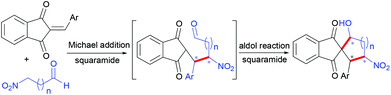
Enantioselective construction of spiro-1,3-indandiones with three stereocenters via organocatalytic Michael-aldol reaction of 2-arylideneindane-1,3-diones and nitro aldehydes†‡
Jindian
Duan
,
Jing
Cheng
and
Pengfei
Li
*
Department of Chemistry, South University of Science and Technology of China, Shenzhen, Guangdong 518055, China. E-mail: lipf@sustc.edu.cn; flyli1980@gmail.com; Fax: (+86) 755-88018304
First published on 19th June 2015
Abstract
Asymmetric domino reaction between 2-arylideneindane-1,3-dione and nitro aldehyde has been developed for the construction of an enantioenriched spiro-1,3-indandione framework with three stereocenters. In the presence of a squaramide-tertiary amine, chiral spiro-1,3-indandione derivatives are obtained in good yields with high enantioselectivities and diastereoselectivities.
The synthesis of multi-substituted spiro-1,3-indandiones has aroused considerable interest because of their abundance in many natural products with useful biological activities,1 such as antitumor, antibiotic,2 and antiproliferative activity on HL60 and apoptosis resistant leukemia cell lines.3 Among various reported methodologies for accessing the spiro-1,3-indandione skeletons,4 organocatalytic domino reactions using readily accessible 2-arylidene-1,3-indandiones have been demonstrated to be a powerful strategy.5–7 Although diversified Michael donors have been employed in the organocascade reactions for the construction of enantioenriched spiro-1,3-indandione skeletons, impressive results (more than 90% ee) are limited to the use of an aldehyde as a Michael donor, which was catalyzed by a chiral amine derived organocatalyst.7i–j The only two exceptions were the primary amine catalyzed asymmetric multicomponent reaction among ynones, aldehydes and indan-1,3-diones7e and the tertiary amine-thiourea catalyzed asymmetric reaction between 2-arylidene-1,3-indandione and nitroalkane.7g Therefore, efforts in developing an efficient organocatalytic methodology for the construction of enantioenriched spiro-1,3-indandiones are in high demand.
Both β- and γ-nitro aldehydes are resourceful moieties in synthetically useful building blocks and have been efficiently utilized in the total synthesis of natural products and functional cyclic skeletons.8 To the best of our knowledge, no results have been reported on the asymmetric domino reaction between nitro aldehyde and 2-arylidene-1,3-indandione for the construction of chiral spiro-1,3-indandione derivatives. As a continuation of our recent efforts,9 herein we disclose the first asymmetric synthesis of chiral multi-substituted spiro-1,3-indandiones from nitro aldehyde and 2-arylidene-1,3-indandione via a Michael-aldol reaction sequence using a squaramide as an organocatalyst (Scheme 1).
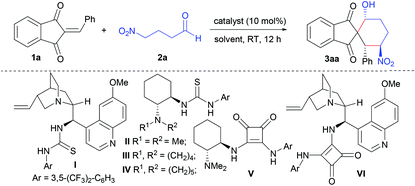
We initiated our studies by investigating the reaction of 2-benzylidene-1,3-indandione (1a) with 4-nitrobutanal (2a) in CH2Cl2 at room temperature in the presence of hydrogen-bonding organocatalysts.10 The results are shown in Table 1. To our gratification, in the presence of 10 mol% quinidine-based thiourea I,11 the Michael-aldol reaction between 1a and 2a proceeded smoothly and the desired product 3aa was obtained as a single diastereomer (>20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr) in 75% yield with 55% ee (Table 1, entry 1). Then some representative bifunctional hydrogen-bonding donor catalysts II–VI were examined in this domino reaction (Table 1, entries 2–6). The results revealed that a squaramide-tertiary amine is more suitable for this asymmetric domino transformation.12 In particular, 84% yield with 88% ee and >20
1 dr) in 75% yield with 55% ee (Table 1, entry 1). Then some representative bifunctional hydrogen-bonding donor catalysts II–VI were examined in this domino reaction (Table 1, entries 2–6). The results revealed that a squaramide-tertiary amine is more suitable for this asymmetric domino transformation.12 In particular, 84% yield with 88% ee and >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr could be obtained when squaramide VI derived from quinidine was employed as an organocatalyst (Table 1, entry 6). To achieve high yield and asymmetric induction, further optimization of reaction conditions was carried out. After screening of common solvents, it was found that reaction media only affected the yield obviously and had less effect on the asymmetric induction (Table 1, entries 7–10). The best yield, 84%, was obtained when the domino reaction was carried out in CH2Cl2 (Table 1, entry 6). Subsequent investigations on the additive indicated that molecular sieves could increase the ee value slightly (Table 1, entries 11 and 12). Under optimized reaction conditions, enantioenriched spiro-1,3-indandione 3aa was obtained in 83% yield with 90% ee and >20
1 dr could be obtained when squaramide VI derived from quinidine was employed as an organocatalyst (Table 1, entry 6). To achieve high yield and asymmetric induction, further optimization of reaction conditions was carried out. After screening of common solvents, it was found that reaction media only affected the yield obviously and had less effect on the asymmetric induction (Table 1, entries 7–10). The best yield, 84%, was obtained when the domino reaction was carried out in CH2Cl2 (Table 1, entry 6). Subsequent investigations on the additive indicated that molecular sieves could increase the ee value slightly (Table 1, entries 11 and 12). Under optimized reaction conditions, enantioenriched spiro-1,3-indandione 3aa was obtained in 83% yield with 90% ee and >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr (Table 1, entry 12). Importantly, lowering catalyst loading from 10 mol% to 5 mol% led to a similar yield without compromising enantioselectivity and diastereoselectivity (Table 1, entry 13).
1 dr (Table 1, entry 12). Importantly, lowering catalyst loading from 10 mol% to 5 mol% led to a similar yield without compromising enantioselectivity and diastereoselectivity (Table 1, entry 13).
| Entry | Catalyst | Solvent | Yieldb (%) | eec (%) | drc |
|---|---|---|---|---|---|
| a Reaction conditions: a mixture of 1a (0.2 mmol), catalyst (10 mol%), and 2a (0.24 mmol) in solvent (2.0 mL) was stirred at room temperature for 12 h. b Isolated yield. c Determined by chiral HPLC analysis. d 4 Å MS (30 mg) was added. e 5 Å MS (30 mg) was added. f Catalyst (5 mol%) was used. | |||||
| 1 | I | CH2Cl2 | 75 | 55 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 2 | II | CH2Cl2 | 60 | 41 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 3 | III | CH2Cl2 | 76 | 27 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 4 | IV | CH2Cl2 | 75 | 3 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 5 | V | CH2Cl2 | 78 | −82 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 6 | VI | CH2Cl2 | 84 | 88 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 7 | VI | Toluene | 76 | 88 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 8 | VI | THF | 72 | 88 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 9 | VI | EtOAc | 50 | 84 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 10 | VI | Et2O | 52 | 88 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 11d | VI | CH2Cl2 | 85 | 89 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 12e | VI | CH2Cl2 | 83 | 90 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 13f | VI | CH2Cl2 | 80 | 90 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
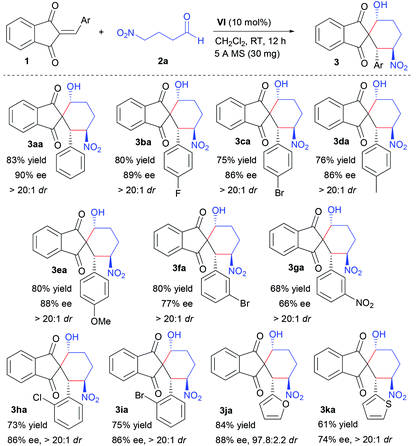
With optimum reaction conditions in hand, the scope of the Michael-aldol domino reactions between various 2-arylidene-1,3-indandiones (1) and 4-nitrobutanal (2a) was investigated, and the results are summarized in Table 2.
| a A mixture of 1 (0.2 mmol), 5 Å MS (30 mg), VI (10 mol%), and 2a (0.24 mmol) in CH2Cl2 (2.0 mL) was stirred at room temperature for 12 h. And products 3 were obtained in isolated yield. Both enantioselectivities and diastereoselectivities were determined by chiral HPLC analysis. |
|---|
|
A set of 2-arylidene-1,3-indandiones (1a–i) with different substitution patterns with respect to electronic properties, steric hindrance, and substitution positions on the aromatic ring were surveyed to react with 4-nitrobutanal (2a) and the desired enantioenriched spiro-1,3-indandiones (Table 2, 3aa–ia) were obtained as single diastereomers (>20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr) in good yields (68–83%) with moderate to good enantioselectivities (66–90% ee). No significant electron effect on the aromatic moiety was observed. The position of the substituents on the aromatic ring had a little influence on the enantioselectivity. Both 2-(4-substituted-arylidene)-1,3-indandiones (1b–e) and 2-(2-substituted-arylidene)-1,3-indandiones (1h, 1i) afforded chiral spiro-1,3-indandiones (Table 2, 3ba–ea, 3ha, 3ia) with moderate to good enantioselectivities (66–90% ee). Comparatively, 2-(3-substituted-arylidene)-1,3-indandiones (1f, 1g) furnished spiro-products with moderate enantioselectivities (Table 2, 3fa, 3ga). To explore the scope of the domino reaction, heteroaromatic 1,3-indandiones were also investigated. The reaction between 2-((furan-2-yl)methylene)-2H-indene-1,3-dione (1j) and 2a proceeded smoothly to afford the corresponding spiro-product in 84% yield with 88% ee and 97.8
1 dr) in good yields (68–83%) with moderate to good enantioselectivities (66–90% ee). No significant electron effect on the aromatic moiety was observed. The position of the substituents on the aromatic ring had a little influence on the enantioselectivity. Both 2-(4-substituted-arylidene)-1,3-indandiones (1b–e) and 2-(2-substituted-arylidene)-1,3-indandiones (1h, 1i) afforded chiral spiro-1,3-indandiones (Table 2, 3ba–ea, 3ha, 3ia) with moderate to good enantioselectivities (66–90% ee). Comparatively, 2-(3-substituted-arylidene)-1,3-indandiones (1f, 1g) furnished spiro-products with moderate enantioselectivities (Table 2, 3fa, 3ga). To explore the scope of the domino reaction, heteroaromatic 1,3-indandiones were also investigated. The reaction between 2-((furan-2-yl)methylene)-2H-indene-1,3-dione (1j) and 2a proceeded smoothly to afford the corresponding spiro-product in 84% yield with 88% ee and 97.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.2 dr (Table 2, 3ja). And 2-((thiophen-2-yl)methylene)-2H-indene-1,3-dione (1k) was found to react with 2a slowly to afford the corresponding product in 61% yield with 74% ee and >20
2.2 dr (Table 2, 3ja). And 2-((thiophen-2-yl)methylene)-2H-indene-1,3-dione (1k) was found to react with 2a slowly to afford the corresponding product in 61% yield with 74% ee and >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr (Table 2, 3ka).
1 dr (Table 2, 3ka).
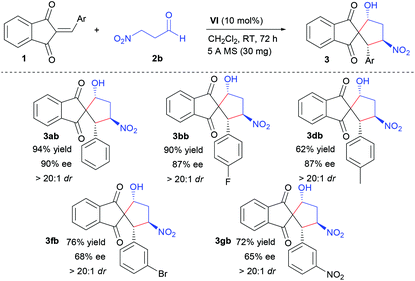
To further explore the scope of the domino reaction, the VI-mediated Michael-aldol reactions between a series of 2-arylidene-1,3-indandiones (1) and 3-nitropropanal (2b) were then probed as shown in Table 3. Under the same optimized conditions, enantioenriched spiro-1,3-indandione-cyclopentanol 3ab was obtained in 94% yield with 90% ee and >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr from the domino reaction between 1a and 2b (Table 3, 3ab). Both electron-withdrawing (Table 3, 3bb, 3fb, 3gb) and electron-donating groups (Table 3, 3db), could be introduced into the aromatic ring of 2-arylidene-1,3-indandione, respectively. And 2-(4-substituted-arylidene)-1,3-indandiones furnished the desired products with higher enantioselectivities than those of the corresponding products from 2-(3-substituted-arylidene)-1,3-indandiones. This is in agreement with the results obtained from the domino reactions between 1a and 2a.
1 dr from the domino reaction between 1a and 2b (Table 3, 3ab). Both electron-withdrawing (Table 3, 3bb, 3fb, 3gb) and electron-donating groups (Table 3, 3db), could be introduced into the aromatic ring of 2-arylidene-1,3-indandione, respectively. And 2-(4-substituted-arylidene)-1,3-indandiones furnished the desired products with higher enantioselectivities than those of the corresponding products from 2-(3-substituted-arylidene)-1,3-indandiones. This is in agreement with the results obtained from the domino reactions between 1a and 2a.
| a A mixture of 1 (0.2 mmol), 5 Å MS (30 mg), VI (10 mol%), and 2b (0.24 mmol) in CH2Cl2 (2.0 mL) was stirred at room temperature for 72 h. And products 3 were obtained in isolated yield. Both enantioselectivities and diastereoselectivities were determined by chiral HPLC analysis. |
|---|
|
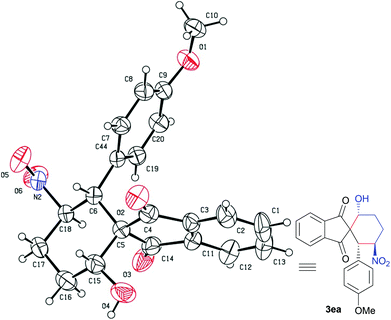
The absolute configuration of adduct 3ea, based on the VI-catalyzed Michael-aldol reaction, was unambiguously determined by single-crystal X-ray crystallography (Fig. 1).13 As shown in Fig. 1, the absolute configuration of C6, C15 and C18 in the structure of 3ea was R-configuration. The stereochemistry of other products was assigned by analogy.
Conclusions
In summary, we have developed a highly efficient asymmetric organocatalytic Michael-aldol domino reaction for the construction of a functionalized enantioenriched spiro-indane-1,3-dione skeleton. In the presence of the available squaramide organocatalyst, various 2-arylidene-1,3-indandiones reacted with 4-nitrobutanal and 3-nitropropanal smoothly to furnish the desired chiral spiro-1,3-indandiones in moderate to high yields with good to high diastereoselectivities and enantiocontrol. Importantly, the spiro ring system containing three stereocenters could be built in one pot via this methodology in a high asymmetric level. Further investigations involving the application of this catalytic approach are currently underway in our laboratory.Acknowledgements
We gratefully acknowledge the start-up fund from the South University of Science and Technology of China (SUSTC) and the National Natural Science Foundation of China (NSFC 21302089) for financial support.Notes and references
- G. S. Singh and Z. Y. Desta, Chem. Rev., 2012, 112, 6104 CrossRef CAS PubMed.
- (a) P. A. Evans and T. A. Brandt, Tetrahedron Lett., 1996, 37, 1367 CrossRef CAS; (b) D. L. J. Clive, X. Kong and C. C. Paul, Tetrahedron, 1996, 52, 6085 CrossRef CAS.
- D. Pizzirani, M. Roberti, S. Grimaudo, A. D. Cristina, R. M. Pipitone, M. Tolomeo and M. Recanatini, J. Med. Chem., 2009, 52, 6936 CrossRef CAS PubMed.
- For selected examples, see: (a) W. Hoeve, H. Wynberg and C. Spiranes, J. Org. Chem., 1979, 44, 1508 CrossRef; (b) W. T. Hoeve and H. Wynberg, J. Org. Chem., 1980, 45, 2925 CrossRef; (c) D. Kuck and H. Bögge, J. Am. Chem. Soc., 1986, 108, 8107 CrossRef CAS; (d) M. J. Rosenfeld, S. B. K. Ravi and H. Shechter, J. Org. Chem., 1988, 53, 2699 CrossRef CAS; (e) A. S. Kende, K. Koch and C. A. Smith, J. Am. Chem. Soc., 1988, 110, 2210 CrossRef CAS; (f) B. Bredenkötter, U. Flörke and D. Kuck, Chem. – Eur. J., 2001, 7, 3387 CrossRef; (g) H. M. Hassaneen, N. M. Elwan and H. M. Hassaneen, Synth. Commun., 2002, 32, 3047 CrossRef CAS; (h) A. R. S. Babu and R. Raghunathan, Tetrahedron Lett., 2006, 47, 9221 CrossRef CAS PubMed; (i) A. R. S. Babu and R. Raghunathan, Synth. Commun., 2007, 37, 451 CrossRef CAS PubMed; (j) A. R. S. Babu and R. Raghunathan, Tetrahedron, 2007, 63, 8010 CrossRef PubMed; (k) I. Yavari, A. Mokhtarporyani-Sanandaj and L. Moradi, Tetrahedron Lett., 2007, 48, 6709 CrossRef CAS PubMed; (l) Z. Ren, W. Cao, W. Tong, J. Chen, H. Deng and D. Wu, Synth. Commun., 2008, 38, 2200 CrossRef CAS PubMed; (m) M. Li, W. Yang, L. Wen and F. Li, Eur. J. Org. Chem., 2008, 2751 CrossRef CAS PubMed; (n) V. V. Shchepin, Y. G. Stepanyan and P. S. Silaichev, Russ. J. Gen. Chem., 2008, 78, 929 CrossRef CAS; (o) A. R. S. Babu and R. Raghunathan, Synth. Commun., 2009, 39, 347 CrossRef PubMed; (p) S. U. Maheswari, K. Balamurugan, S. Perumal, P. Yogeeswari and D. Sriram, Bioorg. Med. Chem. Lett., 2010, 20, 7278 CrossRef CAS PubMed; (q) B. Bredenkötter, B. Neumann, H. G. Stammler and D. Kuck, Eur. J. Org. Chem., 2014, 53 CrossRef PubMed.
- For selected reviews on domino reaction, see: (a) L. F. Tietze, Chem. Rev., 1996, 96, 115 CrossRef CAS PubMed; (b) P. J. Parsons, C. S. Penkett and A. J. Shell, Chem. Rev., 1996, 96, 195 CrossRef CAS PubMed; (c) C. Hulme and V. Gore, Curr. Med. Chem., 2003, 10, 51 CrossRef CAS; (d) D. J. Ramón and M. Yus, Angew. Chem., Int. Ed., 2005, 44, 1602 CrossRef PubMed; (e) H. Pellissier, Tetrahedron, 2006, 62, 1619 CrossRef CAS PubMed; (f) H. Pellissier, Tetrahedron, 2006, 62, 2143 CrossRef CAS PubMed; (g) K. C. Nicolaou, D. J. Edmonds and P. G. Bulger, Angew. Chem., Int. Ed., 2006, 45, 7134 CrossRef CAS PubMed; (h) G. Guillena, D. J. Ramón and M. Yus, Tetrahedron: Asymmetry, 2007, 18, 693 CrossRef CAS PubMed; (i) C. J. Chapman and C. G. Frost, Synthesis, 2007, 1 CAS; (j) D. Enders, C. Grondal and M. R. M. Hüttl, Angew. Chem., Int. Ed., 2007, 46, 1570 CrossRef CAS PubMed; (k) M. Colombo and I. Peretto, Drug Discov. Today, 2008, 13, 677 CrossRef CAS PubMed; (l) B. B. Touré and D. G. Hall, Chem. Rev., 2009, 109, 4439 CrossRef PubMed; (m) K. C. Nicolaou and J. S. Chen, Chem. Soc. Rev., 2009, 38, 2993 RSC; (n) C. Grondal, M. Jeanty and D. Enders, Nat. Chem., 2010, 2, 167 CrossRef CAS PubMed; (o) M. Ruiz, P. López-Alvarado, G. Giorgi and J. C. Menéndez, Chem. Soc. Rev., 2011, 40, 3445 RSC; (p) Ł. Albrecht, H. Jiang and K. A. Jørgensen, Angew. Chem., Int. Ed., 2011, 50, 8492 CrossRef PubMed; (q) H. Pellissier, Adv. Synth. Catal., 2012, 354, 237 CrossRef CAS PubMed; (r) C. de Graaff, E. Ruijter and R. V. A. Orru, Chem. Soc. Rev., 2012, 41, 3969 RSC; (s) C. M. Marson, Chem. Soc. Rev., 2012, 41, 7712 RSC; (t) M. J. Climent, A. Corma and S. Iborra, RSC Adv., 2012, 2, 16 RSC; (u) H. Pellissier, Chem. Rev., 2013, 113, 442 CrossRef CAS PubMed; (v) P. Xie and Y. Huang, Eur. J. Org. Chem., 2013, 6213 CrossRef CAS PubMed; (w) H. Pellissier, Tetrahedron, 2013, 69, 7171 CrossRef CAS PubMed; (x) C. M. R. Volla, I. Atodiresei and M. Rueping, Chem. Rev., 2014, 114, 2390 CrossRef CAS PubMed.
- For organocatalytic non-enantioselective reactions of 2-arylidene-1,3-indandione, see: (a) D. B. Ramachary, N. S. Chowdari and C. F. Barbas III, Synlett, 2003, 1910 CAS; (b) B. Dai, L. Song, P. Wang, H. Yi, W. Cao, G. Jin, S. Zhu and M. Shao, Synlett, 2009, 1842 CAS; (c) S. Roy, M. Amireddy and K. Chen, Tetrahedron, 2013, 69, 8751 CrossRef CAS PubMed; (d) E. Li, Y. Huang, L. Liang and P. Xie, Org. Lett., 2013, 15, 3138 CrossRef CAS PubMed; (e) L. Liang, E. Li, P. Xie and Y. Huang, Chem. – Asian J., 2014, 9, 1270 CrossRef CAS PubMed.
- For organocatalytic enantioselective reactions of 2-arylidene-1,3-indandione, see: (a) D. B. Ramachary, K. Anebouselvy, N. S. Chowdari and C. F. Barbas III, J. Org. Chem., 2004, 69, 5838 CrossRef CAS PubMed; (b) D. B. Ramachary and C. F. Barbas III, Chem. – Eur. J., 2004, 10, 5323 CrossRef CAS PubMed; (c) D. Pizzirani, R. Marinella and M. Recanatini, Tetrahedron Lett., 2007, 48, 7120 CrossRef CAS PubMed; (d) A. Russo, S. Meninno, C. Tedesco and A. Lattanzi, Eur. J. Org. Chem., 2011, 5096 CrossRef CAS PubMed; (e) D. B. Ramachary, C. Venkaiah and P. M. Krishna, Chem. Commun., 2012, 48, 2252 RSC; (f) F. Hu, Y. Wei and M. Shi, Tetrahedron, 2012, 68, 7911 CrossRef CAS PubMed; (g) U. Das, Y. Tsai and W. Lin, Org. Biomol. Chem., 2013, 11, 44 RSC; (h) N. Luo, X. Sun, W. Wei, X. Zhang and M. Yan, Tetrahedron: Asymmetry, 2013, 24, 402 CrossRef CAS PubMed; (i) H. Kuan, C. Chien and K. Chen, Org. Lett., 2013, 15, 2880 CrossRef CAS PubMed; (j) S. Anwar, S. Li and K. Chen, Org. Lett., 2014, 16, 2993 CrossRef CAS PubMed.
- (a) L. Wang, B. Prabhudas and D. L. J. Clive, J. Am. Chem. Soc., 2009, 131, 6005 Search PubMed; (b) D. Enders, R. Hahn and I. Atodiresei, Adv. Synth. Catal., 2013, 355, 1126 CrossRef CAS PubMed; (c) W. Xu, S. Wu, L. Zhou and G. Liang, Org. Lett., 2013, 15, 1978 CrossRef CAS PubMed; (d) L. Zhou, Y. Yao, W. Xu and G. Liang, J. Org. Chem., 2014, 79, 5345 CrossRef CAS PubMed.
- (a) H. Tao, J. Duan and P. Li, Asian J. Org. Chem., 2014, 3, 644 CrossRef CAS PubMed; (b) L. Yu, Q. Yang and P. Li, Eur. J. Org. Chem., 2014, 7499 CrossRef CAS PubMed; (c) J. Duan, J. Cheng and P. Li, Curr. Organocatal., 2015 DOI:10.2174/2213337202666150414201456.
- For selected reviews and examples of hydrogen-bonding catalysis, see: (a) P. R. Schreiner, Chem. Soc. Rev., 2003, 32, 289 RSC; (b) M. S. Taylor and E. N. Jacobsen, Angew. Chem., Int. Ed., 2006, 45, 1520 CrossRef CAS PubMed; (c) K. Etzenbach-Effers and A. Berkessel, Top. Curr. Chem., 2009, 291, 1 CrossRef; (d) R. R. Knowles and E. N. Jacobsen, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 20678 CrossRef CAS PubMed; (e) H. Song, K. Yuan and X. Wu, Chem. Commun., 2011, 47, 1012 RSC; (f) Ł. Albrecht, G. Dickmeiss, F. C. Acosta, C. Rodriguez-Escrich, R. L. Davis and K. A. Jørgensen, J. Am. Chem. Soc., 2012, 134, 2543 CrossRef PubMed; (g) D. Enders, G. Urbanietz, E. Cassens-Sasse, S. Kees and G. Raabe, Adv. Synth. Catal., 2012, 354, 1481 CrossRef CAS PubMed; (h) D. Enders, R. Hahn and I. Atodireseia, Adv. Synth. Catal., 2013, 355, 1126 CrossRef CAS PubMed.
- For reviews on chiral thiourea as an organocatalyst, see: (a) S. J. Connon, Chem. – Eur. J., 2006, 12, 5418 CrossRef PubMed; (b) A. G. Doyle and E. N. Jacobsen, Chem. Rev., 2007, 107, 5713 CrossRef CAS PubMed; (c) S. J. Connon, Chem. Commun., 2008, 2499 RSC; (d) X. Yu and W. Wang, Chem. – Asian J., 2008, 3, 516 CrossRef CAS PubMed; (e) H. Miyabe and Y. Takemoto, Bull. Chem. Soc. Jpn., 2008, 81, 785 CrossRef CAS; (f) Z. Zhang and P. R. Schreiner, Chem. Soc. Rev., 2009, 38, 1187 RSC; (g) Y. Sohtome and K. Nagasawa, Synlett, 2010, 1 CAS; (h) Y. Takemoto, Chem. Pharm. Bull., 2010, 58, 593 CrossRef CAS; (i) W. Siau and J. Wang, Catal. Sci. Technol., 2011, 1, 1298 RSC; (j) L. Lu, X. An, J. Chen and W. Xiao, Synlett, 2012, 490 Search PubMed; (k) Z. Chai and G. Zhao, Catal. Sci. Technol., 2012, 2, 29 RSC; (l) O. V. Serdyuk, C. M. Heckel and S. B. Tsogoeva, Org. Biomol. Chem., 2013, 11, 7051 RSC; (m) S. Narayanaperumal, D. G. Rivera, R. C. Silva and M. W. Paixão, ChemCatChem, 2013, 5, 2756 CrossRef CAS PubMed; (n) T. J. Auvil, A. G. Schafer and A. E. Mattson, Eur. J. Org. Chem., 2014, 2633 CrossRef CAS PubMed.
- For reviews on chiral squaramide as the organocatalyst, see: (a) J. Aleman, A. Parra, H. Jiang and K. A. Jørgensen, Chem. – Eur. J., 2011, 17, 6890 CrossRef CAS PubMed; (b) R. I. Storer, C. Aciro and L. H. Jones, Chem. Soc. Rev., 2011, 40, 2330 RSC; (c) P. Chauhan, S. Mahajan, U. Kaya, D. Hack and D. Enders, Adv. Synth. Catal., 2015, 357, 253 CrossRef CAS PubMed.
- CCDC 1402191.
Footnotes |
| † Dedicated to Professor Zongshi Li on the occasion of his 80th birthday. |
| ‡ Electronic supplementary information (ESI) available. CCDC 1402191. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5qo00166h |
| This journal is © the Partner Organisations 2015 |