Open Access Article
Open Access ArticleAsymmetric aza-Henry reaction to provide oxindoles with quaternary carbon stereocenter catalyzed by a metal-templated chiral Brønsted base†
Ying
Hu
a,
Zijun
Zhou
a,
Lei
Gong
*a and
Eric
Meggers
*ab
aKey Laboratory for Chemical Biology of Fujian Province and Department of Chemical Biology, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen 361005, People's Republic of China. E-mail: gongl@xmu.edu.cn; meggers@chemie.uni-marburg.de
bFachbereich Chemie, Philipps-Universität Marburg, Hans-Meerwein-Strasse, 35043 Marburg, Germany
First published on 22nd June 2015
Abstract
An asymmetric aza-Henry reaction between isatin-derived ketimines and aryl nitromethanes is catalyzed by an inert octahedral chiral-at-metal iridium(III) complex which serves as a chiral Brønsted base. Initially, a kinetically favored diastereomer is formed with high diastereoselectivity and excellent enantioselectivity, and can be epimerized efficiently under base catalysis into the thermodynamically favored diastereomer. The work underscores the potential of our metal-templated approach for the design of high performance asymmetric catalysts.
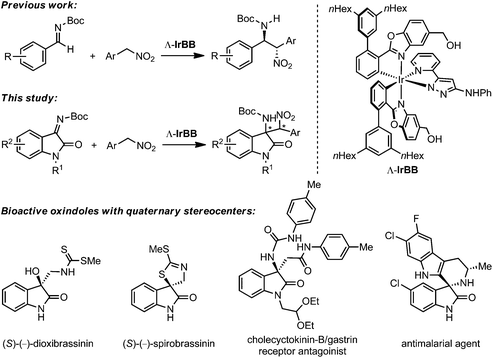
The aza-Henry (nitro-Mannich) reaction, namely the addition of nitronate nucleophiles to imine electrophiles, is a highly useful C–C bond forming reaction and provides straightforward access to a variety of nitrogen-containing chiral building blocks and scaffolds.1 The significant α-C–H acidity of nitroalkanes in combination with the high nucleophilicity of the resulting nitronates offers attractive opportunities to develop asymmetric versions of these reactions through chiral Brønsted base catalysis.2 Furthermore, the ability to increase the reactivity of imines through hydrogen bond interactions provides an additional handle for lowering the energy of the transition state and for controlling the asymmetric induction by means of bifunctional catalysis.2,3
Our group recently introduced a new class of bifunctional chiral Brønsted base catalysts based on chiral octahedral iridium(III) complexes. In these metal-templated catalysts, the metal fulfills a purely structural role and constitutes the exclusive source of chirality (metal-centered chirality),4–8 while the actual catalysis is mediated through carefully arranged functional groups within the organic ligand sphere. Based on this concept, we developed iridium(III) 3-aminopyrazolate complexes as low-loading catalysts for sulfa-Michael (down to 0.02 mol% catalyst loading) and aza-Henry reactions (down to 0.25 mol% catalyst loading).9,10 We believe that the stereochemical complexity of the octahedral scaffold provided us with an advantage with respect to the proper arrangement of functional groups and the intrinsic rigidity facilitated a reduced entropic penalty when reaching the transition state. To further investigate the merit of such metal-templated chiral Brønsted base catalysts in asymmetric catalysis, we herein expand the scope of the previously investigated aza-Henry reaction of benzaldehyde derived imines to isatin ketimine substrates.11,12 Isatin-derived ketimines are highly attractive substrates since they are converted to 2-oxindoles with a quaternary stereocenter in 3-position which constitutes an important chiral structural motif for bioactive natural products and drug candidates (Fig. 1).13,14 A few asymmetric catalysts have been reported recently for the aza-Henry reaction with isatin-derived ketimines, such as quinine-derived bifunctional organocatalysts developed by Zhou and coworkers,12a bis(imidazolidine)pyridine–nickel(II) complexes by Arai's group,12b bisoxazoline copper(II) complexes by Pedro and coworkers,12c and a bifunctional guanidine-amide catalyst by Feng's group.12d However, despite providing excellent results, these reports are limited to nitromethane, nitroethane, and nitropropane, whereas the here presented study deals with the substrate class of aryl nitromethanes.
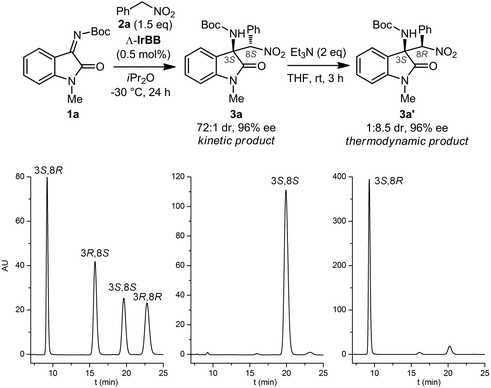
We started our study with the aza-Henry reaction between the isatin N-Boc ketimine 1a and (nitromethyl)benzene 2a (Fig. 2, top). Under optimized reaction conditions, at −30 °C using iPr2O as the solvent (see ESI† for a comparison with other solvents), we found that a catalyst loading of merely 0.5 mol% led to a complete conversion to the C–C-bond formation product 3a after 24 hours. HPLC analysis on chiral stationary phase revealed that the crude compound was formed with high enantioselectivity (96% ee) and high diastereoselectivity (72![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr) (Fig. 2, bottom). Compound 3a could be isolated as a pure single enantiomer (>99% ee, 138
1 dr) (Fig. 2, bottom). Compound 3a could be isolated as a pure single enantiomer (>99% ee, 138![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr) by washing the crude product with a solvent mixture out of toluene and n-hexane (1
1 dr) by washing the crude product with a solvent mixture out of toluene and n-hexane (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). The compound is prone to epimerization and, in our hands, could not be purified by silica gel chromatography. When we treated the crude 3a (96% ee, 72
2). The compound is prone to epimerization and, in our hands, could not be purified by silica gel chromatography. When we treated the crude 3a (96% ee, 72![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr) with triethylamine (2 equiv.) in THF at room temperature, we observed a conversion to the diastereomer 3a′ (1
1 dr) with triethylamine (2 equiv.) in THF at room temperature, we observed a conversion to the diastereomer 3a′ (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
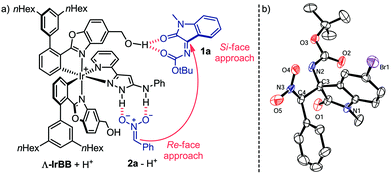
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8.5 dr, 96% ee) whose absolute and relative configuration were assigned by X-ray crystallography of a brominated derivative (Fig. 3b).
8.5 dr, 96% ee) whose absolute and relative configuration were assigned by X-ray crystallography of a brominated derivative (Fig. 3b).
Based on these results, a proposed mechanism involves an initial proton transfer from (nitromethyl)benzene (2a) (pKa = 12.2 in DMSO)15 to the Brønsted base Λ-IrBB (pKa ∼ 16 of protonated Λ-IrBB in MeCN),9 which allows to form a double hydrogen bond between the aminopyrazole unit of the protonated catalyst and the nitronate. Additional attractive electrostatic forces between the cationic protonated catalyst and the nitronate anion will provide an additional stabilization and are optimized by using a fairly nonpolar solvent. Furthermore, it is plausible to assume that a three center hydrogen bond is established between two carbonyl groups of the isatin N-Boc ketimine and one hydroxy group of the catalyst, providing the ternary complex as shown in Fig. 3a.16 We verified the importance of the hydroxy group by using a catalyst devoid of both CH2OH groups and we observed only a sluggish catalysis that required an increased catalyst loading to reach a full conversion and provided the product 3a only with a low diastereo- and enantioselectivity (see ESI† for more details). According to our model for the catalysis with Λ-IrBB, the two substrates are brought into close proximity and preorganized for a Re-face/Si-face attack of the nitronate nucleophile to the ketimine electrophile, thereby being consistent with the observed stereochemistry of 3a. This compound apparently constitutes the kinetically favored product and the high acidity of the proton in α-position of the nitro group allows a base-catalyzed conversion to the thermodynamically more stable diastereomer 3a′.
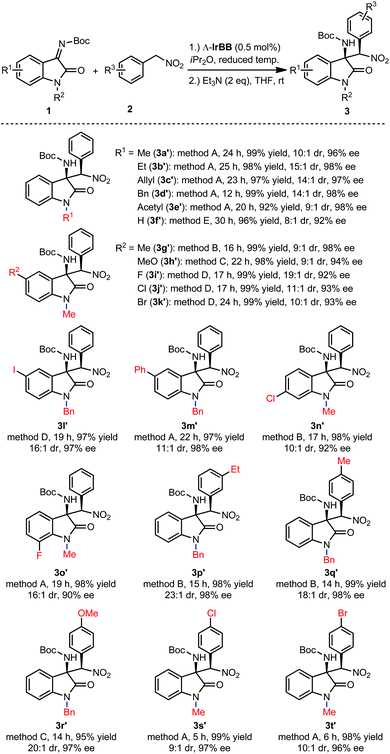
Finally, we investigated the substrate scope of the reaction between isatin N-Boc ketimines and aryl nitromethanes for the formation of the thermodynamically more stable diastereomers (3a′–t′). Fig. 4 demonstrates that the reaction tolerates electron donating and electron withdrawing groups within the indole moiety and within the phenyl group of the nitro substrate, and different substituents on the indole nitrogen. Overall, under optimized conditions with a catalyst loading of 0.5 mol%, excellent yields were observed (92–99%), high enantioselectivities (90–98% ee), whereas diastereoselectivities were found to be in the range between 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 8
1 and 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, which must reflect the relative thermodynamic stabilities of the two diastereomers. It is worth noting that the catalyst loading can even be reduced to 0.25 mol% while only slightly affecting the stereoselectivity. For example, with a loading of 0.25 mol% Λ-IrBB, the kinetic product 3a was obtained with 96% ee and 82
1, which must reflect the relative thermodynamic stabilities of the two diastereomers. It is worth noting that the catalyst loading can even be reduced to 0.25 mol% while only slightly affecting the stereoselectivity. For example, with a loading of 0.25 mol% Λ-IrBB, the kinetic product 3a was obtained with 96% ee and 82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr after an elongated reaction time of 36 h at −30 °C, and subsequently converted to the thermodynamic product upon treatment with Et3N to obtain 3a′ in a yield of 98% with 10
1 dr after an elongated reaction time of 36 h at −30 °C, and subsequently converted to the thermodynamic product upon treatment with Et3N to obtain 3a′ in a yield of 98% with 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr and 95% ee.
1 dr and 95% ee.
In conclusion, we developed a diastereoselective (up to 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr) and highly enantioselective (up to 98% ee) method to oxindoles bearing a quaternary stereocenter in 3-position by using an aza-Henry reaction between isatin N-Boc ketimines and aryl nitromethanes, catalyzed by an inert octahedral bis-cyclometalated iridium(III) complex which served as a chiral Brønsted base. This work underscores the potential of our metal-templated approach for the design of high performance asymmetric catalysts.
1 dr) and highly enantioselective (up to 98% ee) method to oxindoles bearing a quaternary stereocenter in 3-position by using an aza-Henry reaction between isatin N-Boc ketimines and aryl nitromethanes, catalyzed by an inert octahedral bis-cyclometalated iridium(III) complex which served as a chiral Brønsted base. This work underscores the potential of our metal-templated approach for the design of high performance asymmetric catalysts.
Acknowledgements
We gratefully acknowledge funding from the National Natural Science Foundation of P. R. China (grant no. 21272192, 21201143, and 21472154) the Program for Changjiang Scholars and Innovative Research Team of P. R. China (PCSIRT), the National Thousand Talents Program of P. R. China, and the 985 Program of the Chemistry and Chemical Engineering disciplines of Xiamen University.References
- For reviews on asymmetric catalytic aza-Henry reactions, see: (a) B. Westermann, Angew. Chem., Int. Ed., 2003, 42, 151–153 CrossRef CAS PubMed; (b) E. Marqués-López, P. Merino, T. Tejero and R. P. Herrera, Eur. J. Org. Chem., 2009, 2401–2420 CrossRef PubMed; (c) A. Noble and J. C. Anderson, Chem. Rev., 2013, 113, 2887–2939 CrossRef CAS PubMed.
- For enantioselective aza-Henry reactions catalyzed by chiral Brønsted bases, see: (a) T. Okino, S. Nakamura, T. Furukawa and Y. Takemoto, Org. Lett., 2004, 6, 625–627 CrossRef CAS PubMed; (b) K. R. Knudsen and K. A. Jørgensen, Org. Biomol. Chem., 2005, 3, 1362–1364 RSC; (c) L. Bernardi, F. Fini, R. P. Herrera, A. Ricci and V. Sgarzani, Tetrahedron, 2006, 62, 375–380 CrossRef CAS PubMed; (d) M. T. Robak, M. Trincado and J. A. Ellman, J. Am. Chem. Soc., 2007, 129, 15110–15111 CrossRef CAS PubMed; (e) Y.-w. Chang, J.-j. Yang, J.-n. Dang and Y.-x. Xue, Synlett, 2007, 2283–2285 CrossRef CAS; (f) C. Wang, Z. Zhou and C. Tang, Org. Lett., 2008, 10, 1707–1710 CrossRef CAS PubMed; (g) C. Rampalakos and W. D. Wulff, Adv. Synth. Catal., 2008, 350, 1785–1790 CrossRef CAS PubMed; (h) C. Rabalakos and W. D. Wulff, J. Am. Chem. Soc., 2008, 130, 13524–13525 CrossRef CAS PubMed; (i) B. Han, Q.-P. Liu, R. Li, X. Tian, X.-F. Xiong, J.-G. Deng and Y.-C. Chen, Chem. – Eur. J., 2008, 14, 8094–8097 CrossRef CAS PubMed; (j) K. Takada and K. Nagasawa, Adv. Synth. Catal., 2009, 351, 345–347 CrossRef CAS PubMed; (k) X. Jiang, Y. Zhang, L. Wu, G. Zhang, X. Liu, H. Zhang, D. Fu and R. Wang, Adv. Synth. Catal., 2009, 351, 2096–2100 CrossRef CAS PubMed; (l) T. A. Davis, J. C. Wilt and J. N. Johnston, J. Am. Chem. Soc., 2010, 132, 2880–2882 CrossRef CAS PubMed; (m) Z.-X. Jia, Y.-C. Luo and P.-F. Xu, Org. Lett., 2011, 13, 832–835 CrossRef CAS PubMed; (n) T. A. Davis and J. N. Johnston, Chem. Sci., 2011, 2, 1076–1079 RSC; (o) D. Uraguchi, K. Oyaizu and T. Ooi, Chem. – Eur. J., 2012, 18, 8306–8309 CrossRef CAS PubMed; (p) H. Li, X. Zhang, X. Shi, N. Ji, W. He, S. Zhang and B. Zhang, Adv. Synth. Catal., 2012, 354, 2264–2274 CrossRef CAS PubMed; (q) N. R. Amarasinghe, P. Turner and M. H. Todd, Adv. Synth. Catal., 2012, 354, 2954–2958 CrossRef CAS PubMed; (r) B. Zheng, W. Hou and Y. Peng, ChemCatChem, 2014, 6, 2527–2530 CrossRef CAS PubMed.
- For bifunctional asymmetric organocatalysis, see: (a) Y. Takemoto, Org. Biomol. Chem., 2005, 3, 4299–4306 RSC; (b) S. J. Connon, Chem. – Eur. J., 2006, 12, 5418–5427 CrossRef PubMed; (c) A. G. Doyle and E. N. Jacobsen, Chem. Rev., 2007, 107, 5713–5743 CrossRef CAS PubMed; (d) X. Yu and W. Wang, Chem. – Asian J., 2008, 3, 516–532 CrossRef CAS PubMed; (e) D. H. Paull, C. J. Abraham, M. T. Scerba, E. Alden-Danforth and T. Lectka, Acc. Chem. Res., 2008, 41, 655–663 CrossRef CAS PubMed; (f) T. Kano and K. Maruoka, Chem. Commun., 2008, 5465–5473 RSC; (g) X. Liu, L. Lin and X. Feng, Chem. Commun., 2009, 6145–6158 RSC; (h) L.-Q. Lu, X.-L. An, J.-R. Chen and W.-J. Xiao, Synlett, 2012, 490–508 Search PubMed; (i) O. V. Serdyuk, C. M. Heckel and S. B. Tsogoeva, Org. Biomol. Chem., 2013, 11, 7051–7071 RSC; (j) X. Fang and C.-J. Wang, Chem. Commun., 2014, 51, 1185–1197 RSC.
- For metal-templated asymmetric catalysis from our group, see: (a) L.-A. Chen, W. Xu, B. Huang, J. Ma, L. Wang, J. Xi, K. Harms, L. Gong and E. Meggers, J. Am. Chem. Soc., 2013, 135, 10598–10601 CrossRef CAS PubMed; (b) L.-A. Chen, X. Tang, J. Xi, W. Xu, L. Gong and E. Meggers, Angew. Chem., Int. Ed., 2013, 52, 14021–14025 CrossRef CAS PubMed; (c) H. Huo, C. Fu, C. Wang, K. Harms and E. Meggers, Chem. Commun., 2014, 50, 10409–10411 RSC.
- For related chiral-at-metal octahedral iridium(III) and rhodium(III) catalysts from our group, see: (a) H. Huo, C. Fu, K. Harms and E. Meggers, J. Am. Chem. Soc., 2014, 136, 2990–2993 CrossRef CAS PubMed; (b) H. Huo, X. Shen, C. Wang, L. Zhang, P. Röse, L.-A. Chen, K. Harms, M. Marsch, G. Hilt and E. Meggers, Nature, 2014, 515, 100–103 CrossRef CAS PubMed; (c) C. Wang, L.-A. Chen, H. Huo, X. Shen, K. Harms, L. Gong and E. Meggers, Chem. Sci., 2015, 5, 1093–1100 Search PubMed; (d) C. Wang, Y. Zheng, H. Huo, P. Röse, L. Zhang, K. Harms, G. Hilt and E. Meggers, Chem. – Eur. J., 2015, 21, 7355–7359 CrossRef CAS PubMed.
- For the design of metal-templated asymmetric catalysts from other groups, see: (a) Y. N. Belokon, A. G. Bulychev, V. I. Maleev, M. North, I. L. Malfanov and N. S. Ikonnikov, Mendeleev Commun., 2004, 14, 249–250 CrossRef PubMed; (b) Y. N. Belokon, V. I. Maleev, D. A. Kataev, I. L. Mal'fanov, A. G. Bulychev, M. A. Moskalenko, T. F. Saveleva, T. V. Skrupskaya, K. A. Lyssenko, I. A. Godovikov and M. North, Tetrahedron: Asymmetry, 2008, 19, 822–831 CrossRef CAS PubMed; (c) C. Ganzmann and J. A. Gladysz, Chem. – Eur. J., 2008, 14, 5397–5400 CrossRef CAS PubMed; (d) N. Kurono, K. Arai, M. Uemura and T. Ohkuma, Angew. Chem., Int. Ed., 2008, 47, 6643–6646 CrossRef CAS PubMed; (e) N. Kurono, N. Nii, Y. Sakaguchi, M. Uemura and T. Ohkuma, Angew. Chem., Int. Ed., 2011, 50, 5541–5544 CrossRef CAS PubMed; (f) Y. N. Belokon, V. I. Maleev, M. North, V. A. Larionov, T. F. Savel'yeva, A. Nijland and Y. V. Nelyubina, ACS Catal., 2013, 3, 1951–1955 CrossRef CAS; (g) V. I. Maleev, M. North, V. A. Larionov, I. V. Fedyanin, T. F. Savel'yeva, M. A. Moscalenko, A. F. Smolyakov and Y. N. Belokon, Adv. Synth. Catal., 2014, 356, 1803–1810 CrossRef PubMed.
- For reviews covering chiral-at-metal complexes in catalysis, see: (a) H. Brunner, Angew. Chem., Int. Ed., 1999, 38, 1194–1208 CrossRef CAS; (b) P. Knight and P. Scott, Coord. Chem. Rev., 2003, 242, 125–143 CrossRef CAS; (c) M. Fontecave, O. Hamelin and S. Ménage, Top. Organomet. Chem., 2005, 15, 271–288 CAS; (d) E. B. Bauer, Chem. Soc. Rev., 2012, 41, 3153–3167 RSC; (e) L. Gong, L.-A. Chen and E. Meggers, Angew. Chem., Int. Ed., 2014, 53, 10868–10874 CrossRef CAS PubMed; (f) Z.-Y. Cao, W. D. G. Brittain, J. S. Fossey and F. Zhou, Catal. Sci. Technol., 2015 10.1039/C5CY00182J.
- For different aspects of metal-centered chirality, see: (a) U. Knof and A. von Zelewsky, Angew. Chem., Int. Ed., 1999, 38, 302–322 CrossRef; (b) H. Brunner, Angew. Chem., Int. Ed., 1999, 38, 1194–1208 CrossRef CAS; (c) P. D. Knight and P. Scott, Coord. Chem. Rev., 2003, 242, 125–143 CrossRef CAS; (d) M. Fontecave, O. Hamelin and S. Ménage, Top. Organomet. Chem., 2005, 15, 271–288 CAS; (e) H. Amouri and M. Gruselle, Chirality in Transition Metal Chemistry, Wiley, Chichester, UK, 2008 Search PubMed; (f) J. Crassous, Chem. Soc. Rev., 2009, 38, 830–845 RSC; (g) E. Meggers, Eur. J. Inorg. Chem., 2011, 2911–2926 CrossRef CAS PubMed; (h) J. Crassous, Chem. Commun., 2012, 48, 9684–9692 RSC; (i) E. C. Constable, Chem. Soc. Rev., 2013, 42, 1637–1651 RSC.
- J. Ma, X. Ding, Y. Hu, Y. Huang, L. Gong and E. Meggers, Nat. Commun., 2014, 5, 4531 CAS.
- X. Ding, H. Lin, L. Gong and E. Meggers, Asian J. Org. Chem., 2015, 4, 434–437 CrossRef CAS PubMed.
- For asymmetric aza-Henry reactions with ketimines, see: (a) C. Tan, X. Liu, L. Wang, J. Wang and X. Feng, Org. Lett., 2008, 10, 5305–5308 CrossRef CAS PubMed; (b) H. Xie, Y. Zhang, S. Zhang, X. Chen and W. Wang, Angew. Chem., Int. Ed., 2011, 50, 11773–11776 CrossRef CAS PubMed; (c) A. Parra, R. Alfaro, L. Marzo, A. Moreno-Carrasco, J. L. García Ruano and J. Alemán, Chem. Commun., 2012, 48, 9759–9761 RSC; (d) M. G. Núñez, A. J. M. Farley and D. J. Dixon, J. Am. Chem. Soc., 2013, 135, 16348–16351 CrossRef PubMed.
- For asymmetric aza-Henry reactions with isatin ketimines, see: (a) Y.-H. Wang, Y.-L. Liu, Z.-Y. Cao and J. Zhou, Asian J. Org. Chem., 2014, 3, 429–432 CrossRef CAS PubMed; (b) T. Arai, E. Matsumura and H. Masu, Org. Lett., 2014, 16, 2768–2771 CrossRef CAS PubMed; (c) M. Holmquist, G. Blay and J. R. Pedro, Chem. Commun., 2014, 50, 9309–9312 RSC; (d) B. Fang, X. Liu, J. Zhao, Y. Tang, L. Lin and X. Feng, J. Org. Chem., 2015, 80, 3332–3338 CrossRef CAS PubMed.
- For reviews on the asymmetric synthesis of oxindoles with quaternary stereocenter at the 3-position: (a) C. V. Galliford and K. A. Scheidt, Angew. Chem., Int. Ed., 2007, 46, 8748–8758 CrossRef CAS PubMed; (b) F. Zhou, Y.-L. Liu and J. Zhou, Adv. Synth. Catal., 2010, 352, 1381–1407 CrossRef CAS PubMed; (c) N. R. Ball-Jones, J. J. Badillo and A. K. Franz, Org. Biomol. Chem., 2012, 10, 5165–5181 RSC; (d) A. Kumar and S. S. Chimni, RSC Adv., 2012, 2, 9748–9762 RSC; (e) S. Mohammadi, R. Heiran, R. P. Herrera and E. Marqués-López, ChemCatChem, 2013, 5, 2131–2148 CrossRef CAS PubMed.
- Examples of bioactive oxindoles with quaternary stereocenter at the 3-position: (a) M. Ochi, K. Kawasaki, H. Kataoka, Y. Uchio and H. Nishi, Biochem. Biophys. Res. Commun., 2001, 283, 1118–1123 CrossRef CAS PubMed; (b) M. Suchý, P. Kutschy, K. Monde, H. Goto, N. Harada, M. Takasugi, M. Dzurilla and E. Balentová, J. Org. Chem., 2001, 66, 3940–3947 CrossRef PubMed; (c) K. Monde, T. Taniguchi, N. Miura, S.-I. Nishimura, N. Harada, R. K. Dukor and L. A. Nafie, Tetrahedron Lett., 2003, 44, 6017–6020 CrossRef CAS; (d) K. Ding, Y. Lu, Z. Nikolovska-Coleska, S. Qiu, Y. Ding, W. Gao, J. Stuckey, K. Krajewski, P. P. Roller and Y. Tomita, et al. , J. Am. Chem. Soc., 2005, 127, 10130–10131 CrossRef CAS PubMed; (e) M. Rottmann, C. McNamara, B. K. S. Yeung, M. C. S. Lee, B. Zou, B. Russell, P. Seitz, D. M. Plouffe, N. V. Dharia, J. Tan, S. B. Cohen, K. R. Spencer, G. E. Gonzalez-Paez, S. B. Lakshminarayana, A. Goh, R. Suwanarusk, T. Jegla, E. K. Schmitt, H.-P. Beck, R. Brun, F. Nosten, L. Renia, V. Dartois, T. H. Keller, D. A. Fidock, E. A. Winzeler and T. T. Diagana, Science, 2010, 329, 1175–1180 CrossRef CAS PubMed; (f) A. P. Antonchick, C. Gerding-Reimers, M. Catarinella, M. Schürmann, H. Preut, S. Ziegler, D. Rauh and H. Waldmann, Nat. Chem., 2010, 2, 735–740 CrossRef CAS PubMed.
- F. G. Bordwell, J. E. Bares, J. E. Bartmess, G. J. McCollum, M. van der Puy, N. R. Vanier and W. S. Matthews, J. Org. Chem., 1977, 42, 321–325 CrossRef CAS.
- A crystal structure of ketimine 1k confirms the shown syn-configuration at the imine. See ESI† for details.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1060112 and 1060102. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5qo00132c |
| This journal is © the Partner Organisations 2015 |