Improve the photovoltaic performance of new quinoxaline-based conjugated polymers from the view of conjugated length and steric hindrance
Dan
Ouyang
ab,
Manjun
Xiao
c,
Dangqiang
Zhu
ab,
Weiguo
Zhu
c,
Zhengkun
Du
ab,
Ning
Wang
*a,
Yuanhang
Zhou
ab,
Xichang
Bao
a and
Renqiang
Yang
*a
aCAS Key Laboratory of Bio-based Materials, Qingdao Institute of Bioenergy and Bioprocess Technology, Chinese Academy of Sciences, Qingdao 266101, China. E-mail: wangning@qibebt.ac.cn; yangrq@qibebt.ac.cn
bUniversity of Chinese Academy of Sciences, Beijing 100049, China
cCollege of Chemistry, Key Lab of Environment-Friendly Chemistry and Application of the Ministry of Education, Xiangtan University, Xiangtan 411105, China
First published on 29th August 2014
Abstract
In this work, we have synthesized two new quinoxaline derivatives: 2,3-bis(n-octylthiomethyl)-5,8-dibromoquinoxaline (QS) and 2,3-bis[(5-octylthio)thiophen-2-yl]-5,8-dibromoquinoxaline (QTS); in addition, three new donor–acceptor (D–A) copolymers: poly{4,8-bis(2-ethylhexyloxy)-benzo[1,2-b:4,5-b′]-dithiophene-alt-2,3-bis(n-octylthiomethyl)quinoxaline} (PBDTQS), poly{4,8-bis(2-ethylhexyloxy)-benzo[1,2-b:4,5-b′]-dithiophene-alt-2,3-bis[(5-octylthio)thiophen-2-yl]quinoxaline} (PBDTQTS), and poly{2,3-bis[(5-octylthio)thiophen-2-yl]quinoxaline-5,8-diyl-alt-thiophene-2,5-diyl} (PTQTS) were designed from the view of extending the length of conjugated side chain and reducing the steric hindrance of building blocks. Replacing the carbon atom in the side chain of polymer PBDTQS with a thiophene ring could increase the conjugation length and improve the absorption in the visible region of the copolymer PBDTQTS and PTQTS. Furthermore, polymer PTQTS exhibited a more planar backbone and increased intermolecular π-stacking compared to PBDTQTS, which was because the thiophene unit in PTQTS had smaller size and less steric hindrance than those of benzo[1,2-b:4,5-b′]-dithiophene unit in PBDTQTS. For the optimized polymer solar cell of PTQTS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC61BM, PCE of 3.73% with Voc of 0.76 V, Jsc of 9.41 mA cm−2 and FF of 52.33% under an AM 1.5 G solar simulator with an intensity of 100 mW cm−2 was achieved, which was the best performance among the three copolymers. The results implied that the PTQTS with thiophene as the donor unit and QTS as the acceptor unit in the main chain would be a promising donor candidate in the application of polymer solar cells.
PC61BM, PCE of 3.73% with Voc of 0.76 V, Jsc of 9.41 mA cm−2 and FF of 52.33% under an AM 1.5 G solar simulator with an intensity of 100 mW cm−2 was achieved, which was the best performance among the three copolymers. The results implied that the PTQTS with thiophene as the donor unit and QTS as the acceptor unit in the main chain would be a promising donor candidate in the application of polymer solar cells.
Introduction
Polymer solar cells (PSCs) based on the bulk hetero-junction (BHJ) active layers of conjugated polymers and fullerene derivatives have attracted significant attention due to their light weight, low cost, and flexibility.1–4 To obtain donor materials with ideal performance, tremendous efforts have been devoted to tuning their properties through molecular designs.5–8 The key issue in the studies of PSCs is to increase power conversion efficiencies (PCEs) of the devices. Recently, PCEs of the BHJ PSCs have acquired significant improvement due to the development of new polymers with low band gap via intra-molecular charge-transfer (ICT) donor (D)–acceptor (A) strategy.9–11 Numerous conjugated polymers have been developed and various chemical modifications have been used to optimize the physical and photovoltaic properties of the D–A polymers.12–16 PCE has exceeded 9% for single junction solar cells17,18 and 10% for tandem solar cells.19,20It is still very challenging to simultaneously balance the absorptions, energy levels, and charge mobilities of PSCs for linear D–A conjugated polymers. In recent years, side chain conjugated polymers, which had the donor or acceptor building block with conjugated structure in the side chain directions, are attracting attention for modulating the electronic structures of polymers.21–25 Based on this strategy, Hou et al. have successfully proven the benzo[1,2-b:4,5-b′]-dithiophene (BDT)-polymers with conjugated structure in the side chain directions show higher hole transport property and photovoltaic performance.26 In addition, some acceptor building blocks, with conjugated side chain have also been reported.27 For example, a prospecting conjugated polymer poly[2,3-bis(3-(octyloxy)phenyl)quinoxaline-alt-thiophene] (TQ) exhibited excellent photovoltaic performance with a PCE of 6.0%, open-circuit voltage (Voc) of 0.89 V, short-circuit current (Jsc) of 10.5 mA cm−2 and fill factor (FF) of 64%.28 The two substituted benzene groups on the quinoxaline rings could freely rotate, thus potentially hinder the intermolecular packing and further influence charge carrier mobility of the copolymers. Therefore, some groups designed new quinoxaline derivatives by replacing a phenyl group with a thiophene moiety,29,30 which may further improve the absorption capability of the donor materials.
Moreover, it is important to improve the packing orders of the D–A conjugated polymer because the mobility of the polymer film is highly related to the inter-chain π–π stacking of the polymers. Considering the donor–acceptor interaction and the spatial steric hindrance caused by the branched alkyl chains, it could be better that the small donor units dock into the cavity of large acceptor cores.31 This “molecular docking” strategy would reduce the steric hindrance of side chains and increase the inter-chain π–π stacking of the polymer, and improve the carrier mobility of the active layer in devices. Pei's group has introduced a “molecular docking” strategy and demonstrated that polymer symmetry and backbone curvature affect the inter-chain of isoindigo-based polymers in film, ultimately leading to improved device performance.32
Based on the above considerations, a delicate balance between the steric hindrance and conjugated length is needed via judiciously designed polymers. For example, it is well known that increasing the length of the side chains will enhance the steric hindrance, thus increase the solubility of the polymer, which may further lead to better film processability.33 However, it is also obvious that too much steric hindrance will force the backbone of the polymer to bend out of coplanarity, which may influence the mobility and light-harvesting ability of the active layer.34 In the case of conjugated length, a similar problem exists. Large conjugated length will enhance the π–π stacking and decrease the band gap of the polymers, which would surely increase the mobility and light harvesting-ability of the active layer.35 However, if the conjugated length is significantly large, the solubility and film processability of the polymers will be influenced, which may further affect the morphology of the active layer.36 In this context, we tried to improve the photovoltaic performance of quinoxaline-based conjugated polymer from the view of both conjugated length in the side chain direction and steric hindrance of building blocks. Firstly, we introduced an alkylthio substitute on the quinoxaline and synthesized a new low band gap conjugated polymer poly{4,8-bis(2-ethylhexyloxy)-benzo[1,2-b:4,5-b′]-dithiophene-alt-2,3-bis(n-octylthiomethyl)quinoxaline} (PBDTQS). To our knowledge, a quinoxaline segment containing an alkylthio substitute group has not been reported. Sulfur atom has some π-acceptor capability due to the formation of pπ(C)–dπ(S) orbital overlap, where divalent sulfur accepts π-electron from the p-orbital of carbon–carbon double bond into its empty 3d-orbitals.37,38 This causes the polymers containing alkylthio side chain to exhibit unique optoelectronic properties such as broader absorption spectra39 and lower the highest occupied molecular orbital (HOMO) energy level.40,41 In our strategy, the incorporation of alkylthio side chain into quinoxaline monomers may be a promising method to down-shift the HOMO energy level to certain extent.10 To increase the conjugated length in the side chain direction, we replaced a carbon atom with a thiophene unit and synthesized another copolymer, namely, poly{4,8-bis(2-ethylhexyloxy)-benzo[1,2-b:4,5-b′]-dithiophene-alt-2,3-bis[(5-octylthio)thiophen-2-yl]quinoxaline} (PBDTQTS). In our design strategy, the advantage of the alkylthio side chain and the thiophene ring in the quinoxaline are expected to be combined, which would result in the improvement of light-harvest ability and lower the HOMO energy levels of the target polymers. To further improve the performance of our polymer, we changed the BDT donor unit to a simple and small thiophene unit and synthesized the poly{2,3-bis[(5-octylthio)thiophen-2-yl]quinoxaline-5,8-diyl-alt-thiophene-2,5-diyl}(PTQTS) (Scheme 1), which could decrease the steric hindrance and increase the inter-chain π–π stacking of polymer backbones. The UV-vis absorptions, energy levels, morphology and photovoltaic performances of the abovementioned three copolymers were evaluated to understand the relationships between their structures and properties. Using the conventional device structure, the polymer PTQTS displayed a promising PCE of 3.73% with Voc of 0.76 V, Jsc of 9.41 mA cm−2, FF of 52.3% under the illumination of AM 1.5 G 100 mW cm−2 without post-treatment, which was the best performance among the three copolymers because of its good planar and high hole mobility. The results indicated that the polymer PTQTS with thiophene as the donor unit and QTS as the acceptor unit in the main chain, was a promising donor candidate in the application of polymer solar cells.
Results and discussion
Synthesis and structural characterization
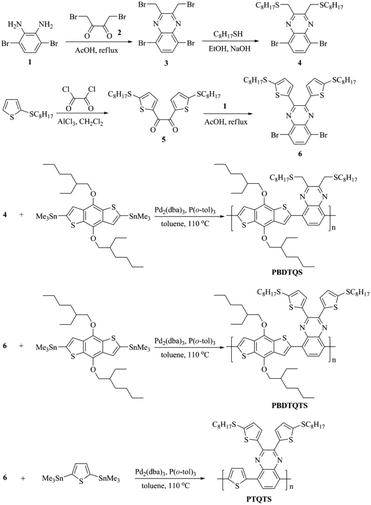
The synthetic route of the monomers and copolymers is outlined in Scheme 1. 3,6-Dibromo-1,2-diamine (1)42 condensed with 1,2-dibromobutane-2,3-dione (2)43 to yield 2,3-bis(bromomethyl)-5,8-dibromoquinoxaline (3). Compound 3 reacted with N-octylmercaptan in a stirred solution of NaOH and ethanol to produce 2,3-bis(n-octylthiomethyl)-5,8-dibromoquinoxaline (4). 2-(octylthio)-thiophene44 was dissolved in a solution of CH2Cl2 containing AlCl3 and oxalyl chloride to give 1,2-bis[(5-octylthio)-thiophen-2-yl]ethane-1,2-dione (5). Then, 2,3-bis[(5-octylthio)thiophen-2-yl]-5,8-dibromoquinoxaline (6) was prepared by closed loop reaction between compound (1) and compound (5) using acetic acid as the solvent and heating at 60 °C for 12 h under nitrogen atmosphere. PBDTQS, PBDTQTS and PTQTS were prepared by Stille cross-coupling reaction with yield of about 78–85%. Further purification was carried out by Soxhlet extraction with methanol, hexane and chloroform, and the product was precipitated from methanol–chloroform system. Molecular weights and molecular weight distributions of these three polymers were measured by gel permeation chromatography (GPC) using tetrahydrofuran (THF) as eluent with narrowly distributed polystyrenes as the calibration standards. PBDTQS showed a number average molecular weight (Mn) of 23.1 kDa and a polydispersity index (PDI) of 1.91. PBDTQTS showed Mn of 19.9 kDa, and PDI of 2.16. PTQTS showed Mn of 24.3 kDa, and PDI of 2.10. All the polymers were fully soluble in common organic solvents such as THF, chloroform, chlorobenzene (CB) and o-dichlorobenzene (ODCB).Thermal properties
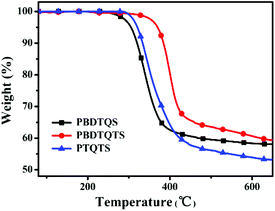
The thermal properties of PBDTQS, PBDTQTS and PTQTS were analyzed by thermo-gravimetric analysis (TGA). The degradation temperature (Td) (with 5% weight lost) was found to be at 304, 370 and 320 °C for PBDTQS, PBDTQTS and PTQTS, respectively (as shown in Fig. 1). Obviously, the thermal stability of these quinoxaline-based polymers is adequate for their applications in PSCs and other optoelectronic devices.Optical properties
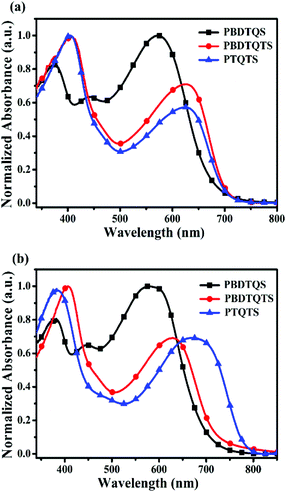
The UV-vis absorption properties of the obtained copolymers were measured in both chloroform solution (Fig. 2a) and as thin films on quartz slides (Fig. 2b). As shown in Fig. 2, all the resulting copolymers had similar absorption profiles and showed two absorption peaks at around 380 and 600 nm, which is a typical feature for D–A copolymers. The absorption peak around 380–410 nm originated from the π–π* transition of the polymer main chain, and the absorption in the wavelength range of 500–800 nm were mainly due to the charge transfer from the donor units to acceptor units. In comparison with PBDTQS, the maximum absorption peaks of PBDTQTS and PTQTS in the long wavelength range were obviously red-shifted, suggesting that thiophene unit could increase the conjugated length in the backbone of the copolymers. A Similar behavior was observed for the absorption spectra of these copolymers as thin solid films (Fig. 2b). Compared to the absorption peaks in solution, the absorption peaks of the films in the wavelength range of 500–800 nm showed larger red-shifts around 25, 8, 51 nm for PBDTQS, PBDTQTS and PTQTS, respectively. The reason could be explained by the formation of π-stacked structures in the solid state which could facilitate charge transportation for photovoltaic applications. Moreover, it also indicated that PTQTS possessed stronger π-stacking ability than the other two copolymers in solid state due to the small thiophene unit, which could decrease the steric hindrance of building blocks and improve the inter-chain π–π stacking. The optical band gaps calculated from the onset of the film absorption spectra were about 1.77, 1.70 and 1.58 eV for PBDTQS, PBDTQTS and PTQTS, respectively. It is worth noting that the Eoptg of PTQTS was considerably lower than those of PBDTQS and PBDTQTS, which would facilitate a high absorption in green-red region of the solar spectrum. Thus, PSCs based on PTQTS and PC61BM probably exhibited a better Jsc value and photovoltaic performance than that of other two polymers.Electrochemical properties
To investigate the differences in electrochemical properties, the redox potentials of PBDTQS, PBDTQTS and PTQTS films deposited onto a platinum electrode were studied by cyclic voltammetry (CV) measurement. The HOMO and LUMO levels of polymers were calculated from their onset oxidation potential (φox) and onset reduction potential (φred) according to the following equations: EHOMO = −e(φox + 4.8 − φ1/2, FeCp2) (eV) and ELUMO = −e(φred + 4.8 − φ1/2, FeCp2) (eV), where the unit of potential is V (vs. SCE).45 As shown in Fig. 3, the onset oxidation potentials of PBDTQS, PBDTQTS and PTQTS were determined to be 0.88, 0.88 and 0.85 V, corresponding to the HOMO energy levels of −5.29, −5.29 and −5.26 eV, respectively. Compared with polymer TTQ (−5.06 eV),28 these three copolymers showed lower HOMO levels due to the introduction of the alkylthio side chain. The onset reduction potentials of PBDTQS, PBDTQTS, and PTQTS were about −0.78, −0.71 and −0.68 V and LUMO energy levels of the corresponding copolymers were estimated to be −3.63, −3.70 and −3.72 eV, respectively. From the onset potentials of the oxidation and reduction processes, the band gaps (Eg) of PBDTQS, PBDTQTS and PTQTS were calculated to be about 1.66, 1.59 and 1.44 eV. The values were different from those obtained by the above-described optical method, which was mainly due to the energy barriers of the charge transfer at the electrodes during the CV measurement.46 The energy levels of the materials used in the OPVs are shown in Fig. 3. The HOMO levels of these three copolymers were lower than that of P3HT,47 as shown in Fig. 3. This implies that these three materials in PSCs might have possessed a higher Voc than the P3HT-based counterparts. The LUMO levels of these three copolymers were higher than that of PC61BM; this induced electron-effective transfer at the donor–acceptor interface. The electrochemical data of the copolymers are summarized in Table 1.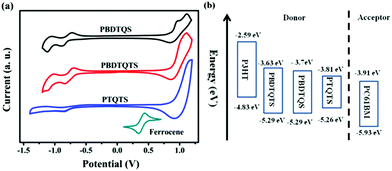 | ||
| Fig. 3 (a) Cyclic voltammograms of the copolymer thin films on Pt electrodes in 0.1 M Bu4NPF6–CH3CN at a scan rate of 50 mV s−1, (b) energy level diagrams of the compounds in BHJ device. | ||
| Polymer | Solution | Film | HOMOb (eV) | LUMOb (eV) |
E
g![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c (eV) c (eV) |
|||
|---|---|---|---|---|---|---|---|---|
| λ max (nm) | λ max (nm) |
E
optg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a (eV) a (eV) |
||||||
| a Band gap estimated from the optical absorption band edge of the film. b HOMO and LUMO levels were estimated from the onset of the oxidation and reduction peaks of cyclic voltammogram. c Electrochemical band gap calculated from the difference of HOMO and LUMO. | ||||||||
| PBDTQS | 374 | 576 | 381 | 591 | 1.77 | −5.29 | −3.63 | 1.66 |
| PBDTQTS | 404 | 628 | 408 | 630 | 1.70 | −5.29 | −3.7 | 1.59 |
| PTQTS | 404 | 628 | 382 | 674 | 1.58 | −5.26 | −3.72 | 1.44 |
Photovoltaic properties
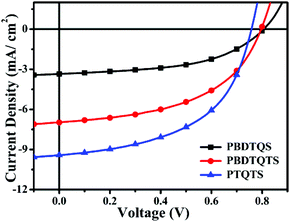
Current density versus voltage (J–V) curves of the PSCs based on PBDTQS, PBDTQTS and PTQTS were shown in Fig. 4. The PSCs devices were fabricated with a typical configuration of ITO/PEDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS/polymers
PSS/polymers![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC61BM/Ca/Al (PEDOT
PC61BM/Ca/Al (PEDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS = poly(3,4-ethylenedioxythiophene)
PSS = poly(3,4-ethylenedioxythiophene)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) poly(styrenesulfonate)). The copolymer film blended with the acceptor PC61BM, which was spin-coated from a CB solution, was used as the active layer of the photovoltaic device. The weight ratios of PBDTQS, PBDTQTS and PTQTS to PC61BM varied from 1
poly(styrenesulfonate)). The copolymer film blended with the acceptor PC61BM, which was spin-coated from a CB solution, was used as the active layer of the photovoltaic device. The weight ratios of PBDTQS, PBDTQTS and PTQTS to PC61BM varied from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 1
1 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 for device optimization; the Voc, Jsc, FF, PCE values of these devices under the illumination of AM 1.5 G 100 mW cm−2 are collected in Table 2. A blend ratio of 1
3 for device optimization; the Voc, Jsc, FF, PCE values of these devices under the illumination of AM 1.5 G 100 mW cm−2 are collected in Table 2. A blend ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 was found to give the best results for all polymers. The three polymers exhibited the similar Voc from 0.81 to 0.76 V, which were higher than those of the reported quinoxaline derivatives without alkylthio substitute groups.27,28 PBDTQS showed PCE of 1.37% with high Voc of 0.81 V, Jsc of 3.35 mA cm−2, and FF of 50.72%. Although the highest Voc (0.81 V) was obtained in the resulting copolymers, the device based on PBDTQS exhibited the lowest PCE of 1.37%. PBDTQTS obtained PCE of 2.78% with high Voc of 0.80 V, Jsc of 6.96 mA cm−2, and FF of 50.28%. Compared with PBDTQS, PBDTQTS showed slightly better photovoltaic performance and a PCE of 2.78% was obtained due to the extended π-conjugated system and the improved planarity of backbone. In addition, the introduction of a thiophene ring could also improve the absorption capability of polymer films. Therefore, the Jsc of the device based on PBDTQTS was increased from 3.61 to 6.96 mA cm−2, which led to an improved PCE (from 1.37% to 2.78%). Compared to PBDTQS and PBDTQTS, PTQTS gave the best PCE of 3.73% with Voc of 0.76 V, Jsc of 9.41 mA cm−2 and FF of 52.3%. This was mainly due to the increased light absorption in long wavelength range of this polymer. It could be seen that replacing the BDT unit in PBDTQS and PBDTQTS with a smaller thiophene unit could reduce the steric hindrance of the backbone of PTQTS, which leads to an enhanced intermolecular π–π stacking. Thus, the Jsc and FF of PTQTS were obviously improved compared with PBDTQS and PBDTQTS. The device performance of our polymer PTQTS, especially the Voc value, was lower than that of the reported polymer TQ1 by Ergang Wang's group,28 which was mainly due to the higher HOMO level of polymer PTQTS because the thiophene substitute group on quinoxaline unit may have stronger electron-donating nature than the phenyl group in TQ1.48
2 was found to give the best results for all polymers. The three polymers exhibited the similar Voc from 0.81 to 0.76 V, which were higher than those of the reported quinoxaline derivatives without alkylthio substitute groups.27,28 PBDTQS showed PCE of 1.37% with high Voc of 0.81 V, Jsc of 3.35 mA cm−2, and FF of 50.72%. Although the highest Voc (0.81 V) was obtained in the resulting copolymers, the device based on PBDTQS exhibited the lowest PCE of 1.37%. PBDTQTS obtained PCE of 2.78% with high Voc of 0.80 V, Jsc of 6.96 mA cm−2, and FF of 50.28%. Compared with PBDTQS, PBDTQTS showed slightly better photovoltaic performance and a PCE of 2.78% was obtained due to the extended π-conjugated system and the improved planarity of backbone. In addition, the introduction of a thiophene ring could also improve the absorption capability of polymer films. Therefore, the Jsc of the device based on PBDTQTS was increased from 3.61 to 6.96 mA cm−2, which led to an improved PCE (from 1.37% to 2.78%). Compared to PBDTQS and PBDTQTS, PTQTS gave the best PCE of 3.73% with Voc of 0.76 V, Jsc of 9.41 mA cm−2 and FF of 52.3%. This was mainly due to the increased light absorption in long wavelength range of this polymer. It could be seen that replacing the BDT unit in PBDTQS and PBDTQTS with a smaller thiophene unit could reduce the steric hindrance of the backbone of PTQTS, which leads to an enhanced intermolecular π–π stacking. Thus, the Jsc and FF of PTQTS were obviously improved compared with PBDTQS and PBDTQTS. The device performance of our polymer PTQTS, especially the Voc value, was lower than that of the reported polymer TQ1 by Ergang Wang's group,28 which was mainly due to the higher HOMO level of polymer PTQTS because the thiophene substitute group on quinoxaline unit may have stronger electron-donating nature than the phenyl group in TQ1.48
Polymer![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC61BM PC61BM |
w/w | V oc (V) | J sc (mA cm−2) | FF | PCE (%) |
|---|---|---|---|---|---|
| PBDTQS | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.80 | 2.44 | 44.43 | 0.86 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
0.81 | 3.35 | 50.72 | 1.37 | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
0.81 | 3.25 | 50.35 | 1.32 | |
| PBDTQTS | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.82 | 4.57 | 38.12 | 1.43 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
0.80 | 6.96 | 50.28 | 2.78 | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
0.75 | 5.39 | 55.95 | 2.26 | |
| PTQTS | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.77 | 9.43 | 48.80 | 3.54 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
0.76 | 9.41 | 52.33 | 3.73 | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
0.75 | 7.20 | 59.52 | 3.22 |
To verify the accuracy of the J–V measurements, the corresponding external quantum efficiencies (EQE) of the devices were measured and shown in Fig. 5. All the three polymers displayed broadened absorption from 350–800 nm. The optimum device PTQTS/PC61BM very efficiently harvests solar light with the maximum EQE of 61.8% at 420 nm, and exhibits a much better photo-response in the range from 350 to 550 nm. Moreover, the calculated Jsc, by integrating the spectral response of the cells, were 3.28, 6.91, and 9.25 for PBDTQS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC61BM, PBDTQTS
PC61BM, PBDTQTS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC61BM and PTQTS
PC61BM and PTQTS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC61BM, respectively. The EQE spectra of the polymers
PC61BM, respectively. The EQE spectra of the polymers![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC61BM (w
PC61BM (w![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) w, 1
w, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) based device agreed well with the absorption spectra of the blends, and the highest EQE supported its highest Jsc value.
2) based device agreed well with the absorption spectra of the blends, and the highest EQE supported its highest Jsc value.
 | ||
Fig. 5 EQE curves of the photovoltaic cells with polymers![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC61BM system illuminated by monochromatic light. PC61BM system illuminated by monochromatic light. | ||
Morphology
In order to understand the effect of active layer morphology on the device performance of PBDTQS, PBDTQTS and PTQTS, tapping mode height images (5 μm × 5 μm) and phase images (5 μm × 5 μm) of copolymers![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC61BM blend films were investigated by atomic force microscopy (AFM). As shown in Fig. 6, the surface of PBDTQS
PC61BM blend films were investigated by atomic force microscopy (AFM). As shown in Fig. 6, the surface of PBDTQS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC61BM blend film exhibited a root-mean-square roughness (RMS) of 2.09 nm and a large phase separation, which was not favorable for the charge transfer from the polymer to PC61BM, thus limited the efficiency of the resulting device.47 The surfaces of PBDTQTS
PC61BM blend film exhibited a root-mean-square roughness (RMS) of 2.09 nm and a large phase separation, which was not favorable for the charge transfer from the polymer to PC61BM, thus limited the efficiency of the resulting device.47 The surfaces of PBDTQTS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC61BM and PTQTS
PC61BM and PTQTS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC61BM blend films spin-coated from CB showed relatively smooth surfaces with RMS of 1.51 and 1.80 nm, respectively. Consequently, the rougher surface of the PBDTQS PC61BM blend film may affect the contact situation between the active layer and other layer of the PBDTQS based device, which may further influence the performance of the device.49,50
PC61BM blend films spin-coated from CB showed relatively smooth surfaces with RMS of 1.51 and 1.80 nm, respectively. Consequently, the rougher surface of the PBDTQS PC61BM blend film may affect the contact situation between the active layer and other layer of the PBDTQS based device, which may further influence the performance of the device.49,50
Hole mobility
In addition to the absorption and energy levels, charge carrier mobility is another crucial factor for achieving high-efficiency devices. The hole mobilities of the three polymers were measured using the space-charge-limited current (SCLC) method, with the device structure of ITO/PEDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS/polymers
PSS/polymers![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC61BM/Au (as shown in Fig. 7). The SCLC is described as
PC61BM/Au (as shown in Fig. 7). The SCLC is described as | (1) |
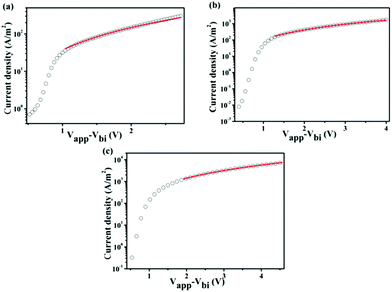 | ||
| Fig. 7 Current voltage characteristics of the SCLC devices of these three copolymer blends: PBDTQS, PBDTQTS and PTQTS, respectively. | ||
Conclusions
In summary, three copolymers (PBDTQS, PBDTQTS and PTQTS) with alkylthio side chain on the quinoxaline acceptor were designed and synthesized. The UV-vis absorptions, thermal stability, energy levels, morphology and photovoltaic characteristics of the three copolymers were systematically evaluated to understand the relationships between the polymer structures and the photovoltaic performances. Replacing the carbon atom in the side chain of polymer PBDTQS with a thiophene ring could increase the conjugation length in the side chain direction and improve the absorption in the visible region for the copolymers PBDTQTS and PTQTS. Furthermore, polymer PTQTS exhibited a more planar backbone and increased intermolecular π-stacking compared to PBDTQTS, which was due to the thiophene unit in PTQTS having a smaller size and less steric hindrance than those of BDT unit in PBDTQTS. The optimized polymer solar cell based on PTQTS showed a PCE of 3.73% with a Voc of 0.76 V, a Jsc of 9.41 mA cm−2 and a FF of 52.33% under AM 1.5 G 100 mW cm−2, which is the best performance among these three copolymers. The results indicated that the polymer PTQTS with thiophene as the donor unit and QTS as the acceptor unit in the main chain, would be a promising donor candidate in the application of polymer solar cells.Experimental section
Materials
All reagents and starting materials were purchased from Energy Chemical, J&K, AlfaAesar and used without further purification, unless otherwise noted. 3,6-Dibromobenzene-1,2-diamine (compound 1 in Scheme 1),38 1,4-dibromobutane-2,3-dione (compound 2 in Scheme 1),39 2-(octylthio)thiophene (compound 5 in Scheme 1)40 were synthesized according to the methods reported in the literature. All air and water sensitive reactions were performed under argon atmosphere. Toluene, tetrahydrofuran (THF), ether were dried over Na/benzophenone and freshly distilled prior to use.Characterization
1H NMR and 13C NMR spectra were recorded on a Bruker DRX-600 spectrometer. APCI-TOF HRMS were performed on Bruker Maxis UHR-TOF (both positive and negative ion reflector mode). The ultraviolet-visible (UV-vis) spectra were measured on a Varian Cary 50 spectrophotometer. Cyclic voltammetry (CV) was performed on a CHI660D electrochemical workstation with a solution of tetrabutylammonium hexafluorophosphate (Bu4NPF6) (0.1M) in acetonitrile. A three-electrode cell consisting of a glassy carbon working electrode, a Pt counter electrode and a saturated calomel reference electrode (SCE) was used with a 100 mV s−1 scan rate. The potential of ferrocene/ferrocenium (Fc/Fc+) was measured to be 0.4 V compared to the SCE electrode under the same conditions. It is assumed that the redox potential of Fc/Fc+ has an absolute energy level of −4.8 eV to vacuum. AFM images were acquired with an Agilent 5400 scanning probe microscope with a nanodrive controller in tapping mode. Thermal gravimetric analysis (TGA) was performed by an ATA409 at a heating rate of 10 °C min−1, under the protection of N2 atmosphere. The molecular weights of polymers were measured by the GPC method, and polystyrene was used as the standard and THF as eluent.Fabrication and characterization of polymer solar cells
PSC devices with the typical structure of ITO/PEDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS/polymers
PSS/polymers![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC61BM/Ca/Al were fabricated under the similar conditions as follows: all cells were fabricated on ITO coated glass substrates with a nominal sheet resistance of 15 Ω per sq. The ITO-coated glass substrates were sequentially washed by ultrasonication in detergent, ultra-pure water, acetone, and isopropyl alcohol for 20 min. The substrates were then oxygen plasma treated for 20 min, spin-coated with a 30 nm layer of PEDOT
PC61BM/Ca/Al were fabricated under the similar conditions as follows: all cells were fabricated on ITO coated glass substrates with a nominal sheet resistance of 15 Ω per sq. The ITO-coated glass substrates were sequentially washed by ultrasonication in detergent, ultra-pure water, acetone, and isopropyl alcohol for 20 min. The substrates were then oxygen plasma treated for 20 min, spin-coated with a 30 nm layer of PEDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS at 4000 rpm, and dried in air for 20 min at 150 °C. The polymers and PC61BM were dissolved in deoxygenated anhydrous chlorobenzene in the weight ratios of 1
PSS at 4000 rpm, and dried in air for 20 min at 150 °C. The polymers and PC61BM were dissolved in deoxygenated anhydrous chlorobenzene in the weight ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, and 1
2, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, respectively, and stirred overnight in a glovebox filled with N2. Typical concentration of the polymers/PC61BM blending solution was 24 mg ml−1. An active layer consisting of the blend of polymer and PC61BM was then spin coated on PEDOT
3, respectively, and stirred overnight in a glovebox filled with N2. Typical concentration of the polymers/PC61BM blending solution was 24 mg ml−1. An active layer consisting of the blend of polymer and PC61BM was then spin coated on PEDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS with a thickness of about 80 nm. Subsequently, Ca (10 nm) and Al (100 nm) were thermally evaporated under a vacuum of 2 × 10−4 Pa on the surface of active layer as a cathode. The cell's active area was 0.1 cm2. The current density–voltage (J–V) characteristics were recorded with a Keithley 2420 source measurement unit under simulated AM 1.5 G, 100 mW cm−2 irradiation from a Newport solar simulator. Light intensity was calibrated with a standard silicon solar cell. The external quantum efficiencies (EQE) of solar cells were analyzed using a certified Newport incident photon conversion efficiency (IPCE) measurement system.
PSS with a thickness of about 80 nm. Subsequently, Ca (10 nm) and Al (100 nm) were thermally evaporated under a vacuum of 2 × 10−4 Pa on the surface of active layer as a cathode. The cell's active area was 0.1 cm2. The current density–voltage (J–V) characteristics were recorded with a Keithley 2420 source measurement unit under simulated AM 1.5 G, 100 mW cm−2 irradiation from a Newport solar simulator. Light intensity was calibrated with a standard silicon solar cell. The external quantum efficiencies (EQE) of solar cells were analyzed using a certified Newport incident photon conversion efficiency (IPCE) measurement system.
Syntheses of the monomers and polymers
MS (MALDI-TOF): calcd for C26H40N2Br2S2 [M+], 604.10; found 604.11.
MS (MALDI-TOF): calcd for C32H40N2Br2S2 [M+], 740.04; found 740.05.
Gel permeation chromatography (tetrahydrofuran, polystyrene standard, 25 °C): number-average molecular weight = 23.1 kDa, polydispersity index = 1.91.
Acknowledgements
This work was supported by National Natural Science Foundation of China (21204097, 51173199 and 61107090), Ministry of Science and Technology of China (2014CB643501, 2010DFA52310), Shandong Provincial Natural Science Foundation (ZR2011BZ007), Qingdao Municipal Science and Technology Program (14-2-4-28-jch and 11-2-4-22-hz).Notes and references
- J. Chen and Y. Cao, Acc. Chem. Res., 2009, 42, 1709–1718 CrossRef CAS PubMed.
- G. Yu, J. Gao, J. Hummelen, F. Wudl and A. Heeger, Science, 1995, 270, 1789–1790 CAS.
- G. Y. Chen, S. C. Lan, P. Y. Lin, C. W. Chu and K. H. Wei, J. Polym. Sci., Part A: Polym. Chem., 2010, 48, 4456–4464 CrossRef CAS.
- H. Wu, B. Qu, Z. Cong, H. Liu, D. Tian, B. Gao, Z. An, C. Gao, L. Xiao, Z. Chen, H. Liu, Q. Gong and W. Wei, React. Funct. Polym., 2012, 72, 897–903 CrossRef CAS PubMed.
- X. X. Sun, W. C. Chen, Z. K. Du, X. C. Bao, G. N. Song, K. Q. Guo, N. Wang and R. Q. Yang, Polym. Chem., 2013, 4, 1317–1322 RSC.
- Y. Li, Acc. Chem. Res., 2012, 45, 723–733 CrossRef CAS PubMed.
- B. S. Rolczynski, J. M. Szarko, H. J. Son, Y. Liang, L. Yu and L. X. Chen, J. Am. Chem. Soc., 2012, 134, 4142–4152 CrossRef CAS PubMed.
- Q. Liu, X. Bao, S. Wen, Z. Du, L. Han, D. Zhu, Y. Chen, M. Sun and R. Yang, Polym. Chem., 2014, 5, 2076–2082 RSC.
- C. J. Brabec, N. S. Sariciftci and J. C. Hummelen, Adv. Funct. Mater., 2001, 11, 15–26 CrossRef CAS.
- X. Guo, C. Cui, M. Zhang, L. Huo, Y. Huang, J. Hou and Y. Li, Energy Environ. Sci., 2012, 5, 7943 CAS.
- C. C. Cui, W. Y. Wong and Y. F. Li, Energy Environ. Sci., 2014, 7, 2276–2284 CAS.
- C. Piliego, T. W. Holcombe, J. D. Douglas, C. H. Woo, P. M. Beaujuge and J. M. Frechet, J. Am. Chem. Soc., 2010, 132, 7595–7597 CrossRef CAS PubMed.
- H. Zhou, L. Yang, A. C. Stuart, S. C. Price, S. Liu and W. You, Angew. Chem., Int. Ed., 2011, 50, 2995–2998 CrossRef CAS PubMed.
- L.-M. Chen, Z. Hong, G. Li and Y. Yang, Adv. Mater., 2009, 21, 1434–1449 CrossRef CAS.
- Z. He, C. Zhang, X. Xu, L. Zhang, L. Huang, J. Chen, H. Wu and Y. Cao, Adv. Mater., 2011, 23, 3086–3089 CrossRef CAS PubMed.
- H. J. Son, B. Carsten, I. H. Jung and L. Yu, Energy Environ. Sci., 2012, 5, 8158–8170 CAS.
- Y. P. Zou, A. Najari, P. Berrouard, S. Beaupre, B. R. Aich, Y. Tao and M. Leclerc, J. Am. Chem. Soc., 2010, 132, 5330–5331 CrossRef CAS PubMed.
- A. Gadisa, W. Mammo, L. M. Andersson, S. Admassie, F. Zhang, M. R. Andersson and O. Inganäs, Adv. Funct. Mater., 2007, 17, 3836–3842 CrossRef CAS.
- J. You, L. Dou, K. Yoshimura, T. Kato, K. Ohya, T. Moriarty, K. Emery, C. C. Chen, J. Gao, G. Li and Y. Yang, Nat. Commun., 2013, 4, 1446 CrossRef PubMed.
- C. C. Chen, W. H. Chang, K. Yoshimura, K. Ohya, J. You, J. Gao, Z. Hong and Y. Yang, Adv. Mater., 2014, 26, 5670–5677 CrossRef CAS PubMed.
- J. Hou, Z. Tan, Y. Yan, Y. He, C. Yang and Y. Li, J. Am. Chem. Soc., 2006, 128, 4911–4916 CrossRef CAS PubMed.
- Y. Li and Y. Zou, Adv. Mater., 2008, 20, 2952–2958 CrossRef CAS.
- L. Han, X. Bao, T. Hu, Z. Du, W. Chen, D. Zhu, Q. Liu, M. Sun and R. Yang, Macromol. Rapid Commun., 2014, 35, 1153–1157 CrossRef CAS PubMed.
- Z. Du, Y. Chen, W. Chen, S. Qiao, S. Wen, Q. Liu, D. Zhu, M. Sun and R. Yang, Chem. – Asian J., 2014, 9, 2621–2627 CrossRef CAS PubMed.
- Y. Chen, Z. Du, W. Chen, Q. Liu, L. Sun, M. Sun and R. Yang, Org. Electron., 2014, 15, 405–413 CrossRef CAS PubMed.
- L. Huo, S. Zhang, X. Guo, F. Xu, Y. Li and J. Hou, Angew. Chem., Int. Ed., 2011, 50, 9697–9702 CrossRef CAS PubMed.
- R. Duan, L. Ye, X. Guo, Y. Huang, P. Wang, S. Zhang, J. Zhang, L. Huo and J. Hou, Macromolecules, 2012, 45, 3032–3038 CrossRef CAS.
- E. Wang, L. Hou, Z. Wang, S. Hellstrom, F. Zhang, O. Inganas and M. R. Andersson, Adv. Mater., 2010, 22, 5240–5244 CrossRef CAS PubMed.
- B. Gao, C. Gao, H. Wu, W. Que and W. Wei, Mater. Lett., 2014, 122, 74–77 CrossRef CAS PubMed.
- R. Kroon, A. Lundin, C. Lindqvist, P. Henriksson, T. T. Steckler and M. R. Andersson, Polymer, 2013, 54, 1285–1288 CrossRef CAS PubMed.
- J. Mei and Z. Bao, Chem. Mater., 2014, 26, 604–615 CrossRef CAS.
- T. Lei, J.-Y. Wang and J. Pei, Chem. Mater., 2014, 26, 594–603 CrossRef CAS.
- R. M. Mairo Souto, K. Hinkelmann, H. Eckert and F. Wudl, Macromolecules, 1990, 23, 1268–1279 CrossRef.
- G. Chen, H. Sasabe, Y. Sasaki, H. Katagiri, X.-F. Wang, T. Sano, Z. Hong, Y. Yang and J. Kido, Chem. Mater., 2014, 26, 1356–1364 CrossRef CAS.
- S. Zhang, L. Ye, W. Zhao, D. Liu, H. Yao and J. Hou, Macromolecules, 2014, 47, 4653–4659 CrossRef CAS.
- W. Y. Zhou, F. Jin, X. B. Huang, X. M. Duan and X. W. Zhan, Macromolecules, 2012, 45, 7823–7828 CrossRef CAS.
- J. A. Schneider, A. Dadvand, W. Wen and D. F. Perepichka, Macromolecules, 2013, 46, 9231–9239 CrossRef CAS.
- C. Pozo-Gonzalo, T. Khan, J. J. W. McDouall, P. J. Skabara, D. M. Roberts, M. E. Light, S. J. Coles, M. B. Hursthouse, H. Neugebauer, A. Cravino and N. S. Sariciftci, J. Mater. Chem., 2002, 12, 500–510 RSC.
- L. Huo, Y. Zhou and Y. Li, Macromol. Rapid Commun., 2009, 30, 925–931 CrossRef CAS PubMed.
- D. Lee, S. W. Stone and J. P. Ferraris, Chem. Commun., 2011, 47, 10987–10989 RSC.
- D. Lee, E. Hubijar, G. J. D. Kalaw and J. P. Ferraris, Chem. Mater., 2012, 24, 2534–2540 CrossRef CAS.
- R. Yang, R. Tian, J. Yan, Y. Zhang, J. Yang, Q. Hou, W. Yang, C. Zhang and Y. Cao, Macromolecules, 2005, 38, 244–253 CrossRef CAS.
- G. Buchi, E. Demole and A. F. Thomas, J. Org. Chem., 1973, 38, 123–125 CrossRef.
- Y. Huang, L. Huo, S. Zhang, X. Guo, C. C. Han, Y. Li and J. Hou, Chem. Commun., 2011, 47, 8904–8906 RSC.
- J. L. Bredas, R. Silbey, D. S. Boudreaux and R. R. Chance, J. Am. Chem. Soc., 1983, 105, 6555–6559 CrossRef CAS.
- S. C. Price, A. C. Stuart, L. Yang, H. Zhou and W. You, J. Am. Chem. Soc., 2011, 133, 4625–4631 CrossRef CAS PubMed.
- B. C. Thompson and J. M. Frechet, Angew. Chem., Int. Ed., 2008, 47, 58–77 CrossRef CAS PubMed.
- B. Sun, W. Hong, Z. Yan, H. Aziz and Y. Li, Adv. Mater., 2014, 2636–2642 CrossRef CAS PubMed.
- Y. Zhang, J. Zou, H.-L. Yip, K.-S. Chen, J. A. Davies, Y. Sun and A. K. Y. Jen, Macromolecules, 2011, 44, 4752–4758 CrossRef CAS.
- N. Wang, L. Sun, X. Zhang, X. Bao, W. Zheng and R. Yang, RSC Adv., 2014, 4, 25886–25891 RSC.
| This journal is © The Royal Society of Chemistry 2015 |