Dispersion and distribution of bimetallic oxides in SBA-15, and their enhanced activity for reverse water gas shift reaction
Baowang
Lu
*a,
Yiwen
Ju
b,
Takayuki
Abe
a and
Katsuya
Kawamoto
c
aHydrogen Isotope Research Center, Organization for Promotion of research, University of Toyama, 3190 Gofuku, Toyama, 930-8555, Japan. E-mail: baowangl@ctg.u-toyama.ac.jp; Tel: +81-76-445-6933
bLaboratory of Computational Geodynamics College of Earth Science, University of Chinese Academy of Sciences, Beijing 100049, China
cGraduate School of Environmental and Life Science, Okayama University, 3-1-1 Tsushima-naka, Kita-ku, Okayama-shi, Okayama, 700-8530, Japan
First published on 18th June 2015
Abstract
We used the direct hydrothermal synthesis method to obtain various well-dispersed bimetallic oxides/SBA-15 for the first time. It is possible that well-dispersed relatively large bimetallic sulfates are formed during the hydrothermal synthesis process and then re-dispersed with difficulty during the heat treatment process resulting in the formation of well-dispersed oxide particles in SBA-15. TEM elemental maps of CuO–NiO/SBA-15 clearly illustrated that CuO and NiO particles were monodispersed in SBA-15. TEM–EDX line analysis revealed that NiO particles were well distributed on the SBA-15 surface, and then covered by CuO particles. TEM elemental maps of CuO–CeO2/SBA-15 clearly showed that CuO and CeO2 particles aggregated slightly in SBA-15. TEM–EDX line analysis showed that CeO2 particles were well distributed on the SBA-15 surface, and then covered by CuO particles. TEM elemental maps of NiO–CeO2/SBA-15 clearly illustrated that NiO and CeO2 particles aggregated slightly in SBA-15. TEM–EDX line analysis revealed that NiO particles were largely mixed with CeO2 on the SBA-15 surface. Therefore, TEM elemental maps can be used to study the dispersion of bimetallic oxides, and TEM–EDX line analysis is very effective for investigating their distribution in SBA-15. Compared with monometallic oxides/SBA-15, the obtained bimetallic oxides/SBA-15 catalysts exhibited excellent efficiency as regards reducing CO2 to CO by the reverse water–gas shift (RWGS) reaction. In particular, the bimetallic oxides/SBA-15 catalysts could result in the high CO2 conversion to CO at low temperature.
1. Introduction
The behaviour of a catalyst is usually influenced by its interaction with other catalyst components such as a second catalyst metal, which could be a promoter. The second metal influences the first metal through electronic interactions or is involved in the reaction by bonding directly to reactants or intermediates. Therefore, bimetallic catalysts are generally used because of the enhanced activity and selectivity that may be achieved by two metals working synergistically. Often, the interactions between the two metals are complex and largely unknown, and the preparation of bimetallic catalysts offers an excellent opportunity for their investigation. Moreover, the work by Sinfelt et al. has generated considerable interest in the formation, structure and further exploitation of the catalytic activity of bimetallic particles.1–6 The deposition of catalytically active nanoparticles on support materials with high dispersion is an important and effective strategy for the design of catalysts. Ordered mesoporous materials with their intrinsically high surface areas have been well studied for this purpose. Multicomponent supported metal catalysts can be prepared by a number of synthesis methods, each with its own distinct advantages.7,8 The most commonly used co-impregnation methods involve a support material that has been modified by the adsorption of metal ion precursors from solution.9–18 The condensation of these centers upon reduction (e.g., in flowing H2 at high temperature) yields the desired bimetallic particle catalysts. Another attractive way of synthesizing supported bimetallic catalysts is to use molecular cluster compounds as precursors.4 However, because the ability to control the size distribution of particles obtained in this way is quite limited, the supported bimetallic nanoparticles typically lack uniformity as regards size and shape, and the nature of the compositional distributions that characterize the sample is often unknown. Therefore, it is a serious technological challenge to design a synthesis approach for preparing supported bimetallic catalysts with a uniform size and distribution.The direct hydrothermal synthesis method is an effective approach for preparing monodispersed metal nanoparticles in SBA-15.19–25 It has been verified that monodispersed metal nanoparticles have enhanced activity and selectivity. However, there has still been no research on the preparation of well-dispersed bimetallic nanoparticles in mesoporous silica by the direct hydrothermal synthesis method. After considering the above options, we first developed a direct hydrothermal synthesis method in a strongly acidic medium to prepare well-dispersed bimetallic nanoparticles in SBA-15. We also investigated the compositional distribution, and catalytic properties compared with well-dispersed monometallic nanoparticles in SBA-15.
2. Experimental
2.1. Synthesis of bimetallic oxides/SBA-15
Block copolymer Pluronic P123 (Aldrich, EO20PO70EO20, Mav = 5800) was dissolved in 105 g of deionized water, and then 19.83 g of sulfuric acid (H2SO4, Wako Chemical, Japan, 95.0 wt%) and an appropriate quantity of nitrate hexahydrate salts, such as nickel(II) nitrate hexahydrate (Ni(NO3)2·6H2O, Wako Chemical, Japan, 98.0 wt%), copper(II) nitrate trihydrate (Cu(NO3)2·3H2O Wako Chemical, Japan, 99.5 wt%), and cerium(III) nitrate hexahydrate (Ce(NO3)3·6H2O Wako Chemical, Japan, 98.0 wt%) were added at room temperature. The mixture was stirred for 30 minutes, and then 8.52 g of tetraethylorthosilicate (Si(OC2H5)4, TEOS, Wako Chemical, Japan, 95.0 wt%) was added. The obtained starting mixture was aged at 60 °C until a white precipitate appeared, and then immediately evaporated at 100 °C overnight (≈14 h). The resulting solid product was dried at 150 °C for 5 h, and then calcined at 500 °C for 10 h and at 800 °C for 2 h.2.2. Characterization
The X-ray diffraction (XRD) patterns of the solid products were collected by using a powder X-ray diffractometer (Bruker AXS D8 Advance) with graphite monochromatized Cu Kα radiation at 40 kV and 40 mA. Nitrogen adsorption isotherms at −196 °C were obtained using a conventional volumetric apparatus (Quadrasorb USA). Before the adsorption measurements were made, the powders (≈0.1 g) were subjected to a temperature of 300 °C for 5 h in a vacuum. The specific surface area was calculated with the Brunauer–Emmett–Teller (BET) method. The pore size was calculated from the desorption branch of nitrogen adsorption–desorption isotherms by the Barrett–Joyner–Halenda (BJH) method. The pore volume was taken at a single point P/P0 = 0.95. The morphology was observed with a scanning electron microscope (SEM, Oxford Instruments). The solid product was observed using a transmission electron microscope (TEM, JEM-2100F) at an electron acceleration of 200 kV. The TEM–EDX analyses were conducted with an electron beam approximately 1 nm in diameter.2.3. Catalytic properties
The RWGS reaction was used as a model reaction to study the catalytic properties of NiO/SBA-15. Before the RWGS reaction, the catalysts were pre-reduced in situ in an 80% H2/N2 stream for 2 h with a total gas flow of 50 ml min−1 at 900 °C and a heating ramp rate of 100 °C h−1. The RWGS reaction was performed at atmospheric pressure in a fixed-bed quartz reactor with 20 mm inner diameter in an initial temperature range of 400 to 900 °C. A 30 mm long catalyst between two layers of quartz wool was loaded into the reactor. A thermocouple was inserted directly into the center of the catalyst bed to measure the actual pre-treatment and reaction temperatures in situ. The reactor was heated in a furnace (KTF-035N, Koyo Thermo Systems, Co., Ltd) equipped with a temperature controller. All reactant gases were monitored with a mass flow meter (E-40) and a controller (PE-D20) (HORIBA STEC, Co., Ltd). The flow of the product was measured with a film flow meter (VP-3, HORIBA STEC, Co., Ltd) and analyzed with a gas chromatography-thermal conductivity detector (GC-TCD) after the RWGS reaction had become stable. The turnover frequencies (TOFs) were calculated from the rate of CO2 conversion divided by the total amount of NiO used.3. Results and discussion
3.1. Synthesis and characterization of bimetallic oxides/SBA-15
As described in detail in the Experimental section, bimetallic oxides/SBA-15 such as CuO–NiO/SBA-15, CuO–CeO2/SBA-15, and NiO–CeO2/SBA-15 were obtained with a direct hydrothermal synthesis method. For comparison, monometallic oxides/SBA-15 used as reference catalysts, such as CuO/SBA-15 and NiO/SBA-15, were also synthesized using a direct hydrothermal synthesis method. The amounts of metallic oxides in bimetallic oxides/SBA-15 and the reference catalyst are shown in Table 1.| Metallic oxides/SBA-15 | CuO | NiO | CeO2 | d 100 (nm) | S BET (m2 g−1) | PVa (cm3 g−1) | PSa (nm) | WTa (nm) | E a (kcal mol−1) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amountb (wt%) | GSa (nm) | Amountb (wt%) | GSa (nm) | Amountb (wt%) | GSa (nm) | ||||||||||
| XRD | TEM | XRD | TEM | XRD | TEM | ||||||||||
| a GS: grain size calculated by XRD and TEM, SBET: surface area calculated using the Brunauer–Emmett–Teller (BET) method in the relative pressure range of p/p0 = 0.05–0.3; PV: pore volume obtained from the volumes of N2 adsorbed at p/p0 = 0.95 or in the vicinity; PD: pore diameter analyzed by the desorption branch of the isotherms using the Barrett–Joyner–Halenda (BJH) method, WT: wall thicknesses: calculated by (2 × d100/√3) − pore size, and Ea: activation energy. b Value in bracket measured by EDX. | |||||||||||||||
| SBA-15 | 0 | — | — | 0 | — | — | 0 | — | — | 10.99 | 678 | 1.15 | 9.15 | 3.54 | — |
| CuO/SBA-15 | 5 (5.02) | 29.6 | 30.1 | 0 | — | — | 0 | — | — | 10.06 | 281 | 0.90 | 9.26 | 2.36 | 72.8 |
| CuO–NiO/SBA-15 | 5 (4.87) | 48.1 | 47.6 | 1 (0.95) | 53.1 | 53.5 | 0 | — | — | 9.80 | 279 | 0.82 | 8.24 | 3.08 | 53.6 |
| CuO/SBA-15 | 10 (9.89) | 36.5 | 37.1 | 0 | — | — | 0 | — | — | 9.79 | 278 | 0.85 | 9.18 | 2.12 | 74.7 |
| CuO–CeO2/SBA-15 | 10 (9.79) | 39.4 | 39.6 | 0 | — | — | 1 (1.02) | 13 | 13.2 | 9.61 | 194 | 0.72 | 8.23 | 2.87 | 63.5 |
| NiO/SBA-15 | 0 | — | — | 1 (0.99) | 5.2 | 5.4 | 0 | — | — | 10.86 | 385 | 1.10 | 9.10 | 3.44 | 27.8 |
| NiO–CeO2/SBA-15 | 0 | — | — | 1 (0.98) | 21.3 | 21.1 | 1 (1.03) | 19.4 | 19.1 | 9.78 | 298 | 1.03 | 7.53 | 3.76 | 9.5 |
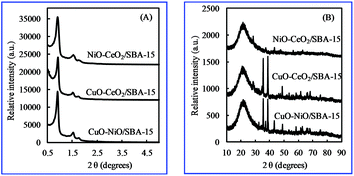
The small-angle XRD patterns (Fig. 1(A)) of bimetallic oxides/SBA-15 showed three well-resolved peaks that could be indexed as (100), (110) and (200) diffraction peaks, indicating the typical two-dimensional hexagonally ordered mesostructure (p6mm) of SBA-15.26,27 In addition, as shown in Fig. 1(B), the wide-angle XRD peaks could be indexed as various bimetallic oxides. Also, a broad peak at around 2θ = 23° caused by the amorphous SiO2 structure of SBA-15 was clearly observed in the wide-angle XRD patterns, indicating that the ordered mesoporous structure was not disturbed by the incorporation of various bimetallic oxides. The Scherrer equation28 was used to calculate the average grain size of various bimetallic oxide particles, which is summarized in Table 1. The average size of metallic oxide particles in bimetallic oxides/SBA-15 was larger than that in monometallic oxides/SBA-15, due to the increased amount of metallic oxides in the limited space, indicating that metal oxides were not located in the channels of SBA-15 with smaller size, but also on the outside of SBA-15 with larger size. In NiO–CeO2/SBA-15, even though the NiO amount was only 1 wt%, the NiO particle size was 21.3 nm, suggesting that the particles were also on the outer SBA-15 surface except in the SBA-15 nano-channels, unlike those in NiO/SBA-15 (only in SBA-15 nano-channels). Therefore, compared with monometallic oxides/SBA-15, both the average size of the metallic oxide particles in bimetallic oxides/SBA-15 became larger, and the distribution was different.
Fig. 2 shows the nitrogen adsorption–desorption isotherms of various bimetallic oxides/SBA-15. The isotherms were similar to the type IV IUPAC classification,29 clearly indicating that these materials had mesoporous structures; a type-H1 hysteresis loop was observed. The isotherm was typical of SBA-15,26,27 indicating the formation of a p6mm structure with open cylindrical mesopores. And there was no blocking effect, unlike that obtained with the post-synthesis method.30,31 This result suggests that NiO particles were dispersed in the SiO2 structure of SBA-15, not in the SBA-15 pores, in contrast with the post-synthesis method.30,31 Also, one narrow peak was observed in the PSD curve (Fig. 2), showing that a unique pore was present.
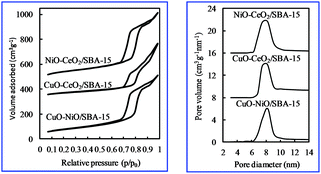 | ||
| Fig. 2 Nitrogen adsorption–desorption isotherms and pore size distribution curves of various bimetallic catalysts. | ||
The characteristics of various bimetallic oxides/SBA-15 obtained by the direct hydrothermal synthesis method are summarized in Table 1. Compared with monometallic oxides/SBA-15 or SBA-15, the surface area, pore volume and pore size all shrank, indicating that the SBA-15 structure was degraded by the incorporation of bimetallic oxides or monometallic oxides. The wall thickness of bimetallic oxides/SBA-15 was thicker than that of monometallic oxides/SBA-15, indicating the reduction of the ordered structure caused by the incorporation of bimetallic oxides.
There have been no reports on the successful synthesis of monodisperse metal particles in strong acidic media using conventional HCl to synthesize SBA-15. Using H2SO4 instead of HCl, we successfully obtained monodisperse NiO particles in SBA-15 and reported it for the first time.25 By dispersing the molten intermediates formed from nitrate during the thermal decomposition process, the oxides can be well dispersed into SBA-15 by heat treatment after mixing the nitrate and SBA-15.32,33 Therefore, the nitrate and its molten intermediates can move during the heat treatment process, resulting in well-dispersed oxides/SBA-15 with severely agglomerated particles. When using H2SO4, the sulphate formed during the hydrothermal synthesis process was relatively larger than nitrate, and so it did not move easily during the heat treatment process, which made it possible to obtain monodisperse metallic oxide particles in SBA-15. H2SO4 was used in this research to obtain various well dispersed bimetallic oxides/SBA-15.
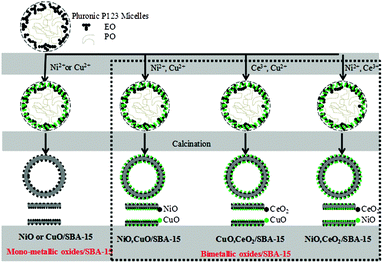
Because nitrate is highly soluble in acidic media, bimetallic ions can interact with the hydrophilic ethylene oxide units of Pluronic P123, with the result that metallic ions are likely to be encapsulated within the core of Pluronic P123 micelles. After silica species have polymerized on the surface of Pluronic P123 micelles, the final product contains bimetallic oxides inside the mesoporous framework. However, we cannot exclude the separate formation of SBA-15 with Pluronic P123 and the adsorption of bimetallic oxides on its surface.
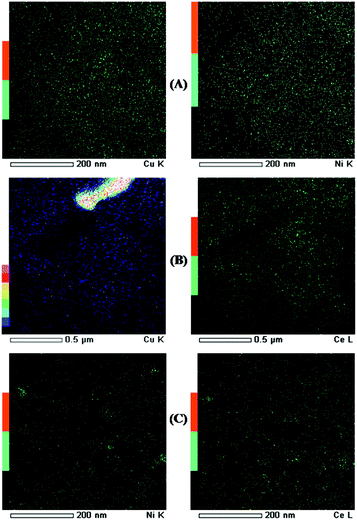
Fig. 3 shows TEM images and SEM image. The morphology of all bimetallic oxides/SBA-15 consisted of rod-like aggregates; and this was the same as that of typical SBA-15, as previously reported.26,27,34,35 All bimetallic oxides/SBA-15 had the ordered mesoporous structure of typical SBA-15. Fig. 4 shows TEM elemental maps of various bimetallic oxides/SBA-15. In CuO–NiO/SBA-15, no aggregates of NiO particles were observed clearly, indicating that the CuO and NiO particles were highly dispersed (monodispersed) into the SBA-15 structure, unlike in previous reports using the post-synthesis method where aggregates were observed.30,31 However, in CuO–CeO2/SBA-15 and NiO–CeO2/SBA-15, there was slight but acceptable aggregation of CuO (due to the large amount), CeO2 (due to its ease of aggregation) and NiO (due to the fact that a small amount disperses into CeO2) particles. Therefore, well-dispersed bimetallic oxides/SBA-15 could be obtained by the direct hydrothermal synthesis method. As shown in Table 1, the average size of bimetallic oxides measured by TEM was similar to that calculated by XRD.
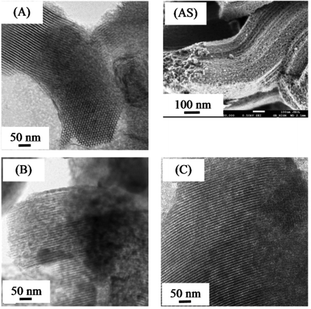 | ||
| Fig. 3 TEM images of (A): CuO–NiO/SBA-15, (B): CuO–CeO2/SBA-15, (C): NiO–CeO2/SBA-15 and the SEM image of (AS): CuO–NiO/SBS-15. | ||
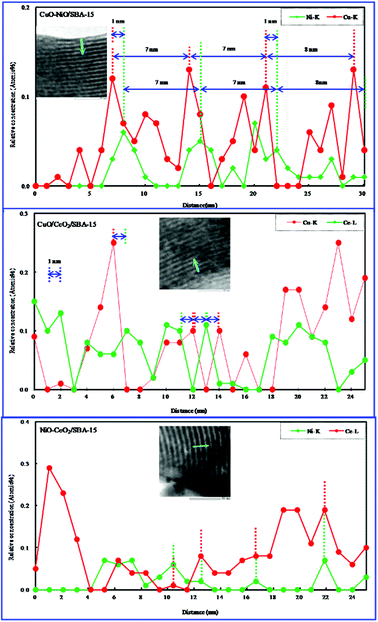
We have investigated the distribution of NiO in NiO/SBA-15 obtained by the direct hydrothermal synthesis method.25 When the NiO amount is low, NiO particles were present in the NiO/SAB-15 pores. As the NiO amount was increased, the NiO particles began to disperse on the outer surface of the NiO/SAB-15. Studying the distribution of bimetallic oxides is very interesting and important. Therefore, we investigated the bimetallic oxide distribution using TEM–EDS line profile analysis. The EDS line profiles are shown in Fig. 5. As shown in Table 1, the actual contents of bimetallic oxides obtained by elemental analysis were close to their initial contents in starting synthesis gel. In CuO–NiO/SBA-15 and CuO–CeO2/SBA-15, the appearance of the maximum relative atom concentration of every metallic oxide was regular, clearly indicating that every metallic oxide was well dispersed. The position of the maximum relative atom concentration of every metallic oxide appeared in turn, and did not overlap in the same place. The interval distance was about 1 nm, suggesting that one metallic oxide was well distributed on the SBA-15 surface, and then covered by another metallic oxide, and they were not simply mixed together. This phenomenon found for supported bimetallic oxides was very interesting. In CuO–NiO/SBA-15, the distance between the two positions at which the maximum relative atom concentration of every metallic oxide appeared was about 7 to 8 nm, which is close to the pore size of bimetallic oxides/SBA-15 measured by N2 adsorption. In addition, in NiO–CeO2/SBA-15, the positions of the maximum relative atom concentrations of NiO and CeO2 appeared at almost the same place and overlapped, similar to the bimetallic oxides/SBA-15 prepared by co-impregnation,36 indicating that the NiO and CeO2 particles were simply mixed (i.e. NiO was dispersed into CeO2), and then dispersed on the SBA-15 surface.
Ni(NO3)2 is hydrophilic relative to Cu(NO3)2 and hydrophobic relative to TEOS. Therefore, TEOS preferentially, and then Ni2+ followed finally by Cu2+ interacted with the hydrophilic ethylene oxide units of Pluronic P123, with the result that the NiO particles were covered with CuO particles in CuO–NiO/SBA-15. In the same way, CeO2 particles were covered by CuO in CuO–CeO2/SBA-15. In NiO–CeO2/SBA-15, the very few NiO and CeO2 particles were present as mixtures and they dispersed into the SBA-15 surface. The images of the bimetallic oxide distribution were considered based on the above results and are shown in Fig. 6.
3.2. Catalytic properties of bimetallic oxides/SBA-15
The conversion of CO2 to valuable chemical materials has been proposed as a possible way of utilizing greenhouse gas CO2.37–42 In terms of CO2 conversion, because CO is a valuable material in many chemical processes, the conversion of CO2 to CO by catalytic hydrogenation is recognized as the most promising process, and the reverse water–gas shift (RWGS) reaction is a highly desirable reaction for CO2 conversion to CO.43–56 Therefore, the RWGS reaction was used as a model reaction with which to study the catalytic properties of various bimetallic oxides/SBA-15.To investigate the catalytic properties, we attempted to perform the RWGS reaction by fixing the CO2/H2 ratio at 1 using various bimetallic oxides/SBA-15. For comparison, monometallic oxides/SBA-15 was also used to investigate the RWGS reaction. The results are shown in Fig. 7. The CO2 conversion increased with increasing temperature, suggesting that high temperature favored CO2 conversion. The CO2 conversion of all bimetallic oxides/SBA-15 was higher than that of monometallic oxides/SBA-15. Therefore, bimetallic oxides enhanced the activity. In particular, the bimetallic oxides/SBA-15 catalysts could result in the high CO2 conversion to CO at low temperature due to the presence of the strong interaction between metal (active center) and oxides (promoter), indicating that the RWGS reaction could occur even at low temperature.
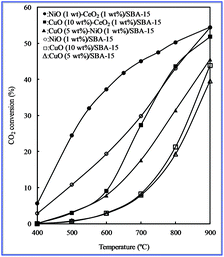 | ||
| Fig. 7 CO2 conversion at different temperatures with various catalysts obtained by the direct synthesis method. | ||
The CO2 conversion of CuO–NiO/SBA-15 was lower than that of NiO/SBA-15, but higher than CuO/SBA-15, indicating that Cu particles may play an important role in CO2 conversion to CO, rather than Ni particles (as promoters), i.e. a large number of NiO particles were covered by CuO particles. This result supported the above EDX line profile analysis. In addition, the large NiO particles formed maybe a result of lower CO2 conversion. The CO2 conversion of CuO–CeO2/SBA-15 or NiO–CeO2/SBA-15 was higher than that of CuO/SBA-15 or NiO/SBA-15, indicating that a large number of CeO2 particles were covered by CuO or mixed with NiO particles. This result also supported the above EDX line profile analysis. In this case CeO2 acted as a promoter, driving force for the CO2 conversion to CO because of the presence of oxygen vacancies. Therefore, these results supported our above conclusion regarding the distribution of bimetallic oxides in SBA-15.
Fig. 8 shows CO selectivity using various bimetallic oxides/SBA-15 and monometallic oxides/SBA-15. Regardless of temperature, all catalysts exhibited a CO selectivity of 100%, except NiO–CeO2/SBA-15. In our previous reports,25,54 NiO particles were monodispersed (isolated) in SBA-15 in the presence of a NiO amount of less than 15 wt%, showing a CO selectivity of close to 100%. Then, Christopher et al. also confirmed that an isolated metal active site can exhibit a CO selectivity of close to 100%.57 However, when NiO–CeO2/SBA-15 was used, the CO selectivity was 100% at high temperature, whereas it was lower than 100% even at low NiO concentrations, due to the formation of methane at low temperatures, different from our previous report,25,54 suggesting that the influence of CeO2 on CO selectivity should be considered. In our previous research,55 NiO particles with low concentrations were monodispersed in CeO2, showing a CO selectivity of close to 100%, while with high concentrations those were aggregated in CeO2, CO selectivity was lower than 100%. Therefore, the possibility is that NiO particles in NiO–CeO2/SBA-15 were not monodispersed.
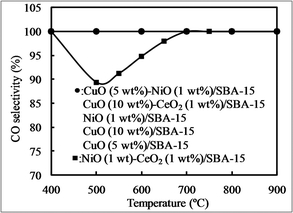 | ||
| Fig. 8 CO selectivity at different temperatures with various catalysts obtained by the direct synthesis method. | ||
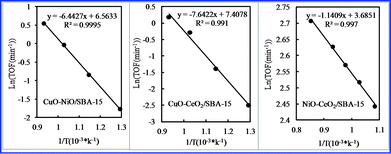
Fig. 9 shows Arrhenius plots for CO2 conversion using various bimetallic oxides/SBA-15 and monometallic oxides/SBA-15. The activation energy for CO2 conversion was calculated, and is summarized in Table 1. The difference in activation energy led to their different catalytic activities. The activation energy of various bimetallic oxides/SBA-15 for CO2 conversion was lower than that of monometallic oxides/SBA-15, suggesting the bimetallic oxides/SBA-15 had enhanced activity due to bimetallic oxide incorporation.
4. Conclusions
In summary, by using the direct hydrothermal synthesis method, various bimetallic oxides/SBA-15 were synthesized for the first time. The dispersion and distribution of bimetallic oxides in SBA-15 were investigated by employing TEM elemental maps and TEM–EDX line analysis. The TEM elemental maps showed that various bimetallic oxides were well dispersed in SBA-15. The TEM–EDX line analysis of CuO–NiO/SBA-15 and CuO–CeO2/SBA-15 showed that the first metallic oxide particles were well distributed on the SBA15 surface, and covered by other metallic oxide particles. In NiO–CeO2/SBA-15, mixtures of NiO and CeO2 particles were well distributed on the SBA-15 surface. Compared with monometallic oxides/SBA-15, the obtained bimetallic oxides/SBA-15 catalysts exhibited excellent efficiency. We can expect this direct synthesis method to be used to prepare other bimetallic oxide particles with various supports, and using TEM elemental maps and TEM–EDX line analysis to investigate dispersion and distribution of bimetallic oxides.Notes and references
- J. H. Sinfelt, Bimetallic Catalystss, Concepts, and Applications, John Wiley & Sons, New York, 1983 Search PubMed.
- J. H. Sinfelt, Int. Rev. Phys. Chem., 1988, 7, 281 CrossRef PubMed.
- J. R. Anderson, Structure of Metallic Catalysts, Academic Press, New York, 1975 Search PubMed.
- K. J. Klabunde and Y.-X. Li, in Selectivity in Catalysis, ed. M. E. Davis and S. L. Suib, ACS Symposium Series 517, American Chemical Society, Washington, DC, 1993, pp. 88–108 Search PubMed.
- K. Kinoshita and P. Stonehart, in Preparation and Characterization of Highly Dispersed Electrocatalytic Materials, ed. K. Kinoshita and P. Stonehart, Plenum, New York, 1977, vol. 12, pp. 183–266 Search PubMed.
- M. Watanabe, M. Uchida and S. Motoo, J. Electroanal. Chem., 1987, 229, 395 CrossRef CAS.
- O. S. Alexeev and B. C. Gates, Ind. Eng. Chem. Res., 2003, 42, 1571 CrossRef CAS.
- B. C. Gates, Chem. Rev., 1995, 95, 511 CrossRef CAS.
- J. Panpranot, J. G. Goodwin and A. Sayari Jr., J. Catal., 2002, 211, 530 CrossRef CAS.
- R. Raja, T. Khimyak, J. M. Thomas, S. Hermans and B. F. G. Johnson, J. Catal., 2003, 213, 78 CrossRef.
- S. N. Pronkin, P. A. Simonov, V. I. Zaikovskii and E. R. Savinova, J. Mol. Catal. A: Chem., 2007, 265, 141 CrossRef CAS PubMed.
- S. S. Itkulova, K. Z. Zhunusova and G. D. Zakumbaeva, World, 2008, 52, 850 Search PubMed.
- M. Dhakada, D. Finob, S. S. Rayalua, R. Kumara, A. Watanabec, H. Hanedac, S. Devottaa, T. Mitsuhashic and N. Labhsetwara, Top. Catal., 2007, 42–43, 273 CrossRef.
- A. Kulkarni and B. C. Gates, Angew. Chem., Int. Ed., 2009, 48, 9697 CrossRef CAS PubMed.
- C. Montesdecorrea and F. Cordobacastrillon, J. Mol. Catal. A: Chem., 2005, 228, 267 CrossRef CAS PubMed.
- M. S. Nashner, A. I. Frenkel, D. L. Adler, J. R. Shapley and R. G. Nuzzo, J. Am. Chem. Soc., 1997, 119, 7760 CrossRef CAS.
- B. F. G. Johnson, J. M. Thomas, G. Sankar, D. Ozkaya, W.-Z. Zhou, R. D. Oldroyd and R. G. Bel, Angew. Chem., Int. Ed. Engl., 1997, 36, 2242 CrossRef PubMed.
- P. Doggali, Y. Teraoka, P. Mungse, I. K. Shah, S. Rayalu and N. Labhsetwar, J. Mol. Catal. A: Chem., 2012, 358, 23 CrossRef CAS PubMed.
- W.-Z. Zhou, J. M. Thomas, D. S. Shephard, B. F. G. Johnson, D. Ozkaya, T. Maschmeyer, R. G. Bell and Q. F. Ge, Science, 1998, 280, 705 CrossRef CAS.
- R. Raja, T. Khimyak, J. M. Thomas, S. Hermans and B. F. G. Johnson, Angew. Chem., Int. Ed., 2001, 40, 4638 CrossRef CAS.
- D. S. Shephard, T. Maschmeyer, G. Sankar, J. M. Thomas, D. Ozkaya, B. F. G. Johnson, R. Raja, R. D. Oldroyd and R. G. Bell, Chem. – Eur. J., 1998, 4, 1214 CrossRef CAS.
- M. Conte, A. F. Carley, G. A. Attard, A. A. Herzing, C. J. Kiely and G. J. Hutchings, J. Catal., 2008, 257, 190 CrossRef CAS PubMed.
- M. S. Nashner, D. M. Somerville, P. D. Lane, D. L. Adler, J. R. Shapley and R. G. Nuzzo, J. Am. Chem. Soc., 1996, 118, 12964 CrossRef CAS.
- H. Song, R. M. Rioux, J. D. Hoefelmeyer, R. Komor, K. Niesz, M. Grass, P. D. Yang and G. A. Somorjai, J. Am. Chem. Soc., 2006, 128, 3027 CrossRef CAS PubMed.
- B.-W. Lu and K. Kawamoto, RSC Adv., 2012, 2, 6800 RSC.
- D. Zhao, J. Feng, Q. Huo, N. Melosh, G. H. Fredrickson, B. F. Chmelka and G. D. Stuchy, Science, 1998, 279, 548 CrossRef CAS.
- D. Zhao, Q. Huo, J. Feng, B. F. Chmelka and G. D. Stuchy, J. Am. Chem. Soc., 1998, 120, 6024 CrossRef CAS.
- A. L. Patterson, Phys. Rev., 1939, 56, 978 CrossRef CAS.
- S. J. Gregg and K. S. W. Sing, Adsorption Surface Area and Porosity, Academic Press, New York, 1982 Search PubMed.
- M.-Y. Cheng, C.-J. Pan and B. J. Hwang, J. Mater. Chem., 2009, 19, 5193 RSC.
- L. Vradman, M. V. Landau, D. Kantorovich, Y. Koltypin and A. Gedanken, Microporous Mesoporous Mater., 2005, 79, 307 CrossRef CAS PubMed.
- T. M. Eggenhuisen, J. P. den Breejen, D. Verdoes, P. E. de Jongh and K. P. de Jong, J. Am. Chem. Soc., 2010, 132, 18318 CrossRef CAS PubMed.
- B.-W. Lu and K. Kawamoto, Fuel, 2013, 103, 699 CrossRef CAS PubMed.
- B.-W. Lu and K. Kawamoto, Mater. Res. Bull., 2012, 47, 1301 CrossRef CAS PubMed.
- B.-W. Lu, Y. Inagi and A. Endo, J. Nanosci. Nanotechnol., 2011, 11, 2361 CrossRef CAS PubMed.
- A. Ungureanu, B. Dragoi, A. Chirieac, S. Royer, D. Duprez and E. Dumitriu, J. Mater. Chem., 2011, 21, 12529 RSC.
- P. G. Jessop, F. Joo and C.-C. Tai, Coord. Chem. Rev., 2004, 248, 2425 CrossRef CAS PubMed.
- T. Sakakura, J.-C. Choi and H. Yasuda, Chem. Rev., 2007, 107, 2365 CrossRef CAS PubMed.
- G. Centi and S. Perathoner, Catal. Today, 2009, 148, 191 CrossRef CAS PubMed.
- M. Bourrez, F. Molton, S. Chardon-Noblat and A. Deronzier, Angew. Chem., Int. Ed., 2011, 50, 9903 CrossRef CAS PubMed.
- C. Federsel, R. Jackstell and M. Beller, Angew. Chem., Int. Ed., 2010, 49, 6254 CrossRef CAS PubMed.
- M. Mikkelsen, M. Jorgensen and F. C. Krebs, Energy Environ. Sci., 2010, 3, 43 CAS.
- C.-S. Chen, W.-H. Cheng and S.-S. Lin, Catal. Lett., 2000, 68, 45 CrossRef CAS.
- C.-S. Chen, W.-H. Cheng and S.-S. Lin, Catal. Lett., 2002, 83, 121 CrossRef CAS.
- C.-S. Chen, W.-H. Cheng and S.-S. Lin, Appl. Catal., A, 2003, 238, 55 CrossRef CAS.
- C.-S. Chen, W.-H. Cheng and S.-S. Lin, Appl. Catal., A, 2004, 257, 97 CrossRef CAS.
- F. S. Stone and D. Waller, Top. Catal., 2003, 22, 305 CrossRef CAS.
- A. Goguet, F. C. Meunier and J. P. Breen, J. Catal., 2004, 226, 382 CrossRef CAS PubMed.
- A. Goguet, F. C. Meunier and D. Tibiletti, J. Phys. Chem. B, 2004, 108, 20240 CrossRef CAS.
- D. Tibiletti, A. Goguet, F. C. Meunier, J. P. Breen and R. Burch, Chem. Commun., 2004, 1636 RSC.
- L.-H. Wang, S.-X. Zhang and Y. Liu, J. Rare Earths, 2008, 26, 66 CrossRef.
- C.-S. Chen, J.-H. Wu and T.-W. Lai, J. Phys. Chem. C, 2010, 114, 15021 CAS.
- C.-S. Chen, C.-C. Chen, C.-T. Chen and H.-M. Kao, Chem. Commun., 2011, 47, 2288 RSC.
- B.-W. Lu and K. Kawamoto, J. Environ. Chem. Eng., 2013, 1, 300 CrossRef CAS PubMed.
- B.-W. Lu and K. Kawamoto, Mater. Res. Bull., 2014, 53, 70 CrossRef CAS PubMed.
- B.-W. Lu and K. Kawamoto, Catal. Sci. Technol., 2014, 4, 4313 CAS.
- J. C. Matsubu, V. N. Yang and P. Christopher, J. Am. Chem. Soc., 2015, 137, 3076 CrossRef CAS PubMed.
| This journal is © the Partner Organisations 2015 |