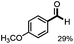
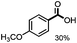
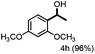
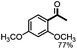
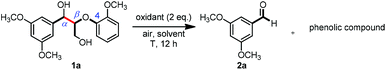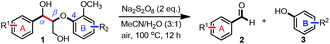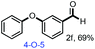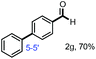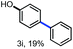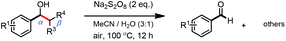Fragmentation of structural units of lignin promoted by persulfate through selective C–C cleavage under mild conditions†
Fei-Xian
Luo
a,
Tai-Gang
Zhou
a,
Xin
Li
a,
Yun-Lei
Luo
b and
Zhang-Jie
Shi
*a
aBeijing National Laboratory of Molecule Science (BNLMS) and Key Laboratory of Bioorganic Chemistry and Molecular Engineering of Ministry of Education, College of Chemistry and Green Chemistry Center, Peking University, Beijing, 100871, China. E-mail: zshi@pku.edu.cn
bSchool of Chemistry and Chemical engineering, Southwest University, Chongqing, 400715, China
First published on 8th July 2015
Abstract
A concise and environmentally benign protocol for lignin model fragmentation was developed by using cheap and commercially available sodium persulfate as an oxidant. The predominant lignin β-O-4 or β-1 model was fragmented into high-value aromatic aldehydes. The other lignin models, such as α-O-4, 4-O-5, and 5–5′, showed good reactivity in this system.
Energy and environmental issues pose serious threats to humans and urgent solutions to these challenges are required by finding renewable energy sources and sustainable synthetic transformations.1 Conversion of non-food biomass lignocellulose is a high-potential alternative for the production of valuable chemicals and fuels. Lignin, as the second most abundant biopolymer, constitutes up to 15–30% of the weight and 40% of the energy content of lignocellulosic biomass. However, the conversion of lignin is far behind that of other members of its family, e.g. cellulose and hemicellulose, due to its structural complexity and stability.2
This predicament of lignin is attributed to the complexity of its irregular structure with methoxyl substituted phenyl and phenolic subunits3 (Fig. 1). Indeed, in recent years, much attention has been paid to lignin fragmentation due to its potential as feedstock for high-value chemicals, pharmaceuticals and fuels.4 Different oxidative,5 reductive,6 and redox-neutral7 lignin protocols have been demonstrated to fragment lignin, native lignin, and lignin model systems. More encouragingly, beautiful examples of promising catalytic lignin degradation have been recently developed by using transition-metal catalysts. Vanadium complexes showed promising activity on the aerobic oxidative degradation of lignin to afford C–C/C–O cleavage products.5g,h,k Aerobic oxidation of lignin models with amide bond formation was developed by using Cu catalysts.8 Cobalt exhibited a credible ability to catalyze the oxidative conversion of lignin models to benzoquinones in an O2 atmosphere.5i During preparation of this manuscript, a chemical selective oxidation through C–O bond cleavage of β-O-4 lignin to isolate phenolic monomers was reported.9 Under reductive conditions, nickel carbene complexes were effective catalysts for the hydrogenolysis of aryl ether bonds in several lignin model compounds under alkaline conditions in a H2 atmosphere.6f Fe catalysts also showed good reductive activity for the hydrogenolysis of aryl ether bonds in lignin models.10 With a hydrogenation transfer strategy, the Ru-catalyzed C–O cleavage of lignin model compounds under neutral conditions has been developed by Bergman's group7a and other groups.7b–f These methods represent diverse strategies toward lignin degradation utilizing transition-metal catalysis.
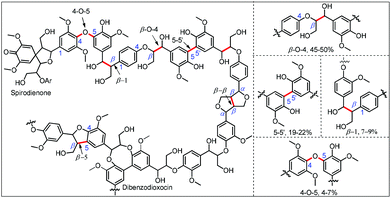 | ||
| Fig. 1 Representative structures of a fragment of lignin and some prevalent chemical linkages characterized in lignin from spruce trees. | ||
Other than transition metal catalysis, Stahl and co-workers developed a chemoselective metal-free aerobic secondary alcohol oxidation in lignin models and further converted the generated ketones to corresponding products in two steps.11 Continuous efforts from Stahl's group have been made to explore formic-acid-induced depolymerization of oxidized lignin to aromatics.12 Interestingly, Stephenson and coworkers developed a photochemical strategy for lignin degradation at room temperature via chemoselective secondary alcohol oxidation and photocatalytic cleavage C–O bond by using an iridium complex photosensitizer.13 More recently, a frustrated Lewis pair B(C6F5)3 as catalyst has been applied to the reductive cleavage of C–O bonds of a β-O-4 model.14 Obviously, the protocols are still facing significant challenges that have yet to be addressed to some extent, such as the step economy and the frequent use of expensive transition-metal catalysts at a high loading. Therefore, new methods to convert the lignin models to valuable chemicals are still appealing. Herein, we report a convenient and transition-metal free protocol for fragmenting lignin models to aromatic aldehydes through selective C–C cleavage in the presence of persulfate.
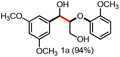
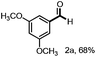
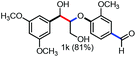
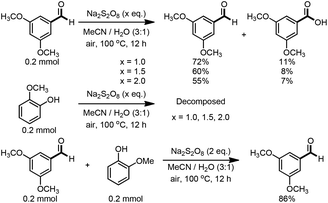
Persulfates are cheap and commercially available inorganic chemicals, which have been widely applied in the chemical industry and laboratory research as radical initiators and/or oxidants.15 We envisaged that the electron-rich lignin models probably transferred an electron to persulfate and formed a radical cation, which could further fragment to small molecules through C–C and/or C–O bond cleavage. To examine our hypothesis, we chose and synthesized the predominant lignin β-O-4 model (1a) as the model compound to screen the reaction conditions (Table 1). To our delight, the lignin β-O-4 model reacted with two equivalents of sodium persulfate in a mixture of MeCN/H2O (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) under air at 80 °C and aromatic aldehyde 2a was isolated in 51% yield (entry 1). Unfortunately, the desired phenolic compound guaiacol failed to be isolated, which may be due to over-oxidization to a water-soluble and/or polymeric species. This observation is consistent with previous reported oxidation systems via radical mechanisms using peroxidase16,17 or Fe/PhI(OAc)2
1, v/v) under air at 80 °C and aromatic aldehyde 2a was isolated in 51% yield (entry 1). Unfortunately, the desired phenolic compound guaiacol failed to be isolated, which may be due to over-oxidization to a water-soluble and/or polymeric species. This observation is consistent with previous reported oxidation systems via radical mechanisms using peroxidase16,17 or Fe/PhI(OAc)2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5e as oxidants. Different persulfates (entries 2–4) were screened and sodium persulfate outperformed the others. Further screening of the reaction temperature found that the best result was obtained at 100 °C (entry 6). Interestingly, air was shown to perform better for this fragmentation than O2 or N2 (entry 6 and entries 8 and 9). To our interest, the ratio of the mixed solvent was critical for high efficiency. Decreasing or increasing the amount of water diminished the efficacy (entries 10 and 11, and entries 11–14 in Table S1†). No desired product 2a was obtained at all when only 4.0 equivalents of water was added (entry 11). After carefully screening other parameters in this transformation (Table 1 and S1†), the best conditions were identified in the presence of 2.0 equivalents of sodium persulfate and 1a was fragmented into an aromatic aldehyde in a mixture of MeCN/H2O (3
5e as oxidants. Different persulfates (entries 2–4) were screened and sodium persulfate outperformed the others. Further screening of the reaction temperature found that the best result was obtained at 100 °C (entry 6). Interestingly, air was shown to perform better for this fragmentation than O2 or N2 (entry 6 and entries 8 and 9). To our interest, the ratio of the mixed solvent was critical for high efficiency. Decreasing or increasing the amount of water diminished the efficacy (entries 10 and 11, and entries 11–14 in Table S1†). No desired product 2a was obtained at all when only 4.0 equivalents of water was added (entry 11). After carefully screening other parameters in this transformation (Table 1 and S1†), the best conditions were identified in the presence of 2.0 equivalents of sodium persulfate and 1a was fragmented into an aromatic aldehyde in a mixture of MeCN/H2O (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) at 100 °C. Encouragingly, when the reaction was conducted in 2.0 mL of MeCN/H2O (3
1, v/v) at 100 °C. Encouragingly, when the reaction was conducted in 2.0 mL of MeCN/H2O (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) with 0.1 mmol of 1a and 0.2 mmol of persulfate at 100 °C for 12 h, the best result was obtained with a 68% isolated yield (entry 16). More importantly, when the product 2a was subjected to the reaction system as substrate, it was successfully recovered in an acceptable isolated yield, accompanied by its oxidized acid product in 7% yield (entry 17). This observation allowed us to conclude that the aldehyde exhibits good compatibility in the oxidation system. Recently, it was reported that lignin, lignin models and hydroxy-aromatics were broken down into C1 and C2 chemicals (mainly methanol, formate, carbonate, and oxalate) via transition-metal free oxidation using persulfate or peroxide as oxidant in alkaline conditions.18 In contrast to this result, we developed an alternative method to fragment the lignin models to high-value aldehydes using transition-metal free persulfate as oxidant in neutral condition. The difference in the results may be attributed to the solvent and the reaction pH.
1, v/v) with 0.1 mmol of 1a and 0.2 mmol of persulfate at 100 °C for 12 h, the best result was obtained with a 68% isolated yield (entry 16). More importantly, when the product 2a was subjected to the reaction system as substrate, it was successfully recovered in an acceptable isolated yield, accompanied by its oxidized acid product in 7% yield (entry 17). This observation allowed us to conclude that the aldehyde exhibits good compatibility in the oxidation system. Recently, it was reported that lignin, lignin models and hydroxy-aromatics were broken down into C1 and C2 chemicals (mainly methanol, formate, carbonate, and oxalate) via transition-metal free oxidation using persulfate or peroxide as oxidant in alkaline conditions.18 In contrast to this result, we developed an alternative method to fragment the lignin models to high-value aldehydes using transition-metal free persulfate as oxidant in neutral condition. The difference in the results may be attributed to the solvent and the reaction pH.
| Entrya | [Oxidant] | T (°C) | Solvent (v/v) | Result |
|---|---|---|---|---|
a 0.05 mmol of lignin model 1a and 0.1 mmol of Na2S2O8, 0.75 mL of organic solvent and 0.25 mL of H2O, 100 °C, 12 h. NMR yield with using CH2Br2 as internal standard reagent.
b DP = decomposed product, no product was identified from the decomposed starting material.
c 0.1 mmol of lignin model 1a and 0.2 mmol of Na2S2O8, 1.5 mL of organic solvent and 0.5 mL of H2O, 100 °C, 12 h.
d Isolated yield.
e 0.2 mmol of 2a and 0.4 mmol of Na2S2O8 in 4.0 mL mixture of MeCN/H2O (v/v = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were reacted at 100 °C for 12 h.
f Isolated yield of the recovery of aldehyde 2a.
g Isolated yield of the corresponding acid of 2a. 1) were reacted at 100 °C for 12 h.
f Isolated yield of the recovery of aldehyde 2a.
g Isolated yield of the corresponding acid of 2a.
|
||||
| 1 | Na2S2O8 | 80 | MeCN/H2O (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
51% |
| 2 | K2S2O8 | 80 | MeCN/H2O (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
41% |
| 3 | (NH4)2S2O8 | 80 | MeCN/H2O (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
42% |
| 4 | Oxone | 80 | MeCN/H2O (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
DPb |
| 5 | Na2S2O8 | 60 | MeCN/H2O (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
34% |
| 6 | Na2S2O8 | 100 | MeCN/H2O (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
61% |
| 7 | Na2S2O8 | 120 | MeCN/H2O (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
50% |
| 8 | Na2S2O8/O2 | 100 | MeCN/H2O (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
53% |
| 9 | Na2S2O8/N2 | 100 | MeCN/H2O (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
44% |
| 10 | Na2S2O8 | 100 | MeCN/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
36% |
| 11 | Na2S2O8 | 100 | MeCN (4 eq. H2O) | DPb |
| 12 | Na2S2O8(1.0 eq.) | 100 | MeCN/H2O (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
29% |
| 13 | Na2S2O8(2.5 eq.) | 100 | MeCN/H2O (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
55% |
| 14 | Na2S2O8 (9 h) | 100 | MeCN/H2O (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
47% |
| 15 | Na2S2O8 (15 h) | 100 | MeCN/H2O (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
55% |
| 16c | Na2S2O8 | 100 | MeCN/H2O (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
65% (68%d) |
| 17e | Na2S2O8 | 100 | MeCN/H2O (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
55%f + 7%g |
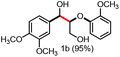
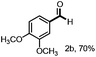
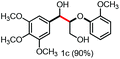
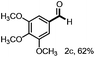
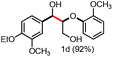
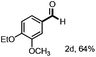
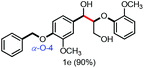
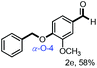
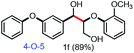
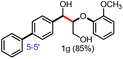
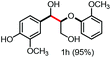
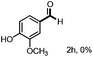
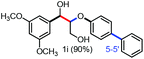
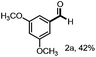
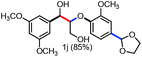
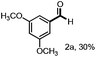
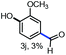
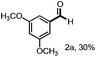
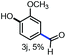
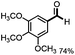
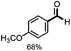
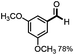
With the optimized conditions in hand, we continued to examine other lignin β-O-4 models with different substituents. As shown in Table 2, different substituents on the left phenyl ring A proceeded smoothly to give the corresponding valuable aromatic aldehyde 2 in good yields. A methoxyl group on different positions of ring A exhibited good activities and gave the corresponding aldehydes in good yields (1a–1c). More importantly, the models with other general substituents on lignin, including ethoxyl (1d), benzoxyl (1e), phenoxyl (1f), and phenyl (1g), were fragmented to high-value aromatic aldehydes in good yields. It is worthy of note that the models with benzoxyl (1e), phenoxyl (1f), and phenyl (1g) substituents belong to the α-O-4, 4-O-5 and 5–5′ lignin models, respectively.2a Our methodology exhibited a high selectivity for β-O-4 lignin models from other structural units of lignin. Unfortunately, the phenolic model (1h) and desired phenol guaiacol decomposed in our system, which is consistent with previously reported results.5e,16,17 Encouragingly, when a phenyl group was inserted at the para-position of the right phenyl ring B (1i), 19% of 4-phenylphenol and 42% of 2a were successfully isolated, thus providing indirect proof for the formation of the corresponding phenol under this condition. Electron withdrawing groups, such as acetyl (1j) or formyl (1k) at the para-position, affected the efficacy of this fragmentation and 2a was isolated in much lower yields with the recovery of the starting materials. The isolation of vanillin, despite the low yield, further validated the formation of phenol to some extent.
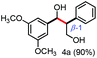
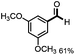
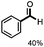
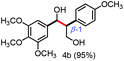
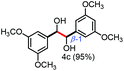
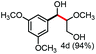
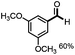
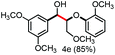
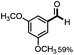
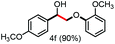
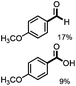
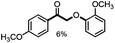
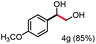
Further investigation was focused on the lignin model analogues with different substituents on the β position of the bridge between two aromatic rings (Table 3). Encouragingly, the β-phenyl substituted models (4a–4c), attributed to the β-1 lignin model,2a,5k were fragmented into two different benzaldehydes in good yields. It was obvious that the β-1 lignin model could be fragmented into different valuable aromatic aldehydes via selective C–C bond cleavage. The β-alkyloxyl substituted models could be also fragmented to aldehydes. The lignin model in which the hydroxymethyl substituent on the β position was replaced with a methoxymethyl group (4e) was converted into aldehyde in 59% yield. Finally, the lignin models with only one substituent on the β position (4f and 4g) were also degraded into 4-methoxy benzaldehyde and 4-methoxy benzoic acid, while the secondary benzyl alcohol (4h) was oxidized to a ketone product in 77% yield.
To highlight our protocol in real lignin fragmentation, we attempted to subject alkaline lignin and dealkaline lignin to our standard reaction system. Unfortunately, real lignin did not dissolve well in this system. We failed to observe and isolate aldehydes or phenols.
To understand the stability of the products, 3,5-dimethoxy benzaldehyde 2a was submitted to the standard conditions. With increasing amounts of persulfate (from 1 equivalent to 2 equivalents), the recovering yield of 2a was reduced, while, to our interest, the oxidized benzoic acid remained at a very low level. Thus, the relatively lower yields in some cases might arise from the over-oxidation of the products. Moreover, when 2-methoxy phenol was delivered into the flask with a different amount of persulfate, the phenol was completely decomposed, which was consistent with the observed results and previous reports.5e,16,17 Finally, when 2a and 2-methoxy phenol were added into the same pot, only 86% of 2a was recovered, which further supported our conclusions. Thus, β-O-4 lignin models were fragmented into arylaldehydes in good yields and the phenols were isolated in poor yields due to over-oxidation.
Conclusions
In conclusion, we successfully developed a concise and environmentally benign protocol to fragment lignin models to valuable aldehydes by using sodium persulfate as oxidant. The predominant lignin β-O-4 model was fragmented into high-value aromatic aldehydes in moderate to good yields. The β-1 lignin model was also converted into two different aromatic aldehydes in excellent yields. The other lignin models, such as α-O-4, 4-O-5, and 5–5′, showed good reactivity in this transformation. Further efforts to optimize the reaction conditions for improving the efficacy and using this protocol on real lignin are underway.Acknowledgements
Support of this work by the “973” Project from the MOST of China (2013CB228102) is acknowledged.Notes and references
- A. J. Ragauskas, C. K. Williams, B. H. Davison, G. Britovsek, J. Cairney, C. A. Eckert, W. J. Frederick, J. P. Hallett, D. J. Leak, C. L. Liotta, J. R. Mielenz, R. Murphy, R. Templer and T. Tschaplinski, Science, 2006, 311, 484 CrossRef CAS PubMed.
- (a) J. Zakzeski, P. C. A. Bruijnincx, A. L. Jongerius and B. M. Weckhuysen, Chem. Rev., 2010, 110, 3552 CrossRef CAS PubMed; (b) Lignin and Lignans: Advances in Chemistry, ed. C. Heitner, D. Dimmel and J. A. Schmidt, CRC Press, Boca Raton, FL, 2010 Search PubMed.
- (a) S. Reale, A. Di Tullio, N. Spreti and F. De Angelis, Mass Spectrom. Rev., 2004, 23, 87 CrossRef CAS PubMed; (b) J. Ralph, K. Lundquist, G. Brunow, F. Lu, H. Kim, P. F. Schatz, J. M. Marita, R. D. Hatfield, S. A. Ralph, J. H. Christensen and W. Boerjan, Phytochem. Rev., 2004, 3, 29 CrossRef CAS.
- (a) A. Corma, S. Iborra and A. Velty, Chem. Rev., 2007, 107, 2411 CrossRef CAS PubMed; (b) D. R. Dodds and R. A. Gross, Science, 2007, 318, 1250 CrossRef CAS PubMed; (c) C. O. Tuck, E. Perez, I. T. Horvath, R. A. Sheldon and M. Poliakoff, Science, 2012, 337, 695 CrossRef CAS PubMed; (d) M. Y. He, Y. H. Sun and B. X. Han, Angew. Chem., Int. Ed., 2013, 52, 9620 CrossRef CAS PubMed; (e) A. J. Ragauskas, G. T. Beckham, M. J. Biddy, R. Chandra, F. Chen, M. F. Davis, B. H. Davison, R. A. Dixon, P. at Gilna, M. Keller, P. Langan, A. K. Naskar, J. N. Saddler, T. J. Tschaplinski, G. A. Tuskan and C. E. Wyman, Science, 2014, 344 DOI:10.1126/science.1246843.
- (a) R. DiCosimo and H. C. Szabo, J. Org. Chem., 1988, 53, 1673 CrossRef CAS; (b) C. Crestini, M. C. Caponi, D. S. Argyropoulos and R. Saladino, Bioorg. Med. Chem., 2006, 14, 5292 CrossRef CAS PubMed; (c) V. T. Oitl and P. R. Rohr, ChemSusChem, 2008, 1, 763 CrossRef PubMed; (d) K. Stärk, N. Taccardi, A. Bosmann and P. Wasserscheid, ChemSusChem, 2010, 3, 719 CrossRef PubMed; (e) D. W. Cho, R. Parthasarathi, A. S. Pimentel, G. D. Maestas, H. J. Park, U. C. Yoon, D. Dunaway-Mariano, S. Gnanakaran, P. Langan and P. S. Mariano, J. Org. Chem., 2010, 75, 6549 CrossRef CAS PubMed; (f) S. K. Badamali, R. Luque, J. H. Clark and S. W. Breeden, Catal. Commun., 2011, 12, 993 CrossRef CAS PubMed; (g) B. Sedai, C. Diaz-Urrutia, R. T. Baker, R. Wu, L. A. Silks and S. K. Hanson, ACS Catal., 2011, 1, 794 CrossRef CAS; (h) S. K. Hanson, R. Wu and L. A. Silks, Angew. Chem., Int. Ed., 2012, 51, 3410 CrossRef CAS PubMed; (i) B. Biannic and J. J. Bozell, Org. Lett., 2013, 15, 2730 CrossRef CAS PubMed; (j) S. K. Lim, K. Nahm, C. S. Ra, D. W. Cho, U. C. Yoon, J. A. Latham, D. Dunaway-Mariano and P. S. Mariano, J. Org. Chem., 2013, 78, 9431 CrossRef CAS PubMed; (k) B. Sedai, C. Diaz-Urrutia, T. Baker, R. Wu, L. A. Silks and S. K. Hanson, ACS Catal., 2013, 3, 3111 CrossRef CAS; (l) F. Napoly, L. Jean-Gérard, C. Goux-Henry, M. Draye and B. Andrioletti, Eur. J. Org. Chem., 2014, 781 CrossRef CAS PubMed; (m) L. J. Mitchell and C. J. Moody, J. Org. Chem., 2014, 79, 11091 CrossRef CAS PubMed. For review see: (n) H. Lange, S. Decina and C. Crestini, Eur. Polym. J., 2013, 49, 1151 CrossRef CAS PubMed; (o) S. R. Collinsona and W. Thielemans, Coord. Chem. Rev., 2010, 254, 1854 CrossRef PubMed.
- (a) E. E. Harris, J. D'Ianni and H. Adkins, J. Am. Chem. Soc., 1938, 60, 1467 CrossRef CAS; (b) A. Cyr, F. Chiltz, P. Jeanson, A. Martel, L. Brossard, J. Lessard and H. Ménard, Can. J. Chem., 2000, 78, 307 CrossRef CAS; (c) N. Yan, C. Zhao, P. J. Dyson, C. Wang, L.-T. Liu and Y. Kou, ChemSusChem, 2008, 1, 626 CrossRef CAS PubMed; (d) M. Nagy, K. David, G. J. P. Britovsek and A. J. Ragauskas, Holzforschung, 2009, 63, 513 CrossRef CAS; (e) K. Barta, T. D. Matson, M. L. Fettig, S. L. Scott, A. V. Iretskii and P. C. Ford, Green Chem., 2010, 12, 1640 RSC; (f) A. G. Sergeev and J. F. Hartwig, Science, 2011, 332, 439 CrossRef CAS PubMed; (g) A. N. Desnoyer, B. Fartel, K. C. MacLeod, B. O. Patrick and K. M. Smith, Organometallics, 2012, 31, 7625 CrossRef CAS; (h) A. G. Sergeev, J. D. Webb and J. F. Hartwig, J. Am. Chem. Soc., 2012, 134, 20226 CrossRef CAS PubMed; (i) T. H. Parsell, B. C. Owen, I. Klein, T. M. Jarrell, C. L. Marcum, L. J. Haupert, L. M. Amundson, H. I. Kenttamaa, F. Ribeiro, J. T. Miller and M. M. Abu-Omar, Chem. Sci., 2013, 4, 806 RSC; (j) Q. Song, F. Wang, J. Y. Cai, Y. H. Wang, J. J. Zhang, W. Q. Yu and J. Xu, Energy Environ. Sci., 2013, 6, 994 RSC; (k) A. Fedorov, A. A. Toutov, N. A. Swisher and R. H. Grubbs, Chem. Sci., 2013, 4, 1640 RSC.
- (a) J. M. Nichols, L. M. Bishop, R. G. Bergman and J. A. Ellman, J. Am. Chem. Soc., 2010, 132, 12554 CrossRef CAS PubMed; (b) S. Son and F. D. Toste, Angew. Chem., Int. Ed., 2010, 49, 3791 CrossRef CAS PubMed; (c) A. Wu, B. O. Patrick, E. Chung and B. R. James, Dalton Trans., 2012, 41, 11093 RSC; (d) J. M. W. Chan, S. Bauer, H. Sorek, S. Sreekumar, K. Wang and F. D. Toste, ACS Catal., 2013, 3, 1369 CrossRef CAS; (e) T. Kleine, J. Buendia and C. Bolm, Green Chem., 2013, 15, 160 RSC; (f) T. Stein, T. Weigand, C. Merkens, J. Klankermayer and W. Leitner, ChemCatChem, 2013, 5, 439 CrossRef PubMed.
- J. Zhang, Y. Liu, S. Chiba and T. P. Loh, Chem. Commun., 2013, 49, 11439 RSC.
- C. S. Lancefield, O. S. Ojo, F. Tran and N. J. Westwood, Angew. Chem., Int. Ed., 2015, 54, 128 CrossRef PubMed.
- Y. Ren, M. Yan, J. Wang, Z. C. Zhang and K. Yao, Angew. Chem., Int. Ed., 2013, 52, 12674 CrossRef CAS PubMed.
- A. Rahimi, A. Azarpira, H. Kim, J. Ralph and S. S. Stahl, J. Am. Chem. Soc., 2013, 135, 6415 CrossRef CAS PubMed.
- A. Rahimi, A. Ulbrich, J. J. Coon and S. S. Stahl, Nature, 2014, 515, 249 CrossRef CAS PubMed.
- J. D. Nguyen, B. S. Matsuura and C. R. J. Stephenson, J. Am. Chem. Soc., 2014, 136, 1218 CrossRef CAS PubMed.
- E. Feghali and T. Cantat, Chem. Commun., 2014, 50, 862 RSC.
- (a) D. A. House, Chem. Rev., 1962, 62, 185 CrossRef CAS; (b) F. Minisci, A. Citterio and C. Giordano, Acc. Chem. Res., 1983, 16, 27 CrossRef CAS; (c) F. Minisci, Acc. Chem. Res., 1975, 8, 165 CrossRef CAS; (d) R. N. Burgin, S. Jones and B. Tarbit, Tetrahedron Lett., 2009, 50, 6772 CrossRef CAS PubMed; (e) R. Xia, M.-S. Xie, H.-Y. Niu, G.-R. Qu and H.-M. Guo, Org. Lett., 2014, 16, 444 CrossRef CAS PubMed; (f) X. Li, H.-Y. Wang and Z.-J. Shi, New J. Chem., 2013, 37, 1704 RSC.
- E. Baciocchi, M. Bietti, M. F. Gerini, O. Lanzalunga and S. Mancinelli, J. Chem. Soc., Perkin Trans. 2, 2001, 1506 RSC.
- E. Baciocchi, C. Fabbri and O. Lanzalunga, J. Org. Chem., 2003, 68, 9061 CrossRef CAS PubMed.
- A. Wu, J. M. Lauzon, I. Andriani and B. R. James, RSC Adv., 2014, 4, 17931 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Lignin meodels preparation procedure and their 1H NMR, 13C NMR and FTMS-ESI data. See DOI: 10.1039/c5qo00116a |
| This journal is © the Partner Organisations 2015 |