Highly efficient pincer nickel catalyzed cross-coupling of aryltrimethylammonium triflates with arylzinc reagents†
Dan
Wu
a,
Jian-Long
Tao
a and
Zhong-Xia
Wang
*ab
aDepartment of Chemistry, University of Science and Technology of China, Hefei, Anhui 230026, China
bCollaborative Innovation Center of Chemical Science and Engineering (Tianjin), Tianjin 300072, China. E-mail: zxwang@ustc.edu.cn; Fax: +86 551 63601592; Tel: +86 551 63603043
First published on 22nd January 2015
Abstract
N,N,P-Pincer-nickel-complex-catalyzed cross-coupling of aryltrimethylammonium triflates with aryl- or heteroaryl-zinc reagents was investigated. The reaction is suitable for a broad scope of substrates, exhibits good functional group compatibility and can be performed under mild conditions with extremely low catalyst loadings.
Introduction
Since the 1970s a series of methodologies have been developed to construct C–C bonds through transition-metal-catalyzed cross-coupling, including Kumada coupling, Negishi coupling, Suzuki coupling, Stille coupling, and so on.1 The electrophiles employed in these coupling reactions are principally organic halides and oxygen-containing compounds such as triflates, tosylates, mesylates, and carboxylates.1–6 It is relatively rare to employ nitrogen-based electrophiles such as aromatic amines in these reactions due to the inertness of the C–N bonds. However, some trials have been performed over the years. An effective tactic is to transform aromatic amines to ammonium salts or diazonium salts to weaken the C–N bonds before catalytic cleavage.7,8 For example, Wenkert and Reeves respectively carried out nickel- or palladium-catalyzed reactions of aryltrimethylammonium salts with Grignard reagents.7a,d Blakey and MacMillan reported cross-coupling of aryltrimethylammonium triflates with arylboronic acids using the Ni(cod)2/IMes catalyst.7b Our group carried out the reaction of aryltrimethylammonium salts with organozinc reagents using Ni(PCy3)2Cl2 or pincer nickel complexes (1–4, Chart 1) as the catalysts.7e–h On the basis of the results achieved, we intended to further develop highly effective catalyst systems for the activation and transformation of C–N bonds of aromatic ammonium salts through tuning electronic and steric effects of the pincer ligands. Herein, we report P,N,N-pincer nickel (5a–5c,3dChart 1) catalyzed cross-coupling of aryltrimethylammonium salts with arylzinc reagents.Results and discussion
We performed a preliminary screen using the 5a-catalyzed cross-coupling of phenyltrimethylammonium triflate with p-MeOC6H4ZnCl as the model reaction (Table 1). We first examined the reaction using similar reaction conditions to those for 5a-catalyzed reaction of aryl chlorides with arylzinc reagents as we reported earlier.3d Thus, the reaction was run in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of THF and NMP with 0.01 mol% catalyst loading. It was found that the reaction could proceed at room temperature and gave the corresponding cross-coupling product in 63% yield in 12 h. The product yield was improved when the reaction temperature was raised, being 92% at 45 °C and 94% at 65 °C (Table 1, entries 1–3). Further enhancing the reaction temperature, prolonging the reaction time or increasing the amount of the zinc reagent did not further improve the yields (Table 1, entries 4–6). Different solvents including a 1
1 mixture of THF and NMP with 0.01 mol% catalyst loading. It was found that the reaction could proceed at room temperature and gave the corresponding cross-coupling product in 63% yield in 12 h. The product yield was improved when the reaction temperature was raised, being 92% at 45 °C and 94% at 65 °C (Table 1, entries 1–3). Further enhancing the reaction temperature, prolonging the reaction time or increasing the amount of the zinc reagent did not further improve the yields (Table 1, entries 4–6). Different solvents including a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of THF and NMP, THF, a 2
1 mixture of THF and NMP, THF, a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of THF and toluene, and a 2
1 mixture of THF and toluene, and a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of THF and DMF were examined. The results showed that these solvents were less effective than the 2
1 mixture of THF and DMF were examined. The results showed that these solvents were less effective than the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of THF and NMP (Table 1, entries 7–10). We also noticed that the zinc reagent prepared from ZnCl2 and the corresponding Grignard reagent in the presence of 2 equiv. of LiCl gave better results than those prepared from ZnCl2 and an equiv. of p-MeOC6H4Li or p-MeOC6H4MgBr in the absence of a LiCl additive (Table 1, entries 11 and 12). This implies that both magnesium ions and lithium ions play important roles in the reaction. The counterion effect of the ammonium salts was also examined with I−, Br−, Cl−, and BF4−, respectively, as counterions in a 2
1 mixture of THF and NMP (Table 1, entries 7–10). We also noticed that the zinc reagent prepared from ZnCl2 and the corresponding Grignard reagent in the presence of 2 equiv. of LiCl gave better results than those prepared from ZnCl2 and an equiv. of p-MeOC6H4Li or p-MeOC6H4MgBr in the absence of a LiCl additive (Table 1, entries 11 and 12). This implies that both magnesium ions and lithium ions play important roles in the reaction. The counterion effect of the ammonium salts was also examined with I−, Br−, Cl−, and BF4−, respectively, as counterions in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of THF and NMP at 65 °C (Table 1, entries 13–16). The iodide salt exhibited the highest reactivity and the tetrafluoroborate salt showed the lowest reactivity. However, each of them was less reactive than the triflate salt. Complexes 5b and 5c exhibited a similar catalytic activity to complex 5a under comparable conditions. Complex 5b led to 93% product yield and complex 5c resulted in 91% product yield under the conditions as shown in Table 1 (entries 17 and 18). It seems that the substituents on the phosphorus atoms of the ligands affect the catalytic properties to a small extent.
1 mixture of THF and NMP at 65 °C (Table 1, entries 13–16). The iodide salt exhibited the highest reactivity and the tetrafluoroborate salt showed the lowest reactivity. However, each of them was less reactive than the triflate salt. Complexes 5b and 5c exhibited a similar catalytic activity to complex 5a under comparable conditions. Complex 5b led to 93% product yield and complex 5c resulted in 91% product yield under the conditions as shown in Table 1 (entries 17 and 18). It seems that the substituents on the phosphorus atoms of the ligands affect the catalytic properties to a small extent.
| Entry | X | Cat. (mol%) | Solvent | T (°C) | Yieldb (%) |
|---|---|---|---|---|---|
| a Unless otherwise specified, the reactions were carried out on a 0.5 mmol scale according to the conditions indicated by the above equation; 0.75 mmol p-MeOC6H4ZnCl was employed. p-MeOC6H4ZnCl was prepared from ZnCl2 and an equiv. of p-MeOC6H4MgBr in the presence of two equiv. of LiCl. b Isolated yield. c The reaction was run for 20 h. d 1.0 mmol p-MeOC6H4ZnCl was employed. e p-MeOC6H4ZnCl was prepared from ZnCl2 and an equiv. of p-MeOC6H4Li. f p-MeOC6H4ZnCl was prepared from ZnCl2 and an equiv. of p-MeOC6H4MgBr. | |||||
| 1 | OTf | 5a | THF–NMP (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
25 | 63 |
| 2 | OTf | 5a | THF–NMP (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
45 | 92 |
| 3 | OTf | 5a | THF–NMP (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
65 | 94 |
| 4 | OTf | 5a | THF–NMP (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
90 | 94 |
| 5 | OTf | 5a | THF–NMP (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
65 | 93c |
| 6 | OTf | 5a | THF–NMP (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
65 | 92d |
| 7 | OTf | 5a | THF–NMP (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
65 | 86 |
| 8 | OTf | 5a | THF | 65 | Trace |
| 9 | OTf | 5a | THF–Toluene (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
65 | Trace |
| 10 | OTf | 5a | THF–DMF (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
65 | 45 |
| 11 | OTf | 5a | THF–NMP (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
65 | 33e |
| 12 | OTf | 5a | THF–NMP (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
65 | 41f |
| 13 | I | 5a | THF–DMF (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
65 | 87 |
| 14 | Br | 5a | THF–NMP (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
65 | 74 |
| 15 | Cl | 5a | THF–NMP (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
65 | 76 |
| 16 | BF4 | 5a | THF–NMP (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
65 | 59 |
| 17 | OTf | 5b | THF–NMP (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
65 | 93 |
| 18 | OTf | 5c | THF–NMP (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
65 | 91 |
Complexes 5a–5c are much more active than the reported catalysts for the cross-coupling of aryltrimethylammonium salts with arylzinc reagents. For example, the reaction of phenyltrimethylammonium salts with p-MeOC6H4ZnCl catalyzed by complexes 1–4 or Ni(PCy3)2Cl2 requires 1–2 mol% catalyst loadings and 65–90 °C reaction temperature, giving 72–99% product yields.7e–h By contrast, the same reaction catalyzed by 5a requires only 0.01 mol% catalyst loading and 45–65 °C reaction temperature, giving 92–94% product yields (Table 1).
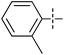
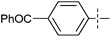
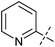
The substrate scope was tested using 5a as the catalyst under the optimized conditions. 1-Naphthyltrimethylammonium triflate is as active as phenyltrimethylammonium triflate in the catalytic reaction. Its reaction with either p-MeOC6H4ZnCl or p-Me2NC6H4ZnCl in the presence of 0.01 mol% 5a gave the corresponding cross-coupling products in excellent yields (Table 2, entries 1 and 2). The electron-rich aryltrimethylammonium triflates involving m-MeOC6H4NMe3+OTf−, m-PivOC6H4NMe3+OTf−, p-MeOC6H4NMe3+OTf−, and o-MeC6H4NMe3+OTf− also reacted smoothly with arylzinc chlorides under the catalysis of 5a (Table 2, entries 3–6). However, the reaction of p-MeOC6H4NMe3+OTf− required a higher catalyst loading (0.05 mol%) to reach completion compared with other substrates. The steric hindrance of the methyl group in o-MeC6H4NMe3+OTf− did not affect the reaction result; the reaction of o-MeC6H4NMe3+OTf− with p-MeOC6H4ZnCl in the presence of 0.01 mol% 5a led to 91% yield of 4′-methoxy-2-methylbiphenyl (Table 2, entry 6). However, more sterically hindered 2,4,6-Me3C6H2NMe3+OTf− showed much lower reactivity in comparison with o-MeC6H4NMe3+OTf−. Its reaction with p-MeOC6H4ZnCl using 0.01 mol% 5a as the catalyst gave only 37% yield of the cross-coupling product, and with p-Me2NC6H4ZnCl in the presence of 0.05 mol% 5a led to 60% yield of the desired product (Table 2, entries 7 and 8). The electron-deficient aryltrimethyl-ammonium triflates involving p-PhC(O)C6H4NMe3+OTf−, p-EtOC(O)C6H4NMe3+OTf−, p-Et2NC(O)C6H4NMe3+OTf−, p-CF3C6H4NMe3+OTf− and p-Et(O)2SC6H4NMe3+OTf− exhibited good reactivity in the 5a-catalyzed reactions with p-MeC6H4ZnCl, p-MeOC6H4ZnCl or p-Me2NC6H4ZnCl (Table 2, entries 9–17), affording the corresponding products in 76–99% yields. Among the reactions, the reaction of p-EtOC(O)C6H4NMe3+OTf− with p-MeC6H4ZnCl can be carried out at room temperature with 0.01 mol% catalyst loading; whereas the reaction of p-CF3C6H4NMe3+OTf− required some higher catalyst loadings (0.03 mol%). The reaction of o-MeC6H4ZnCl with electron-deficient electrophiles such as p-PhC(O)C6H4NMe3+OTf− and p-EtOC(O)C6H4NMe3+OTf− proceeded smoothly and both gave 99% yields of the cross-coupling products. It seems that the steric hindrance of the o-methyl group of o-MeC6H4ZnCl did not affect the reaction results. However, the reaction of electron-rich p-MeOC6H4NMe3+OTf− with o-MeC6H4ZnCl was more difficult to perform. The higher catalyst loading (0.1 mol%) was required and only 67% product yield was achieved. The electron-deficient zinc reagent, p-CF3C6H4ZnCl, was less reactive in the catalytic reaction. Its reaction with the activated aryltrimethylammonium salts proceeded smoothly with 0.01–0.05 mol% catalyst loadings, but resulted in little lower yields compared with those using p-MeC6H4ZnCl (Table 2, entries 23–25). The reaction of p-CF3C6H4ZnCl with m-PivOC6H4NMe3+OTf− also gave a good product yield when 0.05 mol% 5a was employed. However, the reaction with p-MeOC6H4NMe3+OTf− afforded poor results. It required the loading of 1 mol% 5a and gave only 39% product yield (Table 2, entry 27). The catalyst was also demonstrated to be applicable for the reaction of the heteroarylammonium substrate. The reaction of 2-pyridyltrimethylammonium triflate with either p-MeC6H4ZnCl or p-MeOC6H4ZnCl gave desired products in 79% and 81% yields, respectively, but the catalyst loadings were higher than those for electron-deficient substituted phenyl systems (Table 2, entries 28 and 29).
| Entry | Ar | Ar1ZnCl | 5a (mol%) | Yieldb (%) |
|---|---|---|---|---|
| a Unless otherwise specified, the reactions were carried out on a 0.5 mmol scale according to the conditions indicated by the above equation, 1.5 equiv. of ArZnCl were employed. The zinc reagents were prepared from ZnCl2 and an equiv. of Grignard reagents in the presence of two equiv. of LiCl. b Isolated yield. c The reaction was run at 100 °C. d The reaction was run at 25 °C. | ||||
| 1 |

|
p-MeOC6H4ZnCl | 0.01 | 93 |
| 2 |

|
p-Me2NC6H4ZnCl | 0.01 | 99 |
| 3 |

|
p-MeC6H4ZnCl | 0.01 | 95 |
| 4 |

|
p-MeC6H4ZnCl | 0.01 | 84 |
| 5 |

|
p-MeC6H4ZnCl | 0.05 | 87 |
| 6 |
|
p-MeOC6H4ZnCl | 0.01 | 91 |
| 7 |

|
p-MeOC6H4ZnCl | 0.01 | 37 |
| 8 |

|
p-Me2NC6H4ZnCl | 0.05 | 60c |
| 9 |

|
p-MeC6H4ZnCl | 0.01 | 96 |
| 10 |

|
p-MeOC6H4ZnCl | 0.01 | 93 |
| 11 |

|
p-MeC6H4ZnCl | 0.01 | 99d |
| 12 |

|
p-MeOC6H4ZnCl | 0.01 | 93 |
| 13 |

|
p-Me2NC6H4ZnCl | 0.01 | 99 |
| 14 |

|
p-MeC6H4ZnCl | 0.01 | 81 |
| 15 |

|
p-MeOC6H4ZnCl | 0.01 | 88 |
| 16 |
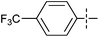
|
p-MeC6H4ZnCl | 0.03 | 92 |
| 17 |

|
p-MeOC6H4ZnCl | 0.03 | 87 |
| 18 |

|
p-MeC6H4ZnCl | 0.01 | 82 |
| 19 |

|
p-MeOC6H4ZnCl | 0.01 | 76 |
| 20 |

|
o-MeC6H4ZnCl | 0.01 | 99 |
| 21 |
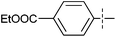
|
o-MeC6H4ZnCl | 0.01 | 99 |
| 22 |
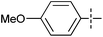
|
o-MeC6H4ZnCl | 0.1 | 67 |
| 23 |
|
p-CF3C6H4ZnCl | 0.01 | 81 |
| 24 |

|
p-CF3C6H4ZnCl | 0.05 | 82 |
| 25 |

|
p-CF3C6H4ZnCl | 0.05 | 79 |
| 26 |

|
p-CF3C6H4ZnCl | 0.05 | 80 |
| 27 |
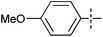
|
p-CF3C6H4ZnCl | 1 | 39 |
| 28 |

|
p-MeC6H4ZnCl | 0.5 | 79 |
| 29 |
|
p-MeOC6H4ZnCl | 0.5 | 81 |
Two electron-rich heteroarylzinc reagents, 2-furylzinc chloride and 2-thienylzinc chloride, were also used in the coupling (Table 3). 2-Furyllithium and 2-thienyllithium are more convenient to prepare than the corresponding magnesium reagents. Hence the zinc reagents were prepared from the heteroaryllithiums and ZnCl2. The reactions using the zinc reagents showed relatively low reaction efficiency (Table 3, entries 1–7). For example, their reactions required higher catalyst loadings and often higher reaction temperatures in comparison with those listed in Table 2. However, one equiv. of MgBr2 and one equiv. of LiCl additives markedly improved the reactions (Table 3, entries 8, 9, 11 and 12). MgBr2 alone as an additive was less effective than the combination of MgBr2 and LiCl (Table 3, entry 10). 2-Thienylzinc chloride exhibited lower reactivity than 2-furylzinc chloride even in the presence of additives, the former requiring higher catalyst loading, higher reaction temperature and gave lower product yields.
| Entry | Ar | HetArZnCl | 5a (mol%) | Yieldb (%) |
|---|---|---|---|---|
| a Unless otherwise specified, the reactions were carried out on a 0.5 mmol scale according to the conditions indicated by the above equation, 1.5 equiv. of ArZnCl were employed. The heteroarylzinc chlorides were prepared from ZnCl2 and an equiv. of heteroaryllithium. b Isolated yield. c The reaction was run at 90 °C. d One equiv. of MgBr2 and one equiv. of LiCl were added. e One equiv. of MgBr2 was added. | ||||
| 1 |
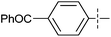
|

|
1 | 86 |
| 2 |

|

|
1 | 80 |
| 3 |
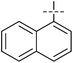
|

|
1 | 77c |
| 4 |
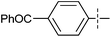
|

|
1 | 41 |
| 5 |

|

|
2 | 45c |
| 6 |
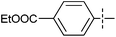
|

|
2 | 72c |
| 7 |
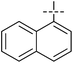
|

|
2 | 86 |
| 8 |

|

|
0.05 | 90d |
| 9 |

|
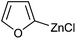
|
0.05 | 88d |
| 10 |

|

|
0.05 | 59e |
| 11 |

|

|
0.5 | 72c,d |
| 12 |
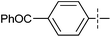
|

|
0.5 | 56c,d |
This experimental fact on the role of additives further shows that both magnesium and lithium ions are important for the success of reactions. The effect of inorganic salt additives for the Negishi reaction has been reported by several groups.9 Recently, studies on the reactivity of RZnCl in the presence of LiCl and MgX2 or of RMgX in the presence of LiCl and ZnCl2 were also reported.10 The enhancement of the reactivity of the zinc reagents by using inorganic salt additives may result from the breaking-down of the ArZnX aggregation through formation of the adduct.9g Whereas in the three-component system Hevia et al. attributed the dramatic increase in the chemoselectivity of the reactions to the existence of a trilateral Li/Mg/Zn synergistic effect.10a In our reaction, the low reactivity of the zinc reagent prepared from a Grignard reagent and ZnCl2 should be due to the aggregation of the arylzinc reagent with the coproduct MgCl2.11 One of the roles of LiCl might be to break the aggregation by forming trimetallic species.10a,b On the other hand, LiCl and MgX2 are likely to enhance the reactivity of ArZnCl by forming the more nucleophilic zincates.9,10a
The exact catalytic cycle is still unknown at present. However, some experimental facts have been derived. The yield of the reaction between PhNMe3+OTf− and p-MeOC6H4ZnCl did not change in the presence of a drop of Hg. This result ruled out the possibility of nickel particles or colloids formed from the decomposition of the nickel complex, as the catalyst.12 The catalytic reaction is inhibited by 1,1-diphenylethene. When 10 mol% of 1,1-diphenylethene was added into the reaction system composed of PhNMe3+OTf−, p-MeOC6H4ZnCl and 0.01 mol% or 1 mol% 5a, no desired cross-coupling product was observed. We thought that the reaction may involve a radical intermediate based on the result. However, we could not confirm the process because the reaction of 1,1-diphenylethene with active nickel species formed in the process of the reaction may also give rise to inactivation of the catalyst. On the other hand, a combination of Ni(COD)2 and [Li{N(2-Ph2PC6H4)(2′-Me2NC6H4)}] exhibited a similar catalytic activity to 5a; 0.01 mol% catalyst loading resulted in 90% product yield for the cross-coupling of PhNMe3+OTf− with p-MeOC6H4ZnCl. This catalytic reaction was also inhibited by the 1,1-diphenylethene additive (10 mol%). Hence the active catalyst may be a Ni(0) species. But we cannot rule out the possibility of a Ni(I) active intermediate13 formed through an electron transfer from Ni(0) to ArNMe3+OTf−.14 Additional studies on the reaction mechanism are underway.
Conclusions
We have demonstrated that N,N,P-pincer nickel complexes, [Ni(Cl){N(2-R2PC6H4)(2′-Me2NC6H4)}] (R = Ph, Pri or Cy) are highly efficient catalysts for the cross-coupling of aryltrimethylammonium triflates and (hetero)arylzinc reagents. In most cases a very small amount of the catalyst and mild reaction conditions are required. The reaction is suitable for a wide scope of substrates, covering electron-rich and electron-deficient electrophiles and nucleophiles. A range of functional groups involving PhC(O), EtOC(O), Et2NC(O), CF3, EtSO2, PivO, OMe, and NMe2 can be tolerated. In comparison with the N,N,N- and P,N,P-pincer nickel complexes [Ni(Cl){N(2-Me2NC6H4)2}] and [Ni(Cl){N(2-R2PC6H4)2}] (R = Ph, Pri or Cy) reported previously,15,16 the N,N,P-pincer nickel complexes 5a–5c feature the aryl C–Cl, C–F or C–N bond activation.3d The N,N,N-pincer nickel complex [Ni(Cl){N(2-Me2NC6H4)2}] is an excellent catalyst for the sp3 C–halide bond activation of alkyl halides; and the P,N,P-pincer nickel complexes [Ni(Cl){N(2-R2PC6H4)2}] can activate only the C–Br or C–I bonds of aryl halides rather than the unreactive C–Cl or C–F bonds. The difference in the catalytic properties of these complexes may result from the electronic effects of the ligands because the N,N,N-pincer ligand is “harder” compared with the “softer” P,N,P-pincer ligands.15c This difference in coordination properties of the ligands may also lead to a different reaction mechanism in the nickel-complex-catalyzed cross-coupling reactions.Experimental section
All reactions were performed under a nitrogen atmosphere using standard Schlenk and vacuum line techniques. THF was distilled under nitrogen over sodium/benzophenone and degassed prior to use. Toluene was distilled under nitrogen over sodium and degassed prior to use. NMP and DMF were dried over 4 Å molecular sieves, fractionally distilled under reduced pressure and stored under a nitrogen atmosphere. LiBun and methyl triflate were purchased from Acros Organics and used as received. CDCl3 was purchased from Cambridge Isotope Laboratories and used as received. Aryldimethylamines were obtained from commercial vendors and purified by distillation under reduced pressure or recrystallization prior to use. Aryltrimethylammonium triflates,7d Grignard reagents,17 2-furyllithium,18 and 2-thienyllithium19 were prepared according to literature procedures. NMR spectra were recorded on a Bruker Avance III 400 NMR spectrometer at room temperature. The chemical shifts of the 1H NMR spectra were referenced to a TMS or solvent residual signal; the chemical shifts of the 13C NMR spectra were referenced to the internal solvent resonances. High-resolution mass spectra (HRMS) were acquired using a Thermo Orbitrap XL ETD mass spectrometer (APCI).General procedure for the cross-coupling of aryltrimethyl-ammonium triflates
A Schlenk tube was charged with aryltrimethylammonium triflate (0.5 mmol), NMP (0.80 cm3) and a solution of complex 5a (0.1 cm3, 0.0005 M solution in THF, 0.00005 mmol). To the stirred mixture ArZnCl solution (1.5 cm3, 0.5 M solution in THF, 0.75 mmol) was added by using a syringe. The reaction mixture was stirred at 65 °C for 12 h. Water (10 cm3) and several drops of hydrochloric acid were successively added. The mixture was extracted with Et2O (3 × 10 cm3). The combined organic phases were dried over anhydrous Na2SO4, concentrated by rotary evaporation, and purified by column chromatography (silica gel).Product characterization
Acknowledgements
This research was supported by the National Natural Science Foundation of China (grant no. 21372212) and the National Basic Research Program of China (grant no. 2015CB856600).Notes and references
-
(a)
Metal-Catalyzed Cross-Coupling Reactions, ed. F. Diederich and P. J. Stang, Wiley-VCH, Weinheim, 1998 Search PubMed
; (b) Metal-Catalyzed Cross-Coupling Reactions, ed. A. de Meijere and F. Diederich, Wiley-VCH, Weinheim, 2nd edn, 2004 Search PubMed
.
-
(a) J. A. Miller and R. P. Farrell, Tetrahedron Lett., 1998, 39, 6441 CrossRef CAS
; (b) S. P. Stanforth, Tetrahedron, 1998, 54, 263 CrossRef CAS
; (c) J. Hassan, M. Sévignon, C. Gozzi, E. Schulz and M. Lemaire, Chem. Rev., 2002, 102, 1359 CrossRef CAS PubMed
; (d) J.-P. Corbet and G. Mignani, Chem. Rev., 2006, 106, 2651 CrossRef CAS PubMed
; (e) C. Wolf and H. Xu, J. Org. Chem., 2008, 73, 162 CrossRef CAS PubMed
; (f) R. Jana, T. P. Pathak and M. S. Sigman, Chem. Rev., 2011, 111, 1417 CrossRef CAS PubMed
; (g) L.-M. Yang, L.-F. Huang and T.-Y. Luh, Org. Lett., 2004, 6, 1461 CrossRef CAS PubMed
; (h) M. Shi, L.-P. Liu and T. Jie, J. Org. Chem., 2005, 70, 10420 CrossRef CAS PubMed
; (i) R. Martin and S. L. Buchwald, J. Am. Chem. Soc., 2007, 129, 3844 CrossRef CAS PubMed
; (j) L. Ackermann, H. Potukuchi, A. Kapdi and C. Schulzke, Chem. – Eur. J., 2010, 16, 3300 CrossRef CAS PubMed
.
-
(a) C. Dai and G. C. Fu, J. Am. Chem. Soc., 2001, 123, 2719 CrossRef CAS PubMed
; (b) L. Ackermann, R. Born, J. H. Spatz, A. Althammer and C. J. Gschrei, Pure Appl. Chem., 2006, 78, 209 CrossRef CAS
; (c) L. Li, S. Zhao, A. Joshi-Pangu, M. Diane and M. R. Biscoe, J. Am. Chem. Soc., 2014, 136, 14027 CrossRef CAS PubMed
; (d) D. Wu and Z.-X. Wang, Org. Biomol. Chem., 2014, 12, 6414 RSC
; (e) M. Giannerini, V. Hornillos, C. Vila, M. Fananas-Mastral and B. L. Feringa, Angew. Chem., Int. Ed., 2013, 52, 13329 CrossRef CAS PubMed
; (f) X. L. Hu, Chimia, 2012, 66, 154 CrossRef CAS PubMed
; (g) C. W. K. Gstöttmayr, V. P. W. Böhm, E. Herdtweck, M. Grosche and W. A. Herrmann, Angew. Chem., Int. Ed., 2002, 41, 1363 CrossRef
; (h) R. Martin and S. L. Buchwald, Acc. Chem. Res., 2008, 41, 1461 CrossRef CAS PubMed
; (i) G. C. Fortman and S. P. Nolan, Chem. Soc. Rev., 2011, 40, 5151 RSC
; (j) C. A. Fleckensteinab and H. Plenio, Chem. Soc. Rev., 2010, 39, 694 RSC
; (k) V. B. Phapale and D. J. Cárdenas, Chem. Soc. Rev., 2009, 38, 1598 RSC
; (l) Z.-X. Wang and N. Liu, Eur. J. Inorg. Chem., 2012, 901 CrossRef CAS
.
-
(a) B. M. Rosen, K. W. Quasdorf, D. A. Wilson, N. Zhang, A.-M. Resmerita, N. K. Garg and V. Percec, Chem. Rev., 2011, 111, 1346 CrossRef CAS PubMed
; (b) B.-J. Li, D.-G. Yu, C.-L. Sun and Z.-J. Shi, Chem. – Eur. J., 2011, 17, 1728 CrossRef CAS PubMed
.
-
(a) K. W. Quasdorf, X. Tian and N. K. Garg, J. Am. Chem. Soc., 2008, 130, 14422 CrossRef CAS PubMed
; (b) B.-T. Guan, Y. Wang, B.-J. Li, D.-G. Yu and Z.-J. Shi, J. Am. Chem. Soc., 2008, 130, 14468 CrossRef CAS PubMed
; (c) A. Antoft-Finch, T. Blackburn and V. Snieckus, J. Am. Chem. Soc., 2009, 131, 17750 CrossRef CAS PubMed
; (d) H. Chen, Z. Huang, X. Hu, G. Tang, P. Xu, Y. Zhao and C.-H. Cheng, J. Org. Chem., 2011, 76, 2338 CrossRef CAS PubMed
; (e) F. Zhao, Y.-F. Zhang, J. Wen, D.-G. Yu, J.-B. Wei, Z. Xi and Z.-J. Shi, Org. Lett., 2013, 15, 3230 CrossRef CAS PubMed
; (f) D.-G. Yu, B.-J. Li and Z.-J. Shi, Acc. Chem. Res., 2010, 43, 1486 CrossRef CAS PubMed
.
-
(a) T. Mesganaw and N. K. Garg, Org. Process Res. Dev., 2013, 17, 29 CrossRef CAS
; (b) F.-S. Han, Chem. Soc. Rev., 2013, 42, 5270 RSC
; (c) G.-J. Chen and F.-S. Han, Eur. J. Org. Chem., 2012, 3575 CrossRef CAS
; (d) K. W. Quasdorf, M. Riener, K. V. Petrova and N. K. Garg, J. Am. Chem. Soc., 2009, 131, 17748 CrossRef CAS PubMed
; (e) K. W. Quasdorf, A. Antoft-Finch, P. Liu, A. L. Silberstein, A. Komaromi, T. Blackburn, S. D. Ramgren, K. N. Houk, V. Snieckus and N. K. Garg, J. Am. Chem. Soc., 2011, 133, 6352 CrossRef CAS PubMed
; (f) M. Baghbanzadeh, C. Pilger and C. O. Kappe, J. Org. Chem., 2011, 76, 1507 CrossRef CAS PubMed
; (g) P. Leowanawat, N. Zhang and V. Percec, J. Org. Chem., 2012, 77, 1018 CrossRef CAS PubMed
; (h) P. Leowanawat, N. Zhang, M. Safi, D. J. Hoffman, M. C. Fryberger, A. George and V. Percec, J. Org. Chem., 2012, 77, 2885 CrossRef CAS PubMed
; (i) N. Zhang, D. J. Hoffman, N. Gutsche, J. Gupta and V. Percec, J. Org. Chem., 2012, 77, 5956 CrossRef CAS PubMed
; (j) T. K. Macklin and V. Snieckus, Org. Lett., 2005, 7, 2519 CrossRef CAS PubMed
; (k) T. M. Gøgsig, A. T. Lindhardt and T. Skrydstrup, Org. Lett., 2009, 11, 4886 CrossRef PubMed
; (l) A. L. Silberstein, S. D. Ramgren and N. K. Garg, Org. Lett., 2012, 14, 3796 CrossRef CAS PubMed
; (m) S. D. Ramgren, A. L. Silberstein, Y. Yang and N. K. Garg, Angew. Chem., Int. Ed., 2011, 50, 2171 CrossRef CAS PubMed
; (n) L. Ackermann, R. Sandmann and W. Song, Org. Lett., 2011, 13, 1784 CrossRef CAS PubMed
; (o) L. Hie, S. D. Ramgren, T. Mesganaw and N. K. Garg, Org. Lett., 2012, 14, 4182 CrossRef CAS PubMed
; (p) N. H. Park, G. Teverovskiy and S. L. Buchwald, Org. Lett., 2014, 16, 220 CrossRef CAS PubMed
; (q) W. Song and L. Ackermann, Angew. Chem., Int. Ed., 2012, 51, 8251 CrossRef CAS PubMed
; (r) L. Ackermann, S. Barfüsser and J. Pospech, Org. Lett., 2010, 12, 724 CrossRef CAS PubMed
; (s) L. Xu, B.-J. Li, Z.-H. Wu, X.-Y. Lu, B.-T. Guan, B.-Q. Wang, K.-Q. Zhao and Z.-J. Shi, Org. Lett., 2010, 12, 884 CrossRef CAS PubMed
; (t) J. F. Cívicos, M. Gholinejad, D. A. Alonso and C. Nájera, Chem. Lett., 2011, 40, 907 CrossRef
; (u) B.-J. Li, Y.-Z. Li, X.-Y. Lu, J. Liu, B.-T. Guan and Z.-J. Shi, Angew. Chem., Int. Ed., 2008, 47, 10124 CrossRef CAS PubMed
.
-
(a) E. Wenkert, A.-L. Han and C.-J. Jenny, J. Chem. Soc., Chem. Commun., 1988, 975 RSC
; (b) S. B. Blakey and D. W. C. MacMillan, J. Am. Chem. Soc., 2003, 125, 6046 CrossRef CAS PubMed
; (c) K. R. Buszek and N. Brown, Org. Lett., 2007, 9, 707 CrossRef CAS PubMed
; (d) J. T. Reeves, D. R. Fandrick, Z. Tan, J. J. Song, H. Lee, N. K. Yee and C. H. Senanayake, Org. Lett., 2010, 12, 4388 CrossRef CAS PubMed
; (e) L.-G. Xie and Z.-X. Wang, Angew. Chem., Int. Ed., 2011, 50, 4901 CrossRef CAS PubMed
; (f) Q. Zhang, X.-Q. Zhang and Z.-X. Wang, Dalton Trans., 2012, 41, 10453 RSC
; (g) X.-Q. Zhang and Z.-X. Wang, J. Org. Chem., 2012, 77, 3658 CrossRef CAS PubMed
; (h) X. Yang and Z.-X. Wang, Organometallics, 2014, 33, 5863 CrossRef CAS
; (i) W.-J. Guo and Z.-X. Wang, Tetrahedron, 2013, 69, 9580 CrossRef CAS PubMed
; (j) X.-Q. Zhang and Z.-X. Wang, Org. Biomol. Chem., 2014, 12, 1448 RSC
; (k) P. Maity, D. M. Shacklady-McAtee, G. P. A. Yap, E. R. Sirianni and M. P. Watson, J. Am. Chem. Soc., 2013, 135, 280 CrossRef CAS PubMed
; (l) D. M. Shacklady-McAtee, K. M. Roberts, C. H. Basch, Y.-G. Song and M. P. Watson, Tetrahedron, 2014, 70, 4257 CrossRef CAS PubMed
.
-
(a) A. Roglans, A. Pla-Quintana and M. Moreno-Mañas, Chem. Rev., 2006, 106, 4622 CrossRef CAS PubMed
; (b) H. Bonin, E. Fouquet and F.-X. Felpinb, Adv. Synth. Catal., 2011, 353, 3063 CrossRef CAS
; (c) E. W. Werner and M. S. Sigman, J. Am. Chem. Soc., 2011, 133, 9692 CrossRef CAS PubMed
; (d) G. Fabrizi, A. Goggiamani, A. Sferrazza and S. Cacchi, Angew. Chem., Int. Ed., 2010, 49, 4067 CrossRef CAS PubMed
; (e) S. Cacchi, G. Fabrizi, A. Goggiamani and D. Persiani, Org. Lett., 2008, 10, 1597 CrossRef CAS PubMed
; (f) K. Cheng, C. Wang, Y. Ding, Q. Song, C. Qi and X.-M. Zhang, J. Org. Chem., 2011, 76, 9261 CrossRef CAS PubMed
; (g) F. Mo, Y. Jiang, D. Qiu, Y. Zhang and J. Wang, Angew. Chem., Int. Ed., 2010, 49, 1846 CrossRef CAS PubMed
; (h) X.-F. Wu, H. Neumann and M. Beller, Angew. Chem., Int. Ed., 2011, 50, 11142 CrossRef CAS PubMed
; (i) F. L. Callonnec, E. Fouquet and F.-X. Felpin, Org. Lett., 2011, 13, 2646 CrossRef PubMed
; (j) X.-F. Wu, H. Neumann and M. Beller, Chem. Commun., 2011, 47, 7959 RSC
.
-
(a) H. Ochiai, M. Jang, K. Hirano, H. Yorimitsu and K. Oshima, Org. Lett., 2008, 10, 2681 CrossRef CAS PubMed
; (b) N. Hadei, G. T. Achonduh, C. Valente, C. J. O'Brien and M. G. Organ, Angew. Chem., Int. Ed., 2011, 50, 3896 CrossRef CAS PubMed
; (c) L. C. McCann, H. N. Hunter, J. A. C. Clyburne and M. G. Organ, Angew. Chem., Int. Ed., 2012, 51, 7024 CrossRef CAS PubMed
; (d) H. N. Hunter, N. Hadei, V. Blagojevic, P. Patschinski, G. Achonduh, S. Avola, D. K. Bohme and M. G. Organ, Chem. – Eur. J., 2011, 17, 7845 CrossRef CAS PubMed
; (e) G. T. Achonduh, N. Hadei, C. Valente, S. Avola and C. J. O'Brien, Chem. Commun., 2010, 46, 4109 RSC
; (f) K. Koszinowski and P. Böhrer, Organometallics, 2009, 28, 771 CrossRef CAS
; (g) L. C. McCann and M. G. Organ, Angew. Chem., Int. Ed., 2014, 53, 4386 CrossRef CAS PubMed
.
-
(a) D. R. Armstrong, W. Clegg, P. García-Álvarez, A. R. Kennedy, M. D. McCall, L. Russo and E. Hevia, Chem. – Eur. J., 2011, 17, 8333 CrossRef CAS PubMed
; (b) M. Hatano, O. Ito, S. Suzuki and K. Ishihara, J. Org. Chem., 2010, 75, 5008 CrossRef CAS PubMed
; (c) M. Hatano, O. Ito, S. Suzuki and K. Ishihara, Chem. Commun., 2010, 46, 2674 RSC
; (d) L.-G. Xie and Z.-X. Wang, Chem. – Eur. J., 2010, 16, 10332 CrossRef CAS PubMed
.
- E. Hevia, J. Z. Chua, P. Garcia-Alvarez, A. R. Kennedy and M. D. McCall, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 5294 CrossRef CAS PubMed
.
-
(a) X. Hu, Chem. Sci., 2011, 2, 1867 RSC
; (b) K. Inamoto, J.-i. Kuroda, K. Hiroya, Y. Noda, M. Watanabe and T. Sakamoto, Organometallics, 2006, 25, 3095 CrossRef CAS
.
-
(a) V. B. Phapale and D. J. Cárdenas, Chem. Soc. Rev., 2009, 38, 1598 RSC
; (b) S. Z. Tasker, E. A. Standley and T. F. Jamison, Nature, 2014, 509, 299 CrossRef CAS PubMed
; (c) A. N. Vedernikov, ChemCatChem, 2014, 6, 2490 CrossRef CAS
; (d) J. Cornella, E. Gómez-Bengoa and R. Martin, J. Am. Chem. Soc., 2013, 135, 1997 CrossRef CAS PubMed
.
- T. T. Tsou and J. K. Kochi, J. Am. Chem. Soc., 1979, 101, 6319 CrossRef CAS
.
-
(a) X. Hu, Chimia, 2010, 64, 231 CrossRef CAS
; (b) Z. Csok, O. Vechorkin, S. B. Harkins, R. Scopelliti and X. Hu, J. Am. Chem. Soc., 2008, 130, 8156 CrossRef CAS PubMed
; (c) O. Vechorkin, Z. Csok, R. Scopelliti and X. Hu, Chem. – Eur. J., 2009, 15, 3889 CrossRef CAS PubMed
.
- L.-C. Liang, P.-S. Chien, J.-M. Lin, M.-H. Huang, Y.-L. Huang and J.-H. Liao, Organometallics, 2006, 25, 1399 CrossRef CAS
.
- L. F. Elson, A. McKillop and E. C. Taylor, Org. Synth., 1976, 55, 48 CrossRef
.
- H. W. Gschwend and H. R. Rodriguez, Org. React., 1979, 26, 1 CAS
.
- E. Jones and I. M. Moodie, Org. Synth., 1970, 50, 104 CrossRef CAS
.
- M. Shi and H. X. Qian, Tetrahedron, 2005, 61, 4949 CrossRef CAS PubMed
.
- H. Gao, Y. Li, Y.-G. Zhou, F.-S. Han and Y.-J. Lin, Adv. Synth. Catal., 2011, 353, 309 CrossRef CAS
.
- C. Desmarets, R. Omar-Amrani, A. Walcarius, J. Lambert, B. Champagne, Y. Fort and R. Schneider, Tetrahedron, 2008, 64, 372 CrossRef CAS PubMed
.
- L. J. Goossen and T. Knauber, J. Org. Chem., 2008, 73, 8631 CrossRef CAS PubMed
.
- W.-J. Guo and Z.-X. Wang, J. Org. Chem., 2013, 78, 1056 Search PubMed
.
- T. Hatakeyama, S. Hashimoto, K. Ishizuka and M. Nakamura, J. Am. Chem. Soc., 2009, 131, 11949 CrossRef CAS PubMed
.
- J. L. Bolliger and C. M. Frech, Chem. – Eur. J., 2010, 16, 11072 CrossRef CAS PubMed
.
- M. Gomberg and J.-C. Pernert, J. Am. Chem. Soc., 1926, 48, 1372 CrossRef CAS
.
- M. B. Andrus and C. Song, Org. Lett., 2001, 3, 3762 Search PubMed
.
- E. Berliner and L.-H. Liu, J. Am. Chem. Soc., 1953, 75, 2417 CrossRef CAS
.
- K. Ueura, T. Satoh and M. Miura, Org. Lett., 2005, 7, 2229 CrossRef CAS PubMed
.
- L. Wang and Z.-X. Wang, Org. Lett., 2007, 9, 4335 CrossRef CAS PubMed
.
- H. Nakamura, C. Wu, S. Inouye and A. Murai, J. Am. Chem. Soc., 2011, 123, 1523 CrossRef
.
- N. Liu, L. Wang and Z.-X. Wang, Chem. Commun., 2011, 47, 1598 RSC
.
- M. Amatore and C. Gosmini, Angew. Chem., Int. Ed., 2008, 47, 2089 CrossRef CAS PubMed
.
- G. A. Molander and F. Beaumard, Org. Lett., 2010, 12, 4022 CrossRef CAS PubMed
.
- J. S. Meek, R. T. Merrow and S. J. Cristol, J. Am. Chem. Soc., 1952, 74, 2667 CrossRef CAS
.
- L. Ackermann, H. Potukuchi, A. Kapdi and C. Schulzke, Chem. – Eur. J., 2010, 16, 3300 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available: 1H and 13C NMR copies of all products. See DOI: 10.1039/c4qo00321g |
| This journal is © the Partner Organisations 2015 |




