Rhodium-catalyzed synthesis of multi-substituted furans from N-sulfonyl-1,2,3-triazoles bearing a tethered carbonyl group†
Wen-Biao
Zhang
,
Shi-Dong
Xiu
and
Chuan-Ying
Li
*
Department of Chemistry, Zhejiang Sci-Tech University, Xiasha West Higher Education District, Hangzhou 310018, China. E-mail: licy@zstu.edu.cn
First published on 28th November 2014
Abstract
The efficient synthesis of highly functionalized furan derivatives from 1-sulfonyl-1,2,3-triazoles with a pendent carbonyl group is reported. The process involves an intramolecular trapping of α-imino carbene and subsequent aromatization.
Functionalized furans occur in a variety of biologically active natural products, pharmaceuticals and functional materials.1 Moreover, they are versatile building blocks for both heterocyclic and acyclic compounds.2 For this reason, the synthesis of multi-substituted furans continues to be a hot topic in modern synthetic chemistry.3 In addition to traditional methods,4 transition-metal-catalyzed reactions5 and organocatalytic approaches6 have been reported during the last several decades. Despite all the achievements, the development of novel and convenient methods for the synthesis of functionalized furans with readily accessible compounds is of great importance.
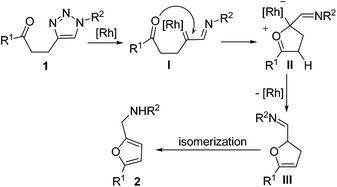
As one of the most important intermediates in organic chemistry, metal carbenes were traditionally prepared by the decomposition of diazo compounds and N-tosylhydrazones.7 In recent years, the application of alkynes as carbene precursors has attracted considerable attention.8 1-Sulfonyl-1,2,3-triazole, which can be easily prepared by CuAAC reaction,9 was used in the formation of α-imino carbene by Fokin and Gevorgyan in 2008.10 Since then, investigation of α-imino carbene derived from N-sulfonyl triazoles has led to the development of a variety of synthetic transformations.11 Cycloaddition,12 ylide formation,13 ring expansion,14 and C–H or heteroatom–H insertion15 were applied in the synthesis of heterocycles or acyclic compounds. It is worth noting that in most cases, the nitrogen atom of the imino group was included in the newly formed heterocycles; the synthesis of furans or thiophenes have not been realized in this area.16 We envisioned that if we use a tethered carbonyl group to trap the α-imino carbene intermediate, furan with a tosylaminomethylene group would be formed after isomerization (Scheme 1). The tosylamino group can be used to introduce other functional groups, which offers more opportunities for the synthesis of complex cyclic compounds.
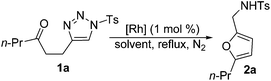
As illustrated in Table 1, the yield of 2a was found to be highly dependent on both the dirhodium catalyst and the solvent. The treatment of a solution of triazole 1a in refluxing DCE with 1 mol% of Rh2(OAc)4 afforded 2,5-disubstituted furan 2a in 80% yield (entry 1). To our surprise, attempts to use Rh2(oct)4 as the catalyst led to only trace furan product (entry 2). The use of Rh2(esp)2 provided the desired product in 89% yield (entry 3). No further improvement was achieved when other catalysts such as Rh2(Piv)4, Rh2(S-pttl)4 or Rh2(S-nttl)4 were used (entries 4–6). Reactions performed in other solvents (entries 7–11) resulted in decreased yield.
| Entry | Catalyst | Solvent | t (h) | Yieldb (%) |
|---|---|---|---|---|
| a 0.2 mmol of 1a and 0.002 mmol of the rhodium(II) catalyst were dissolved in 2 mL solvent. b Isolated yield, average of two runs. | ||||
| 1 | Rh2(OAc)4 | DCE | 1.0 | 80 |
| 2 | Rh2(oct)4 | DCE | 1.0 | Trace |
| 3 | Rh2(esp)2 | DCE | 1.0 | 89 |
| 4 | Rh2(Piv)4 | DCE | 1.0 | 55 |
| 5 | Rh2(S-pttl)4 | DCE | 3.0 | 60 |
| 6 | Rh2(S-nttl)4 | DCE | 3.0 | 62 |
| 7 | Rh2(esp)2 | Toluene | 1.0 | 79 |
| 8 | Rh2(esp)2 | CHCl3 | 3.0 | Trace |
| 9 | Rh2(esp)2 | THF | 3.0 | Trace |
| 10 | Rh2(esp)2 | MeCN | 3.0 | Trace |
| 11 | Rh2(esp)2 | ClCH2CHCl2 | 3.0 | Trace |
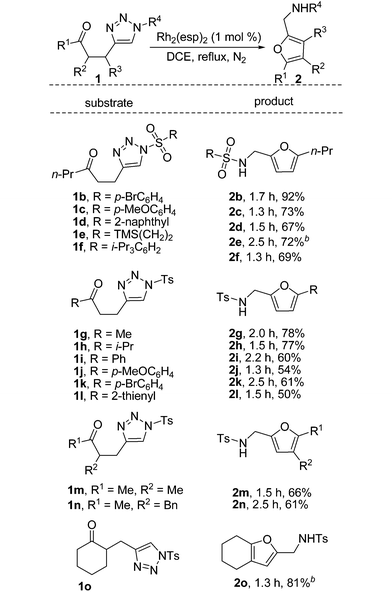
Examination of the reaction scope with respect to the sulfonyl group of the triazole (Table 2, 1b, 1c, 1d, 1e and 1f) revealed that both the electronic nature and the steric hindrance of the substituents have great impact on the reaction. The corresponding furan products were obtained in yields ranging from 67% to 92%. Both arylsulfonyl groups and alkylsulfonyl groups were suitable for this transformation. As for the substituents of the carbonyl group, reaction of 1g and 1h bearing an alkyl chain provided 2,5-disubstituted furans 2g and 2h in good yield, whereas substrates 1i, 1j, 1k and 1l led to the aryl substituted furan in moderate yield. 2,3,5-Trisubstituted furans 2m and 2n were obtained in 66% and 61% yield, respectively, when substrates with a methyl or benzyl group at the α-position of the carbonyl group were used. Substrate 1o can be easily synthesized from cyclohexanone in two steps, and when treated with 1 mol% of Rh2(OAc)4, fused furan 2o was obtained in 81% yield. Of note is that 1,2-H migration products have never been observed in these transformations.
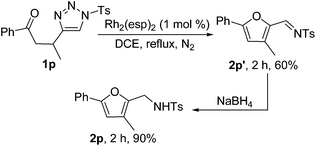
Interestingly, when substrate 1p, which bears a methyl group at the β-position of the carbonyl group, was treated with Rh2(esp)2 in refluxing DCE, furan 2p′ was obtained in 60% yield. 2p′ could be reduced to 2p smoothly by NaBH4 (Scheme 2). We believe that the mechanism of formation of 2p′ is similar to the mechanism proposed by Lin15d and the presence of a methyl group facilitates the elimination of hydride.
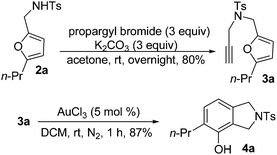
Next, we highlighted the synthetic potential of this transformation with an efficient synthesis of phenol 4a. Treatment of 2a with propargyl bromide led to the formation of 3a, which underwent gold-catalyzed rearrangement to furnish phenol 4a in 87% yield17 (Scheme 3).
Conclusions
In summary, intramolecular trapping of α-imino rhodium carbene with a carbonyl group has been realized; functional furan compounds with different substitution patterns were obtained in high yield. The synthetic utility of this transformation was exemplified by a gold-catalyzed phenol synthesis.Acknowledgements
This work was generously supported by the National Natural Science Foundation of China (21002091, 21372204) and the Zhejiang Sci-Tech University 521 project.Notes and references
- (a) B. A. Keay and P. W. Dibble, Furans and their Benzo Derivatives: Applications, in Comprehensive Heterocyclic Chemistry II, ed. A. R. Katritzky, C. W. Rees and E. F. V. Scriven, Pergamon Press, Oxford, UK, 1996, vol. 2, p. 395 Search PubMed; (b) G. Yin, Z. Wang, A. Chen, M. Gao, A. Wu and Y. Pan, J. Org. Chem., 2008, 73, 3377 CrossRef CAS PubMed; (c) Y. Wang, Y.-C. Luo, X.-Q. Hu and P.-F. Xu, Org. Lett., 2011, 13, 5346 CrossRef CAS PubMed; (d) E. Ripaud, D. Demeter, T. Rousseau, E. Boucard-Cetol, M. Allain, R. Po, P. Leriche and J. Roncali, Dyes Pigm., 2012, 95, 126 CrossRef CAS PubMed.
- For reviews, see: (a) M. Maier, in Organic Synthesis Highlights II, ed. H. Waldmann, VCH, Weinheim, 1995, p. 231 Search PubMed; (b) B. H. Lipshutz, Chem. Rev., 1986, 86, 795 CrossRef CAS. For selected examples, see: (c) A. S. K. Hashmi, J. P. Weyrauch, E. Kurpejovic, T. M. Frost, B. Miehlich, W. Frey and J. W. Bats, Chem. – Eur. J., 2006, 12, 5806 CrossRef CAS PubMed; (d) F. Mo, J. M. Yan, D. Qiu, F. Li, Y. Zhang and J. Wang, Angew. Chem., Int. Ed., 2010, 49, 2028 CrossRef CAS PubMed; (e) A. S. K. Hashmi, M. Ghanbari, M. Rudolph and F. Rominger, Chem. – Eur. J., 2012, 18, 8113 CrossRef CAS PubMed.
- (a) X. L. Hou, H. Y. Cheung, T. Y. Hon, P. L. Kwan, T. H. Lo, S. Y. Tong and H. N. C. Wong, Tetrahedron, 1998, 54, 1955 CrossRef CAS; (b) W. J. Moran and A. Rodríguez, Org. Prep. Proced. Int., 2012, 44, 103 CrossRef CAS.
- (a) C. Paal, Ber. Dtsch. Chem. Ges., 1884, 17, 2756 CrossRef; (b) L. Knorr, Ber. Dtsch. Chem. Ges., 1884, 17, 2863 CrossRef; (c) F. Feist, Ber. Dtsch. Chem. Ges., 1902, 35, 1537 CrossRef CAS; (d) E. Benary, Ber. Dtsch. Chem. Ges., 1911, 44, 489 CrossRef CAS; (e) F. G. González, Adv. Carbohydr. Chem., 1956, 11, 97 Search PubMed.
- For review, see: (a) Y. Lu, F. Song, X. Jia and Y. Liu, Huaxue Jinzhan, 2010, 22, 58 CAS. For selected examples, see: (b) S. Ma and Z. Yu, Angew. Chem., Int. Ed., 2002, 41, 1775 CrossRef CAS; (c) S. Ma and J. Zhang, J. Am. Chem. Soc., 2003, 125, 12386 CrossRef CAS PubMed; (d) A. W. Sromek, M. Rubina and V. Gevorgyan, J. Am. Chem. Soc., 2005, 127, 10500 CrossRef CAS PubMed; (e) Y. Liu, F. Song, Z. Song, M. Liu and B. Yan, Org. Lett., 2005, 7, 5409 CrossRef CAS PubMed; (f) A. Aponick, C.-Y. Li, J. Malinge and E. F. Marques, Org. Lett., 2009, 11, 4624 CrossRef CAS PubMed; (g) K.-G. Ji, X.-Z. Shu, J. Chen, S.-C. Zhao, Z.-J. Zheng, X.-Y. Liu and Y.-M. Liang, Org. Biomol. Chem., 2009, 7, 2501 RSC; (h) H. Cao, H. Jiang, G. Yuan, Z. Chen, C. Qi and H. Huang, Chem. – Eur. J., 2010, 16, 10553 CrossRef CAS PubMed; (i) Y. Xia, S. Qu, Q. Xiao, Z.-X. Wang, P. Qu, L. Chen, Z. Liu, L. Tian, Z. Huang, Y. Zhang and J. Wang, J. Am. Chem. Soc., 2013, 135, 13502 CrossRef CAS PubMed; (j) F. Hu, Y. Xia, C. Ma, Y. Zhang and J. Wang, Org. Lett., 2014, 16, 4082 CrossRef CAS PubMed.
- (a) C.-K. Jung, J.-C. Wang and M. J. Krische, J. Am. Chem. Soc., 2004, 126, 4118 CrossRef CAS PubMed; (b) H. Kuroda, E. Hanaki, H. Izawa, M. Kano and H. Itahashi, Tetrahedron, 2004, 60, 1913 CrossRef CAS PubMed; (c) Ł. Albrecht, L. K. Ransborg, B. Gschwend and K. A. Jorgensen, J. Am. Chem. Soc., 2010, 132, 17886 CrossRef PubMed; (d) J. S. Clark, A. Boyer, A. Aimon, P. E. García, D. M. Lindsay, A. D. F. Symington and Y. Danoy, Angew. Chem., Int. Ed., 2012, 51, 12128 CrossRef CAS PubMed.
- (a) T. Ye and M. A. McKervey, Chem. Rev., 1994, 94, 1091 CrossRef CAS; (b) M. P. Doyle and D. C. Forbes, Chem. Rev., 1998, 98, 911 CrossRef CAS PubMed; (c) H. Lebel, J. F. Marcoux, C. Molinaro and A. B. Charette, Chem. Rev., 2003, 103, 977 CrossRef CAS PubMed; (d) V. K. Aggarwal and C. L. Winn, Acc. Chem. Res., 2004, 37, 611 CrossRef CAS PubMed; (e) Q. Xiao, Y. Zhang and J. Wang, Acc. Chem. Res., 2013, 46, 236 CrossRef CAS PubMed; (f) X. Guo and W. Hu, Acc. Chem. Res., 2013, 46, 2427 CrossRef CAS PubMed; (g) X. Xu and M. P. Doyle, Acc. Chem. Res., 2014, 47, 1396 CrossRef CAS PubMed.
- For reviews, see: (a) J. Xiao and X. Li, Angew. Chem., Int. Ed., 2011, 50, 7226 CrossRef CAS PubMed; (b) L. Zhang, Acc. Chem. Res., 2014, 47, 877 CrossRef CAS PubMed; (c) H.-S. Yeom and S. Shin, Acc. Chem. Res., 2014, 47, 966 CrossRef CAS PubMed. For publications of our group, see: (d) C.-F. Xu, M. Xu, Y.-X. Jia and C.-Y. Li, Org. Lett., 2011, 13, 1556 CrossRef CAS PubMed; (e) M. Xu, T.-T. Ren and C.-Y. Li, Org. Lett., 2012, 14, 4902 CrossRef CAS PubMed; (f) L.-Q. Yang, K.-B. Wang and C.-Y. Li, Eur. J. Org. Chem., 2013, 2775 CrossRef CAS; (g) K.-B. Wang, R.-Q. Ran, S.-D. Xiu and C.-Y. Li, Org. Lett., 2013, 15, 2374 CrossRef CAS PubMed; (h) M. Xu, T.-T. Ren, K.-B. Wang and C.-Y. Li, Adv. Synth. Catal., 2013, 355, 2488 CrossRef CAS.
- (a) E. J. Yoo, M. Ahlquist, S. H. Kim, I. Bae, V. V. Fokin, K. B. Sharpless and S. Chang, Angew. Chem., Int. Ed., 2007, 46, 1730 CrossRef CAS PubMed; (b) J. Raushel and V. V. Fokin, Org. Lett., 2010, 12, 4952 CrossRef CAS PubMed; (c) Y. Liu, X. Wang, J. Xu, Q. Zhang, Y. Zhao and Y. Hu, Tetrahedron, 2011, 67, 6294 CrossRef CAS PubMed.
- (a) T. Horneff, S. Chuprakov, N. Chernyak, V. Gevorgyan and V. V. Fokin, J. Am. Chem. Soc., 2008, 130, 14972 CrossRef CAS PubMed; (b) S. Chuprakov, F. W. Hwang and V. Gevorgyan, Angew. Chem., Int. Ed., 2007, 46, 4757 CrossRef CAS PubMed.
- (a) B. Chattopadhyay and V. Gevorgyan, Angew. Chem., Int. Ed., 2012, 51, 862 CrossRef CAS PubMed; (b) A. V. Gulevich and V. Gevorgyan, Angew. Chem., Int. Ed., 2013, 52, 1371 CrossRef CAS PubMed; (c) H. M. L. Davies and J. S. Alford, Chem. Soc. Rev., 2014, 43, 5151 RSC.
- (a) T. Miura, M. Yamauchi and M. Murakami, Chem. Commun., 2009, 1470 RSC; (b) B. Chattopadhyay and V. Gevorgyan, Org. Lett., 2011, 13, 3746 CrossRef CAS PubMed; (c) S. Chuprakov, S. W. Kwok and V. V. Fokin, J. Am. Chem. Soc., 2013, 135, 4652 CrossRef CAS PubMed; (d) E. E. Schultz and R. Sarpong, J. Am. Chem. Soc., 2013, 135, 4696 CrossRef CAS PubMed; (e) B. T. Parr, S. A. Green and H. M. L. Davies, J. Am. Chem. Soc., 2013, 135, 4716 CrossRef CAS PubMed; (f) J. E. Spangler and H. M. L. Davies, J. Am. Chem. Soc., 2013, 135, 6802 CrossRef CAS PubMed; (g) M. Zibinsky and V. V. Fokin, Angew. Chem., Int. Ed., 2013, 52, 1507 CrossRef CAS PubMed; (h) J. S. Alford, J. E. Spangler and H. M. L. Davies, J. Am. Chem. Soc., 2013, 135, 11712 CrossRef CAS PubMed; (i) T. Miura, K. Hiraga, T. Biyajima, T. Nakamuro and M. Murakami, Org. Lett., 2013, 15, 3298 CrossRef CAS PubMed; (j) Y. Shi and V. Gevorgyan, Org. Lett., 2013, 15, 5394 CrossRef CAS PubMed; (k) T. Miura, Y. Funakoshi and M. Murakami, J. Am. Chem. Soc., 2014, 136, 2272 CrossRef CAS PubMed; (l) S. W. Kwok, L. Zhang, N. P. Grimster and V. V. Fokin, Angew. Chem., Int. Ed., 2014, 53, 3452 CrossRef CAS PubMed; (m) K. Chen, Z.-Z. Zhu, Y.-S. Zhang, X.-Y. Tang and M. Shi, Angew. Chem., Int. Ed., 2014, 53, 6645 CrossRef CAS PubMed; (n) C.-E. Kim, S. Park, D. Eom, B. Seo and P. H. Lee, Org. Lett., 2014, 16, 1900 CrossRef CAS PubMed; (o) R.-Q. Ran, J. He, S.-D. Xiu, K.-B. Wang and C.-Y. Li, Org. Lett., 2014, 16, 3704 CrossRef CAS PubMed; (p) B. T. Parr and H. M. L. Davies, Angew. Chem., Int. Ed., 2013, 52, 10044 CrossRef CAS PubMed; (q) T. Miura, T. Tanaka, K. Hiraga, S. G. Stewart and M. Murakami, J. Am. Chem. Soc., 2013, 135, 13652 CrossRef CAS PubMed; (r) H. Shang, Y. Wang, Y. Tian, J. Feng and Y. Tang, Angew. Chem., Int. Ed., 2014, 53, 5662 CrossRef CAS PubMed; (s) E. E. Schultz, V. N. G. Lindsay and R. Sarpong, Angew. Chem., Int. Ed., 2014, 53, 9904 CrossRef CAS PubMed; (t) S. Kim, J. Mo, J. Kim, T. Ryu and P. H. Lee, Asian J. Org. Chem., 2014, 3, 926 CrossRef CAS.
- (a) T. Miura, T. Tanaka, A. Yada and M. Murakami, Chem. Lett., 2013, 42, 1308 CrossRef CAS; (b) D. Yadagiri and P. Anbarasan, Chem. – Eur. J., 2013, 19, 15115 CrossRef CAS PubMed; (c) A. Boyer, Org. Lett., 2014, 16, 1660 CrossRef CAS PubMed; (d) F. Medina, C. Besnard and J. Lacour, Org. Lett., 2014, 16, 3232 CrossRef CAS PubMed; (e) D. J. Lee, H. S. Han, J. Shin and E. J. Yoo, J. Am. Chem. Soc., 2014, 136, 11606 CrossRef CAS PubMed.
- (a) T. Miura, Y. Funakoshi, M. Morimoto, T. Biyajima and M. Murakami, J. Am. Chem. Soc., 2012, 134, 17440 CrossRef CAS PubMed; (b) N. Selander, B. T. Worrell and V. V. Fokin, Angew. Chem., Int. Ed., 2012, 51, 13054 CrossRef CAS PubMed; (c) R. Liu, M. Zhang, G. Winston-McPherson and W. Tang, Chem. Commun., 2013, 49, 4376 RSC.
- (a) S. Chuprakov, J. A. Malik, M. Zibinsky and V. V. Fokin, J. Am. Chem. Soc., 2011, 133, 10352 CrossRef CAS PubMed; (b) J.-M. Yang, C.-Z. Zhu, X.-Y. Tang and M. Shi, Angew. Chem., Int. Ed., 2014, 53, 5142 CAS; (c) D. Yadagiri and P. Anbarasan, Org. Lett., 2014, 16, 2510 CrossRef CAS PubMed; (d) B. Rajagopal, C.-H. Chou, C.-C. Chung and P.-C. Lin, Org. Lett., 2014, 16, 3752 CrossRef CAS PubMed; (e) J. S. Alford and H. M. L. Davies, J. Am. Chem. Soc., 2014, 136, 10266 CrossRef CAS PubMed; (f) T. Miura, T. Biyajima, T. Fuji and M. Murakami, J. Am. Chem. Soc., 2012, 134, 194 CrossRef CAS PubMed; (g) T. Miura, T. Tanaka, T. Biyajima, A. Yada and M. Murakami, Angew. Chem., Int. Ed., 2013, 52, 3883 CrossRef CAS PubMed; (h) S. Chuprakov, B. T. Worrell, N. Selander, R. K. Sit and V. V. Fokin, J. Am. Chem. Soc., 2014, 136, 195 CrossRef CAS PubMed; (i) S. Park, W.-S. Yong, S. Kim and P. H. Lee, Org. Lett., 2014, 16, 4468 CrossRef CAS PubMed.
- For the synthesis of dihydrofuran-3-imine, see ref. 13c.
- A. S. K. Hashmi, T. M. Frost and J. W. Bats, J. Am. Chem. Soc., 2000, 122, 11553 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, characterization data, and NMR spectra for new compounds. See DOI: 10.1039/c4qo00273c |
| This journal is © the Partner Organisations 2015 |