophenone (2a), [Ir(ppy)3], Ag2CO3 and 36 W compact fluorescent light, alcohols 1b–d, which contain two same substituted aryl groups, including two 4-MePh, two 4-ClPh and 4-BrPh groups, on the α-position chemospecifically furnished 3ba–3da in moderate to good yields. Alcohols 1e–s, which contain two different substituted aryl groups, were also viable substrates for the alkylation/1,2-aryl migration reaction, and selectivity of their products 3ea–3sa toward the migrating aryl group relied on the electronic and steric hindrance properties of the α-substituted aryl groups. For alcohols 1e–f, the Ph group was the major migration group in alcohols 1e and 1f (products 3ea and 3fa![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12c,j) (product 3ea, two regioisomers are not separated by silica gel column chromatography). Alcohols 1g–h, containing other two substituents – a α-Ph group and a α-4-substituted Ph group – provided the α-4-substituted Ph migrating products 3ga–3ha12c,j as the major isomers in high yield. While the α-Ph group and the α-3-MePh group in alcohol 1i had the same migrating chance (product 3ia), in alcohol 1j the migration of the α-3-CF3Ph group had precedence over the α-Ph group (product 3ja6a,12f–h). For the α-Ph group vs. α-3,4-disubstituted Ph groups, the former was a major migration group (product 3ka6a,12f–h) and the latter was a major migration group (product 3la12c,j). Notably, the sterically hindered α-2-substituted Ph groups were not good migrating groups: the migration of the α-Ph group or the α-4-ClPh group was found to be preferred during the reaction of alcohols 1m–q (products 3ma–qa).12c,j Using 1-(naphthalen-2-yl)-1-phenylprop-2-en-1-ol (1r) to react with 2-bromoacetophenone (2a) delivered the α-naphthalen group migrating product 3ra
12c,j) (product 3ea, two regioisomers are not separated by silica gel column chromatography). Alcohols 1g–h, containing other two substituents – a α-Ph group and a α-4-substituted Ph group – provided the α-4-substituted Ph migrating products 3ga–3ha12c,j as the major isomers in high yield. While the α-Ph group and the α-3-MePh group in alcohol 1i had the same migrating chance (product 3ia), in alcohol 1j the migration of the α-3-CF3Ph group had precedence over the α-Ph group (product 3ja6a,12f–h). For the α-Ph group vs. α-3,4-disubstituted Ph groups, the former was a major migration group (product 3ka6a,12f–h) and the latter was a major migration group (product 3la12c,j). Notably, the sterically hindered α-2-substituted Ph groups were not good migrating groups: the migration of the α-Ph group or the α-4-ClPh group was found to be preferred during the reaction of alcohols 1m–q (products 3ma–qa).12c,j Using 1-(naphthalen-2-yl)-1-phenylprop-2-en-1-ol (1r) to react with 2-bromoacetophenone (2a) delivered the α-naphthalen group migrating product 3ra![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12p as the major product in 58% yield. The α-Ph- and α-thiophen-2-yl-substituted alcohol 1s also worked well and mainly provided the thiophen-2-yl-migrating product 3sa in 85% yield. Interestingly, the reaction was applicable to 2-phenylbut-3-en-2-ol (1t), exclusively giving the Ph-migrating product 3ta in 75% yield.
12p as the major product in 58% yield. The α-Ph- and α-thiophen-2-yl-substituted alcohol 1s also worked well and mainly provided the thiophen-2-yl-migrating product 3sa in 85% yield. Interestingly, the reaction was applicable to 2-phenylbut-3-en-2-ol (1t), exclusively giving the Ph-migrating product 3ta in 75% yield.
The rule of α-substituted aryl group migration applies to alcohols 1 with other α-carbonyl alkyl bromides. For example, the α-Ph- and α-2-Cl-substituted alcohol 1n reacted with methyl 2-bromopropanoate (2h), diethyl 2-bromo-2-methylmalonate (2l) or 2-bromo-2,2-difluoroacetate (2m) and mainly delivered the Ph-migrating products 3nh, 3nl and 3nm in 75–85% yield. Notably, the reaction with diethyl 2-bromo-2-methylmalonate (2l) was finished quickly (18 h) in contrast with the results of Yang and Xia's group (143 h).12p
As shown in Scheme 2, a control experiment using a mixture of two different α-aryl allylic alcohols 1a and 1c reacted with 2-bromoacetophenone (2a) under the optimal conditions was conducted to gain some mechanistic insight into the alkylation/1,2-aryl migration protocol. The results demonstrated that no cross aryl-migrating product 3ga was observed, suggesting that the 1,2-aryl migration proceeds via an intramolecular process. Notably, three radical inhibitors (2.5 equiv.), TEMPO, hydroquinone and BHT, were added to the reaction of alcohol 1a with 2-bromoacetophenone (2a) and resulted in no detectable product 3aa, implying that this current reaction includes a radical process.
An off/on light profile over time was also illustrated to understand the mechanism of this photoredox alkylation/1,2-aryl migration insertion (Fig. S1 in the ESI†). The results show that the additional visible light is necessary for the current reaction: the reaction successfully proceeds upon irradiation with light, but the absence of the additional visible light results in no further conversion. These results suggest that the current reaction follows a photoredox mechanism.
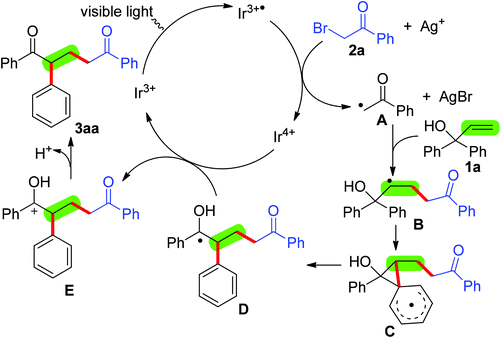
Consequently, a possible mechanism outlined in Scheme 3 was proposed on the basis of the above results as well as previous studies.8–12 Initially, the active Ir3+ species is irradiated to the excited state Ir3+˙ species by visible light.8–11 Single-electron transfer (SET) between the Ir3+˙ species and 2-bromoacetophenone (2a) readily takes place to form alkyl radical A and the Ir4+ species. Subsequently, the addition of alkyl radical A to the C–C double bond of 1,1-diphenylprop-2-en-1-ol (1a) leads to a new alkyl radical intermediate B. Within intermediate B, 1,2-migration of the aryl group occurs via spiro2,5 octadienyl radical C, giving intermediate D.12 Finally, intermediate D is oxidized by the Ir4+ species to afford the cationic intermediate E and regenerate the active Ir3+ species, followed by deprotonation of the cationic intermediate E giving the desired product 3aa.
Conclusions
In summary, we have illustrated a highly efficient and practical alkylation/1,2-aryl migration of α-aryl allylic alcohols with α-carbonyl alkyl bromides through visible-light photoredox catalysis. This new method successfully works with primary, secondary and tertiary α-bromoalkyl carbonyl compounds or 2-bromo-2,2-difluoroacetate to produce 1,5-dicarbonyl compounds with different substitution patterns in good yields, which represents the first visible-light-induced alkylarylation of alkenes using the 1,2-aryl migration strategy with a broad substrate scope, excellent selectivity and mild reaction conditions.Experimental section
General considerations
The 1H and 13C NMR spectra were recorded in CDCl3 solvent on a NMR spectrometer using TMS as the internal standard. LRMS was performed on a GC-MS instrument and HRMS was recorded on an electrospray ionization (ESI) apparatus using time-of-flight (TOF) mass spectrometry. Melting points are uncorrected.Typical experimental procedure for alkylation/1,2-aryl migration of α-aryl allylic alcohols with α-carbonyl alkyl bromides using visible-light photoredox catalysis
To a Schlenk tube were added α-aryl allylic alcohols 1 (0.2 mmol), α-carbonyl alkyl bromides 2 (0.4 mmol), [Ir(ppy)3] (2 mol%), Ag2CO3 (1.2 equiv.), and MeCN (2 mL). Then the tube was charged with argon, and was stirred at 50 °C (oil bath temperature) with 36 W compact fluorescent light under an argon atmosphere for the indicated time until complete consumption of the starting material as monitored by TLC and/or GC-MS analysis. After the reaction was finished, the reaction mixture was cooled to room temperature, diluted in diethyl ether, and washed with brine. The aqueous phase was re-extracted with diethyl ether. The combined organic extracts were dried over Na2SO4 and concentrated in vacuum, and the resulting residue was purified by silica gel column chromatography (hexane/ethyl acetate) to afford the desired products 3.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2; 26.9 mg, 48%; Colorless oil; 1H NMR (400 MHz, CDCl3) δ: 7.97–7.92 (m, 2.0H), 7.48 (t, J = 7.2 Hz, 1.0H), 7.38 (t, J = 7.2 Hz, 2.0H), 7.30–7.21 (m, 5.0H), 4.66–4.61 (m, 1.0H), 2.49–2.39 (m, 1.3H), 2.19 (t, J = 6.8 Hz, 1.0H), 2.13 (s, 1.8H), 2.03 (s, 1.2H), 1.90–1.82 (m, 0.7H), 1.16 (d, J = 5.6 Hz, 1.2H), 1.09 (d, J = 6.0 Hz, 1.8H); 13C NMR (100 MHz, CDCl3) δ: 212.4, 212.3, 199.5, 199.4, 139.2, 138.9, 136.6, 136.5, 133.0 (2C), 129.1, 129.0, 128.7, 128.7, 128.6, 128.5, 128.3, 128.1, 127.3, 127.2, 51.1, 50.8, 45.1, 44.4, 36.7, 36.2, 28.4, 28.2, 17.2, 16.9; IR (KBr, cm−1): 1686, 1660; LRMS (EI, 70 eV) m/z (%): 381 (M+ + 1, 8), 380 (M+, 3), 176 (21), 105 (100); HRMS m/z (ESI) calcd for C19H21O2 ([M + H]+) 281.1549, found 281.1536.
2; 26.9 mg, 48%; Colorless oil; 1H NMR (400 MHz, CDCl3) δ: 7.97–7.92 (m, 2.0H), 7.48 (t, J = 7.2 Hz, 1.0H), 7.38 (t, J = 7.2 Hz, 2.0H), 7.30–7.21 (m, 5.0H), 4.66–4.61 (m, 1.0H), 2.49–2.39 (m, 1.3H), 2.19 (t, J = 6.8 Hz, 1.0H), 2.13 (s, 1.8H), 2.03 (s, 1.2H), 1.90–1.82 (m, 0.7H), 1.16 (d, J = 5.6 Hz, 1.2H), 1.09 (d, J = 6.0 Hz, 1.8H); 13C NMR (100 MHz, CDCl3) δ: 212.4, 212.3, 199.5, 199.4, 139.2, 138.9, 136.6, 136.5, 133.0 (2C), 129.1, 129.0, 128.7, 128.7, 128.6, 128.5, 128.3, 128.1, 127.3, 127.2, 51.1, 50.8, 45.1, 44.4, 36.7, 36.2, 28.4, 28.2, 17.2, 16.9; IR (KBr, cm−1): 1686, 1660; LRMS (EI, 70 eV) m/z (%): 381 (M+ + 1, 8), 380 (M+, 3), 176 (21), 105 (100); HRMS m/z (ESI) calcd for C19H21O2 ([M + H]+) 281.1549, found 281.1536.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2; 41.4 mg, 70%; Yellow oil; 1H NMR (400 MHz, CDCl3) δ: 7.95 (t, J = 6.8 Hz, 2.0H), 7.47 (d, J = 6.8 Hz, 1.0H), 7.39 (d, J = 7.6 Hz, 2.0H), 6.31 (d, J = 10.0 Hz, 2.0H), 7.28 (s, 4.0H), 7.21 (s, 1.0H), 4.67 (s, 1.0H), 3.67 (s, 1.8H), 3.57 (s, 1.2H), 2.52–2.45 (m, 0.8H), 2.34 (m, 1.2H), 2.16–2.10 (m, 0.4H), 2.02–1.96 (m, 0.6H), 1.21 (d, J = 6.8 Hz, 1.2H), 1.15 (d, J = 6.8 Hz, 1.8H); 13C NMR (100 MHz, CDCl3) δ: 199.2 (2C), 176.8, 176.7, 143.5, 139.1, 138.7, 136.7, 136.4, 132.9, 129.0, 128.7, 128.6, 128.5, 128.4, 128.1 (2C), 127.2 (2C), 126.9, 51.6, 51.5, 51.3, 51.2, 37.9, 37.6, 37.4, 36.9, 18.0, 17.6; IR (KBr, cm−1): 1728, 1686; LRMS (EI, 70 eV) m/z (%): 297 (M+ + 1, 16), 296 (M+, 8), 209 (8), 105 (100); HRMS m/z (ESI) calcd for C19H21O3 ([M + H]+) 297.1497, found 297.1485.
2; 41.4 mg, 70%; Yellow oil; 1H NMR (400 MHz, CDCl3) δ: 7.95 (t, J = 6.8 Hz, 2.0H), 7.47 (d, J = 6.8 Hz, 1.0H), 7.39 (d, J = 7.6 Hz, 2.0H), 6.31 (d, J = 10.0 Hz, 2.0H), 7.28 (s, 4.0H), 7.21 (s, 1.0H), 4.67 (s, 1.0H), 3.67 (s, 1.8H), 3.57 (s, 1.2H), 2.52–2.45 (m, 0.8H), 2.34 (m, 1.2H), 2.16–2.10 (m, 0.4H), 2.02–1.96 (m, 0.6H), 1.21 (d, J = 6.8 Hz, 1.2H), 1.15 (d, J = 6.8 Hz, 1.8H); 13C NMR (100 MHz, CDCl3) δ: 199.2 (2C), 176.8, 176.7, 143.5, 139.1, 138.7, 136.7, 136.4, 132.9, 129.0, 128.7, 128.6, 128.5, 128.4, 128.1 (2C), 127.2 (2C), 126.9, 51.6, 51.5, 51.3, 51.2, 37.9, 37.6, 37.4, 36.9, 18.0, 17.6; IR (KBr, cm−1): 1728, 1686; LRMS (EI, 70 eV) m/z (%): 297 (M+ + 1, 16), 296 (M+, 8), 209 (8), 105 (100); HRMS m/z (ESI) calcd for C19H21O3 ([M + H]+) 297.1497, found 297.1485.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; 54.2 mg, 73%; Colorless oil; 1H NMR (400 MHz, CDCl3) δ: 8.03 (d, J = 7.6 Hz, 1.5H), 7.94 (d, J = 7.6 Hz, 0.5H), 7.49 (t, J = 6.8 Hz, 0.5H), 7.41 (t, J = 6.8 Hz, 1.5H), 7.31–7.24 (m, 4.5H), 7.20 (s, 3.5H), 7.17–7.11 (m, 1.0H), 6.74 (s, 2.0H), 4.82–4.78 (m, 0.8H), 4.63–4.59 (m, 0.3H), 3.25 (s, 2.2H), 3.18 (s, 0.8H), 2.50–2.43 (m, 1.0H), 2.36–2.28 (m, 1.0H), 2.02–1.95 (m, 1.0H), 1.07 (d, J = 6.8 Hz, 0.8H), 0.96 (d, J = 6.8 Hz, 2.3H); 13C NMR (100 MHz, CDCl3) δ: 199.9, 199.0, 176.2, 175.7, 143.4, 143.4, 139.4, 138.6, 136.7, 136.5, 136.4, 133.0, 132.8, 129.5, 129.3, 128.8 (2C), 128.7 (2C), 128.5 (2C), 127.9, 127.6, 127.5, 127.2, 127.0, 126.9 (2C), 50.9, 50.6, 38.5 (2C), 37.4, 36.8, 34.7, 34.2, 18.8, 18.5; IR (KBr, cm−1): 1686, 1640; LRMS (EI, 70 eV) m/z (%): 372 (M+ + 1, 13), 371 (M+, 18), 256 (32), 105 (100); HRMS m/z (ESI) calcd for C25H26NO2 ([M + H]+) 372.1946, found 372.1958.
1; 54.2 mg, 73%; Colorless oil; 1H NMR (400 MHz, CDCl3) δ: 8.03 (d, J = 7.6 Hz, 1.5H), 7.94 (d, J = 7.6 Hz, 0.5H), 7.49 (t, J = 6.8 Hz, 0.5H), 7.41 (t, J = 6.8 Hz, 1.5H), 7.31–7.24 (m, 4.5H), 7.20 (s, 3.5H), 7.17–7.11 (m, 1.0H), 6.74 (s, 2.0H), 4.82–4.78 (m, 0.8H), 4.63–4.59 (m, 0.3H), 3.25 (s, 2.2H), 3.18 (s, 0.8H), 2.50–2.43 (m, 1.0H), 2.36–2.28 (m, 1.0H), 2.02–1.95 (m, 1.0H), 1.07 (d, J = 6.8 Hz, 0.8H), 0.96 (d, J = 6.8 Hz, 2.3H); 13C NMR (100 MHz, CDCl3) δ: 199.9, 199.0, 176.2, 175.7, 143.4, 143.4, 139.4, 138.6, 136.7, 136.5, 136.4, 133.0, 132.8, 129.5, 129.3, 128.8 (2C), 128.7 (2C), 128.5 (2C), 127.9, 127.6, 127.5, 127.2, 127.0, 126.9 (2C), 50.9, 50.6, 38.5 (2C), 37.4, 36.8, 34.7, 34.2, 18.8, 18.5; IR (KBr, cm−1): 1686, 1640; LRMS (EI, 70 eV) m/z (%): 372 (M+ + 1, 13), 371 (M+, 18), 256 (32), 105 (100); HRMS m/z (ESI) calcd for C25H26NO2 ([M + H]+) 372.1946, found 372.1958.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; 50.3 mg, 72%; White solid; mp 115.1–116.3 °C (uncorrected); 1H NMR (400 MHz, CDCl3) δ: 8.00 (d, J = 7.2 Hz, 2.0H), 7.95 (d, J = 7.6 Hz, 0.4H), 7.46 (t, J = 6.8 Hz, 1.2H), 7.40–7.33 (m, 3.0H), 7.30–7.24 (m, 4.6H), 7.20 (t, J = 6.0 Hz, 1.2H), 4.73 (t, J = 4.8 Hz, 1.2H), 3.67 (t, J = 6.8 Hz, 1.0H), 3.53 (t, J = 6.8 Hz, 1.2H), 3.37 (t, J = 7.6 Hz, 0.2H), 3.22 (d, J = 4.8 Hz, 2.0H), 3.08 (d, J = 4.8 Hz, 0.4H), 2.78–2.73 (m, 0.2H), 2.52–2.46 (m, 2.0H), 2.30–2.14 (m, 0.5H), 1.98 (t, J = 8.8 Hz, 1.0H), 1.63–1.57 (m, 4.4H), 1.43 (s, 2.8H), 1.14 (d, J = 6.8 Hz, 0.6H), 1.08 (d, J = 6.0 Hz, 3.0H); 13C NMR (100 MHz, CDCl3) δ: 199.8, 173.8, 139.6, 138.9, 136.5, 133.0, 132.8, 128.8, 128.6, 128.5 (2C), 128.4, 128.1, 127.1, 50.9, 50.7, 46.2, 42.8, 38.7, 37.3, 33.1, 32.7, 26.3, 25.7, 25.5, 24.6, 24.4, 18.1; IR (KBr, cm−1): 1686, 1651; LRMS (EI, 70 eV) m/z (%): 350 (M+ + 1, 19), 349 (M+, 23), 243 (16), 105 (100); HRMS m/z (ESI) calcd for C23H28NO2 ([M + H]+) 350.2128, found 350.2115.
1; 50.3 mg, 72%; White solid; mp 115.1–116.3 °C (uncorrected); 1H NMR (400 MHz, CDCl3) δ: 8.00 (d, J = 7.2 Hz, 2.0H), 7.95 (d, J = 7.6 Hz, 0.4H), 7.46 (t, J = 6.8 Hz, 1.2H), 7.40–7.33 (m, 3.0H), 7.30–7.24 (m, 4.6H), 7.20 (t, J = 6.0 Hz, 1.2H), 4.73 (t, J = 4.8 Hz, 1.2H), 3.67 (t, J = 6.8 Hz, 1.0H), 3.53 (t, J = 6.8 Hz, 1.2H), 3.37 (t, J = 7.6 Hz, 0.2H), 3.22 (d, J = 4.8 Hz, 2.0H), 3.08 (d, J = 4.8 Hz, 0.4H), 2.78–2.73 (m, 0.2H), 2.52–2.46 (m, 2.0H), 2.30–2.14 (m, 0.5H), 1.98 (t, J = 8.8 Hz, 1.0H), 1.63–1.57 (m, 4.4H), 1.43 (s, 2.8H), 1.14 (d, J = 6.8 Hz, 0.6H), 1.08 (d, J = 6.0 Hz, 3.0H); 13C NMR (100 MHz, CDCl3) δ: 199.8, 173.8, 139.6, 138.9, 136.5, 133.0, 132.8, 128.8, 128.6, 128.5 (2C), 128.4, 128.1, 127.1, 50.9, 50.7, 46.2, 42.8, 38.7, 37.3, 33.1, 32.7, 26.3, 25.7, 25.5, 24.6, 24.4, 18.1; IR (KBr, cm−1): 1686, 1651; LRMS (EI, 70 eV) m/z (%): 350 (M+ + 1, 19), 349 (M+, 23), 243 (16), 105 (100); HRMS m/z (ESI) calcd for C23H28NO2 ([M + H]+) 350.2128, found 350.2115.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; 35.2 mg, 52%; Colorless oil; 1H NMR (400 MHz, CDCl3) δ: 7.90 (d, J = 8.4 Hz, 3.0H), 7.49–7.43 (m, 2.0H), 7.39–7.34 (m, 3.0H), 7.30–7.23 (m, 6.0H), 7.21–71.5 (m, 1.5H), 4.74–4.71 (m, 0.5H), 4.68–4.65 (m, 1.0H), 3.60 (s, 3.0H), 3.41 (s, 1.5H), 2.33–2.19 (m, 3.0H), 2.09 (d, J = 4.8 Hz, 4.5H), 2.06–1.95 (m, 2.0H), 1.90–1.81 (m, 1.0H), 0.80–0.76 (m, 4.6H); 13C NMR (100 MHz, CDCl3) δ: 206.0, 205.2, 198.9, 198.7, 172.8, 172.7, 140.0, 139.7, 136.6, 136.4, 132.9, 132.8, 129.1, 129.0, 128.7 (2C), 128.5 (2C), 128.3, 128.2, 127.2, 127.1, 63.3, 63.2, 52.2, 52.1, 49.3, 48.9, 35.1, 34.8, 26.9, 26.8, 26.4, 25.9, 8.4, 8.3; IR (KBr, cm−1): 1721, 1686, 1668; LRMS (EI, 70 eV) m/z (%): 353 (M+ + 1, 19), 352 (M+, 14), 248 (18), 105 (100); HRMS m/z (ESI) calcd for C14H18NO3 ([M + H]+) 353.1747, found 353.1742.
1; 35.2 mg, 52%; Colorless oil; 1H NMR (400 MHz, CDCl3) δ: 7.90 (d, J = 8.4 Hz, 3.0H), 7.49–7.43 (m, 2.0H), 7.39–7.34 (m, 3.0H), 7.30–7.23 (m, 6.0H), 7.21–71.5 (m, 1.5H), 4.74–4.71 (m, 0.5H), 4.68–4.65 (m, 1.0H), 3.60 (s, 3.0H), 3.41 (s, 1.5H), 2.33–2.19 (m, 3.0H), 2.09 (d, J = 4.8 Hz, 4.5H), 2.06–1.95 (m, 2.0H), 1.90–1.81 (m, 1.0H), 0.80–0.76 (m, 4.6H); 13C NMR (100 MHz, CDCl3) δ: 206.0, 205.2, 198.9, 198.7, 172.8, 172.7, 140.0, 139.7, 136.6, 136.4, 132.9, 132.8, 129.1, 129.0, 128.7 (2C), 128.5 (2C), 128.3, 128.2, 127.2, 127.1, 63.3, 63.2, 52.2, 52.1, 49.3, 48.9, 35.1, 34.8, 26.9, 26.8, 26.4, 25.9, 8.4, 8.3; IR (KBr, cm−1): 1721, 1686, 1668; LRMS (EI, 70 eV) m/z (%): 353 (M+ + 1, 19), 352 (M+, 14), 248 (18), 105 (100); HRMS m/z (ESI) calcd for C14H18NO3 ([M + H]+) 353.1747, found 353.1742.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; 42.4 mg, 62%; White solid; mp 88.3–89.6 °C (uncorrected); 1H NMR (400 MHz, CDCl3) δ: 7.97 (d, J = 7.6 Hz, 1H), 7.91–7.87 (m, 3H), 7.57–7.50 (m, 1H), 7.48–7.34 (m, 4H), 7.32–7.25 (m, 2H), 7.21–7.16 (m, 2H), 7.08 (d, J = 8.0 Hz, 1H), 4.77–4.71 (m, 1H), 3.05–2.88 (m, 2H), 2.60–2.54 (m, 1H), 2.33 (s, 2H), 2.31–2.22 (m, 2H); 13C NMR (100 MHz, CDCl3) δ: 200.0, 199.9, 199.7, 199.2, 143.7, 139.4, 136.8, 136.8, 136.6, 136.0, 134.1, 133.0, 132.8, 129.7, 129.2, 129.0, 128.9, 128.8, 128.7, 128.6, 128.5 (2C), 128.4, 128.3, 128.1, 128.0, 127.1, 126.8, 52.3, 52.0, 36.0, 36.0, 28.3, 28.2, 21.5, 21.0; IR (KBr, cm−1): 1690, 1674; LRMS (EI, 70 eV) m/z (%): 343 (M+ + 1, 16), 342 (M+, 12), 223 (9), 119 (100); HRMS m/z (ESI) calcd for C24H23O2 ([M + H]+) 343.1689, found 343.1691.
1; 42.4 mg, 62%; White solid; mp 88.3–89.6 °C (uncorrected); 1H NMR (400 MHz, CDCl3) δ: 7.97 (d, J = 7.6 Hz, 1H), 7.91–7.87 (m, 3H), 7.57–7.50 (m, 1H), 7.48–7.34 (m, 4H), 7.32–7.25 (m, 2H), 7.21–7.16 (m, 2H), 7.08 (d, J = 8.0 Hz, 1H), 4.77–4.71 (m, 1H), 3.05–2.88 (m, 2H), 2.60–2.54 (m, 1H), 2.33 (s, 2H), 2.31–2.22 (m, 2H); 13C NMR (100 MHz, CDCl3) δ: 200.0, 199.9, 199.7, 199.2, 143.7, 139.4, 136.8, 136.8, 136.6, 136.0, 134.1, 133.0, 132.8, 129.7, 129.2, 129.0, 128.9, 128.8, 128.7, 128.6, 128.5 (2C), 128.4, 128.3, 128.1, 128.0, 127.1, 126.8, 52.3, 52.0, 36.0, 36.0, 28.3, 28.2, 21.5, 21.0; IR (KBr, cm−1): 1690, 1674; LRMS (EI, 70 eV) m/z (%): 343 (M+ + 1, 16), 342 (M+, 12), 223 (9), 119 (100); HRMS m/z (ESI) calcd for C24H23O2 ([M + H]+) 343.1689, found 343.1691.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of 3ia and a compound tentatively assigned as 3ia′ based on the methyl peak at δ 2.34 for 3ia and at δ 2.28 for 3ia′. 35.6 mg, 52%; White solid; mp 92.7–94.1 °C (uncorrected); 1H NMR (400 MHz, CDCl3) δ: 7.98 (d, J = 7.2 Hz, 1.0H), 7.90 (d, J = 7.6 Hz, 2.0H), 7.77 (t, J = 6.4 Hz, 1.0H), 7.53 (t, J = 7.2 Hz, 1.0H), 7.48–7.35 (m, 3.5H), 7.32–7.25 (m, 3.0H), 7.23–7.17 (m, 1.0H), 7.11 (s, 1.0H), 7.01 (d, J = 7.2 Hz, 0.5H), 4.79–4.71 (m, 1.0H), 3.05–2.87 (m, 2.0H), 2.63–2.53 (m, 1.0H), 2.31–2.22 (m, 2.5H); 13C NMR (100 MHz, CDCl3) δ: 200.0, 199.9, 199.8, 199.6, 139.2, 139.0, 138.7, 138.3, 136.8, 136.6, 133.7, 133.0, 132.9, 129.2, 129.0, 128.8 (2C), 128.5 (2C), 128.3 (2C), 128.0, 127.2, 126.0, 125.5, 52.4 (2C), 36.0, 28.3, 21.4, 21.3; IR (KBr, cm−1): 1688, 1674; LRMS (EI, 70 eV) m/z (%): 343 (M+ + 1, 33), 342 (M+, 15), 223 (13), 119 (100); HRMS m/z (ESI) calcd for C24H23O2 ([M + H]+) 343.1689, found 343.1693.
1 mixture of 3ia and a compound tentatively assigned as 3ia′ based on the methyl peak at δ 2.34 for 3ia and at δ 2.28 for 3ia′. 35.6 mg, 52%; White solid; mp 92.7–94.1 °C (uncorrected); 1H NMR (400 MHz, CDCl3) δ: 7.98 (d, J = 7.2 Hz, 1.0H), 7.90 (d, J = 7.6 Hz, 2.0H), 7.77 (t, J = 6.4 Hz, 1.0H), 7.53 (t, J = 7.2 Hz, 1.0H), 7.48–7.35 (m, 3.5H), 7.32–7.25 (m, 3.0H), 7.23–7.17 (m, 1.0H), 7.11 (s, 1.0H), 7.01 (d, J = 7.2 Hz, 0.5H), 4.79–4.71 (m, 1.0H), 3.05–2.87 (m, 2.0H), 2.63–2.53 (m, 1.0H), 2.31–2.22 (m, 2.5H); 13C NMR (100 MHz, CDCl3) δ: 200.0, 199.9, 199.8, 199.6, 139.2, 139.0, 138.7, 138.3, 136.8, 136.6, 133.7, 133.0, 132.9, 129.2, 129.0, 128.8 (2C), 128.5 (2C), 128.3 (2C), 128.0, 127.2, 126.0, 125.5, 52.4 (2C), 36.0, 28.3, 21.4, 21.3; IR (KBr, cm−1): 1688, 1674; LRMS (EI, 70 eV) m/z (%): 343 (M+ + 1, 33), 342 (M+, 15), 223 (13), 119 (100); HRMS m/z (ESI) calcd for C24H23O2 ([M + H]+) 343.1689, found 343.1693.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of 3ka and a compound tentatively assigned as 3ka′ based on the methyl peak at δ 2.16 for 3ka and at δ 2.10 for 3ka′. 32.8 mg, 46%; White solid; mp 98.4–99.3 °C (uncorrected); 1H NMR (400 MHz, CDCl3) δ: 8.06 (d, J = 7.2 Hz, 0.5H), 7.89 (d, J = 7.2 Hz, 1.0H), 7.81 (d, J = 7.6 Hz, 2.0H), 7.68 (s, 1.0H), 7.63 (d, J = 7.6 Hz, 1.0H), 7.55–7.51 (m, 1.0H), 7.44 (t, J = 7.2 Hz, 2.0H), 7.35–7.29 (m, 5.0H), 7.24–7.16 (m, 4.0H), 7.12–7.07 (m, 2.0H), 7.03 (d, J = 7.6 Hz, 1.0H), 6.96 (d, J = 7.2 Hz, 1.0H), 4.67 (t, J = 7.2 Hz, 1.0H), 4.61 (t, J = 7.2 Hz, 0.5H), 2.98–2.89 (m, 2.0H), 2.86–2.79 (m, 2.0H), 2.54–2.45 (m, 2.0H), 2.16 (d, J = 2.0 Hz, 6.0H), 2.10 (d, J = 3.6 Hz, 3.0H); 13C NMR (100 MHz, CDCl3) δ: 200.0 (2C), 199.7, 199.4, 142.5, 139.5, 136.8 (2C), 136.4, 134.5, 133.0, 132.8, 130.2, 129.9 (2C), 129.7, 129.3, 128.9 (2C), 128.8, 128.5 (2C), 128.4, 128.2, 128.0, 127.8, 127.1, 126.8, 126.5, 125.8, 52.2, 52.0, 48.2, 36.1, 34.3, 29.7, 28.3, 19.9, 19.7, 19.3; IR (KBr, cm−1): 1696, 1674; LRMS (EI, 70 eV) m/z (%): 357 (M+ + 1, 19), 356 (M+, 10), 223 (3), 133 (100); HRMS m/z (ESI) calcd for C25H25O2 ([M + H]+) 357.1873, found 357.1861.
1 mixture of 3ka and a compound tentatively assigned as 3ka′ based on the methyl peak at δ 2.16 for 3ka and at δ 2.10 for 3ka′. 32.8 mg, 46%; White solid; mp 98.4–99.3 °C (uncorrected); 1H NMR (400 MHz, CDCl3) δ: 8.06 (d, J = 7.2 Hz, 0.5H), 7.89 (d, J = 7.2 Hz, 1.0H), 7.81 (d, J = 7.6 Hz, 2.0H), 7.68 (s, 1.0H), 7.63 (d, J = 7.6 Hz, 1.0H), 7.55–7.51 (m, 1.0H), 7.44 (t, J = 7.2 Hz, 2.0H), 7.35–7.29 (m, 5.0H), 7.24–7.16 (m, 4.0H), 7.12–7.07 (m, 2.0H), 7.03 (d, J = 7.6 Hz, 1.0H), 6.96 (d, J = 7.2 Hz, 1.0H), 4.67 (t, J = 7.2 Hz, 1.0H), 4.61 (t, J = 7.2 Hz, 0.5H), 2.98–2.89 (m, 2.0H), 2.86–2.79 (m, 2.0H), 2.54–2.45 (m, 2.0H), 2.16 (d, J = 2.0 Hz, 6.0H), 2.10 (d, J = 3.6 Hz, 3.0H); 13C NMR (100 MHz, CDCl3) δ: 200.0 (2C), 199.7, 199.4, 142.5, 139.5, 136.8 (2C), 136.4, 134.5, 133.0, 132.8, 130.2, 129.9 (2C), 129.7, 129.3, 128.9 (2C), 128.8, 128.5 (2C), 128.4, 128.2, 128.0, 127.8, 127.1, 126.8, 126.5, 125.8, 52.2, 52.0, 48.2, 36.1, 34.3, 29.7, 28.3, 19.9, 19.7, 19.3; IR (KBr, cm−1): 1696, 1674; LRMS (EI, 70 eV) m/z (%): 357 (M+ + 1, 19), 356 (M+, 10), 223 (3), 133 (100); HRMS m/z (ESI) calcd for C25H25O2 ([M + H]+) 357.1873, found 357.1861.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 mixture of 3pa and a compound tentatively assigned as 3pa′ based on the methine peak at δ 5.13 for 3pa and at δ 4.63 for 3pa′. 58.2 mg, 80%; Colorless oil; 1H NMR (400 MHz, CDCl3) δ: 7.99 (d, J = 7.6 Hz, 1.2H), 7.92–7.88 (m, 2.0H), 7.56–7.50 (m, 1.6H), 7.44–7.42 (m, 3.6H), 7.30–7.20 (m, 2.0H), 7.12–7.07 (m, 0.4H), 7.04–6.96 (m, 1.6H), 6.90–6.84 (m, 1.6H), 5.13 (t, J = 7.2 Hz, 0.6H), 4.63 (t, J = 7.6 Hz, 0.4H), 3.10–2.88 (m, 2.0H), 2.64–2.56 (m, 1.0H), 2.29–2.19 (m, 1.0H); 13C NMR (100 MHz, CDCl3) δ: 199.6, 199.2, 198.5 (2C), 137.6, 136.7 (2C), 135.9, 133.4, 133.1, 133.0, 128.9, 128.7, 128.6 (2C), 128.5, 128.0 (2C), 127.5, 121.0, 120.9, 120.8, 120.7, 118.2, 118.1, 117.9 (2C), 117.1, 117.0, 116.9, 116.8, 116.7, 116.6, 115.7 (2C), 115.6 (2C), 115.4 (4C), 56.5, 56.4, 43.7, 36.0, 35.7, 27.7, 27.2.; 19F NMR (375 MHz, CDCl3) δ: −115.3 (d, J = 18.4 Hz, 1F), −117.3 (d, J = 17.6 Hz, 1F), −117.6 (d, J = 18.8 Hz, 1F), −124.1 (d, J = 28.9 Hz, 1F); IR (KBr, cm−1): 1710, 1674; LRMS (EI, 70 eV) m/z (%): 365 (M+ + 1, 16), 364 (M+, 9), 224 (11), 105 (100); HRMS m/z (ESI) calcd for C23H19F2O2 ([M + H]+) 365.1353, found 365.1369.
2 mixture of 3pa and a compound tentatively assigned as 3pa′ based on the methine peak at δ 5.13 for 3pa and at δ 4.63 for 3pa′. 58.2 mg, 80%; Colorless oil; 1H NMR (400 MHz, CDCl3) δ: 7.99 (d, J = 7.6 Hz, 1.2H), 7.92–7.88 (m, 2.0H), 7.56–7.50 (m, 1.6H), 7.44–7.42 (m, 3.6H), 7.30–7.20 (m, 2.0H), 7.12–7.07 (m, 0.4H), 7.04–6.96 (m, 1.6H), 6.90–6.84 (m, 1.6H), 5.13 (t, J = 7.2 Hz, 0.6H), 4.63 (t, J = 7.6 Hz, 0.4H), 3.10–2.88 (m, 2.0H), 2.64–2.56 (m, 1.0H), 2.29–2.19 (m, 1.0H); 13C NMR (100 MHz, CDCl3) δ: 199.6, 199.2, 198.5 (2C), 137.6, 136.7 (2C), 135.9, 133.4, 133.1, 133.0, 128.9, 128.7, 128.6 (2C), 128.5, 128.0 (2C), 127.5, 121.0, 120.9, 120.8, 120.7, 118.2, 118.1, 117.9 (2C), 117.1, 117.0, 116.9, 116.8, 116.7, 116.6, 115.7 (2C), 115.6 (2C), 115.4 (4C), 56.5, 56.4, 43.7, 36.0, 35.7, 27.7, 27.2.; 19F NMR (375 MHz, CDCl3) δ: −115.3 (d, J = 18.4 Hz, 1F), −117.3 (d, J = 17.6 Hz, 1F), −117.6 (d, J = 18.8 Hz, 1F), −124.1 (d, J = 28.9 Hz, 1F); IR (KBr, cm−1): 1710, 1674; LRMS (EI, 70 eV) m/z (%): 365 (M+ + 1, 16), 364 (M+, 9), 224 (11), 105 (100); HRMS m/z (ESI) calcd for C23H19F2O2 ([M + H]+) 365.1353, found 365.1369.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; 49.5 mg, 75%; Yellow oil; 1H NMR (400 MHz, CDCl3) δ: 7.32 (d, J = 7.6 Hz, 1.0H), 7.28–7.21 (m, 4.0H), 7.19–7.10 (m, 3.0H), 7.10–7.06 (m, 1.0H), 4.52–4.48 (m, 1.0H), 3.67 (s, 1.5H), 3.60 (s, 1.5H), 2.57–2.46 (m, 1.0H), 2.38–2.20 (m, 1.6H), 2.07–2.00 (m, 0.6H), 1.22 (d, J = 7.2 Hz, 1.5H), 1.17 (d, J = 6.8 Hz, 1.5H); 13C NMR (100 MHz, CDCl3) δ: 202.6 (2C), 176.7, 176.5, 139.4, 139.3, 137.0, 136.9, 131.2, 131.1, 130.4 (2C), 130.2, 130.1, 128.9, 128.8 (2C), 128.6, 127.5 (2C), 126.5 (2C), 55.7, 55.6, 51.6, 51.5, 37.4, 36.8, 36.0, 35.9, 18.0, 17.4; IR (KBr, cm−1): 1711, 1704; LRMS (EI, 70 eV) m/z (%): 332 (M+ + 2, 6), 332 (M+, 16), 236 (10), 139 (100); HRMS m/z (ESI) calcd for C19H20ClO3 ([M + H]+) 331.1101, found 331.1109.
1; 49.5 mg, 75%; Yellow oil; 1H NMR (400 MHz, CDCl3) δ: 7.32 (d, J = 7.6 Hz, 1.0H), 7.28–7.21 (m, 4.0H), 7.19–7.10 (m, 3.0H), 7.10–7.06 (m, 1.0H), 4.52–4.48 (m, 1.0H), 3.67 (s, 1.5H), 3.60 (s, 1.5H), 2.57–2.46 (m, 1.0H), 2.38–2.20 (m, 1.6H), 2.07–2.00 (m, 0.6H), 1.22 (d, J = 7.2 Hz, 1.5H), 1.17 (d, J = 6.8 Hz, 1.5H); 13C NMR (100 MHz, CDCl3) δ: 202.6 (2C), 176.7, 176.5, 139.4, 139.3, 137.0, 136.9, 131.2, 131.1, 130.4 (2C), 130.2, 130.1, 128.9, 128.8 (2C), 128.6, 127.5 (2C), 126.5 (2C), 55.7, 55.6, 51.6, 51.5, 37.4, 36.8, 36.0, 35.9, 18.0, 17.4; IR (KBr, cm−1): 1711, 1704; LRMS (EI, 70 eV) m/z (%): 332 (M+ + 2, 6), 332 (M+, 16), 236 (10), 139 (100); HRMS m/z (ESI) calcd for C19H20ClO3 ([M + H]+) 331.1101, found 331.1109.
Acknowledgements
We thank the Natural Science Foundation of China (no. 21172060), Specialized Research Fund for the Doctoral Program of Higher Education (no. 20120161110041), and Hunan Provincial Natural Science Foundation of China (no. 13JJ2018) for financial support.Notes and references
- For selected reviews on alkene functionalization: (a) A. de Meijere and F. E. Meyer, Angew. Chem., Int. Ed. Engl., 1994, 33, 2379 CrossRef PubMed; (b) M. Oestreich, The Mizoroki-Heck reaction, Wiley, Hoboken, NJ, 2008 Search PubMed; (c) I. P. Beletskaya and A. V. Cheprakov, Chem. Rev., 2000, 100, 3009 CrossRef CAS PubMed; (d) D. Mc Cartney and P. J. Guiry, Chem. Soc. Rev., 2011, 40, 5122 RSC; (e) M. Beller, J. Seayad, A. Tillack and H. Jiao, Angew. Chem., Int. Ed., 2004, 43, 3368 CrossRef CAS PubMed; (f) S. Sumino, A. Fusano, T. Fukuyama and I. Ryu, Acc. Chem. Res., 2014, 47, 1563 CrossRef CAS PubMed; (g) G. Liu and Y. Wu, Top. Curr. Chem., 2010, 292, 195 CrossRef CAS; (h) W. Oppolzer, Pure Appl. Chem., 1988, 60, 39 CrossRef CAS; (i) J. J. Brunet, D. Neibecker and F. Niedercorn, J. Mol. Catal., 1989, 49, 235 CrossRef CAS; (j) J. Lindley and T. Mason, J. Educ. Chem., 1981, 18, 78 CAS.
- For selected reviews on alkene difunctionalization: (a) K. Muñiz, Chem. Soc. Rev., 2004, 33, 166 RSC; (b) V. Kotov, C. C. Scarborough and S. S. Stahl, Inorg. Chem., 2007, 46, 1910 CrossRef CAS PubMed; (c) R. I. McDonald, G. Liu and S. S. Stahl, Chem. Rev., 2011, 111, 2981 CrossRef CAS PubMed; (d) S. R. Chemler and M. T. Bovino, ACS Catal., 2013, 3, 1076 CrossRef CAS; (e) J. P. Wolfe, Angew. Chem., Int. Ed., 2012, 51, 10224 CrossRef CAS PubMed; (f) Y. Shimizu and M. Kanai, Tetrahedron Lett., 2014, 55, 3727 CrossRef CAS PubMed; (g) B. Jacques and K. Muñiz, in Catalyzed Carbon-Heteroatom Bond Formation, ed. A. K. Yudin, Wiley-VCH, Weinheim, 2010, pp. 119–135 Search PubMed; (h) H. C. Kolb, M. S. Van-Nieuwenhze and B. K. Sharpless, Chem. Rev., 1994, 94, 2483 CrossRef CAS; (i) G. Li, T.-T. Chang and B. K. Sharpless, Angew. Chem., Int. Ed. Engl., 1996, 35, 451 CrossRef CAS PubMed; (j) V. K. Reddy, B. Haritha and M. Yamashita, Lett. Org. Chem., 2005, 2, 128 CrossRef CAS.
- (a) T. Wu, X. Mu and G.-S. Liu, Angew. Chem., Int. Ed., 2011, 50, 12578 CrossRef CAS PubMed; (b) H. Zhang, P. Chen and G. Liu, Synlett, 2012, 2749 CAS.
- (a) W.-T. Wei, M.-B. Zhou, J.-H. Fan, W. Liu, R.-J. Song, Y. Liu, M. Hu, P. Xie and J.-H. Li, Angew. Chem., Int. Ed., 2013, 52, 3638 CrossRef CAS PubMed; (b) Y. Nishikawa and H. Yamamoto, J. Am. Chem. Soc., 2011, 133, 8432 CrossRef CAS PubMed.
- (a) T. Wu, H. Zhang and G.-S. Liu, Tetrahedron, 2012, 68, 5229 CrossRef CAS PubMed; (b) Q. Dai, J. Yu, Y. Jiang, S. Guo, H. Yang and J. Cheng, Chem. Commun., 2014, 50, 3865 RSC; (c) J.-H. Fan, M.-B. Zhou, W. Liu, W.-T. Wei, X.-H. Ouyang, R.-J. Song and J.-H. Li, Synlett, 2014, 657 CAS; (d) D. Azarifar and K. Khosravi, Synlett, 2010, 2755 CrossRef CAS.
- (a) X.-Q. Chu, H. Meng, Y. Zi, X.-P. Xu and S.-J. Ji, Chem. Commun., 2014, 50, 9718 RSC; (b) G. Joyram, D. S. Suman, G. Stefan and S. Armido, Angew. Chem., Int. Ed., 2008, 47, 8727 CrossRef PubMed.
- J.-H. Fan, W.-T. Wei, M.-B. Zhou, R.-J. Song and J.-H. Li, Angew. Chem., Int. Ed., 2014, 53, 6650 CrossRef CAS PubMed.
- For special reviews on visible light photoredox catalysis: (a) P. Melchiorre, Angew. Chem., Int. Ed., 2009, 48, 1360 CrossRef CAS PubMed; (b) K. Zeitler, Angew. Chem., Int. Ed., 2009, 48, 9785 CrossRef CAS PubMed; (c) T. P. Yoon, M. A. Ischay and J. Du, Nat. Chem., 2010, 2, 527 CrossRef CAS PubMed; (d) J. M. R. Narayanam and C. R. J. Stephenson, Chem. Soc. Rev., 2011, 40, 102 RSC; (e) F. Teplý and C. Czech, Chem. Commun., 2011, 76, 859 Search PubMed; (f) D. Ravelli and M. Fagnoni, ChemCatChem, 2012, 4, 169 CrossRef CAS PubMed; (g) J. Xuan and W.-J. Xiao, Angew. Chem., Int. Ed., 2012, 51, 6828 CrossRef CAS PubMed; (h) J. Xuan, L.-Q. Lu, J.-R. Chen and W.-J. Xiao, Eur. J. Org. Chem., 2013, 2071 Search PubMed; (i) Y.-Q. Zou, J.-R. Chen and W.-J. Xiao, Angew. Chem., Int. Ed., 2013, 52, 11701 CrossRef CAS PubMed; (j) C. K. Prier, D. A. Rankic and D. W. C. MacMillan, Chem. Rev., 2013, 113, 5322 CrossRef CAS PubMed; (k) J. J. Douglas, J. D. Nguyen, K. P. Cole and C. R. J. Stephenson, Aldrichimica Acta, 2014, 47, 15 CAS; (l) M. N. Hopkin-son, B. Sahoo, J. Li and F. Glorius, Chem. – Eur. J., 2014, 20, 3874 CrossRef CAS PubMed; (m) E. Jahn and U. Jahn, Angew. Chem., Int. Ed., 2014, 53, 13326 CrossRef CAS PubMed; (n) J.-R. Chen, X.-Y. Yu and W.-J. Xiao, Synthesis, 2015, 604 Search PubMed.
- (a) J. D. Nguyen, J. W. Tucker, M. D. Konieczynska and C. R. J. Stephenson, J. Am. Chem. Soc., 2011, 133, 4160 CrossRef CAS PubMed; (b) C.-J. Wallentin, J. D. Nguyen, P. Finkbeiner and C. R. J. Stephenson, J. Am. Chem. Soc., 2012, 134, 8875 CrossRef CAS PubMed; (c) J. W. Tucker, Y. Zhang, T. F. Jamison and C. R. J. Stephenson, Angew. Chem., Int. Ed., 2012, 51, 4144 CrossRef CAS PubMed.
- (a) R. S. Andrews, J. J. Becker and M. R. Gagné, Angew. Chem., Int. Ed., 2010, 49, 7274 CrossRef CAS PubMed; (b) J. W. Tucker, J. D. Nguyen, J. M. R. Narayanam, S. W. Krabbe and C. R. J. Stephenson, Chem. Commun., 2010, 46, 4985 RSC; (c) R. S. Andrews, J. J. Becker and M. R. Gagné, Angew. Chem., Int. Ed., 2012, 51, 4140 CrossRef CAS PubMed; (d) H. Kim and C. Lee, Angew. Chem., Int. Ed., 2012, 51, 12303 CrossRef CAS PubMed.
- (a) H. Jiang, C. Huang, J. Guo, C. Zeng, Y. Zhang and S. Yu, Chem. – Eur. J., 2012, 18, 15158 CrossRef CAS PubMed; (b) Q. Liu, H. Yi, J. Liu, Y. Yang, X. Zhang, Z. Zeng and A. Lei, Chem. – Eur. J., 2013, 19, 5120 CrossRef CAS PubMed.
- The neophyl rearrangement: (a) C. Rüchardt and R. Hecht, Chem. Ber., 1965, 98, 2471 CrossRef PubMed; (b) C. S. A. Antunes, M. Bietti, G. Ercolani, O. Lanzalunga and M. Salamone, J. Org. Chem., 2005, 70, 3884 CrossRef CAS PubMed. For recent examples of 1,2-aryl migration involving allylic alcohols: (c) X. Liu, F. Xiong, X. Huang, L. Xu, P. Li and X. Wu, Angew. Chem., Int. Ed., 2013, 52, 6962 CrossRef CAS PubMed; (d) H. Egami, R. Shimizu, Y. Usui and M. Sodeoka, Chem. Commun., 2013, 49, 7346 RSC; (e) Z.-M. Chen, W. Bai, S.-H. Wang, B.-M. Yang, Y.-Q. Tu and F.-M. Zhang, Angew. Chem., Int. Ed., 2013, 52, 9781 CrossRef CAS PubMed; (f) X.-Q. Chu, Y. Zi, H. Meng, X.-P. Xu and S.-J. Ji, Chem. Commun., 2014, 50, 7642 RSC; (g) X.-Q. Chu, H. Meng, Y. Zi, X.-P. Xu and S.-J. Ji, Chem. – Eur. J., 2014, 20, 17198 CrossRef CAS PubMed; (h) X.-Q. Chu, H. Meng, Y. Zi, X.-P. Xu and S.-J. Ji, Org. Chem. Front., 2015, 2, 216 RSC; (i) J. Zhao, H. Fang, R. Song, J. Zhou, J. Han and Y. Pan, Chem. Commun., 2015, 51, 599 RSC; (j) Y. Yu and U. K. Tambar, Chem. Sci., 2015, 6, 2777 RSC; (k) Z.-M. Chen, Z. Zhang, Y.-Q. Tu, M.-H. Xu, F.-M. Zhang, C.-C. Li and S.-H. Wang, Chem. Commun., 2014, 50, 10805 RSC; (l) Y. Li, B. Liu, H.-B. Li, Q. Wang and J.-H. Li, Chem. Commun., 2015, 51, 1024 RSC; (m) R.-J. Song, Y.-Q. Tu, D.-Y. Zhu, F.-M. Zhang and S.-H. Wang, Chem. Commun., 2015, 51, 749 RSC; (n) A. Bunescu, Q. Wang and J. Zhu, Angew. Chem., Int. Ed., 2015, 54, 3132 CrossRef CAS PubMed. For a review on the Tu-type semipinacol rearrangement: (o) B. Wang and Y.-Q. Tu, Acc. Chem. Res., 2011, 44, 1207 CrossRef CAS PubMed. During our preparation of this manuscript, the group of Yang/Xia reported a mild photocatalytic process for the selective arylalkylation of allylic alcohols with a-bromo diethyl malonate via unique 1,2-arylmigration using fac-Ir(ppy)3 (0.005 mmol) with 1W blue LEDs; however, the reaction required a longer reaction time (143 h) and was limited to only α-bromo diethyl malonates: (p) H.-L. Huang, H. Yan, C. Yang and W. Xia, Chem. Commun., 2015, 51, 4910 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Copies of 1H and 13C spectra. CCDC 1407703. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5qo00220f |
| This journal is © the Partner Organisations 2015 |