Doubly thermo-responsive nanoparticles constructed with two diblock copolymers prepared through the two macro-RAFT agents co-mediated dispersion RAFT polymerization†
Quanlong
Li
,
Xin
He
,
Yongliang
Cui
,
Pengfei
Shi
,
Shentong
Li
and
Wangqing
Zhang
*
Key Laboratory of Functional Polymer Materials of the Ministry of Education, State Key Laboratory and Institute of Elemento-Organic Chemistry, Collaborative Innovation Center of Chemical Science and Engineering (Tianjin), Institute of Polymer Chemistry, Nankai University, Tianjin 300071, China. E-mail: wqzhang@nankai.edu.cn; Fax: +86-22-23503510; Tel: +86-22-23509794
First published on 4th September 2014
Abstract
A new strategy to prepare doubly thermo-responsive nanoparticles constructed with the two diblock copolymers poly(N-isopropylacrylamide)-block-polystyrene (PNIPAM-b-PS) and poly[N,N-(dimethylamino) ethyl methacrylate]-block-polystyrene (PDMAEMA-b-PS) through the two macro-RAFT agents co-mediated dispersion polymerization is proposed. In this two macro-RAFT agents co-mediated dispersion polymerization, two macro-RAFT agents are simultaneously adopted, and the two in situ synthesized diblock copolymers PNIPAM-b-PS and PDMAEMA-b-PS co-assemble into nanoparticles containing a PNIPAM/PDMAEMA mixed corona and a common PS core. It is found that the molecular weight of PNIPAM-b-PS and PDMAEMA-b-PS in the mixed corona–core nanoparticles increases with the monomer conversion, and the size of the mixed corona–core nanoparticles increases with the PS block extension during the two macro-RAFT agents co-mediated dispersion polymerization. In water, the mixed corona–core nanoparticles exhibit two separate phase transition temperatures of 44 °C and 56 °C, corresponding to the PNIPAM and PDMAEMA blocks, respectively, which is confirmed by turbidity analysis, 1H NMR analysis and TEM observation. Our strategy named the two macro-RAFT agents co-mediated dispersion polymerization is believed to be a valid method to prepare multi-thermo-responsive nano-objects constructed with two or more than two thermo-responsive diblock copolymers.
1 Introduction
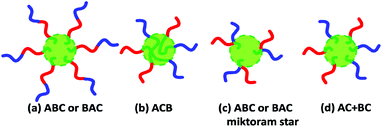
In recent years, thermo-responsive block copolymer nano-objects have aroused great attention because of their potential use in drug delivery, biological separation and tissue engineering scaffolds.1,2 Of all the thermo-responsive block copolymer nano-objects, the doubly thermo-responsive ones containing two thermo-responsive blocks are interesting.3–13 Based on the topology of block copolymers, four kinds of doubly thermo-responsive block copolymer nanoparticles as shown in Scheme 1 are generally clarified. In the first case of the linear ABC or BAC triblock terpolymer (note: A and B represent the two thermo-responsive blocks and C represents the solvophobic block throughout this article), two thermo-responsive blocks of A and B are located at the same side of the solvophobic core-forming C block, and the triblock terpolymer nanoparticles have corona–core structure. In the second case of the linear ACB triblock terpolymer, the thermo-responsive A block is located at one side and the thermo-responsive B block is located at the other side of the central solvophobic core-forming C block, and the corona–core nanoparticles have a mixed A/B corona. In the third case of the ABC or BAC miktoarm terpolymer, the two thermo-responsive blocks of A and B are joined at the same terminal of the core-forming C block. In the last case, the nanoparticles are constructed with the two thermo-responsive diblock copolymers AC and BC, in which the C block coming from both AC and BC forms the core and the two thermo-responsive blocks of A and B form the mixed corona.Up to now, most research has focused on the doubly thermo-responsive nano-objects of ABC and ACB triblock terpolymers through their micellization in the selective solvent for A and B blocks,3,4 and no example of the doubly thermo-responsive nano-objects of ABC or BAC miktoarm terpolymers has been reported, possibly due to the difficult or laborious synthesis of such complex structures. Compared with ABC triblock terpolymers or ABC miktoarm terpolymers,3,4,14–19 thermo-responsive AC or BC diblock copolymers can be conveniently prepared.20–23 However, preparation of well-defined nanoparticles constructed with the two diblock copolymers AC and BC by co-micellization or blending of AC and BC diblock copolymers is not an easy task,24–38 since this strategy unavoidably leads to nonergodic micelles constructed with one block copolymer.38 Besides, since the micelle exchange dynamics is generally very slow due to the high polymer molecular weight,35–38 translating nonergodic micelles into mixed micelles by inter-micelle macromolecular exchange is very difficult. Thus, it is necessary to discover a convenient synthesis of well-defined doubly thermo-responsive block copolymer nano-objects.
The macromolecular RAFT (macro-RAFT) agent mediated dispersion polymerization is demonstrated to be a valid method to prepare block copolymer nano-objects.39–50 Following this method, a soluble macro-RAFT agent, an initiator and a monomer are one-pot added, and polymerization under dispersion conditions is performed, and block copolymer nano-objects with polymer concentration up to 30% can be prepared. Recently, we have prepared doubly thermo-responsive ABC triblock terpolymer nanoparticles of poly(N-isopropylacrylamide)-block-poly[N,N-(dimethylamino) ethyl methacrylate]-block-polystyrene and poly[poly(ethylene glycol) methyl ether vinylphenyl]-block-poly(N-isopropylacrylamide)-block-polystyrene by dispersion RAFT polymerization,3,4 which show two separate phase transition temperatures (PTTs) in water. In this contribution, we focus on the convenient preparation of doubly thermo-responsive nanoparticles constructed with two diblock copolymers through a new strategy named the two macro-RAFT agents co-mediated dispersion polymerization. Different from the general macro-RAFT agent mediated dispersion polymerization,3,4,39–50 two different thermo-responsive macro-RAFT agents, namely poly(N-isopropylacrylamide) trithiocarbonate (PNIPAM-TTC) and poly[N,N-(dimethylamino) ethyl methacrylate] trithiocarbonate (PDMAEMA-TTC), are simultaneously adopted in the dispersion RAFT polymerization. This two macro-RAFT agents co-mediated dispersion polymerization affords the simultaneous synthesis of the two thermo-responsive diblock copolymers poly(N-isopropylacrylamide)-block-polystyrene (PNIPAM-b-PS) and poly[N,N-(dimethylamino) ethyl methacrylate]-block-polystyrene (PDMAEMA-b-PS) and their co-assembly into nanoparticles containing a mixed corona of PNIPAM/PDMAEMA and a PS core, which exhibit two separate PTTs of 44 °C and 56 °C in water.
2 Experimental
2.1 Materials
The monomer of N-isopropylacrylamide (NIPAM, >99%, Acros Organics) was purified by recrystallization in an acetone–n-hexane mixture (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 by volume). The monomer of N,N-(dimethylamino) ethyl methacrylate (DMAEMA, 98%, Alfa, Scheme S1†) was dried with CaH2 overnight and distilled under reduced pressure prior to use. Styrene (St, >98%, Tianjin Chemical Company) was distilled under vacuum and stored at −5 °C prior to use. 2,2′-Azobis(2-methylpropionitrile) (AIBN, >99%, Tianjin Chemical Company) was recrystallized from ethanol before being used. The RAFT agent 4-cyano-4-(dodecylsulfanylthiocarbonyl) sulfanyl pentanoic acid (CDTPA, Scheme S1†) was synthesized as discussed elsewhere.51 Other chemical reagents were of analytic grade and were used as received. Deionized water was used in the present experiments.
50 by volume). The monomer of N,N-(dimethylamino) ethyl methacrylate (DMAEMA, 98%, Alfa, Scheme S1†) was dried with CaH2 overnight and distilled under reduced pressure prior to use. Styrene (St, >98%, Tianjin Chemical Company) was distilled under vacuum and stored at −5 °C prior to use. 2,2′-Azobis(2-methylpropionitrile) (AIBN, >99%, Tianjin Chemical Company) was recrystallized from ethanol before being used. The RAFT agent 4-cyano-4-(dodecylsulfanylthiocarbonyl) sulfanyl pentanoic acid (CDTPA, Scheme S1†) was synthesized as discussed elsewhere.51 Other chemical reagents were of analytic grade and were used as received. Deionized water was used in the present experiments.
2.2 Synthesis of PNIPAM-TTC
The PNIPAM-TTC macro-RAFT agent was synthesized by solution RAFT polymerization. In a 100 mL Schlenk flask with a magnetic bar, NIPAM (10.0 g, 88.4 mmol), CDTPA (356.7 mg, 0.88 mmol), and AIBN (36.3 mg, 0.22 mmol) dissolved in 1,4-dioxane (35.0 g) were added. The solution was degassed with nitrogen at 0 °C, and then the flask content was immersed in a preheated oil bath at 65 °C for 150 min. The polymerization was quenched by rapid cooling upon immersion of the flask in iced water. A monomer conversion of 92% was determined by 1H NMR analysis. The synthesized polymer was precipitated in iced diethyl ether and then dried under vacuum at room temperature overnight to afford a yellow powder of PNIPAM-TTC (8.4 g, 87% yield).2.3 Synthesis of PDMAEMA-TTC
The PDMAEMA-TTC macro-RAFT agent was synthesized by RAFT polymerization in 1,4-dioxane using AIBN as an initiator and CDTPA as a RAFT agent. In a 50 mL Schlenk flask with a magnetic bar, DMAEMA (10.0 g, 6.37 mmol), CDTPA (0.321 g, 0.80 mmol), and AIBN (16.4 mg, 0.10 mmol) dissolved in 1,4-dioxane (10.0 g) were added. The solution was degassed with nitrogen at 0 °C, and then the flask content was immersed in a preheated oil bath at 70 °C for 4.5 h. The polymerization was quenched by rapid cooling upon immersion of the flask in iced water. To detect the monomer conversion, a drop of the polymerization solution was dripped into CDCl3 and subjected to 1H NMR analysis. The monomer conversion of 61% was calculated by comparing the integral areas of the protons of the double-bond peaks at δ = 5.56 ppm with reference to the proton peaks of the methylene at δ = 4.07 ppm. To collect the polymer, the flask content was precipitated in n-hexane at 0 °C, and dried under vacuum at room temperature overnight to afford a yellow powder of PDMAEMA-TTC (5.5 g, 53% yield).2.4 Two macro-RAFT agents co-mediated dispersion polymerization and synthesis of mixed corona–core nanoparticles
The two macro-RAFT agents co-mediated dispersion polymerization of styrene was performed in a 85/15 methanol–water mixture at 70 °C under [St]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [PNIPAM-TTC]0
[PNIPAM-TTC]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [PDMAEMA-TTC]0
[PDMAEMA-TTC]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [AIBN]0 = 1800
[AIBN]0 = 1800![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 with a constant weight ratio of the fed styrene monomer to the solvent at 15%. In a Schlenk flask with a magnetic bar, PDMAEMA-TTC (0.200 g, 0.020 mmol), PNIPAM-TTC (0.219 g, 0.020 mmol), St (1.250 g, 12.0 mmol), and AIBN (1.10 mg, 0.0066 mmol) dissolved in the 85/15 methanol–water mixture (8.32 g) were added. The solution was degassed with nitrogen at 0 °C, and then the polymerization was performed at 70 °C under vigorous stirring. After a given time, the polymerization was quenched by rapid cooling upon immersion of the flask in iced water to afford the mixed corona–core nanoparticles constructed with the two diblock copolymers PNIPAM-b-PS/PDMAEMA-b-PS.
1 with a constant weight ratio of the fed styrene monomer to the solvent at 15%. In a Schlenk flask with a magnetic bar, PDMAEMA-TTC (0.200 g, 0.020 mmol), PNIPAM-TTC (0.219 g, 0.020 mmol), St (1.250 g, 12.0 mmol), and AIBN (1.10 mg, 0.0066 mmol) dissolved in the 85/15 methanol–water mixture (8.32 g) were added. The solution was degassed with nitrogen at 0 °C, and then the polymerization was performed at 70 °C under vigorous stirring. After a given time, the polymerization was quenched by rapid cooling upon immersion of the flask in iced water to afford the mixed corona–core nanoparticles constructed with the two diblock copolymers PNIPAM-b-PS/PDMAEMA-b-PS.
The monomer conversion in the two macro-RAFT agents co-mediated dispersion polymerization was detected by UV-vis analysis at 245 nm as discussed elsewhere.49 To remove the residual St monomer in the colloidal dispersion, the colloidal dispersion was dialyzed against water at room temperature (20–25 °C) for three days (molecular weight cutoff: 7000 Da) to afford the aqueous dispersion of the mixed corona–core nanoparticles of PNIPAM-b-PS/PDMAEMA-b-PS (the polymer concentration at 1–10 wt% dependent on the monomer conversion). To collect the polymer for further gel permeation chromatography (GPC) analysis and 1H NMR analysis, part of the aqueous colloidal dispersion was extracted with dichloromethane, and then the organic phase was collected and dried over anhydrous magnesium sulfate overnight. After filtration of magnesium sulfate and removal of the solvent, the polymer was collected and dried under vacuum at room temperature overnight to afford a pale yellow powder of the PNIPAM-b-PS/PDMAEMA-b-PS mixture.
2.5 Characterizations
The GPC analysis was performed on a Waters 600E GPC system equipped with three TSK-GEL columns and a Waters 2414 refractive index detector, where THF containing 3 wt% triethylamine was used as an eluent at a flow rate of 0.5 mL min−1 at 30.0 °C and the narrow-polydispersity polystyrene (molecular weight: 500–280![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 Da) was used as the calibration standard, from which the polymer molecular weights Mn and Mw or its distribution Đ (Đ = Mw/Mn) were obtained. The triethylamine in the eluent of THF was to reduce the interaction of the N-containing polymer with the GPC columns as discussed elsewhere.52 The 1H NMR analysis was performed on a Bruker Avance III 400 MHz NMR spectrometer. For polymers dissolved in CDCl3, the proton signal at δ = 7.26 ppm of the internal solvent was used as the standard, and for polymers dissolved in D2O, the chemical shift of 1,3,5-trioxane at δ = 5.20 ppm was locked and the signal was used as the reference. The styrene monomer conversion in the dispersion RAFT polymerization was determined by UV-vis analysis, in which a given volume of the colloidal dispersion (1.0 mL) was filtered twice with a 0.22 μm nylon filter, diluted with alcohol, and analysed by UV-vis analysis at 245 nm. The PTT of the thermo-responsive polymers was determined by turbidity analysis at 500 nm on a Varian 100 UV-vis spectrophotometer equipped with a thermo-regulator (±0.1 °C) with a heating rate of 1 °C min−1. The PTT was determined at the middle point of the transmittance change. The transmission electron microscopy (TEM) observation was performed using a Tecnai G2 F20 electron microscope at an acceleration of 200 kV, whereby a small drop of the preheated dispersion of the synthesized nanoparticles was dripped onto a piece of preheated copper grid till the solvent was evaporated at a given temperature.
500 Da) was used as the calibration standard, from which the polymer molecular weights Mn and Mw or its distribution Đ (Đ = Mw/Mn) were obtained. The triethylamine in the eluent of THF was to reduce the interaction of the N-containing polymer with the GPC columns as discussed elsewhere.52 The 1H NMR analysis was performed on a Bruker Avance III 400 MHz NMR spectrometer. For polymers dissolved in CDCl3, the proton signal at δ = 7.26 ppm of the internal solvent was used as the standard, and for polymers dissolved in D2O, the chemical shift of 1,3,5-trioxane at δ = 5.20 ppm was locked and the signal was used as the reference. The styrene monomer conversion in the dispersion RAFT polymerization was determined by UV-vis analysis, in which a given volume of the colloidal dispersion (1.0 mL) was filtered twice with a 0.22 μm nylon filter, diluted with alcohol, and analysed by UV-vis analysis at 245 nm. The PTT of the thermo-responsive polymers was determined by turbidity analysis at 500 nm on a Varian 100 UV-vis spectrophotometer equipped with a thermo-regulator (±0.1 °C) with a heating rate of 1 °C min−1. The PTT was determined at the middle point of the transmittance change. The transmission electron microscopy (TEM) observation was performed using a Tecnai G2 F20 electron microscope at an acceleration of 200 kV, whereby a small drop of the preheated dispersion of the synthesized nanoparticles was dripped onto a piece of preheated copper grid till the solvent was evaporated at a given temperature.
3 Results and discussion
3.1 Synthesis of the PNIPAM-TTC and PDMAEMA-TTC macro-RAFT agents
The PNIPAM-TTC macro-RAFT agent was synthesized by solution RAFT polymerization of NIPAM in 1,4-dioxane at [NIPAM]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [CDTPA]0
[CDTPA]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [AIBN]0 = 400
[AIBN]0 = 400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
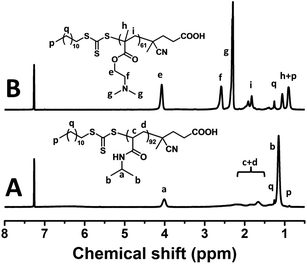
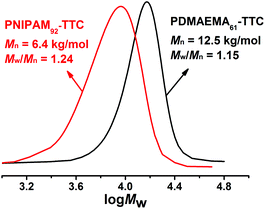
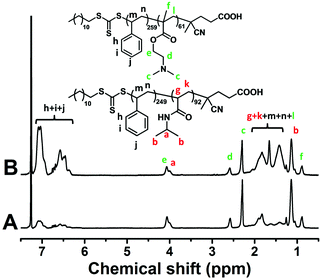
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The solution RAFT polymerization of NIPAM runs smoothly and 92% monomer conversion was achieved in 150 min. The synthesized PNIPAM-TTC was characterized by 1H NMR analysis (Fig. 1A) and GPC analysis (Fig. 2). Based on the proton resonance signals at δ = 0.88 ppm corresponding to the RAFT agent terminal and δ = 4.00 ppm corresponding to the polymer main chains shown in Fig. 1A, the molecular weight Mn,NMR of the synthesized PNIPAM-TTC of 9.6 kg mol−1 was calculated. The molecular weight Mn,GPC of PNIPAM-TTC by GPC analysis is 6.4 kg mol−1, and the molecular weight is narrowly dispersed with Đ = 1.24. It is found that Mn,NMR of PNIPAM-TTC is very close to the theoretical molecular weight Mn,th, which is 10.9 kg mol−1 as calculated by the monomer conversion following eqn (1) as described elsewhere.53 In the next discussion, PNIPAM-TTC is labeled as PNIPAM92-TTC, in which the polymerization degree (DP) is determined by Mn,th.
1. The solution RAFT polymerization of NIPAM runs smoothly and 92% monomer conversion was achieved in 150 min. The synthesized PNIPAM-TTC was characterized by 1H NMR analysis (Fig. 1A) and GPC analysis (Fig. 2). Based on the proton resonance signals at δ = 0.88 ppm corresponding to the RAFT agent terminal and δ = 4.00 ppm corresponding to the polymer main chains shown in Fig. 1A, the molecular weight Mn,NMR of the synthesized PNIPAM-TTC of 9.6 kg mol−1 was calculated. The molecular weight Mn,GPC of PNIPAM-TTC by GPC analysis is 6.4 kg mol−1, and the molecular weight is narrowly dispersed with Đ = 1.24. It is found that Mn,NMR of PNIPAM-TTC is very close to the theoretical molecular weight Mn,th, which is 10.9 kg mol−1 as calculated by the monomer conversion following eqn (1) as described elsewhere.53 In the next discussion, PNIPAM-TTC is labeled as PNIPAM92-TTC, in which the polymerization degree (DP) is determined by Mn,th.
The PDMAEMA-TTC macro-RAFT agent was synthesized by the RAFT polymerization of DMAEMA in 1,4-dioxane using CDTPA as a RAFT agent and AIBN as an initiator. The PDMAEMA-TTC macro-RAFT agent was also characterized by 1H NMR analysis (Fig. 1B) and GPC analysis (Fig. 2). The molecular weight Mn,NMR of the PDMAEMA-TTC macro-RAFT agent, 8.7 kg mol−1, was calculated by comparing the proton resonance signals at δ = 4.07 ppm and δ = 1.26 ppm. The molecular weight Mn,GPC of PDMAEMA-TTC by GPC analysis is 12.5 kg mol−1, and the molecular weight narrowly dispersed with Đ = 1.15. It is found that the molecular weight Mn,NMR of PDMAEMA-TTC by 1H NMR analysis is close to Mn,th, which is 10.0 kg mol−1 as calculated by the monomer conversion following eqn (1), and Mn,GPC is larger than Mn,th. The slight difference between Mn,NMR, Mn,GPC and Mn,th is possibly ascribed to the non-polar polystyrene standard employed in the GPC analysis. In the next discussion, PDMAEMA-TTC is labeled as PDMAEMA61-TTC, in which the DP is determined by Mn,th.
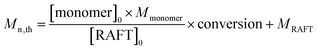 | (1) |
3.2 Two macro-RAFT agents co-mediated dispersion polymerization and synthesis of mixed corona–core nanoparticles
The two macro-RAFT agents co-mediated dispersion polymerization of styrene was performed in an 85/15 methanol–water mixture at [St]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [PNIPAM-TTC]0
[PNIPAM-TTC]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [PDMAEMA-TTC]0
[PDMAEMA-TTC]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [AIBN]0 = 1800
[AIBN]0 = 1800![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
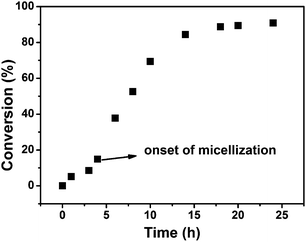
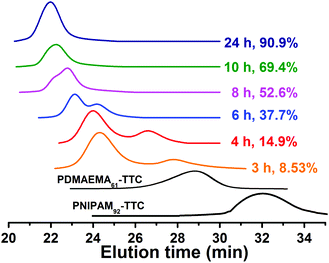
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The 85/15 methanol–water solvent mixture was chosen, since it is a good solvent for the two macro-RAFT agents of PNIPAM-TTC and PDMAEMA-TTC and the St monomer but is a non-solvent of the PS block, which is essential for the polymerization-induced self-assembly of PNIPAM-b-PS/PDMAEMA-b-PS. It was optically observed that the dispersion RAFT polymerization underwent an initial homogeneous stage below 4 h and a subsequent heterogeneous stage, which is very similar to the individual macro-RAFT agent mediated dispersion polymerizations reported by Armes and by our research group.41–49 This two-stage polymerization is due to the PNIPAM-b-PS/PDMAEMA-b-PS diblock copolymers synthesized in the initial stage being molecularly soluble in the polymerization medium and becoming insoluble with the extension of the solvophobic PS block in the later stage. As shown in Fig. 3, the monomer conversion increases steadily in the initial 4 h at the homogeneous stage and increases quickly to 84.3% in 14 h, and then increases slowly to 90.9% when the polymerization is extended to 24 h, which is very similar to those in the individual macro-RAFT agent mediated dispersion polymerization under similar conditions (Fig. S1†). The ln([M]0/[M]) vs. polymerization time plot is shown in Fig. S2,† in which a two-stage plot containing a gradient linear stage corresponding to the initial homogeneous polymerization and a steep linear one corresponding to the later heterogeneous polymerization is observed. This clearly suggests that the two macro-RAFT agents co-mediated dispersion RAFT polymerization undergoes a similar polymerization kinetics to those in the presence of an individual macro-RAFT agent.
1. The 85/15 methanol–water solvent mixture was chosen, since it is a good solvent for the two macro-RAFT agents of PNIPAM-TTC and PDMAEMA-TTC and the St monomer but is a non-solvent of the PS block, which is essential for the polymerization-induced self-assembly of PNIPAM-b-PS/PDMAEMA-b-PS. It was optically observed that the dispersion RAFT polymerization underwent an initial homogeneous stage below 4 h and a subsequent heterogeneous stage, which is very similar to the individual macro-RAFT agent mediated dispersion polymerizations reported by Armes and by our research group.41–49 This two-stage polymerization is due to the PNIPAM-b-PS/PDMAEMA-b-PS diblock copolymers synthesized in the initial stage being molecularly soluble in the polymerization medium and becoming insoluble with the extension of the solvophobic PS block in the later stage. As shown in Fig. 3, the monomer conversion increases steadily in the initial 4 h at the homogeneous stage and increases quickly to 84.3% in 14 h, and then increases slowly to 90.9% when the polymerization is extended to 24 h, which is very similar to those in the individual macro-RAFT agent mediated dispersion polymerization under similar conditions (Fig. S1†). The ln([M]0/[M]) vs. polymerization time plot is shown in Fig. S2,† in which a two-stage plot containing a gradient linear stage corresponding to the initial homogeneous polymerization and a steep linear one corresponding to the later heterogeneous polymerization is observed. This clearly suggests that the two macro-RAFT agents co-mediated dispersion RAFT polymerization undergoes a similar polymerization kinetics to those in the presence of an individual macro-RAFT agent.
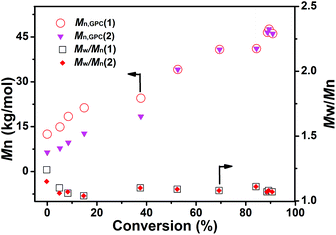
The polymer synthesized through the two macro-RAFT agents co-mediated dispersion polymerization at different polymerization times was characterized by GPC analysis and 1H NMR analysis. From the GPC traces shown in Fig. 4, bimodal GPC traces of the synthesized polymer below 8 h of polymerization, unimodal GPC traces after 8 h of polymerization, and a clear shift from low to high molar mass with increasing monomer conversion are observed. Fig. 5 shows the 1H NMR spectra of the typical polymers synthesized at 4 h and 24 h, from which the characteristic chemical shifts due to the PNIPAM block, the PDMAEMA block and the PS block are clearly discerned at the two cases of polymerization time, suggesting the formation of the two diblock copolymers PNIPAM-b-PS and PDMAEMA-b-PS. By checking the GPC traces of the two macro-RAFT agents and the two diblock copolymers PNIPAM-b-PS and PDMAEMA-b-PS synthesized in the initial RAFT polymerization, it is deemed that the right peak in the bimodal GPC traces is ascribed to PNIPAM-b-PS and the left peak is ascribed to PDMAEMA-b-PS. To further clarify which peak in the bimodal GPC traces belongs to PNIPAM-b-PS or PDMAEMA-b-PS, the separation of the individual diblock copolymers from the PNIPAM-b-PS/PDMAEMA-b-PS mixture was attempted. Since PDMAEMA and PS are soluble in iced diethyl ether, whereas PNIPAM is insoluble in iced diethyl ether, the separation of diblock copolymers by firstly dissolving the polymer in dichloromethane and then depositing in iced diethyl ether was performed (see the separation details in ESI†). However, successful separation was not achieved even after several dissolving/precipitation cycles. The unsuccessful separation is possibly due to the co-precipitation of PNIPAM-b-PS and PDMAEMA-b-PS in iced diethyl ether. Besides, since the PNIPAM-b-PS diblock copolymer containing a relatively long PS block is somewhat soluble in iced diethyl ether, this also causes trouble in the separation. However, by comparing the 1H NMR spectra and the GPC traces of the precipitate with those of the PNIPAM-b-PS/PDMAEMA-b-PS mixture before separation, weakening signals due to the PDMAEMA-b-PS diblock copolymer in both 1H NMR spectra and GPC traces have been detected (Fig. S3†), and therefore the above speculation is confirmed. Based on the GPC analysis, the molecular weight Mn,GPC of the individual diblock copolymers in the PNIPAM-b-PS/PDMAEMA-b-PS mixture prepared at different polymerization times and the molecular weight distribution, Đ, are obtained and summarized in Fig. 6. Note that in the case of the unimodal GPC traces, the same molecular weight Mn,GPC of PNIPAM-b-PS and PDMAEMA-b-PS is approximately assumed. It is found that the molecular weight of PNIPAM-b-PS or PDMAEMA-b-PS increases with increasing monomer conversion, which is similar to those in the individual macro-RAFT agent mediated polymerization.3,4,39–50
 | ||
| Fig. 4 The GPC traces of the synthesized PNIPAM-b-PS/PDMAEMA-b-PS mixture and the macro-RAFT agents of PNIPAM92-TTC and PDMAEMA61-TTC. | ||
 | ||
| Fig. 5 The 1H NMR spectra of the synthesized PNIPAM-b-PS/PDMAEMA-b-PS mixture at polymerization times of 4 h (A) and 24 h (B). | ||
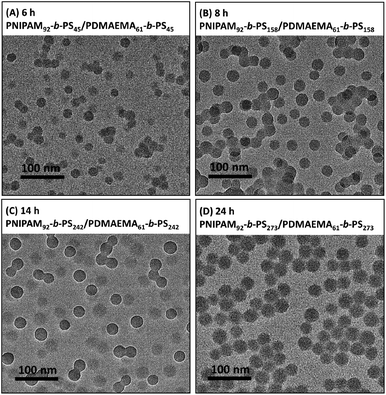
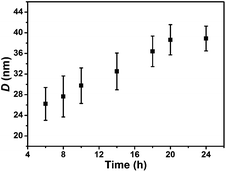
Fig. 7 shows the TEM images of the mixed corona–core nanoparticles dispersed in water prepared through the two macro-RAFT agents co-mediated dispersion polymerization, from which uniform nanoparticles are observed. By statistical analysis of the above 100 particles, the average diameter (D) of the mixed corona–core nanoparticles is obtained and the results are summarized in Fig. 8. The results show that the average diameter (D) of the mixed corona–core nanoparticles increases with the polymerization time, which is similar to those of diblock copolymer nanoparticles in the individual macro-RAFT agent mediated dispersion polymerization.3,4,46–49 The typical mixed corona–core nanoparticles of PNIPAM92-b-PS273/PDMAEMA61-b-PS273 are compared with the two nano-objects constructed with one individual diblock copolymer, the PNIPAM92-b-PS275 nanoparticles (Fig. S4A†) and the PDMAEMA61-b-PS265 nanoparticles (Fig. S4B†), which were prepared through the individual macro-RAFT agent mediated dispersion polymerization under the same conditions such as a constant polymerization time of 24 h at [St]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [macro-RAFT]0
[macro-RAFT]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [AIBN]0 = 1800
[AIBN]0 = 1800![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. It is found that the three nanoparticles have a similar diameter at 32–35 nm. This is not surprising, since the DP of the PS block in the three nanoparticles is very similar to each other.
1. It is found that the three nanoparticles have a similar diameter at 32–35 nm. This is not surprising, since the DP of the PS block in the three nanoparticles is very similar to each other.
3.3 Double thermo-response of the mixed corona–core nanoparticles
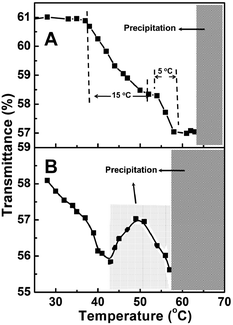
Since the mixed corona–core nanoparticles contain two thermo-responsive blocks of PNIPAM and PDMAEMA, their temperature-sensitive response in water is expected. Fig. 9A shows the temperature dependent transmittance of the aqueous dispersion of the typical mixed corona–core nanoparticles of PNIPAM92-b-PS273/PDMAEMA61-b-PS273, from which two PTTs of 44 °C and 56 °C have been observed, and therefore the double thermo-response of the mixed corona–core nanoparticles is concluded. By checking the thermo-response of the reference polymers PNIPAM92-TTC and PDMAEMA61-TTC (Fig. S5†), it is concluded that the first PTT of 44 °C is ascribed to the PNIPAM block and the second PTT of 56 °C is ascribed to the PDMAEMA block, respectively. Compared with the soluble-to-insoluble phase transition of the reference polymers PNIPAM92-TTC at 30 °C and PDMAEMA61-TTC at 42 °C, two differences in the thermo-response of the mixed corona–core nanoparticles are detected. First, the PTT of the PNIPAM block and the PDMAEMA block in the mixed corona–core nanoparticles is higher than those of the reference thermo-responsive polymers. Second, the phase transition of the PNIPAM block or the PDMAEMA block in the mixed corona–core nanoparticles occurs within a wide temperature range, e.g. 15 °C for the PNIPAM block and 5 °C for the PDMAEMA block, respectively. The reason for the increase in the PTTs of the PNIPAM and PDMAEMA blocks in the mixed corona–core nanoparticles is possibly due to the steric repulsion among the crowded polymer chains tethered on the PS core, which retards the soluble-to-insoluble transition of the tethered PNIPAM and PDMAEMA chains as discussed elsewhere.54The thermo-response of the mixed corona–core nanoparticles of PNIPAM92-b-PS273/PDMAEMA61-b-PS273 was further checked by variable temperature 1H NMR analysis. As shown by the 1H NMR spectra in Fig. 10, the proton signals assigned to the PNIPAM and PDMAEMA blocks are clearly discerned at a temperature of 35 °C, suggesting that both the PNIPAM and PDMAEMA blocks are soluble at this temperature below the PTT of the PNIPAM block. Note that the PS block is insoluble in D2O and a very faint signal is detected, and therefore is not discussed herein. When the temperature increases from 35 to 50 °C, a gradual decrease of the typical proton signal at δ = 3.86 ppm [f, CH(CH3)2] assigned to the PNIPAM block as indicated by a green rectangle in Fig. 10 is detected, suggesting the soluble-to-insoluble phase transition of the PNIPAM block. When the temperature further increases from 50 to 60 °C, a gradual decrease of the typical proton signal at δ = 4.16 ppm (c, COOCH2) assigned to the PDMAEMA block as indicated by a red rectangle is detected, suggesting the soluble-to-insoluble phase transition of the PDMAEMA block. These results confirm the two-step phase transition of the PNIPAM block and the PDMAEMA block in the mixed corona–core nanoparticles.
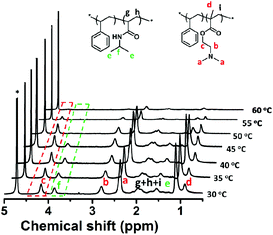 | ||
| Fig. 10 Temperature-dependent 1H NMR spectra of the mixed corona–core nanoparticles of PNIPAM92-b-PS273/PDMAEMA61-b-PS273 dispersed in D2O (* = D2O). | ||
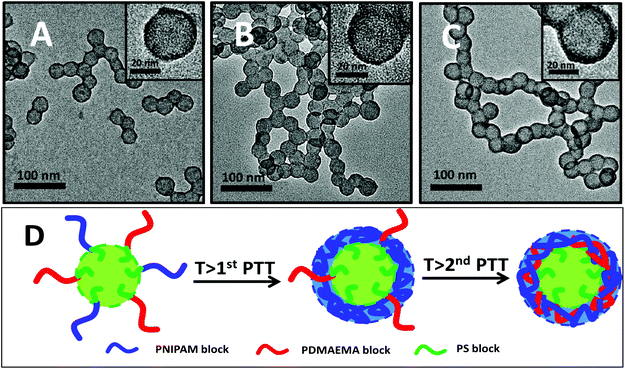
The thermo-response of the PNIPAM and PDMAEMA blocks is expected to lead to morphological changes of the mixed corona–core nanoparticles of PNIPAM92-b-PS273/PDMAEMA61-b-PS273. That is, in water at temperatures below the first PTT of the PNIPAM block, the PNIPAM and PDMAEMA blocks are soluble, and therefore the nanoparticles contain a solvophobic PS core and a PNIPAM/PDMAEMA mixed corona; at temperatures above the first PTT of the PNIPAM block, the PNIPAM block deposits onto the PS core and meanwhile the PDMAEMA block remains soluble; at temperatures above the second PTT of the PDMAEMA block, the PDMAEMA block further deposits onto the PS core, and therefore the nanoparticles contain a solvophobic PS core and an insoluble shell of PNIPAM/PDMAEMA. To detect the morphological changes, three samples of the diluted aqueous dispersion of the mixed corona–core nanoparticles were kept at temperatures of 25 °C (below the first PTT), 50 °C (above the first PTT) and 60 °C (above the second PTT) for 30 min, and then the morphology of the nanoparticles was checked by TEM. From the TEM images shown in Fig. 11, uniform nanoparticles with the diameters at 33 nm (Fig. 11A), 35 nm (Fig. 11B), and 36 nm (Fig. 11C) have been observed at three cases of temperature. Note that the solvophobic core-forming block is visible and the soluble corona-forming block is invisible in the TEM images as discussed elsewhere.55 This slight increase in the diameter of the nanoparticles possibly suggests the two-step soluble-to-insoluble phase transition of the PNIPAM block and the PDMAEMA block on the PS core. By assuming that the dark shell is constructed with the PNIPAM chains or the PNIPAM/PDMAEMA chains and the shell density is equal to the bulk polymer, the shell thickness of the nanoparticles, 1.0 nm at 50 °C and 1.8 nm at 60 °C, can be approximately calculated; thus the theoretical diameter of the nanoparticles, 35 nm at 50 °C (above the first PTT) and 36.6 nm at 60 °C (above the second PTT), is calculated, which is well consistent with those by TEM. Besides, a slight difference in the thickness of the dark periphery of the nanoparticles, 2 nm at 25 °C, 3 nm at 50 °C and 5 nm at 60 °C, is observed. Note that the dark periphery is ascribed to the deposited PNIPAM or PNIPAM/PDMAEMA chains, since they have relatively higher electron density compared with the PS block. Despite the approximate estimation of the dark periphery of the nanoparticles being seriously affected by the TEM sampling, an increase in the thickness of the dark periphery of the nanoparticles with increasing temperature is concluded. Based on the TEM observation, the thermo-response of the mixed corona–core nanoparticles as schematically shown in Fig. 11D is therefore concluded.
Lastly, the thermo-response of the mixed corona–core nanoparticles of PNIPAM92-b-PS273/PDMAEMA61-b-PS273 is compared with the mixture of PNIPAM92-b-PS275 nanoparticles and PDMAEMA61-b-PS265 nanoparticles. This mixture was prepared by blending the two samples of the aqueous dispersion of PNIPAM92-b-PS275 nanoparticles and PDMAEMA61-b-PS265 nanoparticles (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 by volume and 1/1 by polymer weight), in which the composition of the two diblock copolymers is similar to those in the mixed corona–core nanoparticles. As shown in Fig. 9B, the transmittance of the mixture of two diblock copolymer nanoparticles initially decreases from 28 °C to 43 °C, which can be ascribed to the phase transition of the PNIPAM block in the PNIPAM92-b-PS273 nanoparticles at a PTT of 36 °C. When the temperature increases from 43 °C to 49 °C, the colloids become unstable and a little precipitate deposited on the bottom of the glass cell is observed, which leads to the slight increase in the transmittance as shown in Fig. 9B. When the temperature increases from 49 °C to 57 °C, a phase transition of the PDMAEMA block in the PDMAEMA61-b-PS265 nanoparticles takes place and the transmittance decreases again as shown in Fig. 9B. A further increase in temperature leads to almost complete precipitation of the nanoparticles. By comparing the thermo-response of the mixed corona–core nanoparticles and the mixture of two individual diblock copolymer nanoparticles, two differences in the thermo-response are observed. First, the PTTs of the PNIPAM block and the PDMAEMA block in the mixed corona–core nanoparticles are higher than those in the mixture of two individual nanoparticles (44 °C vs. 36 °C for the PNIPAM block and 56 °C vs. 53 °C for the PDMAEMA block). The reason is possibly due to the much crowded corona-forming thermo-responsive chains on the PS core in the mixed corona–core nanoparticles, which makes the phase transition occur at a higher temperature in the mixed corona–core nanoparticles than in the mixture of two individual nanoparticles.54 Second, the mixed corona–core nanoparticles are more stable than the mixture of two individual nanoparticles at temperatures above the PTT of the PNIPAM block. For example, the mixed corona–core nanoparticles keep suspending in water at temperatures above PTT of the PNIPAM block, since the corona-forming PDMAEMA block remains soluble in water, whereas due to the dehydration of the PNIPAM block at this temperature, part of the PNIPAM92-b-PS275 nanoparticles in the mixture of two individual nanoparticles deposit as shown in Fig. 9. The different thermo-response further confirms that the mixed corona–core nanoparticles are constructed with two diblock copolymers.
1 by volume and 1/1 by polymer weight), in which the composition of the two diblock copolymers is similar to those in the mixed corona–core nanoparticles. As shown in Fig. 9B, the transmittance of the mixture of two diblock copolymer nanoparticles initially decreases from 28 °C to 43 °C, which can be ascribed to the phase transition of the PNIPAM block in the PNIPAM92-b-PS273 nanoparticles at a PTT of 36 °C. When the temperature increases from 43 °C to 49 °C, the colloids become unstable and a little precipitate deposited on the bottom of the glass cell is observed, which leads to the slight increase in the transmittance as shown in Fig. 9B. When the temperature increases from 49 °C to 57 °C, a phase transition of the PDMAEMA block in the PDMAEMA61-b-PS265 nanoparticles takes place and the transmittance decreases again as shown in Fig. 9B. A further increase in temperature leads to almost complete precipitation of the nanoparticles. By comparing the thermo-response of the mixed corona–core nanoparticles and the mixture of two individual diblock copolymer nanoparticles, two differences in the thermo-response are observed. First, the PTTs of the PNIPAM block and the PDMAEMA block in the mixed corona–core nanoparticles are higher than those in the mixture of two individual nanoparticles (44 °C vs. 36 °C for the PNIPAM block and 56 °C vs. 53 °C for the PDMAEMA block). The reason is possibly due to the much crowded corona-forming thermo-responsive chains on the PS core in the mixed corona–core nanoparticles, which makes the phase transition occur at a higher temperature in the mixed corona–core nanoparticles than in the mixture of two individual nanoparticles.54 Second, the mixed corona–core nanoparticles are more stable than the mixture of two individual nanoparticles at temperatures above the PTT of the PNIPAM block. For example, the mixed corona–core nanoparticles keep suspending in water at temperatures above PTT of the PNIPAM block, since the corona-forming PDMAEMA block remains soluble in water, whereas due to the dehydration of the PNIPAM block at this temperature, part of the PNIPAM92-b-PS275 nanoparticles in the mixture of two individual nanoparticles deposit as shown in Fig. 9. The different thermo-response further confirms that the mixed corona–core nanoparticles are constructed with two diblock copolymers.
4 Conclusions
Doubly thermo-responsive nanoparticles constructed with the two diblock copolymers PNIPAM-b-PS and PDMAEMA-b-PS are prepared through the two macro-RAFT agents co-mediated dispersion polymerization. In this two macro-RAFT agents co-mediated dispersion polymerization, two macro-RAFT agents are simultaneously adopted, and the two in situ synthesized thermo-response diblock copolymers PNIPAM-b-PS and PDMAEMA-b-PS are simultaneously co-assembled into mixed nanoparticles constructed with PNIPAM-b-PS and PDMAEMA-b-PS. It is found that the two macro-RAFT agents co-mediated dispersion polymerization undergoes a polymerization kinetics similarly to the individual macro-RAFT agent mediated dispersion polymerization. The molecular weight of PNIPAM-b-PS and PDMAEMA-b-PS in the mixed nanoparticles increases with the monomer conversion, and the size of the mixed nanoparticles increases with the PS block extension during the dispersion RAFT polymerization. In water, the mixed nanoparticles have a corona–core structure, in which the PNIPAM and PDMAEMA blocks form the mixed core, and the common PS block in the two diblock copolymers forms the core. The double thermo-response of the mixed corona–core nanoparticles is confirmed by turbidity analysis, 1H NMR analysis and TEM observation. Our strategy named the two macro-RAFT agents co-mediated dispersion polymerization is believed to be a valid method to prepare multi-thermo-responsive nano-objects constructed with two or more than two thermo-responsive diblock copolymers, which is still ongoing in our lab.Acknowledgements
The financial support provided by the National Science Foundation of China (no. 21274066 and 21474054) and PCSIRT (IRT1257) is gratefully acknowledged.Notes and references
- D. Roy, W. L. A. Brooks and B. S. Sumerlin, Chem. Soc. Rev., 2013, 42, 7214–7243 RSC.
- V. Aseyev, H. Tenhu and F. M. Winnik, Adv. Polym. Sci., 2011, 242, 29–89 CrossRef CAS.
- Q. Li, F. Huo, Y. Cui, C. Gao, S. Li and W. Zhang, J. Polym. Sci., Part A: Polym. Chem., 2014, 52, 2266–2278 CrossRef CAS.
- Q. Li, C. Gao, S. Li, F. Huo and W. Zhang, Polym. Chem., 2014, 5, 2961–2972 RSC.
- J. Xu, S. Luo, W. Shi and S. Liu, Langmuir, 2006, 22, 989–997 CrossRef CAS PubMed.
- H. Wei, S. Perrier, S. Dehn, R. Ravarian and F. Dehghani, Soft Matter, 2012, 8, 9526–9528 RSC.
- C. Li, N. J. Buurma, I. Haq, C. Turner, S. P. Armes, V. Castelletto, I. W. Hamley and A. L. Lewis, Langmuir, 2005, 21, 11026–11033 CrossRef CAS PubMed.
- F. D. Jochum, P. J. Roth, D. Kessler and P. Theato, Biomacromolecules, 2010, 11, 2432–2439 CrossRef CAS PubMed.
- P. J. Roth, T. P. Davis and A. B. Lowe, Macromolecules, 2012, 45, 3221–3230 CrossRef CAS.
- H.-N. Lee, Z. Bai, N. Newell and T. P. Lodge, Macromolecules, 2010, 43, 9522–9528 CrossRef CAS.
- S. Li, F. Huo, Q. Li, C. Gao, Y. Su and W. Zhang, Polym. Chem., 2014, 5, 3910–3918 RSC.
- M. Dan, Y. Su, X. Xiao, S. Li and W. Zhang, Macromolecules, 2013, 46, 3137–3146 CrossRef CAS.
- Y. Su, Q. Li, S. Li, M. Dan, F. Huo and W. Zhang, Polymer, 2014, 55, 1955–1963 CrossRef CAS PubMed.
- A. Hanisch, A. H. Gröschel, M. Förtsch, M. Drechsler, H. Jinnai, T. M. Ruhland, F. H. Schacher and A. H. E. Müller, ACS Nano, 2013, 7, 4030–4041 CrossRef CAS PubMed.
- C. Liu, M. A. Hillmyer and T. P. Lodge, Langmuir, 2008, 24, 12001–12009 CrossRef CAS PubMed.
- J. Mao, P. Ni, Y. Mai and D. Yan, Langmuir, 2007, 23, 5127–5134 CrossRef CAS PubMed.
- L. Wang, R. Xu, Z. Wang and X. He, Soft Matter, 2012, 8, 11462–11470 RSC.
- Z. Ge and S. Liu, Macromol. Rapid Commun., 2009, 30, 1523–1532 CrossRef CAS PubMed.
- Y. Zhang, H. Liu, J. Hu, C. Li and S. Liu, Macromol. Rapid Commun., 2009, 30, 941–947 CrossRef CAS PubMed.
- N. J. Warren, O. O. Mykhaylyk, D. Mahmood, A. J. Ryan and S. P. Armes, J. Am. Chem. Soc., 2014, 136, 1023–1033 CrossRef CAS PubMed.
- L. A. Fielding, J. A. Lane, M. J. Derry, O. O. Mykhaylyk and S. P. Armes, J. Am. Chem. Soc., 2014, 136, 5790–5798 CrossRef CAS PubMed.
- M. Topuzogullari, V. Bulmus, E. Dalgakiran and S. Dincer, Polymer, 2014, 55, 525–534 CrossRef CAS PubMed.
- I. Berndt, J. S. Pedersen and W. Richtering, Angew. Chem., Int. Ed., 2006, 45, 1737–1741 CrossRef CAS PubMed.
- L. Cheng, X. Lin, F. Wang, B. Liu, J. Zhou, J. Li and W. Li, Macromolecules, 2013, 46, 8644–8648 CrossRef CAS.
- X. Liu, H. Gao, F. Huang, X. Pei, Y. An, Z. Zhang and L. Shi, Polymer, 2013, 54, 3633–3640 CrossRef CAS PubMed.
- M. Štěpánek, K. Podhájecká, E. Tesarová, K. Procházka, Z. Tuzar and W. Brown, Langmuir, 2001, 17, 4240–4244 CrossRef.
- F. J. Esselink, E. E. Dormidontova and G. Hadziioannou, Macromolecules, 1998, 31, 4873–4878 CrossRef CAS PubMed.
- R. Zheng, G. Liu and X. Yan, J. Am. Chem. Soc., 2005, 127, 15358–15359 CrossRef CAS PubMed.
- F. Schacher, E. Betthausen, A. Walther, H. Schmalz, D. V. Pergushov and A. H. E. Müller, ACS Nano, 2009, 3, 2095–2102 CrossRef CAS PubMed.
- E. W. Price, Y. Guo, C.-W. Wang and M. G. Moffitt, Langmuir, 2009, 25, 6398–6406 CrossRef CAS PubMed.
- D. A. Christian, A. Tian, W. G. Ellenbroek, I. Levental, K. Rajagopal, P. A. Janmey, A. J. Liu, T. Baumgart and D. E. Discher, Nat. Mater., 2009, 8, 843–849 CrossRef CAS PubMed.
- J. Zhu and R. C. Hayward, Macromolecules, 2008, 41, 7794–7797 CrossRef CAS.
- J.-F. Gohy, N. Lefèvre, C. D'Haese, S. Hoeppener, U. S. Schubert, G. Kostov and B. Améduri, Polym. Chem., 2011, 2, 328–332 RSC.
- G. Srinivas and J. W. Pitera, Nano Lett., 2008, 8, 611–618 CrossRef CAS PubMed.
- T. Nicolai, O. Colombani and C. Chassenieux, Soft Matter, 2010, 6, 3111–3118 RSC.
- Z. Li, M. A. Hillmyer and T. P. Lodge, Macromolecules, 2006, 39, 765–771 CrossRef CAS.
- D. J. Pochan, J. Zhu, K. Zhang, K. L. Wooley, C. Miesch and T. Emrick, Soft Matter, 2011, 7, 2500–2506 RSC.
- S. I. Yoo, B.-H. Sohn, W.-C. Zin, J. C. Jung and C. Park, Macromolecules, 2007, 40, 8323–8328 CrossRef CAS.
- B. Charleux, G. Delaittre, J. Rieger and F. D'Agosto, Macromolecules, 2012, 45, 6753–6765 CrossRef CAS.
- J.-T. Sun, C.-Y. Hong and C.-Y. Pan, Soft Matter, 2012, 8, 7753–7767 RSC.
- N. J. Warren and S. P. Armes, J. Am. Chem. Soc., 2014, 136, 10174–10185 CrossRef CAS PubMed.
- M. Semsarilar, V. Ladmiral, A. Blanazs and S. P. Armes, Langmuir, 2012, 28, 914–922 CrossRef CAS PubMed.
- A. Blanazs, J. Madsen, G. Battaglia, A. J. Ryan and S. P. Armes, J. Am. Chem. Soc., 2011, 133, 16581–16587 CrossRef CAS PubMed.
- X. Wang, S. Li, Y. Su, F. Huo and W. Zhang, J. Polym. Sci., Part A: Polym. Chem., 2013, 51, 2188–2198 CrossRef CAS.
- J. Xu, X. Xiao, Y. Zhang, W. Zhang and P. Sun, J. Polym. Sci., Part A: Polym. Chem., 2013, 51, 1147–1161 CrossRef CAS.
- Y. Su, X. Xiao, S. Li, M. Dan, X. Wan and W. Zhang, Polym. Chem., 2014, 5, 578–587 RSC.
- M. Dan, F. Huo, X. Zhang, X. Wang and W. Zhang, J. Polym. Sci., Part A: Polym. Chem., 2013, 51, 1573–1584 CrossRef CAS.
- X. Xiao, S. He, M. Dan, Y. Su, F. Huo and W. Zhang, J. Polym. Sci., Part A: Polym. Chem., 2013, 51, 3177–3190 CrossRef CAS.
- X. Wang, J. Xu, Y. Zhang and W. Zhang, J. Polym. Sci., Part A: Polym. Chem., 2012, 50, 2452–2462 CrossRef CAS.
- Y. Pei, N. C. Dharsana, J. A. van Hensbergen, R. P. Burford, P. J. Roth and A. B. Lowe, Soft Matter, 2014, 10, 5787–5796 RSC.
- G. Moad, Y. K. Chong, A. Postma, E. Rizzardo and S. H. Thang, Polymer, 2005, 46, 8458–8468 CrossRef CAS PubMed.
- A. P. Narrainen, A. Pascual and D. M. Haddleton, J. Polym. Sci., Part A: Polym. Chem., 2002, 40, 439–450 CrossRef CAS.
- H. de Brouwer, M. A. J. Schellekens, B. Klumperman, M. J. Monteiro and A. L. German, J. Polym. Sci., Part A: Polym. Chem., 2000, 38, 3596–3603 CrossRef CAS.
- C. Yang, J. N. Kizhakkedathu, D. E. Brooks, F. Jin and C. Wu, J. Phys. Chem. B, 2004, 108, 18479–18484 CrossRef CAS.
- A. H. Gröschel, F. H. Schacher, H. Schmalz, O. V. Borisov, E. B. Zhulina, A. Walther and A. H. E. Müller, Nat. Commun., 2012, 3, 1–10 Search PubMed.
Footnote |
† Electronic supplementary information (ESI) available: Text showing experimental procedures of the separation of the PNIPAM-b-PS/PDMAEMA-b-PS mixture, Scheme S1 showing the chemical structure of DMAEMA and CDTPA, Fig. S1 showing the polymerization kinetics of the PNIPAM-TTC mediated dispersion polymerization and the PDMAEMA-TTC mediated dispersion polymerization, Fig. S2 showing the ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ([M]0/[M]) time plots of the two macro-RAFT agents co-mediated dispersion polymerization, Fig. S3 showing the typical PNIPAM-b-PS/PDMAEMA-b-PS mixture prepared at 4 h before and after separation, Fig. S4 showing the TEM images of the PNIPAM92-b-PS275 nanoparticles and the PDMAEMA61-b-PS265 nanoparticles, and Fig. S5 showing the transmittance versus temperature plots for the aqueous solution of PNIPAM92-TTC and PDMAEMA61-TTC. See DOI: 10.1039/c4py00998c ([M]0/[M]) time plots of the two macro-RAFT agents co-mediated dispersion polymerization, Fig. S3 showing the typical PNIPAM-b-PS/PDMAEMA-b-PS mixture prepared at 4 h before and after separation, Fig. S4 showing the TEM images of the PNIPAM92-b-PS275 nanoparticles and the PDMAEMA61-b-PS265 nanoparticles, and Fig. S5 showing the transmittance versus temperature plots for the aqueous solution of PNIPAM92-TTC and PDMAEMA61-TTC. See DOI: 10.1039/c4py00998c |
| This journal is © The Royal Society of Chemistry 2015 |