A fluorescent supramolecular polymer with aggregation induced emission (AIE) properties formed by crown ether-based host–guest interactions†
Dong
Chen
a,
Jiayi
Zhan
a,
Mingming
Zhang
b,
Jing
Zhang
a,
Jiaju
Tao
a,
Danting
Tang
a,
Ailin
Shen
a,
Huayu
Qiu
a and
Shouchun
Yin
*a
aCollege of Material, Chemistry and Chemical Engineering, Hangzhou Normal University, Hangzhou 310036, P. R. China. E-mail: yinsc@hznu.edu.cn; Fax: +86 57128867899; Tel: +86 57128867899
bDepartment of Biochemistry and Chemistry, University of Maryland, College Park, 20740, USA
First published on 25th September 2014
Abstract
A fluorescent supramolecular polymer with aggregation induced emission properties formed by crown ether-based host–guest interactions was prepared. It can be used as a fluorescent sensor for Pd2+ ions.
Supramolecular polymers, obtained as a result of the integration of polymer science and supramolecular chemistry, have received considerable attention during the last decade.1 Compared with the traditional covalent bonds linked polymers, supramolecular polymers whose repeating units are joined together by non-covalent bonds often exhibit some interesting properties such as stimuli-responsiveness,2 self-healing,3 and shape memory4 due to their dynamic nature. Different kinds of supramolecular polymers were prepared based on different non-covalent interactions such as hydrogen bonding,5 metal–ligand,6 π–π stacking,7 charge transfer,8 and host–guest interactions.9 Nowadays, the studies of supramolecular polymers have gradually turned from the preparation and characterization of supramolecular polymers10 to the functionality of supramolecular polymers, which is of significant importance for their future applications.
Tetraphenylethylene (TPE) is a fluorescent chromophore which shows bright fluorescence emissions with an increase in the concentration or in the solid state.11 Compared with the conventional organic chromophores that display strong fluorescence in dilute solutions and are non-emissive at high concentrations or in the solid state, the aggregation induced emission (AIE) properties of TPE make it an excellent fluorescent molecule to dock with supramolecular polymers, because supramolecular polymers only form at relative high concentrations. Herein, we report a supramolecular polymer formed by crown ether-based host–guest interactions with tetraphenylethylene moieties as the chromophore. It exhibits much higher fluorescence emissions than its monomer due to the AIE properties of TPE. Interestingly, the fluorescence intensity of the supramolecular polymer decreased dramatically by the addition of Pd2+ ions, hence it can be used in the detection of Pd2+ ions.
As shown in Scheme S1,† monomer 1 was synthesized via a click reaction from one equivalent of TPE unit with two azide groups and two equivalents of dibenzo-24-crown-8 (DB24C8) moieties with one acetylene group. A supramolecular polymer was obtained by mixing equal molar ratios of monomer 1 and linker 2, a bisammonium salt with two dibenzylammonium (DBA) units in solution based on the DB24C8/DBA recognition motif, which has been widely used in the construction of molecular machines and supramolecular gels due to its responsiveness.9 At the same time, the aggregated TPE molecules endowed the supramolecular polymer with strong fluorescence at high concentrations (Scheme 1).
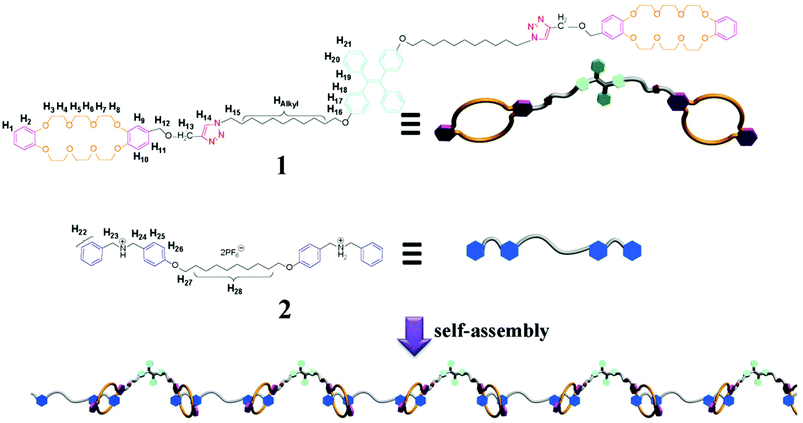 | ||
| Scheme 1 Chemical structures of monomer 1 and linker 2 and cartoon representations of the formation of the fluorescent supramolecular polymer. | ||
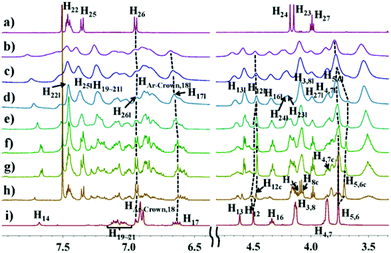
Concentration-dependent 1H NMR measurements (Fig. 1) were first carried out to study the formation of the supramolecular polymers. As the concentrations of monomers 1 and 2 increased, noticeable chemical shift changes were observed for both DB24C8 and DBA. The NMR spectra became complicated and each of the protons of DB24C8 and DBA split into two signals, because this complexation is a slow exchange system. The aromatic protons H22, H25, H26 and benzyloxymethylene protons H27 of 2 shifted downfield, while upfield chemical shifts were found for the benzyl protons H23 and H24. Moreover, the proton signals became more and more broad and a conversion from cyclic oligomers to linear polymers was observed as the concentration increased, indicating a ring-chain mechanism for the formation of this kind of supramolecular polymers.5a
To further confirm the formation of linear polymeric aggregates, viscosity measurements, a reliable method to reflect the property of polymers, was performed in CH3CN–CHCl3 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) using a semi-micro dilution viscometer. From Fig. 2a, it can be seen that the slope of the curve was 1.41 in the low concentration range, while a sudden rise in the viscosity (slope of 2.25) was observed when the concentration exceeded the critical polymerization concentration (CPC, 70 mM), indicating the transition from cyclic oligomers to linear supramolecular polymers. Two-dimensional diffusion-ordered NMR (DOSY) experiments were also carried out to test the mobility of the supramolecular polymer in solution. As the concentration of the equimolar solutions of 1 and 2 increased from 5.00 to 200 mM, the measured weight average diffusion coefficient D decreased from 4.42 × 10−10 to 2.90 × 10−11 m2 s−1 (Fig. 2b). This result suggested that the intermolecular interactions were weak in the low concentration range, while they became strong in the high concentration range, providing another evidence for the formation of supramolecular polymers.
1, v/v) using a semi-micro dilution viscometer. From Fig. 2a, it can be seen that the slope of the curve was 1.41 in the low concentration range, while a sudden rise in the viscosity (slope of 2.25) was observed when the concentration exceeded the critical polymerization concentration (CPC, 70 mM), indicating the transition from cyclic oligomers to linear supramolecular polymers. Two-dimensional diffusion-ordered NMR (DOSY) experiments were also carried out to test the mobility of the supramolecular polymer in solution. As the concentration of the equimolar solutions of 1 and 2 increased from 5.00 to 200 mM, the measured weight average diffusion coefficient D decreased from 4.42 × 10−10 to 2.90 × 10−11 m2 s−1 (Fig. 2b). This result suggested that the intermolecular interactions were weak in the low concentration range, while they became strong in the high concentration range, providing another evidence for the formation of supramolecular polymers.
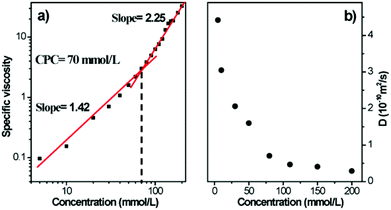 | ||
Fig. 2 (a) Specific viscosity of the linear supramolecular polymer (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 CH3CN–CHCl3, 298 K); (b) concentration dependence of the diffusion coefficient D (1 1 CH3CN–CHCl3, 298 K); (b) concentration dependence of the diffusion coefficient D (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 CD3CN–CDCl3, 293 K, 500 MHz). 1 CD3CN–CDCl3, 293 K, 500 MHz). | ||
Due to the pH-responsive complexation of DB24C8 and DBA, the conversion between the supramolecular polymers and monomers 1 and 2 can be reversibly realized by using NEt3 and CF3COOH to adjust the pH of the solution, and this process was confirmed by 1H NMR (Fig. S9, ESI†).6g
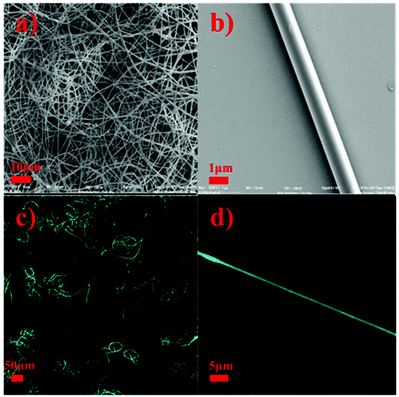
Electrospinning is a convenient technique to obtain nanofibers, and it requires molecular chain entanglements in the charged fluid to avoid the formation of droplets. Therefore, whether low molecular weight molecules can generate nanofibers by electrospinning is dependent on the existence of their intermolecular interactions. Overlapped nanofibers were obtained by electrospinning as shown in the SEM images (Fig. 3a), and their average diameter was about 1.0 μm (Fig. 3b), indicating the formation of supramolecular polymers with a high degree of chain entanglements in solution. It can be clearly seen that the fibers show strong blue fluorescence when they were irradiated at 350 nm (Fig. 3c and d), which is consistent with the AIE properties of the TPE molecules, because in the fibers the concentration of TPE molecules is very high. Consequently, a fluorescent supramolecular polymer was obtained by docking fluorescent tetraphenylethylene molecules with crown ether-based host–guest interactions.
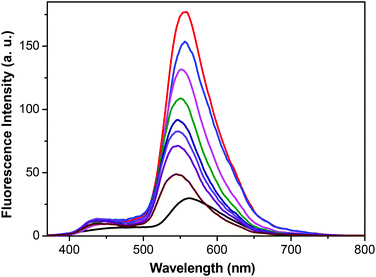
Then, we investigated the concentration dependent fluorescent intensity of the formed linear supramolecular polymer. Fig. 4 shows the fluorescent response of monomer 1 and the linear supramolecular polymer at different concentrations. Monomer 1 shows a very weak fluorescence peak only at 560 nm even at a high concentration (200 mM). Interestingly, the fluorescent intensity of the corresponding linear supramolecular polymer at the same concentration was enhanced by 6-fold, which is due to the greater restriction of the intramolecular torsional/rotational motions of TPE group in the linear supramolecular polymers.11 As the concentration of the linear supramolecular polymers increased from 75 mM to 200 mM (above CPC of the supramolecular polymer), the fluorescent intensity did not decrease, but increased along with a 13 nm red-shift, which is consistent with other reported AIE systems.11 The fluorescence intensity was further enhanced with a 79 nm blue-shift when the liner supramolecular polymer was in the solid state (Fig. S10, ESI†). The blue-shift could be explained by the morphological change of the aggregates from the amorphous to crystalline state.12 These results indicate that the linear supramolecular polymer exhibit AIE properties both in solution and in the solid state, making it possible to use this linear supramolecular polymer as a solid supramolecular material.
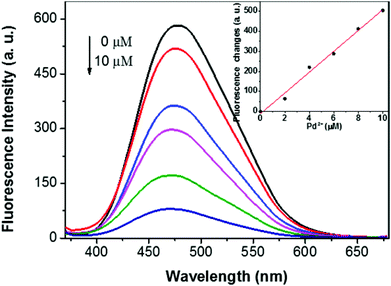
Since 1,2,3-triazole units can form metal–ligand complexes with some metal ions, we further studied whether the supramolecular polymer can act as a supramolecular sensor. Fig. 5 shows the fluorescence response of the fluorescent supramolecular polymer to various metal cations. Upon excitation at λex = 350 nm, the supramolecular polymer in the solid state displayed a strong fluorescence peak at 477 nm. No obvious fluorescence intensity changes were observed in the presence of the following metal ions: Na+, Mg2+, Ca2+, Co2+, Ba2+, Zn2+, Cd2+, Fe2+, Hg2+, Pb2+, and Ag+. However, a dramatic decrease in the fluorescence intensity along with an 8 nm blue-shift was found when 9 μM Pd2+ ions were added, which was caused by the energy transfer from the supramolecular polymer to Pd2+ by the coordination of Pd2+ with the 1,2,3-triazole group.4f,13 The fluorescence spectral changes of the supramolecular polymer as a function of the Pd2+ concentration in the solid state are shown in Fig. 6. As the concentration of Pd2+ increased, the fluorescence intensity gradually decreased. A plot of the fluorescence intensity changes versus the concentration of Pd2+ was obtained and was found to fit linearly. Thus, the resulting supramolecular polymer can act as a fluorescent sensor for Pd2+ ions.
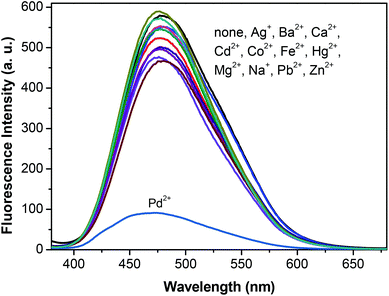 | ||
| Fig. 5 Fluorescence spectra of the linear supramolecular polymer with different metal ions in the solid state. | ||
Conclusions
In summary, a linear fluorescent supramolecular polymer formed by crown ether-based host–guest interactions was prepared using TPE as the chromophore. The supramolecular polymer exhibits AIE properties resulting from the TPE group not only in solution, but also in the solid state, and both of them are higher than that of its monomer 1. Moreover, the fluorescence intensity decreased dramatically on the addition of Pd2+ due to the coordination interactions of Pd2+ with the 1,2,3-triazole group, which allows the fluorescent supramolecular polymer to be applied as a fluorescent sensor for Pd2+. This study on supramolecular sensors based on fluorescent supramolecular polymers will enrich the applications of supramolecular polymers as solid supramolecular materials.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (91127032, 21174035, 21274034), Program for Changjiang Scholars and Innovative Research Team in Chinese University (IRT 1231), Excellent Young Teachers in Zhejiang Province and Hangzhou Normal University (HNUEYT 2011-01-019), the Opening Foundation of Zhejiang Provincial Top Key Discipline (no. 20121109), National Training Programs of Innovation and Entrepreneurship for Undergraduates (201310346014) and Zhejiang Province's Xinmiao Talent Plan (2014R421064).Notes and references
-
(a) S. Yang, A. V. Ambade and M. Week, Chem. Soc. Rev., 2011, 40, 129 RSC
; (b) T. Aida, E. W. Meijer and S. I. Stupp, Science, 2012, 335, 813 CrossRef CAS PubMed
; (c) B. Zheng, F. Wang, S. Dong and F. Huang, Chem. Soc. Rev., 2012, 41, 1621 RSC
; (d) D. Guo and Y. Liu, Chem. Soc. Rev., 2012, 41, 5907 RSC
; (e) F. Wang, C. Han, C. He, Q. Zhou, J. Zhang, C. Wang, N. Li and F. Huang, J. Am. Chem. Soc., 2008, 130, 11254 CrossRef CAS PubMed
; (f) S. Dong, Y. Luo, X. Yan, B. Zheng, X. Ding, Y. Yu, Z. Ma, Q. Zhao and F. Huang, Angew. Chem., Int. Ed., 2011, 50, 1905 CrossRef CAS PubMed
; (g) X. Yan, D. Xu, X. Chi, J. Chen, S. Dong, X. Ding, Y. Yu and F. Huang, Adv. Mater., 2012, 24, 362 CrossRef CAS PubMed
; (h) Z. Zhang, Y. Luo, J. Chen, S. Dong, Y. Yu, Z. Ma and F. Huang, Angew. Chem., Int. Ed., 2011, 50, 1397 CrossRef CAS PubMed
.
-
(a) Q. Zhang, D. Qu, X. Ma and H. Tian, Chem. Commun., 2013, 49, 9800 RSC
; (b) S. Dong, L. Gao, J. Li, D. Xu and Q. Zhou, Polym. Chem., 2013, 4, 3968 RSC
.
-
(a) M. Burnworth, L. Tang, J. R. Kumpfer, A. J. Duncan, F. L. Beyer, G. L. Fiore, S. J. Rowan and C. Weder, Nature, 2011, 472, 334 CrossRef CAS PubMed
; (b) M. Zhang, D. Xu, X. Yan, J. Chen, S. Dong, B. Zheng and F. Huang, Angew. Chem., Int. Ed., 2012, 51, 7011 CrossRef CAS PubMed
; (c) Y. Yang and M. W. Urban, Chem. Soc. Rev., 2013, 42, 7446 RSC
; (d) L. R. Hart, J. L. Harries, B. W. Greenland, H. M. Colquhoun and W. Hayes, Polym. Chem., 2013, 4, 4860 RSC
.
-
(a) X. Chi, D. Xu, X. Yan, J. Chen, M. Zhang, B. Hu, Y. Yu and F. Huang, Polym. Chem., 2013, 4, 2767 RSC
; (b) J. R. Kumpfer and S. J. Rowan, J. Am. Chem. Soc., 2011, 133, 12866 CrossRef CAS PubMed
; (c) Y. Cui, M. Tan, A. Zhu and M. Guo, J. Mater. Chem. B, 2014, 2, 2978 RSC
; (d) W. Nan, W. Wang, H. Gao and W. Liu, Soft Matter, 2013, 9, 132 RSC
; (e) A. Yasih, H. Li, Z. Lu, S. Rehman, M. Siddiq and H. Yang, Soft Matter, 2014, 10, 972 RSC
; (f) J. Zhan, M. Zhang, M. Zhou, B. Liu, D. Chen, Y. Liu, Q. Chen, H. Qiu and S. Yin, Macromol. Rapid Commun., 2014, 35, 1424 CrossRef CAS PubMed
.
-
(a) L. Brunsveld, B. J. B. Folmer, E. W. Meijer and R. P. Sijbesma, Chem. Rev., 2001, 101, 4071 CrossRef CAS PubMed
; (b) D. G. Rodrŕguez and A. P. H. J. Schenning, Chem. Mater., 2011, 23, 310 CrossRef
; (c) S. J. George, R. D. Bruijn, Ž. Tomović, B. V. Averbeke, D. Beljonne, R. Lazzaroni, A. P. H. J. Schenning and E. W. Meijer, J. Am. Chem. Soc., 2012, 134, 17789 CrossRef CAS PubMed
; (d) T. Xiao, X. Feng, S. Ye, Y. Guan, S. Li, Q. Wang, Y. Ji, D. Zhu, X. Hu, C. Lin, Y. Pan and L. Wang, Macromolecules, 2012, 45, 9585 CrossRef CAS
; (e) A. Bertrand, F. Lortie and J. Bernard, Macromol. Rapid Commun., 2012, 33, 2062 CrossRef CAS PubMed
; (f) J. Xu, Y. Chen, D. Wu, L. Wu, C. H. Tung and Q. Yang, Angew. Chem., Int. Ed., 2013, 52, 9738 CrossRef CAS PubMed
.
-
(a) U. S. Schubert and C. Eschbaumer, Angew. Chem., Int. Ed., 2002, 41, 2892 CrossRef CAS
; (b) J. B. Beck and S. J. Rowan, J. Am. Chem. Soc., 2003, 125, 13922 CrossRef CAS PubMed
; (c) G. R. Whittell and I. Manners, Adv. Mater., 2007, 19, 3439 CrossRef CAS
; (d) F. Han, M. Higuchi and D. G. Kurth, J. Am. Chem. Soc., 2008, 130, 2073 CrossRef CAS PubMed
; (e) X. Yan, B. Jiang, T. R. Cook, Y. Zhang, J. Li, Y. Yu, F. Huang, H. Yang and P. J. Stang, J. Am. Chem. Soc., 2013, 135, 16813 CrossRef CAS PubMed
; (f) Y. Ding, P. Wang, Y. Tian, Y. Tian and F. Wang, Chem. Commun., 2013, 49, 5951 RSC
; (g) J. Zhan, Q. Li, Q. Hu, Q. Wu, H. Qiu, M. Zhang and S. Yin, Chem. Commun., 2014, 50, 722 RSC
.
-
(a) R. Fang, Y. Liu, Z. Wang and X. Zhang, Polym. Chem., 2013, 4, 900 RSC
; (b) R. Marty, R. Nigon, D. Leite and H. Frauenrath, J. Am. Chem. Soc., 2014, 136, 3919 CrossRef CAS PubMed
.
-
(a) Y. Liu, Y. Yu, J. Gao, Z. Wang and X. Zhang, Angew. Chem., Int. Ed., 2010, 49, 6576 CrossRef CAS PubMed
; (b) K. Liu, C. Wang, Z. Li and X. Zhang, Angew. Chem., Int. Ed., 2011, 50, 4952 CrossRef CAS PubMed
; (c) B. Schulze, C. Friebe, S. Hoeppener, G. M. Pavlov, A. Winter, M. D. Hager and U. S. Schubert, Macromol. Rapid Commun., 2012, 33, 597 CrossRef CAS PubMed
; (d) Y. Tian, Y. Shi, Z. Yang and F. Wang, Angew. Chem., Int. Ed., 2014, 53, 6090 CrossRef CAS PubMed
.
-
(a) A. Harada, A. Hashidzume, H. Yamaguchi and Y. Takashima, Chem. Rev., 2009, 109, 5974 CrossRef CAS PubMed
; (b) G. Chen and M. Jiang, Chem. Soc. Rev., 2011, 40, 2254 RSC
; (c) X. Ma, R. Sun, W. Li and H. Tian, Polym. Chem., 2011, 2, 1068 RSC
; (d) H. Zhao, D. Guo, L. Wang, H. Qian and Y. Liu, Chem. Commun., 2012, 48, 11319 RSC
; (e) L. Chen, Y. Tian, Y. Ding, Y. Tian and F. Wang, Macromolecules, 2012, 45, 8412 CrossRef CAS
; (f) Y. Liu, C. Yu, H. Jin, B. Jiang, X. Zhu, Y. Zhou, Z. Lu and D. Yan, J. Am. Chem. Soc., 2013, 135, 4765 CrossRef CAS PubMed
; (g) X. Ji, S. Dong, P. Wei, D. Xia and F. Huang, Adv. Mater., 2013, 25, 5725 CrossRef CAS PubMed
; (h) Y. Liu, Z. Huang, X. Tan, Z. Wang and X. Zhang, Chem. Commun., 2013, 49, 5766 RSC
; (i) X. Hu, X. Wu, S. Wang, D. Chen, W. Xia, C. Lin, Y. Pan and L. Wang, Polym. Chem., 2013, 4, 4292 RSC
; (j) M. Zhang, X. Yan, F. Huang, Z. Niu and H. W. Gibson, Acc. Chem. Res., 2014, 47, 1995 CrossRef CAS PubMed
; (k) F. Zeng, Z. Meng, Y. Han and C. Chen, Chem. Commun., 2014, 50, 7611 RSC
; (l) F. Zeng, Y. Shen and C. Chen, Soft Matter, 2013, 9, 4875 RSC
; (m) F. Zeng, Y. Han, Z. Yan, C. Liu and C. Chen, Polymer, 2013, 54, 6929 CrossRef CAS PubMed
; (n) S. Li, H. Lu, Y. Shen and C. Chen, Macromol. Chem. Phys., 2013, 214, 1596 CrossRef CAS
; (o) Y. Han, Z. Meng, Y. Ma and C. Chen, Acc. Chem. Res., 2014, 47, 2026 CrossRef CAS PubMed
.
-
(a) Y. Liu, Z. Wang and X. Zhang, Chem. Soc. Rev., 2012, 41, 5922 RSC
; (b) G. Yu, X. Yan, C. Han and F. Huang, Chem. Soc. Rev., 2013, 42, 6697 RSC
.
-
(a) Y. Hong, J. W. Y. Lam and B. Z. Tang, Chem. Soc. Rev., 2011, 40, 5361 RSC
; (b) J. Wang, J. Mei, R. Hu, J. Sun, A. Qin and B. Z. Tang, J. Am. Chem. Soc., 2012, 134, 9956 CrossRef CAS PubMed
; (c) Z. Zhao, J. W. Y. Lam and B. Z. Tang, J. Mater. Chem., 2012, 22, 23726 RSC
; (d) Z. Wang, S. Chen, J. W. Y. Lam, W. Qin, R. T. K. Kwok, N. Xie, Q. Hu and B. Z. Tang, J. Am. Chem. Soc., 2013, 135, 8238 CrossRef CAS PubMed
; (e) X. Yang, Y. Jiang, B. Shen, F. Dong, K. Yu, B. Yang and Q. Liu, Polym. Chem., 2013, 4, 5591 RSC
; (f) J. Tong, Y. Wang, J. Mei, J. Wang, A. Qin, J. Sun and B. Z. Tang, Chem. – Eur. J., 2014, 20, 1 CrossRef
; (g) H. Feng, S. Song, Y. Chen, C. Shen and Y. Zheng, J. Mater. Chem. C, 2014, 2, 2353 RSC
; (h) R. Hu, N. C. Leung and B. Z. Tang, Chem. Soc. Rev., 2014, 43, 4494 RSC
; (i) Z. Zhao, Z. Chang, B. He, B. Chen, C. Deng, P. Lu, H. Qiu and B. Z. Tang, Chem. – Eur. J., 2013, 19, 11512 CrossRef CAS PubMed
; (j) C. W. T. Leung, Y. Hong, S. Chen, E. Zhao, J. W. Y. Lam and B. Z. Tang, J. Am. Chem. Soc., 2013, 135, 62 CrossRef CAS PubMed
.
-
(a) J. Huang, N. Sun, Y. Q. Dong, R. L. Tang, P. Lu, P. Cai, Q. Q. Li, D. G. Ma, J. G. Qin and Z. Li, Adv. Funct. Mater., 2013, 23, 2329 CrossRef CAS
; (b) H. Tong, Y. Q. Dong, Y. N. Hong, M. Häußler, J. W. Y. Lam, H. H. Y. Sung, X. M. Yu, J. X. Sun, I. D. Williams, H. S. Kwok and B. Z. Tang, J. Phys. Chem. C, 2007, 111, 2287 CrossRef CAS
.
-
(a) F. Wang, J. Zhang, X. Ding, S. Dong, M. Liu, B. Zheng, S. Li, K. Zhu, L. Wu, Y. Yu, H. W. Gibson and F. Huang, Angew. Chem., Int. Ed., 2010, 49, 1090 CrossRef CAS PubMed
; (b) J. Huang, S. Wang, G. Wu, L. Yan, L. Dong, X. Lai, S. Yin and B. Song, Soft Matter, 2014, 10, 1018 RSC
.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures and full characterization of monomer 1, the conversion and fluorescence spectra of monomer 1 and the linear supramolecular polymer. See DOI: 10.1039/c4py01206b |
| This journal is © The Royal Society of Chemistry 2015 |