Efficient catalytic enantioselective Nazarov cyclizations of divinyl ketoesters†
Zhou
Xu
ab,
Hai
Ren
a,
Lijia
Wang
*a and
Yong
Tang
*a
aState Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai 200032, China. E-mail: tangy@mail.sioc.ac.cn; wanglijia@sioc.ac.cn
bXuzhou Medical College, 209 Tongshan Road, Xuzhou 221004, China
First published on 29th April 2015
Abstract
An efficient catalytic enantioselective Nazarov cyclization of divinyl ketoesters was developed using a chiral BOX/Cu(II) complex, which provides facile access to a variety of optically active multi-substituted cyclopent-2-enone esters in 78–95% yields with 78–90% ee.
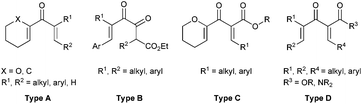
Multi-substituted five membered carbocyclic skeletons are widely found in natural products and other biologically active compounds.1 Nazarov cyclization reaction2–4 represents one of the most effective methods for the construction of five membered carbocyclic rings, and it has been applied in the total synthesis of many useful natural products.5 However, in the asymmetric catalytic Nazarov reaction, which has attracted increasing attention from chemists, it is quite difficult to achieve high enantioselectivity. Only a few successful examples have been reported.6–9 In 2004, Trauner et al. developed the first highly efficient scandium–pybox catalyzed asymmetric Nazarov reactions of divinyl ketones bearing an oxygen at the α-position of the vinyl nucleophile (Type A, Fig. 1).6b In 2013, Rawal et al. documented the Cr(III)/salen promoted enantioselective Nazarov cyclizations of dienones (Type A, Fig. 1), giving rise to cyclopentenoids in 80 − 96% ee.6f In 2010, an elegant bifunctional thiourea promoted organocatalytic asymmetric Nazarov cyclization of diketoesters (Type B, Fig. 1) was realized by Tius et al., affording the α-hydroxycyclopentenones in 42–95% yields with 80–97% ee.7a In the same year, our group reported a highly regio-, diastereo-, and enantioselective Nazarov reaction of alkoxy divinyl ketoesters (Type C, Fig. 1) catalyzed by a chiral trisoxazoline/copper(II) system.8
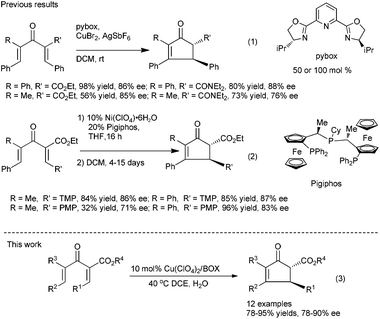
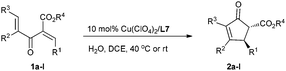
On the other hand, for acyclic divinyl ketoesters (Type D, Fig. 1) as substrates,10d,e the racemic studies on the Nazarov cyclization10 have achieved important breakthrough in recent years; however, successful examples of an asymmetric version are still limited. In 2003, Aggarwal et al. developed the first asymmetric Nazarov cyclization promoted by stoichiometric or semi-stoichiometric chiral Cu(II)–pybox complexes, achieving up to 88% enantiomeric excess (eqn (1), Scheme 1).9a Togni et al. reported a chiral tridentate phosphine Pigiphos/Ni(II) catalyzed process of divinyl ketoesters containing an activated trimethoxyphenyl (TMP) group or a 4-methoxyphenyl (PMP) group, affording the products in 32–97% yields with 45–88% ee after 4–15 days (eqn (2), Scheme 1).9b Despite these great efforts, challenging problems, such as reactivity, stereoselectivity and substrate scope generality in this process, have not been well resolved yet. Recently, we have developed an efficient catalytic enantioselective Nazarov cyclization of divinyl ketoesters, which provides facile access to the optically active multi-substituted cyclopent-2-enones in high yields with good to excellent ee values (eqn (3), Scheme 1). In this communication, we wish to report the preliminary results.
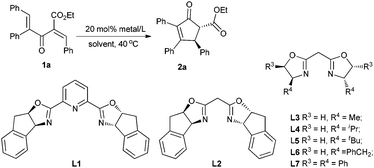
Initially, the enantioselective Nazarov cyclization of substrate 1a was carried out with 20 mol% of copper complex in a chloroform solution at 40 °C. The pyridyl bisoxazolines were documented as effective chiral ligands in the asymmetric Nazarov reactions.6b,e,9a,c However, as to substrate 1a,11 with L1 the reaction could not occur (entry 1, Table 1). Then we tried to use BOX ligand L2, and found that the cyclization proceeded smoothly producing 2a in 86% yield with 51% ee (entry 2). Changing the solvent to 1,2-dichloroethane (DCE) led to a better level of enantioselectivity (61% ee, entry 3). Next, we turned to investigate a series of BOX ligands bearing various chiral backbones.12 With the L-Ala derived BOX ligand L3, the product 2a was obtained in 84% yield with 47% ee (entry 4). A more hindered iPr group was beneficial to the enantioselectivity (67% ee, entry 5). However, on continuing to increase the hindrance, L5 led to a dramatic drop of the enantioselectivity (23% ee entry 6). Meanwhile, when the L-Phe derived BOX ligand L6 was employed, 2a was produced in 93% yield with 58% ee (entry 7). Under optimal conditions, we finally found that chiral ligand L7 could promote the reaction very efficiently, affording 2a in 92% yield with 90% ee after 10 h (entry 8), better than pybox/Cu(SbF6)2 in both selectivity and catalyst loading. We also examined the counter ion effect of this reaction. As shown in entries 8–10, ClO4− proved to be the best one. However, when the catalyst loading was further reduced to 10 mmol%, the ee value dropped to 84% ee (entry 11). In order to raise the efficiency of this reaction system, additives were examined. Interestingly, 4 Å molecular sieves destroyed the reaction, while a trace amount of water could promote the reaction to give 92% yield and 90% ee (entry 12 vs. 13).13
| Entry | Metal salts | L | Solvent | Time (h) | Yieldb (%) | eec (%) |
|---|---|---|---|---|---|---|
| a Reactions were carried out with metal salts (0.04 mmol), ligand (0.04 mmol) and 1a (0.2 mmol) in solvent (4.0 mL) under an Ar atmosphere. b Isolated yield. c Determined by chiral HPLC. d The reaction was carried out with 10 mol% catalyst loading. e 4 Å molecular sieves were added. f H2O (0.12 mmol, 2.3 μL) was added. | ||||||
| 1 | Cu(ClO4)2·6H2O | L1 | CHCl3 | 24 | 0 | — |
| 2 | Cu(ClO4)2·6H2O | L2 | CHCl3 | 17 | 86 | 51 |
| 3 | Cu(ClO4)2·6H2O | L2 | DCE | 14 | 90 | 62 |
| 4 | Cu(ClO4)2·6H2O | L3 | DCE | 12 | 84 | 47 |
| 5 | Cu(ClO4)2·6H2O | L4 | DCE | 14 | 90 | 67 |
| 6 | Cu(ClO4)2·6H2O | L5 | DCE | 16 | 95 | 23 |
| 7 | Cu(ClO4)2·6H2O | L6 | DCE | 14 | 93 | 58 |
| 8 | Cu(ClO4)2·6H2O | L7 | DCE | 10 | 92 | 90 |
| 9 | Cu(SbF6)2 | L7 | DCE | 8 | 94 | 89 |
| 10 | Cu(OTf)2 | L7 | DCE | 10 | 86 | 88 |
| 11d | Cu(ClO4)2·6H2O | L7 | DCE | 14 | 91 | 84 |
| 12d,e | Cu(ClO4)2·6H2O | L7 | DCE | 24 | 0 | — |
| 13d,f | Cu(ClO4)2·6H2O | L7 | DCE | 24 | 92 | 90 |
Under the optimized reaction conditions (entry 8, Table 1), we next investigated the substrate scope (Table 2). Divinyl ketoesters 1b–d bearing the R1 group with –Br substituted at the para-, meta- and ortho- positions underwent cyclization with high enantioselectivity (85–88% ee) and a decline of the reactivity (entries 2–4). When R1 was a 4-PhC6H5– group, 20% catalyst loading was required to produce the cyclization product 2e in 92% yield with 88% ee (entry 5). Cyclic enone products 2g and 2h bearing both 1- and 2-naphthyl groups could be easily accessed in 89% yield with 86% ee, and in 93% yield with 87% ee, respectively (entries 7 and 8). The catalyst system was even competent with electron deficient substrate 1f, affording the product 2f in 83% yield with 84% ee (entry 6). As to the electron-rich substrates 1i and 1j, the reactions proceeded very fast and were complete within 2 h at room temperature, giving the corresponding products 2i and 2j in high yields with good enantioselectivities (entries 9 and 10). Thus, the current catalyst system is tolerated for both electron rich and poor substrates. In addition, by changing the ester group from ethyl to methyl, both the reactivity and the enantioselectivity are maintained (entry 11). Moreover, for the 4-IC6H5 substituted divinyl ketoester 1l, a pleasing result of 87% yield with 90% ee was obtained (entry 12).
| Entry | R1; R2; R3; R4 | 2 | Time (h) | Yieldb (%) | eec (%) |
|---|---|---|---|---|---|
| a Reactions were carried out with Cu(ClO4)2·6H2O (0.02 mmol), L7 (0.02 mmol), 1 (0.2 mmol) and H2O (0.12 mmol) in DCE (4.0 mL) under an Ar atmosphere. b Isolated yield. c Determined by chiral HPLC. d The reaction was carried out with 20 mol% catalyst loading. e The absolute configuration of the major enantiomer is (1R,5S) by the comparison of the reported data.9c f The reaction was carried out at rt. | |||||
| 1 | Ph; Ph; Ph; Et | 2a | 24 | 92 | 90e |
| 2 | 4-BrC6H5; Ph; Ph; Et | 2b | 45 | 81 | 88 |
| 3 | 3-BrC6H5; Ph; Ph; Et | 2c | 55 | 80 | 85 |
| 4 | 2-BrC6H5; Ph; Ph; Et | 2d | 60 | 78 | 86 |
| 5 | 4-PhC6H5; Ph; Ph; Et | 2e | 22 | 92 | 84 (88)d |
| 6 | 4-CF3C6H5; Ph; Ph; Et | 2f | 72 | 83 | 84 |
| 7 | 1-Naphthyl; Ph; Ph; Et | 2g | 24 | 89 | 86 |
| 8 | 2-Naphthyl; Ph; Ph; Et | 2h | 24 | 93 | 87 |
| 9f | Ph; 4-MeOC6H5; Ph; Et | 2i | 2 | 91 | 78 |
| 10f | Ph; 4-MeOC6H5; 4-MeOC6H5; Et | 2j | 2 | 90 | 82 |
| 11 | Ph; Ph; Ph; Me | 2k | 20 | 95 | 90 |
| 12 | 4-IC6H5; Ph; Ph; Me | 2l | 30 | 87 | 90 |
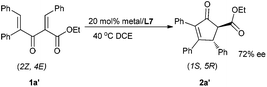
Interestingly, when substrate 1a′ was employed with the current catalytic system, the absolute configuration of the major enantiomer reversed to (1S,5R), leading to the product 2a′ in 72% ee (Scheme 2). Thus, under the same catalyst system, both the enantiomers could be obtained in terms of changing the Z/E configuration of substrates.
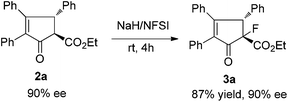
Fluorine-containing chiral cyclic ketoesters are potentially useful building blocks for the synthesis of natural products and medicines. Ma et al. reported an elegant copper-catalyzed tandem Nazarov cyclization–electrophilic fluorination reaction in the stereoselective synthesis of highly substituted indanones.14 We found that under mild reaction conditions, compound 2a was easily transferred to fluorine substituted ketoester 3a in 87% yield without loss of optical purity with stereospecific diastereoselectivity (Scheme 3).7,9c
Conclusions
In conclusion, we have developed an efficient catalytic enantioselective Nazarov cyclization of divinyl ketoesters by a chiral BOX/Cu(II) complex, which provides facile access to the optically active cyclopent-2-enone esters with functional diversity in 78–95% yields with 78–90% ee. There are several remarkable features of the method, such as mild reaction conditions, high catalytic efficiency and simple procedure, that make the current reaction practically useful. A study on the application of this method to the total synthesis of natural products is ongoing in our lab.Acknowledgements
We are grateful for the financial support from the National Natural Sciences Foundation of China (no. 21121062 and 21272250), and the Chinese Academy of Sciences. We thank Dr Xue-bing, Leng (SIOC) and Mr Jie, Sun (SIOC) for X-ray crystal analysis.Notes and references
- For recent reviews on five-membered carbocycles, see: (a) S. E. Gibson, S. E. Lewis and N. Mainolfi, J. Organomet. Chem., 2004, 689, 3873 CrossRef CAS PubMed; (b) V. B. Kurteva and C. A. M. Afonso, Chem. Rev., 2009, 109, 6809 CrossRef CAS PubMed.
- For books and reviews on the Nazarov reaction: (a) S. E. Denmark, in Comprehensive Organic Synthesis, ed. B. M. Trost and I. Fleming, Pergamon, Oxford, 1991, vol. 5, p. 751 Search PubMed; (b) C. Santelli-Rouvier and M. Santelli, Synthesis, 1983, 429 CrossRef CAS; (c) M. Ramaiah, Synthesis, 1984, 529 CrossRef CAS; (d) K. L. Habermas, S. E. Denmark and T. K. Jones, Org. React., 1994, 45, 1 CAS; (e) M. A. Tius, Acc. Chem. Res., 2003, 36, 284 CrossRef CAS PubMed; (f) H. Pellissier, Tetrahedron, 2005, 61, 6479 CrossRef CAS PubMed; (g) A. J. Frontie and C. Collison, Tetrahedron, 2005, 61, 7577 CrossRef PubMed; (h) M. A. Tius, Eur. J. Org. Chem., 2005, 2193 CrossRef CAS PubMed; (i) N. Grant, C. J. Rieder and F. G. West, Chem. Commun., 2009, 5676 RSC; (j) S. Thompson, A. G. Coyne, P. C. Knipe and M. D. Smith, Chem. Soc. Rev., 2011, 40, 4217 RSC; (k) C. Schotes and A. Mezzetti, ACS Catal., 2012, 2, 528 CrossRef CAS.
- For selected racemic examples, see: (a) S. E. Denmark and T. K. Jones, J. Am. Chem. Soc., 1982, 104, 2642 CrossRef CAS; (b) T. K. Jones and S. E. Denmark, Helv. Chim. Acta, 1983, 66, 2397 CrossRef CAS PubMed; (c) T. K. Jones and S. E. Denmark, Helv. Chim. Acta, 1983, 66, 2377 CrossRef CAS PubMed; (d) W. He, X. F. Sun and A. J. Frontier, J. Am. Chem. Soc., 2003, 125, 14278 CrossRef CAS PubMed; (e) M. Janka, W. He, A. J. Frontier and R. Eisenberg, J. Am. Chem. Soc., 2004, 126, 6864 CrossRef CAS PubMed; (f) A. R. Banaag and M. A. Tius, J. Org. Chem., 2008, 73, 8133 CrossRef CAS PubMed; (g) A. K. Basak and M. A. Tius, Org. Lett., 2008, 10, 4073 CrossRef CAS PubMed; (h) T. N. Grant and F. G. West, J. Am. Chem. Soc., 2006, 128, 9348 CrossRef CAS PubMed; (i) M. Janka, W. He, I. E. Haedicke, F. R. Fronczek, A. J. Frontier and R. Eisenberg, J. Am. Chem. Soc., 2006, 128, 5312 CrossRef CAS PubMed; (j) D. Song, A. Rostami and F. G. West, J. Am. Chem. Soc., 2007, 129, 12019 CrossRef CAS PubMed; (k) F. Dhoro, T. E. Kristensen, V. Stockmann, G. P. A. Yap and M. A. Tius, J. Am. Chem. Soc., 2007, 129, 7256 CrossRef CAS PubMed; (l) A. R. Banaag and M. A. Tius, J. Am. Chem. Soc., 2007, 129, 5328 CrossRef CAS PubMed; (m) J. Huang and A. J. Frontier, J. Am. Chem. Soc., 2007, 129, 8060 CrossRef CAS PubMed; (n) W. He, I. R. Herrick, T. A. Atesin, P. A. Caruana, C. A. Kellenberger and A. J. Frontier, J. Am. Chem. Soc., 2008, 130, 1003 CrossRef CAS PubMed.
- (a) P. Cordier, C. Aubert, M. Malacria, E. Lacte and V. Gandon, Angew. Chem., Int. Ed., 2009, 48, 8757 CrossRef CAS PubMed; (b) C. J. Rieder, K. J. Winberg and F. G. West, J. Am. Chem. Soc., 2009, 131, 7504 CrossRef CAS PubMed; (c) P. Cao, X.-L. Sun, B.-H. Zhu, Q. Shen, Z. Xie and Y. Tang, Org. Lett., 2009, 11, 3048 CrossRef CAS PubMed; (d) F. De Simone, J. Gertsch and J. Waser, Angew. Chem., Int. Ed., 2010, 49, 5767 CrossRef CAS PubMed; (e) V. M. Marx and D. J. Burnell, J. Am. Chem. Soc., 2010, 132, 1685 CrossRef CAS PubMed; (f) J. L. Brooks, P. A. Caruana and A. J. Frontier, J. Am. Chem. Soc., 2011, 133, 12454 CrossRef CAS PubMed; (g) C. J. Hastings, M. P. Backlund, R. G. Bergman and K. N. Raymond, Angew. Chem., Int. Ed., 2011, 50, 10570 CrossRef CAS PubMed; (h) V. M. Marx, R. L. Stoddard, G. S. Heverly-Coulson and D. J. Burnell, Chem. – Eur. J., 2011, 17, 8098 CrossRef CAS PubMed; (i) J. Barluenga, A. Alvarez-Fernandez, A. L. Suarez-Sobrino and M. Tomas, Angew. Chem., Int. Ed., 2012, 51, 183 CrossRef CAS PubMed; (j) J. L. Brooks and A. J. Frontier, J. Am. Chem. Soc., 2012, 134, 16551 CrossRef CAS PubMed; (k) D. J. Kerr, M. Miletic, J. H. Chaplin, J. M. White and B. L. Flynn, Org. Lett., 2012, 14, 1732 CrossRef CAS PubMed; (l) L. H. Zhu, Z. G. Xi, J. Lv and S. Z. Luo, Org. Lett., 2013, 15, 4496 CrossRef CAS PubMed.
- For selected examples, see: (a) G. O. Berger and M. A. Tius, J. Org. Chem., 2007, 72, 6473 CrossRef CAS PubMed; (b) L. Wan and M. A. Tius, Org. Lett., 2007, 9, 647 CrossRef CAS PubMed; (c) W. He, J. Huang, X. Sun and A. J. Frontier, J. Am. Chem. Soc., 2007, 129, 498 CrossRef CAS PubMed; (d) D. R. Williams, L. A. Robinson, C. R. Nevill and J. P. Reddy, Angew. Chem., Int. Ed., 2007, 46, 915 CrossRef CAS PubMed; (e) W. He, J. Huang, X. Sun and A. J. Frontier, J. Am. Chem. Soc., 2008, 130, 300 CrossRef CAS PubMed; (f) J. A. Malona, K. Cariou and A. J. Frontier, J. Am. Chem. Soc., 2009, 131, 7560 CrossRef CAS PubMed; (g) S. Gao, Q. Wang and C. Chen, J. Am. Chem. Soc., 2009, 131, 1410 CrossRef CAS PubMed; (h) A. Y. Bitar and A. J. Frontier, Org. Lett., 2009, 11, 49 CrossRef CAS PubMed; (i) H. M. Cheng, W. W. Tian, P. A. Peixoto, B. Dhudshia and D. Y. K. Chen, Angew. Chem., Int. Ed., 2011, 50, 4165 CrossRef CAS PubMed; (j) D. J. Kerr and B. L. Flynn, Org. Lett., 2012, 14, 1740 CrossRef CAS PubMed; (k) P. Magnus, W. A. Freund, E. J. Moorhead and T. Rainey, J. Am. Chem. Soc., 2012, 134, 6140 CrossRef CAS PubMed; (l) J. A. Malona, K. Cariou, W. T. Spencer and A. J. Frontier, J. Org. Chem., 2012, 77, 1891 CrossRef CAS PubMed; (m) J. C. P. Reyes and D. Romo, Angew. Chem., Int. Ed., 2012, 51, 6870 CrossRef CAS PubMed; (n) C. J. Song, H. Liu, M. L. Hong, Y. Y. Liu, F. F. Jia, L. Sun, Z. L. Pan and J. B. Chang, J. Org. Chem., 2012, 77, 704 CrossRef CAS PubMed; (o) D. H. Dethe and G. Murhade, Org. Lett., 2013, 15, 429 CrossRef CAS PubMed; (p) D. J. Kerr, M. Miletic, N. Manchala, J. M. White and B. L. Flynn, Org. Lett., 2013, 15, 4118 CrossRef CAS PubMed; (q) B. J. Moritz, D. J. Mack, L. C. Tong and R. J. Thomson, Angew. Chem., Int. Ed., 2014, 53, 2988 CrossRef CAS PubMed; (r) A. Shvartsbart and A. B. Smith, J. Am. Chem. Soc., 2014, 136, 870 CrossRef CAS PubMed.
- (a) G. Liang, S. N. Gradl and D. Trauner, Org. Lett., 2003, 5, 4931 CrossRef CAS PubMed; (b) G. Liang and D. Trauner, J. Am. Chem. Soc., 2004, 126, 9544 CrossRef CAS PubMed; (c) M. Rueping, W. Ieawsuwan, A. P. Antonchick and B. J. Nachtsheim, Angew. Chem., Int. Ed., 2007, 46, 2097 CrossRef CAS PubMed; (d) M. Rueping and W. Ieawsuwan, Adv. Synth. Catal., 2009, 351, 78 CrossRef CAS PubMed; (e) K. Yaji and M. Shindo, Synlett, 2009, 2524 CAS; (f) G. E. Hutson, Y. E. Türkmen and V. H. Rawal, J. Am. Chem. Soc., 2013, 135, 4988 CrossRef CAS PubMed.
- (a) A. K. Basak, N. Shimada, W. F. Bow, D. A. Vicic and M. A. Tius, J. Am. Chem. Soc., 2010, 132, 8266 CrossRef CAS PubMed; (b) N. Shimada, C. Stewart, W. F. Bow, A. Jolit, K. Wong, Z. Zhou and M. A. Tius, Angew. Chem., Int. Ed., 2012, 51, 5727 CrossRef CAS PubMed; (c) A. Jolit, S. Vazquez-Rodriguez, G. P. A. Yap and M. A. Tius, Angew. Chem., Int. Ed., 2013, 52, 11102 CrossRef CAS PubMed; (d) A. Jolit, P. M. Walleser, G. P. A. Yap and M. A. Tius, Angew. Chem., Int. Ed., 2014, 53, 6180 CrossRef CAS PubMed.
- P. Cao, C. Deng, Y. Y. Zhou, X. L. Sun, J. C. Zheng, Z. W. Xie and Y. Tang, Angew. Chem., Int. Ed., 2010, 49, 4463 CrossRef CAS PubMed.
- (a) V. K. Aggarwal and A. J. Belfield, Org. Lett., 2003, 5, 5075 CrossRef CAS PubMed; (b) I. Walz and A. Togni, Chem. Commun., 2008, 4315 RSC; (c) M. Kawatsura, K. Kajita, S. Hayase and T. Itoh, Synlett, 2010, 1243 CrossRef CAS PubMed.
- (a) W. He, I. R. Herrick, T. A. Atesin, P. A. Caruana, C. A. Kellenberger and A. J. Frontier, J. Am. Chem. Soc., 2008, 130, 1003 CrossRef CAS PubMed; (b) T. Vaidya, A. C. Atesin, I. R. Herrick, A. J. Frontier and R. Eisenberg, Angew. Chem., Int. Ed., 2010, 49, 3363 CrossRef CAS PubMed; (c) J. Huang, D. Leboeuf and A. J. Frontier, J. Am. Chem. Soc., 2011, 133, 6307 CrossRef CAS PubMed; (d) D. Leboeuf, V. Gandon, J. Ciesielski and A. J. Frontier, J. Am. Chem. Soc., 2012, 134, 6296 CrossRef CAS PubMed; (e) Y. Kwon, R. McDonald and F. G. West, Angew. Chem., Int. Ed., 2013, 52, 8616 CrossRef CAS PubMed.
- CCDC 1029676 (2a) contains the supplementary crystallographic data for this paper. See the ESI.†.
- For recent reviews on bisoxazoline and oxazoline-containing ligands in asymmetric catalysis, see: (a) C. Foltz, B. Stecker, G. Marconi, S. Bellemin-Laponnaz, H. Wadepohl and L. H. Gade, Chem. – Eur. J., 2007, 13, 9912 CrossRef CAS PubMed; (b) S. Bellemin-Laponnaz and L. H. Gade, Actual. Chim., 2007, 16 CAS; (c) G. C. Hargaden and P. J. Guiry, Chem. Rev., 2009, 109, 2505 CrossRef CAS PubMed; (d) G. Desimoni, G. Faita and K. A. Jørgensen, Chem. Rev., 2011, 111, 284 CrossRef PubMed; (e) J. Zhou and Y. Tang, Top. Organomet. Chem., 2011, 36, 287 CrossRef CAS; (f) S. H. Liao, X. L. Sun and Y. Tang, Acc. Chem. Res., 2014, 47, 2260 CrossRef CAS PubMed; (g) Q.-H. Deng, R. L. Melen and L. H. Gade, Acc. Chem. Res., 2014, 47, 3162 CrossRef CAS PubMed. For selected recent studies, see: (h) J. Choi and G. C. Fu, J. Am. Chem. Soc., 2012, 134, 9102 CrossRef CAS PubMed; (i) J. Li, S. Liao, H. Xiong, Y.-Y. Zhou, X.-L. Sun, Y. Zhang, X.-G. Zhou and Y. Tang, Angew. Chem., Int. Ed., 2012, 51, 8838 CrossRef CAS PubMed; (j) C. Deng, L. Wang, J. Zhu and Y. Tang, Angew. Chem., Int. Ed., 2012, 51, 11620 CrossRef CAS PubMed; (k) D. Leboeuf, V. Gandon, J. Ciesielski and A. J. Frontier, J. Am. Chem. Soc., 2012, 134, 6296 CrossRef CAS PubMed; (l) Y.-Y. Zhou, L. Wang, J. Li, X.-L. Sun and Y. Tang, J. Am. Chem. Soc., 2012, 134, 9066 CrossRef CAS PubMed; (m) X.-G. Song, S.-F. Zhu, X.-L. Xie and Q.-L. Zhou, Angew. Chem., Int. Ed., 2013, 52, 2555 CrossRef CAS PubMed; (n) H. Xiong, H. Xu, S. Liao, Z. Xie and Y. Tang, J. Am. Chem. Soc., 2013, 135, 7851 CrossRef CAS PubMed; (o) T. Kusakabe, T. Takahashi, R. Shen, A. Y. Ikeda, D. Dhage, Y. Kanno, Y. Inouye, H. Sasai, T. Mochida and K. Kato, Angew. Chem., Int. Ed., 2013, 52, 7845 CrossRef CAS PubMed; (p) J. Choi, P. Martin-Gago and G. C. Fu, J. Am. Chem. Soc., 2014, 136, 12161 CrossRef CAS PubMed; (q) X.-L. Xie, S.-F. Zhu, J.-X. Guo, Y. Cai and Q.-L. Zhou, Angew. Chem., Int. Ed., 2014, 53, 2978 CrossRef CAS PubMed; (r) J.-J. Shen, S.-F. Zhu, Y. Cai, H. Xu, X.-L. Xie and Q.-L. Zhou, Angew. Chem., Int. Ed., 2014, 53, 13188 CrossRef CAS PubMed.
- For details, see the ESI.†.
- J. Nie, H.-W. Zhu, H.-F. Cui, M.-Q. Hua and J.-A. Ma, Org. Lett., 2007, 9, 3053 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures and the characterization data of new compounds. See DOI: 10.1039/c5qo00099h |
| This journal is © the Partner Organisations 2015 |