Solar ultraviolet radiation in Africa: a systematic review and critical evaluation of the health risks and use of photoprotection
Robyn M.
Lucas
a,
Mary
Norval
b and
Caradee Y.
Wright
*c
aNational Centre for Epidemiology and Population Health, The Australia National University, Canberra, Australia
bBiomedical Sciences, University of Edinburgh, Edinburgh, UK
cSouth African Medical Research Council and University of Pretoria, Pretoria, South Africa. E-mail: cwright@mrc.ac.za
First published on 26th November 2015
Abstract
Most information on the harmful health effects of solar ultraviolet radiation (UVR) has been obtained in populations in which the majority has fair skin. Here a systematic review of evidence on diseases related to solar UVR in Africa was undertaken, and the appropriateness of effective photoprotection for these people considered. There are few population-based studies on UV-induced skin cancers (melanoma, squamous and basal cell carcinomas) in Africa, although limited reports indicated that they occur, even in people with deeply pigmented skin. The incidence of melanoma is particularly high in the white population living in the Western Cape of South Africa and has increased significantly in recent years. Cataract is extremely common in people of all skin colours and is a frequent cause of blindness, particularly in the elderly. For both skin cancer and cataract, the proportion of the disease risk that is attributable to exposure to solar UVR in African populations, and therefore the health burden caused by UV irradiation is unclear. There was little published information on the use of sun protection in Africa. The potential disease burden attributable to solar UVR exposure of Africans is high, although accurate data to quantify this are sparse. Information is required on the incidence, prevalence and mortality for the range of UV-related diseases in different populations living throughout Africa. Photoprotection is clearly required, at least for those subpopulations at particularly high risk, but may be limited by cost and cultural acceptability.
Introduction
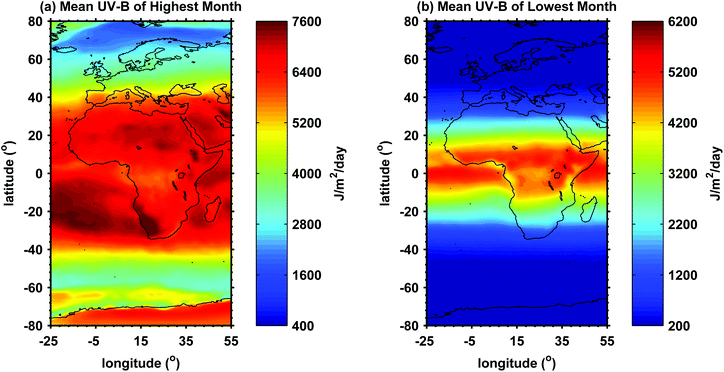
Africa comprises more than 55 countries and spans latitudes between 40° North and 34° South. Its topographical landscape includes mountain ranges, coastal plains and high altitude plateaus. Maximum ambient daytime temperatures (excluding humidity effects) may exceed 35 °C. Cloud and rainfall are highly variable. The levels of ambient solar ultraviolet radiation (UVR) throughout most of the year over much of the African continent are high (Fig. 1), with the UV Index (UVI) being frequently extreme (11+).1,2 In 2010, about 60% of Africans lived within rural areas3 and were therefore likely to be out-of-doors for most of the daylight hours. The health consequences of this combination of high ambient UVR and long periods outdoors depend on individual skin phototypes and sun protection behaviours. The peoples of Africa belong to several thousand ethnic groups with a wide range of skin colours. Sub-Saharan Africa is dominated by Black populations and northern Africa by Arabs. In some sub-Saharan countries, White people of European origin account for a significant proportion of the population, for example 8.8% in South Africa.Sun exposure has both beneficial and adverse effects on health. The major positive outcome is the production of vitamin D which is required for bone health and possibly for protection against a range of diseases.4 Here we focus on the most common negative health outcomes of exposure to solar UVR within the context of the varying climate and diverse peoples of Africa. Strategies for, and use of, personal photoprotection of African populations are also reviewed.
Review methods
A systematic review of the evidence on skin and eye diseases known to be caused by sun exposure in Africa, and on practices relating to photoprotection was conducted using the PRISMA guidelines.5 PubMed was searched for studies with full texts in English, published between 1990 and 2014. When none or very few articles were found in this time period, earlier years were included. The terms listed in Table 1 were used for the separate searches, and Africans were defined as people living in Africa. We also searched the reference lists of included papers to ensure that no studies were omitted.| Term Group 1 | Term Group 2 | Term Group 3 | Combinations |
|---|---|---|---|
| Skin cancer OR Melanoma OR Non-melanoma skin cancer OR Basal cell carcinoma OR Squamous cell carcinoma OR Cataract OR Pterygium OR Eye tumour OR Sunburn OR Polymorphic light eruption | Sun protection OR Sun exposure OR Shade OR Sunglasses OR Sunscreen OR Umbrella | Africa OR African OR Name of each African country | Term from Group 1 plus Term from Group 3 until all combinations exhausted |
| Term from Group 2 plus Term from Group 3 until all combinations exhausted |
Table 2 shows the number of records identified from the database search and the number of studies included in the review, using an adapted schematic from Moher et al.5 The search yielded many articles on skin cancer and ocular effects in Africa but the majority were not relevant as they consisted solely of case reports or clinical management, or were part of reviews. All studies that provided estimates of incidence or prevalence in defined populations groups were included. Several included a small number of participants, and were limited to people with a specific underlying disorder such as oculocutaneous albinism (OCA).6 Only three studies reported on the acute health effects of sun exposure.7–9 These are considered separately in the following section and are not included in Table 2. There was a notable lack of studies about sun protection practices in Africa.
| Skin cancer | Ocular effects | Sun protection | ||
|---|---|---|---|---|
| Identification | Records identified by title through database searching | 852 | 1079 | 640 |
| Records identified through other sources | 30 | 35 | 16 | |
| Screening | Records after duplicated records removed | 294 | 471 | 132 |
| Records screened by Abstract | 200 | 317 | 68 | |
| Records excluded | 94 | 54 | 64 | |
| Eligibility | Full-text articles assessed for eligibility | 50 | 154 | 31 |
| Full text articles excluded (case report, clinical management or review) | 26 | 41 | 13 | |
| Included | Studies included in qualitative synthesis | 24 | 113 | 12 |
Acute adverse health effects of sun exposure
Photoconjunctivitis and photokeratitis
Photokeratitis, inflammation of the cornea, and photoconjunctivitis, inflammation of the conjunctiva, are considered as sunburn of the tissues on the surface of the eyeball and eyelids. No information on the incidence of either disorder in African populations was located. Nevertheless, considering the high solar UVR and the clear skies and reflective terrain over parts of Africa, acute ocular damage is likely to occur. In support, in a preliminary study based in South Africa, outdoor workers reported painful eyes and blurring of vision during the hottest weather which may indicate ocular sunburn.7 Conjunctivitis (diagnosed as allergic conjunctivitis) was significantly more common in technical/outdoor workers than in non-technical/indoor workers in the Delta State of Nigeria which lies near the Equator,9 and thus may represent photoconjunctivitis due to high dose exposure to solar UVR.Light-sensitive dermatoses
The light-sensitive dermatoses, such as polymorphic light eruption, are less common in people with pigmented skin and rarely occur in regions near the Equator; therefore they are unlikely to constitute a major health problem. Indeed in the only report found from an African country, as few as 0.4% (64 cases) of patients attending a dermatology clinic in Lagos, Nigeria, had light sensitive dermatoses, the majority being hydroquinone-induced.16Chronic adverse health effects of sun exposure
The 24 reports which investigated skin cancer in African countries are listed in Table 3, together with a summary of each set of findings.23–46 The majority comprised a small number of individuals, sometimes of a single ethnicity, living in a geographically defined area of one country. Generally, SCCs were the commonest dermatological malignancy found in black people, and were most frequently located on the lower limb in sites of chronic ulceration and previous burns.24,26,33,36–42,45,46 CMs were the next most common in black people and were mainly acral, located on the foot. Thus there are marked differences between the body sites of SCCs and CMs in those with black and fair skin in Africa. This could be due to the more frequent occurrence of chronic scarring following burns and injury and of inflammation caused by parasitic and other skin infections on the feet and legs in Blacks compared with Whites which may provide an opportunity for tumour development in these body sites. In contrast to the relative proportions of the different types of skin cancer in Whites, BCCs were the least frequent, although had a similar presentation and body site distribution in Blacks as in Caucasians. The SCCs and CMs tended to present late and were frequently more aggressive and prone to metastasise in Blacks compared with Caucasians.36,40,44,45 This may be explained by the difficulty in diagnosing tumours in black skin, the lack of self-examination and screening, and for cultural and financial reasons plus the shortage of clinical facilities for many Black people.
| Reference | Study years | Location | Study population/sample | Main findings |
|---|---|---|---|---|
| BCC basal cell carcinoma, CM cutaneous melanoma, NMSC non-melanoma skin cancer, OCA oculocutaneous albinism, SCC squamous cell carcinoma. | ||||
| I. Descriptive studies of skin cancer incidence: | ||||
| 23 | 1968–1997 | Cancer Registry, Kenya | 74 cases BCC | Extrapolated to calculate race-specific annual incidence rate per million population in Kenya as 58.5 in Caucasians, 0.065 in Africans |
| 24 | 1966–1975 | Soweto, South Africa | Survey of skin tumours in Blacks; 101 cases SCC, 83 CM, 9 BCC | SCC mainly on head/neck/lower limbs; 84% CM on lower limbs. Average annual incidence of CM per 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 black population of Soweto estimated as 0.78 in men, 0.87 in women 000 black population of Soweto estimated as 0.78 in men, 0.87 in women |
| 25 | 1990–1995 | Cape Town, South Africa | 595 primary invasive CMs in whites | Annual age-standardised incidence per 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 white population in Cape Town estimated as 27.2 in men, 22.2 in women. Most on lower extremity and trunk, with superficial spreading type commonest 000 white population in Cape Town estimated as 27.2 in men, 22.2 in women. Most on lower extremity and trunk, with superficial spreading type commonest |
| 26 | 2000–2004 | Cancer Registry, South Africa | All histologically confirmed cases of skin cancer, totalling 44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 716 716 |
See Table 5 for incidence of SCC, BCC and CM in the 4 population groups of South Africa |
| II. Studies of determinants of skin cancer: | ||||
| 27 | 1992 | Alexandria, Egypt | Case-control study: 136 NMSCs, 145 controls with black, brown, olive and fair skin | Increased relative risk of NMSC with fair skin, ease of sun burning, outdoor occupation, degree of sun exposure and lack of clothing |
| 28 | 1962–1972 | Sudan | 1225 skin cancer biopsies | 63% SCC (North Sudan, light brown skin, most on head/neck; South Sudan, black skin, most on legs in pre-existing ulcers); 19% CM (almost all in North Sudan with 61% on soles of feet); 15% BCC (73% on head/neck) |
| 29 | 2001–2010 | Northwestern Tanzania | 64 OCAs with histopathological diagnosis of skin cancer | Median age 30 years; 84% outdoor workers, late presentation common, head/neck most frequent body site; 75% SCC, 23% BCC, 2% CM |
| III. Descriptive studies of type, histology and body site of skin cancers: | ||||
| 30 | 1985–2004 | Cotonou, Benin | 16 cases of skin cancer | Skin cancer rare; 5 CM, 6 SCC and 5 BCC in 5 OCAs, 1 White and 10 Blacks |
| 31 | 1989–2004 | Upper Egypt | 262 histologically confirmed cases of skin cancer in Arabs with light to olive skin | 77% BCC (most of face), 15% SCC (most on face and extremities), 8% CM (on face and lower limbs) |
| 32 | 1999–2005 | Addis Ababa, Ethiopia | 50 cases primary CM | 96% nodular; 64% on foot; poor prognosis |
| 33 | 2007–2013 | Addis Ababa, Ethiopia | 2343 cutaneous biopsies | 22% malignant of which 8% SCC (in sites of chronic ulceration/previous burns), 4% CM, 3% BCC |
| 34 | 2007–2010 | Lilongwe, Malawi | 406 surgical oncology cases | 6% CM; women![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) men 2 men 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 35 | 1960–1967 | Ibadan, Nigeria | 435 epidermal cancers in Blacks | 67% SCC (25% on head/neck), 24% CM (most on foot), 5% BCC |
| 36 | 1984–1987 | Port Harcourt, Nigeria | 18 cases of histologically confirmed skin cancer including in 3 OCAs | SCC (on several body sites, 3 OCA participants), 22% BCC (on face); late presentation very common |
| 37 | 1978–1989 | Zaria, Nigeria | 775 histologically confirmed skin cancers | 67% SCC (13% on face, 58% on extremities, most on leg), 19% CM (89% on foot), 2% BCC (on head and neck); late presentation very common |
| 38 | 2000–2004 | Calabar, Nigeria | 63 histologically confirmed skin cancers | 37% SCC (commonest on lower limb), 8% CM (commonest on lower limb), 8% BCC (commonest on head/neck/upper limb) |
| 39 | 2006–2007 | Calabar, Nigeria | 19 Blacks (including 2 OCAs) with histological diagnosis of SCC | SCCs represented 51% of all skin malignancies; 58% SCC on lower limbs in Blacks in site of chronic ulceration/inflammation but on head in OCAs; poor prognosis due to late presentation |
| 40 | 2000–2009 | Calabar, Nigeria | Histologically confirmed skin cancers in 146 Blacks and 16 OCAs | In Blacks, 36% SCC, 10% CM, 1% BCC (49% on lower limb, 22% on head/neck). In OCAs, 56% SCC, 38% BCC and 6% CM |
| 41 | 2001–2013 | Lagos, Nigeria | 197 skin tumour biopsies | 31% malignant with SCC commonest, followed by CM |
| 42 | 1982–2007 | Benin City, Nigeria | 694 skin biopsies | 27% malignant of which 33% CM, 24% SCC, 10% BCC; leg and foot commonest sites; increasing number of cases with time |
| 43 | 1950–1970 | Dakar, Senegal | 972 skin cancer biopsies from Blacks | 79% SCC (80% on lower limbs), 9% CM (67% on foot), 2% BCC (89% on head/neck) |
| 44 | 1972–1985 | Cape Town, South Africa | 40 Blacks with CM | 68% acral lentiginous CM, delay in presentation/advanced disease; 5-year survival rate of 25% |
| 45 | 1969–1983 | Pretoria, South Africa | 175 Blacks with acral lentiginous CM, confirmed by histology | 98% CM on foot; relatively advanced stage at initial presentation; 73% died within 1 year of presentation, 17% survived more than 3 years |
| 46 | 1971–1983 | Nyankunde, Zaire | 794 biopsies from patients with malignant diseases (skin colour not specified but probably all black) | 24% skin cancers of which 9% SCC, 6% CM, 1% BCC; 43% occurred in sites of trauma/chronic ulcer |
Four studies included data on the incidence of skin cancer (Table 3, section I). The earliest, based on 83 histologically confirmed cases of CM in Blacks living in Soweto, South Africa, in 1966–75 gave an estimated annual incidence per 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Blacks in Soweto (population approximately one million) of 0.78 for men and 0.87 for women.24 Based on 595 histology reports, Saxe et al.25 estimated the age-standardised incidence rate in 1990–1995 for primary invasive CM in the White population of Cape Town (approximately 490
000 Blacks in Soweto (population approximately one million) of 0.78 for men and 0.87 for women.24 Based on 595 histology reports, Saxe et al.25 estimated the age-standardised incidence rate in 1990–1995 for primary invasive CM in the White population of Cape Town (approximately 490![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) as 27.5 per 100
000) as 27.5 per 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 for men and 22.2 per 100
000 for men and 22.2 per 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 women. No change in the annual incidence rate over the period of the study occurred. In a retrospective study of all BCCs documented in the Kenya Cancer Registry from 1968 to 1997, the race-specific mean annual incidence rates of BCC per million of the Kenyan population were 58.5 for Caucasians and 0.065 for Blacks.23 However this calculation was based on only a small number of BCC cases: 35 in Caucasians and 39 in Blacks.
000 women. No change in the annual incidence rate over the period of the study occurred. In a retrospective study of all BCCs documented in the Kenya Cancer Registry from 1968 to 1997, the race-specific mean annual incidence rates of BCC per million of the Kenyan population were 58.5 for Caucasians and 0.065 for Blacks.23 However this calculation was based on only a small number of BCC cases: 35 in Caucasians and 39 in Blacks.
The results of a survey of all histologically confirmed cases of skin cancer (n = 44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 716) in South Africa that were reported to the National Cancer Registry between 2000 and 2004 are shown in Table 4.26 The South African population is divided into four to reflect the pre-1994 legislated groupings: Black, White, Coloured (mixed ancestry between White and Black or between Black and Asian/Indian with skin colour ranging from pale to dark brown), and Asian/Indian (commonly called Asian in South Africa). There was no change in the incidence rates over the years of the survey. The highest incidence of all three skin cancers occurred in the Whites, followed by the Coloured, then Asian/Indian, and then Black. CM was the least frequent skin tumour and BCC the most frequent, except in black people. It is noteworthy that the incidence of CM in Blacks was about half of the incidences of SCC and BCC, which was a higher proportion than in the other three population groups. This difference could be explained by acral lentiginous melanoma being the commonest melanoma type only in the Black population where it is unlikely that solar UVR present a direct risk factor.26 It is also possible that there are a higher number of undiagnosed BCCs and SCCs in the Black compared with the other three population groups, resulting in different ratios. As Blacks comprise almost 80% of the population of South Africa, even the low incidences of SCC, BCC and CM in this group may be significant in terms of national health burden. Although accurate data were not available after 2004, the South African Melanoma Advisory Board estimated an incidence in 2009 of 69 cases of CM per 100
716) in South Africa that were reported to the National Cancer Registry between 2000 and 2004 are shown in Table 4.26 The South African population is divided into four to reflect the pre-1994 legislated groupings: Black, White, Coloured (mixed ancestry between White and Black or between Black and Asian/Indian with skin colour ranging from pale to dark brown), and Asian/Indian (commonly called Asian in South Africa). There was no change in the incidence rates over the years of the survey. The highest incidence of all three skin cancers occurred in the Whites, followed by the Coloured, then Asian/Indian, and then Black. CM was the least frequent skin tumour and BCC the most frequent, except in black people. It is noteworthy that the incidence of CM in Blacks was about half of the incidences of SCC and BCC, which was a higher proportion than in the other three population groups. This difference could be explained by acral lentiginous melanoma being the commonest melanoma type only in the Black population where it is unlikely that solar UVR present a direct risk factor.26 It is also possible that there are a higher number of undiagnosed BCCs and SCCs in the Black compared with the other three population groups, resulting in different ratios. As Blacks comprise almost 80% of the population of South Africa, even the low incidences of SCC, BCC and CM in this group may be significant in terms of national health burden. Although accurate data were not available after 2004, the South African Melanoma Advisory Board estimated an incidence in 2009 of 69 cases of CM per 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Caucasians living in the Western Cape47 (white population around 1.4 million); this rate is amongst the highest in the world and is almost double the annual incidence reported in 2000 to 2004 in white South Africans.26
000 Caucasians living in the Western Cape47 (white population around 1.4 million); this rate is amongst the highest in the world and is almost double the annual incidence reported in 2000 to 2004 in white South Africans.26
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 persons in the Black, Asian/Indian, Coloured and White population groups of South Africa, 2000–2004
000 persons in the Black, Asian/Indian, Coloured and White population groups of South Africa, 2000–2004![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 41
41
| Black | Asian/Indian | Coloured | White | All | |
|---|---|---|---|---|---|
| Basal cell carcinoma | 4.7 | 13.0 | 85.7 | 311.1 | 76.7 |
| Squamous cell carcinoma | 4.6 | 7.0 | 41.5 | 101.3 | 29.3 |
| Cutaneous melanoma | 2.2 | 1.8 | 10.0 | 37.0 | 9.2 |
In contrast to the studies based in sub-Saharan Africa where the majority of the population has deeply pigmented skin, similar work in Egypt, where people of Arab ancestry with brown/olive skin predominate, presents a different picture. Here BCCs were the most common skin cancer, followed by SCC and then CM very rarely.31 In Alexandria, Egypt, the relative risk of developing a non-melanoma skin cancer (NMSC) (BCC and SCC combined) was calculated as 2.3 for olive-skinned and 3.8 for fair/medium-skinned individuals compared with brown-skinned individuals.27 There was a fifteen-fold increased risk of NMSC for fair/medium skinned people with extensive exposure compared with brown/black skinned people with light or moderate sun exposure. It was concluded that over 60% of the risk of developing NMSC could be attributed to sun exposure and about 45% to skin colour.27 In North Sudan where most people have light brown skin, SCCs tended to occur on the head and neck which are amongst the most frequently sun exposed body sites, while in South Sudan where most have black skin, SCCs tended to occur on the legs in sites of pre-existing ulcers or trauma.28 In the latter, exposure to solar UVR may not represent a strong direct risk factor for the development of these tumours. The incidence of skin tumours in the Northern African countries has not been recorded.
Some ethnic groups in Africa have a significant proportion of people who have OCA, for example 1/1000 in the Tonga tribe in Zimbabwe,48 and 1/1970 in the Vhavenda tribe in South Africa.49 Individuals with this disorder have impaired formation of the UV-protective pigment, melanin, and are fair-skinned with light-coloured eyes, making them particularly sensitive to UVR.6 As they have a considerably higher than normal risk of developing skin cancer, particularly SCCs,6,29,50,51 the prevalence of OCA may thus affect estimates of skin cancer incidence in Black populations. Furthermore, the high prevalence of HIV infection in sub-Saharan Africa, currently estimated as 10% of the Black population, may lead to a higher-than-expected incidence of SCC in Blacks, probably due to the lack of an effective immune response to combat the initial or a progressive stage in tumour development.52,53
Eye diseases
Chronic UVR exposure is a major risk factor for several eye conditions that become more common with increasing age (and thus longer sun exposure) and may cause loss of vision. Pterygium is a wing-shaped invasive growth of the conjunctiva that can impinge on the cornea. A proportion of pterygia contain dysplastic and neoplastic changes, and pterygium may represent a continuum with SCC of the cornea or conjunctiva (SCCC).54,55 The main risk factors for pterygium are repeated exposures to UVR and to dust, with both together, such as found in desert areas, presenting the highest risk. Pterygium is relatively common in Africa (Table 5)9,56–70 with a higher prevalence in outdoor workers,58 some occupational groups,9,57,61–62,66 or in association with HIV infection.68| Reference | Location | Study population | Prevalence of pterygium |
|---|---|---|---|
| 9 | Warri, Nigeria | Petroleum industry workers | 20% technical workers, 8% non-technical |
| 56 | Douala, Cameroon | New patients | 1.1% (mean age 41.2 y) |
| 57 | Tarkwa, Ghana | Industrial gold mine workers | 25.8% |
| 58 | Ibadan, Nigeria | New cases at the eye clinic | 9%; 65% of patients outdoor workers; 40% had recurrence post-op |
| 59 | Imo State, Nigeria | New patients at eye clinic | 11.5% |
| 60 | Anambra State, Nigeria | 3 rural villages, adults 18–49 y | 8.2% |
| 61 | Enugu State, Nigeria, | Cement factory, coal mine, saw mill & iron/steel workers | 27.7% (pinguecula and pterygium) |
| 62 | Benin City, Nigeria | Commercial motorcyclists | 25.7% of eyes |
| 63 | Ibadan, Nigeria | University drivers | 5.6% (age 31–64 y) |
| 64 | Kano, Nigeria | Red eye presenting to eye clinic | 11% due to inflamed pterygium |
| 65 | Nigeria | Elderly patients presenting at primary care (60+ y) | 6.4%. |
| 66 | Osun State, Nigeria | Welders | 17.5% (mean age 38 y). |
| 67 | Lagos, Nigeria | Presentations with conjunctival masses | 89.5% |
| 68 | Lagos, Nigeria | Seropositive HIV/AIDS patients >15 y | 19.0% |
| 69 | Kigali, Rwanda | Eye outpatients | 4.4% |
| 70 | Harare, Zimbabwe | Patients with conjunctival tumours | 53% ocular surface squamous neoplasia, 42% pterygium, 5% other |
SCCC is a rare tumour caused by excessive exposure to UVR![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70,71 which is more common at lower latitudes.72 There is a significantly increased risk of SCCC in association with HIV/AIDS in both the USA73 and Africa.74,75 For example, the incidence rate (per 100
70,71 which is more common at lower latitudes.72 There is a significantly increased risk of SCCC in association with HIV/AIDS in both the USA73 and Africa.74,75 For example, the incidence rate (per 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 person years) rose from 0.2 in 1960–71 to 2.1 in 1995–7 in Uganda,76 and from 0.17 in 1990 to 1.8 in 1999 in Zimbabwe,77 believed to be due to the increased prevalence of HIV/AIDS. Evidence is equivocal currently regarding UVR as a risk factor for malignant melanoma of the eye,78 an uncommon tumour in both Black and White populations.
000 person years) rose from 0.2 in 1960–71 to 2.1 in 1995–7 in Uganda,76 and from 0.17 in 1990 to 1.8 in 1999 in Zimbabwe,77 believed to be due to the increased prevalence of HIV/AIDS. Evidence is equivocal currently regarding UVR as a risk factor for malignant melanoma of the eye,78 an uncommon tumour in both Black and White populations.
Cataracts account for 50% of cases of blindness world-wide, and sub-Saharan Africa has the highest regional burden of blindness (20% of world's blindness and only 11% of world's population).79 Solar UVR is a risk factor for cortical cataract, with weaker evidence for a similar role in nuclear cataract. There is a high prevalence of avoidable blindness across Africa, with “senile” cataract the main cause in most locations (Table 6).80–133 It is not possible to define the contribution of UVR exposure to the cataract burden, although in studies from Nigeria, mixed and cortical cataracts were the most prevalent in those with visual impairment.134,135 Cataract begins earlier in African populations than in comparable populations in the USA or India.97 It is more common in rural than urban areas,105,107 and the effects on vision are compounded by poorer access to surgical services in these regions. In addition to the prevalence estimates, it is worth considering the absolute numbers of people affected by cataract that is at least partly caused by solar UVR exposure: over one million in Nigeria alone.105
| Reference | Study year (when stated) | Location | Age (y years) | Prevalence of bilateral blindness (<3/60 in better eye) | Main cause |
|---|---|---|---|---|---|
| NS not stated. | |||||
| 80 | 1991 | Mmankgodi, Botswana | 60+ y | 11% | Cataract (34%) |
| 81 | NS | Botswana | 50+ y | 3.7% | Cataract (46.9%) |
| 82 | NS | Two rural provinces, Burundi | 50+ y | 1.1%. | Cataract (55%) |
| 83 | NS | Limbe urban area, Cameroon | 40+ y | 1.1% | Posterior segment disease (29%), cataract (21%) |
| 84 | 2005 | Muyuka, South West Cameroon | 40+ y | 1.6% | Cataract (62.1%) |
| 85 | 1992 | North Province Cameroon | 6+ y | 1.2% | Cataract |
| 86 | 1998 | Cape Verde Islands | All | 0.8% all ages; 11.4% | Cataract (57.7%) |
| >70 y | |||||
| 87 | 1994 | Bossangoa, Central African Republic | All | 2.2% all ages; 11.4% | Onchocerciasis (73.1%) |
| 50+ y | Cataract (16.4%) | ||||
| 88 | 2007–8 | Upper Egypt | ≥40 y | 9.3%. | Cataract (60%) |
| 89 | 2008 | Eritrea | 50+ y | 7.5% | Cataract (55.1%) |
| 90 | NS | Ethiopia | All | 1.6% | Cataract (49.9%) |
| 91 | 1989 | Hamar tribe, Ethiopia | All | 1.9% all ages; for 40+ y, <1% of men, 13% of women | Cataract |
| 92 | NS | Gurage Zone, Central Ethiopia | 40+ y | 7.9% | Cataract (46.1%) |
| 93 | 2006 | Gurage Zone, Central Ethiopia | 40+ y | 3.5%. | Cataract (59.0%) |
| 94 | 1994–5 | Jimma Zone, SW Ethiopia | All | 0.85% | Cataract (56.8%) |
| 95 | 1996 | Gambia | All | 0.43% all ages; 4.08% 50+ y | Cataract (48%) |
| 96 | 2006–8 | Tema, Ghana | 40+ y | 1.2% | Cataract (44.2%) |
| 97 | NS | Turkana tribe, NW Kenya | All | 1.1% | Cataract in >45 y |
| 98 | 2005 | Nakuru District, Kenya | 50+ y | 2.0% | Cataract (42.0%) |
| 99 | NS | Rural areas, Kenya | All | 0.7% all ages; 6.5% men, 9.4% women 60+ y | Cataract (36%) |
| 100 | 2010 | 4 regions, Libya | 50+ y | 3.15% | Cataract (29%) |
| 101 | 2011 | Atsinanana region, Madagascar | 50+ y | 1.96% | Cataract (64%) |
| 102 | 2009–10 | Southern Malawi | 50+ y | 3.3% | Cataract (48.2%) |
| 103 | 1990 | Rural Segou region, Mali | All | 1.7% | Cataract (54%) |
| 104 | 1995 | Dambatta, Kano State, Nigeria | 1.14% | Cataract (54%) | |
| 105 | 2005–7 | Nigeria | ≥40 y | 4.2% | Cataract (43%) |
| 106 | 2002 | Ife-Ijesha, Osun State, Nigeria | 60+ y | 5.6% | Cataract (42.3%) |
| 107 | 2002 | Rural south-west Nigeria | All | 1.0% (mainly in the 50+ age group) | Cataract (44.4%) |
| 108 | NS | Egbedore, Osun State, Nigeria | All | 1.18% | Cataract (47.4%) |
| 109 | 1991 | Akinalu-Ashipa, Osun State, Nigeria | All | 0.9% | Cataract (48.1%) |
| 110 | NS | Anambra State, Nigeria | All | 0.33% all ages; 2.62% 50+ y | Cataract (70.6%) |
| 111 | NS | SW Nigeria | 60+ y | 28.6% | Cataract (32.3%) |
| 112 | NS | Egbedore, Osun State, Nigeria | 50+ y | 6.3% | Cataract (56.0%) |
| 113 | 2005 | Egbedore, Osun State, Nigeria | 50+ y | Cataract-related blindness: 2.0% | |
| 114 | 2006–7 | Akinyele, SW Nigeria | 50+ y | Cataract blindness 2.0% | |
| 115 | 2005 | Sokoto State, Nigeria | All | 1.9% | Cataract (51.6%) |
| 116 | 2007 | Plateau State, Nigeria | 50+ y | Cataract blindness 2.1% | |
| 117 | NS | Atakunmosa West, Nigeria | 5–120 y | 1.1% | Cataract (57.2%) |
| 118 | NS | Ozoro, Delta State, Nigeria | 40+ y | 6.3% | Cataract (60%) |
| 119 | 2004 | Kaduna State, Nigeria | All | 0.6% | Cataract (37.8%) |
| 120 | 2005–7 | National Blindness Survey, Nigeria | 40+ y | 4.2% | Cataract (43%) |
| 121 | 2006 | Western Rwanda | 50+ y | 1.8% | Cataract (65%) |
| 122 | 2010 | Cape Town, South Africa | 50+ y | 1.4% | Cataract (27%) |
| 123 | NS | Ingwavuma, KwaZulu-Natal, South Africa | All | 1.0% | Cataract (59.0%) |
| 124 | 2006 | South Africa | 60+ y women, 65+ y men | Cataract blindness 15.6% | |
| 125 | 2005 | Mankien, Southern Sudan | 5+ y | 4.1% | Cataract (41.2%) |
| 126 | 2007 | Kilimanjaro region, Tanzania | 50+ y | 2.4% | Cataract (52.4%) |
| 127 | 1986 | Central Tanzania | 7+ y | 1.26% | Cataract (22%) |
| 128 | 2002 | Southern Togo | All | 2.47% | Cataract (44.4%) |
| 129 | 1999 | Sudanese refugees in Uganda | All | 21% | Cataract (42%) |
| 130 | NS | SW Uganda | 13+ y | 1.6%4% | Glaucoma (38.5%) |
| Cataract (23.1%) | |||||
| 131 | 1996 | Mbandaka, Iboko and Lukolela, SW Equator, Zaire | 20% | Cataract (54%) | |
| 132 | 2010 | Southern Zambia | 50+ y | 2.2% | Cataract (47.2%) |
| 133 | 1990–2010 | North Africa and Middle East countries | All | 2.1% (1990) 1.1% (2010) | Cataract 29.2% (1990) 23.4% (2010) |
Any dependence of ocular damage on skin or eye colour is not known and, while some UV-induced eye disorders are more common in black than in white populations, the relationship to biological differences rather than to lifestyle differences has not been investigated.
Strategies for sun protection
Personal photoprotection can decrease the solar UVR dose and thus ameliorate its harmful effects. Strategies include wearing protective clothing, wide-brimmed hats and sunglasses, seeking shade and applying sunscreen. Few reports were found on photoprotection in the context of Africa (Table 7).136–146| Reference | Study years | Location | Study population | Main findings |
|---|---|---|---|---|
| OCA oculocutaneous albinism. | ||||
| 8 | 2012 | All nine provinces, South Africa | 707 schoolchildren from 24 government primary schools | 56% reported sunburn last summer; 50% ever wore a hat; 66% ever used sunscreen |
| 136 | 2010–2011 | Tizi-Ouzou, Algeria | 435 children, aged 5–15 years | Majority spent >60 min outside per day at end of both summer and winter |
| 137 | 2008–2009 | Cairo, Egypt | 75 girls, aged 14–17 years | Mean daily sun exposure 22 min; mean body surface exposed <20% |
| 138 | 2007 | Rabat, Morocco | 411 adults (84% female), mean age 40 years | 38% low levels of knowledge of sun exposure risks and sun protection options; 50% used sunscreens; sunscreen cost noted as prohibitive |
| 139 | 2008 | Rabat, Morocco | 2896 adults stratified by origin (urban/rural) and gender | 52% regularly exposed to the sun for >2 hours during peak UVR times; 16% of them used no photoprotection; women, rural residents and workers used more photoprotection than their counterparts |
| 140 | 1990 | Cape Town, South Africa | 231 White adult beachgoers at 3 Cape Peninsula beaches | Half (more females) used sunscreen ≥ SPF15; 90% cited skin cancer as a potential consequence of excess sun exposure |
| 141 | 2002 | Durban, South Africa | 30 children and adolescents, aged 4–14 years | Children received an average of 4.6% of the total daily ambient solar UVR each day; boys received higher sun exposure than girls |
| 142 | Not stated | Limpopo, South Africa | 90 children with OCA (mean age 11.8 years) attending a special needs school for the visually impaired | Children with OCA had at least one hat; girls sought shade more than boys; 38% used sunscreen |
| 143 | Not stated | Limpopo, South Africa | 38 children with OCA, mean age 13 years | Sun protection practices used: 71% sunscreen, 100% hats, 21% sunglasses, 73% long skirts/trousers and 34% stayed indoors |
| 144 | 1997 | Kilimanjaro region, Tanzania | 94 individuals with OCA attending outreach clinics, median age 16 years | 50% had read about skin cancer risk, 63% knew skin cancers were related to sun exposure and 78% knew that skin cancer was preventable; 96% applied sunscreen daily |
| 145 | Not stated | 13 locations in northern Tanzania | 164 individuals with OCA, 52% <16 years | Risk factors for sun damage: 50% spent >6 hours per day in the sun, 62% had no hat, 80% wore short sleeves |
| 146 | 2005–2006 | Tunis, Tunisia | 197 patients with melasma, aged 19–60 years, 95% women | Sun exposure reported as a triggering factor for melasma by 51% women and an aggravating factor by 84% |
Shade
Trees can provide effective shade, depending on the denseness of their foliage.147 In Africa, tree species and their leaf canopies are variable, ranging from the thorny acacia to large baobab. Man-made shade, such as awnings, can be effective depending on design, quality and ease of use.148 The shade should be effective at preventing exposure to both direct and diffuse solar UVR149 and additionally in Africa should provide temperature control for heat relief. Umbrellas are a handheld form of shade, and are used in many African countries. Depending on diameter, fabric colour and type, and holding position, an umbrella may block up to 90% of solar UVR exposure of the head.150 Given the cost of an umbrella, this is unlikely to be a feasible option for many Black people in Africa.Hats and clothing
Clothing can provide protection against solar UVR exposure depending on the extent of coverage, textile colour, weave, porosity and weight.151 In some African countries, traditional female dress requires full body coverage (with the face covered/uncovered). Headgear, such as veils, kufi (brimless, rounded cap worn by men in Western Africa) and mokorotlo (traditional Basothu hat), differs between cultures and tribes. Wearing a broad-brimmed hat (brim > 10 cm) or a hat with flaps over the ears and neck is an effective way to reduce head and neck exposure. Nevertheless, uptake of such hats is challenged by fashion, culture, cost, and temperature-related factors. In northern Tanzania, many individuals with OCA do not wear hats and most wear short-sleeved shirts.145 A Moroccan survey found that 70% of adults were regularly exposed to the sun during midday hours, with 66% wearing no hat and 16% using no photoprotection.139As agriculture is an important economic activity in Africa, the health risks of farm workers due to chronic exposure to high solar UVR merit consideration, but have not been documented. While most African countries have occupational health and safety legislation for the protection of staff, specific mention of the hazards of solar UVR is unlikely. In addition whether African outdoor workers would wear sun protective clothing and hats is unknown.
Sunglasses
Sunglasses can provide excellent eye protection from both direct and diffuse/reflected UVR, but are costly and may not be culturally acceptable by some African populations. How commonly eye protection is used in Africa has not been systematically investigated. Among Moroccans, sunglasses as a photoprotection option was used by only 19% of the study population.138Sunscreen
Topically applied sunscreens are used widely for photoprotection, particularly in fair-skinned individuals, in developed countries. They are highly effective in decreasing the risk of sunburn, particularly if combined with wearing protective clothing and seeking shade. In addition, there is emerging evidence that regular sunscreen use lowers skin cancer risk and photoageing.152 Sunscreens with high SPFs, 50+, are now available.153 These may be useful in environments where the UVI is high or extreme, circumstances that are frequent throughout Africa. It is particularly important that African people with fair skin adopt adequate sun protection strategies. A 1990 survey of fair-skinned South Africans found that only 5% used sunscreen with SPF 15 or higher at the beach.140 In some African countries, such as South Africa, individuals with OCA are provided with sunscreen by the government free of charge. In addition to supply, detailed information and advice on the use of such sunscreens are required.142,144While the purchase of sunscreens per capita in Europe, North America, Latin America and Asia is known,153 such data are not available for African countries. Commercial sunscreens are unlikely to be in common use in Africa because sunburn occurs less frequently in pigmented skin than in white skin, the cost of sunscreens may be beyond the means of the majority, sunscreens are not widely available, and/or there is little public health guidance to promote their use.
Some rural tribes in southern Africa have traditional methods for skin protection which may fulfil a similar function to sunscreen. These come in the form of clays, found locally and rich in the mineral kaolinite, which are applied to the face. Two such clays were shown recently to have SPFs around 3 with broad spectrum protection.154 These may provide cost-effective, easily obtainable and culturally-acceptable alternatives to conventional sunscreens in Africa.
Information for the general public
The WHO INTERSUN programme provides information and practical advice on the health effects of solar UVR exposure. It recommends use of the UVI, providing estimates of predicted levels of solar UVR, to guide sun exposure, and suggests that personal photoprotection is used when the UVI is 3 or greater. However the UVI is not well known or understood, even in countries where awareness campaigns have been extensive,155 and its effectiveness in influencing behaviour has not been assessed. Furthermore, a simple message using the UVI to provide recommendations on the amount of time that can be spent outdoors safely is not appropriate for African countries in which the population is highly heterogeneous, comprising a wide range of ethnic groups, skin types and photosensitivities. A very recent survey found that people with deeply pigmented skin, particularly in South Africa, were less familiar with the term “sunburn” than population groups with less pigmented skin; when it was explained as a change in skin colour associated with pain or tenderness, many agreed that they had in fact experienced sunburn.156 Special attention is warranted to protect children, those with compromised immunity and people with OCA from over-exposure to the sun, and to encourage changes in lifestyle that promote safe sun behaviour throughout life. Only two African Cancer Associations, Namibia and South Africa, mention on their websites that they run skin cancer prevention and sun awareness programmes. In parallel, Vision 2020 strives to reduce the burden of eye disease and blindness in Africa.157 Beyond the health benefits of such campaigns, they are likely to strengthen national economies by reducing the financial burden to health care systems caused by eye and skin cancer therapies. In the USA, for every dollar invested in the SunWise programme, 2–4 dollars in medical care costs and productivity is saved.158Future research
Few reports were found on the prevalence and incidence of diseases associated with exposure to solar UVR in African countries in comparison with studies in many other parts of the world, despite the potential for high-dose exposure to solar UVR. In addition such investigations frequently comprised a small number of participants, and only a minority involved work carried out in the last 10 years. Thus there is a pressing need to collect data on a large, preferably country-wide scale, regarding the frequency of SCC, BCC, CM and cataract in populations of varying skin colours living in different locations throughout Africa. Only then can an estimate of the disease burden be made and any trends in incidence determined. It is particularly important to monitor any changes in CM incidence and mortality data in the fair skinned population living in South Africa. Over time, it is likely that changes in personal sun exposure will occur, for example, due to migration from rural to urban areas potentially leading to a reduction in solar UVR overall and possibly more intense “recreational” intermittent exposure, or wearing less clothing potentially leading to greater exposure to solar UVR with increased risk of sunburn. Legislation and policy changes are required to ensure appropriate shade is incorporated into the design of buildings in Africa, especially for health clinics and school playgrounds. Knowledge about sunglasses, improvements in the acceptability of wearing them and their provision at low cost would contribute significantly to the reduction of eye disease and blindness in Africa. Finally the impact of public health messages regarding “safe” sun exposure needs to be monitored, together with an estimate of the cost-effectiveness of such programmes in Africa.Conflict of interest statement
The authors declare no conflicts of interest.References
- http://www.who.int/uv/intersunprogramme/activities/uv_index/en/index3.html .
- http://www.weather2travel/climate-guides .
- http://www.geohive.com/earth/pop_urban.aspx .
- U. Grober, J. Spitz, J. Reichrach, K. Kisters and M. G. Holick, Vitamin D: update: from rickets prophylaxis to general preventative health care, Dermatoendocrinology, 2013, 5, 331–347 CrossRef PubMed.
- D. Moher, A. Liberati, J. Tetzlaff and D. G. Altman, PRISMA Group, Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement, PLoS Med., 2009, 6, e1000097 Search PubMed.
- C. Y. Wright, M. Norval and R. W. Hertle, Oculocutaneous albinism in Sub-Sahara Africa: adverse sun-associated health effects and photoprotection, Photochem. Photobiol., 2015, 91, 27–32 CrossRef CAS PubMed.
- A. Mathee, J. Oba and A. Rose, Climate change impacts on working people (the HOTHAPS initiative): findings of the South African pilot study, Global Health Action, 2010, 3 DOI:10.3402/gha.v3i0.5612.
- C. Y. Wright, P. N. Albers, M. A. Oosthuizen and N. Phala, Self-reported knowledge, attitudes and behaviours among schoolchildren attending South African primary schools, Photodermatol. Photoimmunol. Photomed., 2014, 30, 266–276 CrossRef PubMed.
- A. E. Omoti, J. M. Waziri-Erameh and M. E. Enock, Ocular disorders in a petroleum industry in Nigeria, Eye, 2008, 22, 925–929 CrossRef CAS PubMed.
- B. L. Diffey, Solar ultraviolet radiation effects on biological systems, Phys. Med. Biol., 1991, 36, 299–328 CrossRef CAS PubMed.
- C. Battie, M. Gohara, M. Verschoore and W. Roberts, Skin cancer in skin of color: an update on current facts, trends, and misconceptions, J. Drugs Dermatol., 2013, 12, 194–198 Search PubMed.
- Centres for Disease Control and Prevention, Sunburn and sun protective behaviours among adults aged 18–29 years – United States, 2000–2010, MMWR Morb. Mortal. Wkly. Rep., 2012, 61, 317–322 Search PubMed.
- G. S. Munavalli, R. A. Weiss and R. M. Halder, Photoaging and nonablative photorejuvenation in ethnic skin, Dermatol. Surg., 2005, 31, 1250–1260 CrossRef CAS PubMed.
- L. K. Dennis, M. J. Vanbeek, L. E. Beane Freeman, B. J. Smith, D. V. Dawson and J. A. Coughlin, Sunburns and risk of cutaneous melanoma: does age matter? A comprehensive meta-analysis, Ann. Epidemiol., 2008, 18, 614–627 CrossRef PubMed.
- C. Dessinioti, C. Antoniou, A. Katsambas and A. J. Stratigos, Basal cell carcinoma: what's new under the sun, Photochem. Photobiol., 2010, 86, 481–491 CrossRef CAS PubMed.
- Y. M. Olumide, Photodermatoses in Lagos, Int. J. Dermatol., 1987, 25, 295–299 CrossRef.
- R. M. Halder and S. Bridgeman-Shah, Skin cancer in African Americans, Cancer, 1995, 75(Suppl 2), 667–673 CrossRef CAS PubMed.
- H. M. Gloser and K. Neal, Skin cancer in skin of color, J. Am. Acad. Dermatol., 2006, 55, 741–760 CrossRef PubMed.
- P. T. Bradford, Skin cancer in skin of color, Dermatol. Nurs., 2009, 2, 170–178 Search PubMed.
- X. C. Wu, M. J. Eide and J. King, et al., Racial and ethnic variations in incidence and survival of cutaneous melanoma in the United States, 1995–2006, J. Am. Acad. Dermatol., 2011, 65, S26–S37 CrossRef PubMed.
- O. N. Agbai, K. Buster and M. Sanchez, et al., Skin cancer and photoprotection in people of color: a review and recommendations for physicians and the public, J. Am. Acad. Dermatol., 2014, 70, 748–762 CrossRef PubMed.
- F. El Ghissassi, R. Baan and K. Straif, et al., A review of human carcinogens – part D: radiation, Lancet Oncol., 2009, 10, 751–752 CrossRef PubMed.
- T. M. Munyao and N. A. Othieno-Abinya, Cutaneous basal cell carcinoma in Kenya, East Afr. Med. J., 1999, 76, 97–100 CAS.
- C. Isaacson, Cancer of the skin in urban blacks of South Africa, Br. J. Dermatol., 1979, 100, 347–350 CrossRef CAS PubMed.
- N. Saxe, M. Hoffman and J. E. Krige, et al., Malignant melanoma in Cape Town, South Africa, Br. J. Dermatol., 1998, 138, 998–1002 CrossRef CAS PubMed.
- M. Norval, P. Kellett and C. V. Wright, The incidence and body site of skin cancers in the population groups of South Africa, Photodermatol. Photoimmunol. Photomed., 2014, 30, 262–265 CrossRef PubMed.
- F. El Khwsky, R. Bedwani and B. D'Avanzo, et al., Risk factors for non-melanoma skin cancer in Alexandria, Egypt, Int. J. Cancer, 1994, 56, 375–378 CrossRef CAS.
- M. O. Malik, A. Hidaytalla, E. H. Daoud and A. M. el-Hassan, Superficial cancer in the Sudan. A study of 1225 primary malignant superficial tumours, Br. J. Cancer, 1974, 30, 355–364 CrossRef CAS PubMed.
- J. B. Mabula, P. L. Chalya and M. D. Mchembe, et al., Skin cancers among albinos at a university teaching hospital in Northwestern Tanzania: a retrospective review of 64 cases, BMC Dermatol., 2012, 12, 5 CrossRef PubMed.
- H. Adegbibi, H. Yedomon and F. Atadokpede, et al., Skin cancers at the National University Hospital of Cotonou from 1985 to 2004, Int. J. Dermatol., 2007, 46(Suppl 1), 26–29 CrossRef PubMed.
- M. R. Hussein, Skin cancer in Egypt: a word in your ear, Cancer Biol. Ther., 2005, 4, 593–595 CrossRef PubMed.
- T. B. Legesse and J. Schneider, Primary cutaneous malignant melanoma in Ethiopian patients histopathologic study of 50 cases from Tikur Anbessa Hospital, Ethiop. Med. J., 2011, 49, 313–322 Search PubMed.
- D. C. Gimbel and T. B. Legesse, Dermatopathology practice in Ethiopia, Arch. Pathol. Lab. Med., 2013, 137, 798–804 CrossRef PubMed.
- C. E. Kendig, J. C. Samuel and A. F. Tyson, et al., Cancer treatment in Malawi: a disease of palliation, World J. Oncol., 2013, 4, 142–146 Search PubMed.
- J. O. Oluwasanmi, A. O. Williams and A. E. Alli, Superficial cancer in Nigeria, Br. J. Cancer, 1969, 23, 714–728 CrossRef CAS PubMed.
- D. D. Datudo-Brown, Primary malignant skin tumors in Nigerians, J. Natl. Med. Assoc., 1991, 83, 345–348 Search PubMed.
- A. Yakubu and O. A. Mabogunje, Skin cancer in Zaria, Nigeria, Trop. Doct., 1995, 25(Suppl 1), 63–67 Search PubMed.
- M. E. Asuquo, O. Ngim, G. Ugare, J. Omotoso and G. Ebughe, Major dermatological malignancies encountered in a teaching hospital surgical department in South Nigeria, Am. J. Clin. Dermatol., 2008, 9, 383–387 CrossRef PubMed.
- M. E. Asuquo, I. A. Ikpeme, E. E. Bassey and G. Ebughe, Squamous cell carcinoma in South Eastern equatorial rain forest in Calabar, Nigeria, Eplasty, 2009, 16, e53 Search PubMed.
- M. E. Asuquo and G. Ebughe, Major dermatological maliganacies encountered in the University of Calabar teaching hospital, Calabar, southern Nigeria, Int. J. Dermatol., 2012, 51(Suppl 1), 32–36 CrossRef PubMed.
- O. Ayanlowo, A. O. Daramola and A. Akinkugbe, et al., Skin tumors at the Lagos University Teaching Hospital, Nigeria, West Afr. J. Med., 2013, 32, 286–290 CAS.
- G. D. Forae and A. N. Olu-Eddo, Malignant skin tumors in Benin city, South-South, Nigeria, Oman Med. J., 2013, 28, 311–315 CrossRef PubMed.
- R. Camain, A. J. Tuyns, H. Sarrat, C. Quenum and I. Faye, Cutaneous cancer in Dakar, J. Natl. Cancer Inst., 1972, 48, 33–49 CAS.
- D. A. Hudson and J. E. Krige, Planta melanoma in black South Africans, Br. J. Surg., 1993, 80, 992–994 CrossRef CAS PubMed.
- J. V. Lodder, W. Simson and P. J. Becker, Malignant melanoma of the skin in black South Africans: a 15-year experience, S. Afr. J. Surg., 2010, 48, 76–79 CAS.
- K. R. Oates, A survey of malignant disease in Zaire, Fam. Pract., 1986, 3, 102–106 CrossRef CAS PubMed.
- http://www.melanoma.co.za/D_MFS.asp .
- P. M. Lund, N. Puri, D. Durham-Pierre, R. A. King and M. H. Brilliant, Oculocutaneous albinism in an isolated Tonga community in Zimbabwe, J. Med. Genet., 1997, 34, 733–735 CrossRef CAS PubMed.
- J. G. Kromberg and T. Jenkins, Prevalence of albinism in the South African Negro, S. Afr. Med. J., 1982, 61, 383–386 CAS.
- K. O. Opara and B. C. Jiburum, Skin cancers in albinos in a teaching hospital in eastern Nigeria – presentation and challenges of care, World J. Surg. Oncol., 2010, 8, 73 CrossRef PubMed.
- M. E. Asuquo, O. O. Otei, J. Omotoso and E. E. Bassey, Letter: Skin cancer in albinos at the University of Calabar Teaching Hospital, Calabar, Nigeria, Dermatol. Online J., 2010, 16, 14 CAS.
- L. Stein, M. I. Urban and D. O'Connell, et al., The spectrum of human immunodeficiency virus-associated cancers in a South African black population: results from a case-control study, 1995–2004, Int. J. Cancer, 2008, 122, 2260–2265 CrossRef CAS PubMed.
- P. M. Nthumba, P. C. Cavadas and L. Landin, Primary cutaneous malignancies in sub-Saharan Africa, Ann. Plast. Surg., 2011, 66, 1267–1274 CrossRef PubMed.
- P. Artornsombudh, A. Sanpavat, U. Tinnungwattana, V. Tongkhomsai, L. Sansopha and W. Tulvatana, Prevalence and clinicopathologic findings of conjunctival epithelial neoplasia in pterygia, Ophthalmology, 2013, 120, 1337–1340 CrossRef PubMed.
- P. Oellers, C. L. Karp and A. Sheth, et al., Prevalence, treatment, and outcomes of coexistent ocular surface squamous neoplasia and pterygium, Ophthalmology, 2013, 120, 445–450 CrossRef PubMed.
- C. Ebana Mvogo, A. Bella-Hiag, A. Ngosso and A. Ellong, Pterygium: epidemiological, clinical and therapeutical aspects at the Douala General Hospital, Rev. Int. Trach. Pathol. Ocul. Trop. Subtrop. Sante Publique, 1995, 72, 151–161 CAS.
- G. Ovenseri-Ogbomo, S. Ocansey, E. Abu, S. Kyei and S. Boadi-Ksi, Oculo-visual findings among industrial mine workers at Goldfields Ghana Limited, Tarkwa, Ophthalmol. Eye Dis., 2012, 4, 35–42 Search PubMed.
- A. O. Ashaye, Pterygium in Ibadan, West Afr. J. Med., 1991, 10, 232–243 CAS.
- E. Anyanwu and J. N. Nnadozie, The frequency distribution of ocular disease by age in Imo State Nigeria, J. Am. Optom. Assoc., 1993, 64, 704–708 CAS.
- S. N. Nwosu, Ocular problems of young adults in rural Nigeria, Int. Ophthalmol., 1998, 22, 259–263 CrossRef CAS PubMed.
- O. I. Okoye and R. E. Umeh, Eye health of industrial workers in Southeastern Nigeria, West Afr. J. Med., 2002, 21, 132–137 CAS.
- C. U. Ukponmwan, O. A. Dawodu, O. F. Edema and O. Okojie, Prevalence of pterygium and pingueculum among motorcyclists in Nigeria, East Afr. Med. J., 2007, 84, 516–521 CAS.
- C. O. Bekibele, R. Ajav and M. C. Asuzu, Eye health of professional drivers of a Nigerian University, Nig. Postgrad. Med. J., 2009, 16, 256–259 CAS.
- A. Lawan, Causes of red eye in Aminu Kano Teaching Hospital, Kano-Nigeria, Niger. J. Med., 2009, 18, 184–185 CAS.
- L. A. Adebusoye, E. T. Owoaje, M. M. Ladipo and A. O. Adeniji, Visual morbidites among elderly patients presenting at a primary care clinic in Nigeria, West Afr. J. Med., 2011, 30, 118–120 CAS.
- A. Ajayi Iyiade and J. Omotoye Olusola, Pattern of eye diseases among welders in a Nigeria community, Afr. Health Sci., 2012, 12, 210–216 CAS.
- F. B. Akinsola, C. A. Mbadugha, A. O. Onokoya, A. O. Adefule-Ositelu, O. T. Aribaba and A. Rotimi-Samuel, APattern of conjunctival masses seen at Guiness Eye Centre Luth Idi-Araba, Nig. Q. J. Hosp. Med., 2012, 22, 39–43 CAS.
- A. O. Onakoya, M. G. Odeyemi, O. T. Aribaba and F. B. Akjinsola, Ocular findings in acquired immunodeficiency syndrome patients in Lagos, Nigeria, Nig. Q. J. Hosp. Med., 2012, 22, 52–57 CAS.
- H. Forsius, K. Maertens and J. Fellman, Changes of the eye caused by the climate in Rwanda, Africa, Ophthalmic Epidemiol., 1995, 2, 107–113 CrossRef CAS PubMed.
- E. C. Pola, R. Masanganise and S. Rusakaniko, The trend of ocular surface squamous neoplasia among ocular surface tumour biopsies submitted for histology from Sekuru Kaguvi Eye Unit, Harare between 1996 and 2000, Cent. Afr. J. Med., 2003, 49, 1–4 CAS.
- K. M. Waddell, R. G. Downing, S. B. Lucas and R. Newton, Corneo-conjunctival carcinoma in Uganda, Eye, 2006, 20, 893–899 CrossRef CAS PubMed.
- R. Newton, A review of the aetiology of squamous cell carcinoma of the conjunctiva, Br. J. Cancer, 1996, 74, 1511–1513 CrossRef CAS PubMed.
- M. Guech-Ongey, A. E. Engels, J. J. Goedert, R. J. Biggar and S. M. Mbulaiteye, Elevated risk for squamous cell carcinoma of the conjunctiva among adults with AIDS in the United States, Int. J. Cancer, 2008, 122, 2590–2593 CrossRef CAS PubMed.
- T. S. Chinogurei, R. Masanganise, S. Rusakaniko and E. Sibanda, Ocular surface squamous neoplasia (OSSN) and human immunodeficiency virus at Sekuru Kaguvi Eye Unit in Zimbabwe: the role of operational research studies in a resource poor environment?, Cent. Afr. J. Med., 2006, 52, 56–58 CAS.
- G. Furahini and S. Lewallen, Epidemiology and management of ocular surface squamous neoplasia in Tanzania, Ophthalmic Epidemiol., 2010, 17, 171–176 CrossRef PubMed.
- D. M. Parkin, H. Wabinga, S. Nambooze and F. Wabwire-Mangen, AIDS-related cancers in Africa: maturation of the epidemic in Uganda, AIDS, 1999, 13, 2563–2570 CrossRef CAS PubMed.
- R. Masanganise, S. Rusakaniko and R. Makunike, et al., A historical perspective of registered cases of malignant ocular tumors in Zimbabwe (1990 to 1999). Is HIV infection a factor?, Cent. Afr. J. Med., 2008, 54, 28–32 CAS.
- M. Norval, R. M. Lucas and A. P. Cullen, et al., The human health effects of ozone depletion and interactions with climate change, Photochem. Photobiol. Sci., 2011, 10, 199–225 CAS.
- S. Resnikoff, D. Pascolini and D. Etya'ale, et al., Global data on visual impairment in the year 2002, Bull. World Health Organ., 2004, 82, 844–851 Search PubMed.
- F. Clausen, E. Sandberg, B. ingstad and P. Hjortgahl, Morbidity and health care utilisation among elderly people in Mmankgodi village, Botswana, J. Epidemiol. Community Health, 2000, 54, 58–63 CrossRef CAS PubMed.
- O. Nkomazana, Disparity in access to cataract surgical services leads to higher prevalence of blindness in women as compared with men: results of a national survey of visual impairment, Health Care Women Int., 2009, 30, 228–229 CrossRef PubMed.
- L. Kandeke, W. Mathenge and C. Giramahoro, et al., Rapid assessment of avoidable blindness in two northern provinces of Burundi without eye services, Ophthalmic Epidemiol., 2012, 19, 211–215 CrossRef PubMed.
- J. E. Oye and H. Kuper, Prevalence and causes of blindness and visual impairment in Limbe urban area, South West Province, Cameroon, Br. J. Ophthalmol., 2007, 91, 1435–1439 CrossRef PubMed.
- J. E. Oye, H. Kuper, B. Dineen, R. Befidi and A. Foster, Prevalence and causes of visual impairment in Myuka: a rural health district in South West Province, Cameroon, Br. J. Ophthalmol., 2006, 90, 538–542 CrossRef CAS PubMed.
- M. R. Wilson, M. Mansour and D. Ross-Degnan, et al., Prevalence and causes of low vision and blindness in the Extreme North Province of Cameroon, West Africa, Ophthalmic Epidemiol., 1996, 3, 23–33 CrossRef CAS PubMed.
- J. F. Schemann, F. Inocencio and M. de Loudes Monteiro, et al., Blindness and low vision in Cape Verde Islands: results of a national eye survey, Ophthalmic Epidemiol., 2006, 13, 219–226 CrossRef PubMed.
- E. C. Schwartz, R. Huss and A. Hopkins, et al., Blindness and visual impairment in a region endemic for onchocerciasis in the Central African Republic, Br. J. Ophthalmol., 1997, 81, 443–447 CrossRef CAS PubMed.
- A. Mousa, P. Courtright, A. Kazanjian and K. Bassett, Prevalence of visual impairment and blindness in Upper Egypt: a gender-based perspective, Ophthalmic Epidemiol., 2014, 21, 190–196 CrossRef PubMed.
- A. Muller, M. Zerom and H. Limburg, et al., Results of a rapid assessment of avoidable blindness (RAAB) in Eritrea, Ophthalmic Epidemiol., 2011, 18, 103–108 CrossRef PubMed.
- Y. Berhane, A. Worku and A. Bejiga, et al., Prevalence and causes of blindness and low vision in Ethiopia, Ethiopian J. Health Develop., 2007, 21, 204–210 Search PubMed.
- P. Courtright, P. Klungsoyr, S. Lewallen and T. H. Henriksen, The epidemiology of blindness and visual loss in Hamar tribesmen of Ethiopia. The role of gender, Trop. Geogr. Med., 1993, 45, 168–170 CAS.
- M. Melese, W. Alemayehu and S. Bayu, et al., Low vision and blindness in adults in Gurage Zone, central Ethiopia, Br. J. Ophthalmol., 2003, 87, 677–680 CrossRef CAS PubMed.
- A. Woldeyes and Y. Adamu, Gender differences in adult blindness and low vision, Central Ethiopia, Ethiop. Med. J., 2008, 4, 211–218 Search PubMed.
- N. Zerihun and D. Mabey, Blindness and low vision in Jimma Zone, Ethopia: results of a population based survey, Opthalmic Epidemiol., 1997, 4, 19–26 CrossRef CAS.
- B. Dineen, A. Foster and H. Faal, A proposed rapid methodology to assess the prevalence and causes of blindness and visual impairment, Ophthalmic Epidemiol., 2006, 13, 31–34 CrossRef PubMed.
- D. L. Budenz, J. R. Bandi and K. Barton, et al., Blindness and visual impairment in an urban West African population: the Tema Eye Survey, Ophthalmology, 2012, 119, 1744–1753 CrossRef PubMed.
- R. Loewenthal and J. Pe'er, A prevalence survey of ophthalmic diseases among the Turkana tribe in north-west Kenya, Br. J. Ophthalmol., 1990, 74, 84–88 CrossRef CAS PubMed.
- W. Mathenge, H. Kuper and H. Limburg, et al., Rapid assessment of avoidable blindness in Nakuru district, Kenya, Ophthalmology, 2007, 114, 599–605 CrossRef PubMed.
- R. Whitfield, L. Schwab, D. Ross-Degnan, P. Steinkuller and J. Swartwood, Blindness and eye disease in Kenya: ocular status survey results from the Kenya Rural Blindness Prevention Project, Br. J. Ophthalmol., 1990, 74, 333–340 CrossRef CAS PubMed.
- M. M. Rabiu, M. Jenf, S. Fituri, A. Choudhury, J. Agbabiaka and A. Mousa, Prevalence and causes of visual impairment and blindness, cataract surgical coverage and outcomes of cataract surgery in Libya, Ophthalmic Epidemiol., 2013, 20, 26–32 CrossRef PubMed.
- J. B. Randrianaivo, R. M. Anholt, D. L. Tendrisoa, N. J. Margiano, P. Courtright and S. Lewallen, Blindness and cataract surgical services in Atsinanana region, Madagascar, Middle East Afr. J. Ophthalmol., 2014, 21, 153–157 CrossRef PubMed.
- K. Kalua, R. Lindfield, M. Mtupanyama, D. Mtumodzi and V. Msiska, Findings from a rapid assessment of avoidable blindness (RAAB) in Southern Malawi, PLoS One, 2011, 6, e19226 CAS.
- C. Kortlang, J. C. Koster, S. Coulbaly and R. P. Dubbeldam, Prevalence of blindness and visual impairment in the region of Segou, Mali. A baseline survey for a primary eye care programme, Trop. Med. Int. Health, 1996, 1, 314–319 CAS.
- L. Abdu, Prevalence and causes of blindness and low vision in Dambatta local government area, Kano State, Nigeria, Niger. J. Med., 2002, 11, 108–112 CAS.
- M. M. Abdull, S. Sivasubramaniam and G. V. Murthy, et al., Causes of blindness and visual impairment in Nigeria: the Nigeria national blindness and visual impairment survey, Ophthalmol. Vis. Sci. Invest., 2009, 50, 4114–4120 CrossRef PubMed.
- B. O. Adegbehingbe, B. R. Fajemilehin, E. O. Ojofeitimi and L. A. Bisiriyu, Blindness and visual impairment among the elderly in Ife-Ijesha zone of Osun State, Nigeria, Indian J. Ophthalmol., 2006, 54, 59–62 CrossRef CAS PubMed.
- B. O. Adegbehingbe and T. O. Majengbasan, Ocular health status of rural dwellers in south-western Nigeria, Aust. J. Rural Health, 2007, 15, 269–272 CrossRef PubMed.
- C. O. Adeoti, Prevalence and causes of blindness in a tropical African population, West Afr. J. Med., 2004, 23, 249–252 Search PubMed.
- A. Adeoye, Survey of blindness in rural communities of south-western Nigeria, Trop. Med. Int. Health, 1996, 1, 672–676 CrossRef CAS PubMed.
- U. F. Ezepue, Magnitude and causes of blindness and low vision in Anambra State of Nigeria (results of 1992 point prevalence survey), Public Health, 1997, 111, 305–309 CrossRef CAS PubMed.
- O. F. Fafowora and O. O. Osuntokun, Age-related eye disease in the elderly members of rural African community, East Afr. Med. J., 1997, 74, 435–437 CAS.
- O. U. Kolawole, A. O. Ashaye, C. O. Adeoti and A. O. Mahmound, Survey of blindness and low vision in Egbedore, South-Western Nigeria, West Afr. J. Med., 2010, 29, 327–331 CAS.
- O. U. Kolawole, A. O. Ashaye, A. O. Mahmoud and C. O. Adeoti, Cataract blindness in Osun state, Nigeria: results of a survey, Middle East Afr. J. Med., 2012, 19, 364–371 Search PubMed.
- O. O. Komolafe, A. O. Ashaye, B. G. Ajayi and C. O. Bekibele, Visual impairment from age-related cataract among an indigenous African population, Eye, 2010, 24, 53–58 CrossRef CAS PubMed.
- N. Muhammad, R. M. Mansur, A. M. Dantani, E. Elhassan and S. Isiyaku, Prevalence and causes of blindness and visual impairment in Sokoto State, Nigeria: baseline data for vision 2020: the right to sight eye care programme, Middle East Afr. J. Ophthalmol., 2011, 18, 123–128 CrossRef PubMed.
- O. I. Okoye and R. E. Umeh, Eye health of industrial workers in Southeastern Nigeria, West Afr. J. Med., 2002, 21, 132–137 CAS.
- O. H. Onakpoya, A. O. Adeoye, F. B. Akinsola and B. O. Adegbehingbe, Prevalence of blindnessand visual impairment in Atakunmosa West Local Government area of southwestern Nigeria, Tanzan. Health Res. Bull., 2007, 9, 126–131 CAS.
- G. Patrick-Ferife, A. O. Ashaye and B. M. Qureshi, Blindness and low vision in adults in Ozoro, a rural community in Delta State, Nigeria, Niger. J. Med., 2005, 14, 390–395 CAS.
- M. M. Rabiu, Prevalence of blindness and low vision in north central, Nigeria, West Afr. J. Med., 2008, 27, 238–244 Search PubMed.
- M. M. Rabiu, M. Jenf, S. Fituri, A. Choudhury, I. Agbabiaka and A. Mousa, Prevalence and causes of visual impairment and blindness, cataract surgical coverage and outcomes of cataract surgery in Libya, Ophthalmic Epidemiol., 2013, 20, 26–32 CrossRef PubMed.
- W. Mathenge, J. Nkurikiye, H. Limburg and H. Kuper, Rapid assessment of avoidable blindness in Western Rwanda: blindness in a postconflict setting, PLoS Med., 2007, 4, e217 Search PubMed.
- N. Cockburn, D. Steven and K. Lecuona, et al., Prevalence, causes and socio-economic determinants of vision loss in Cape Town, South Africa, PLoS One, 2012, 7, e30718 CAS.
- C. D. Cook, S. E. Knight and I. Crofton-Brown, Prevalence and causes of low vision and blindness in northern KwaZulu, S. Afr. Med. J., 1993, 83, 590–593 CAS.
- C. Cook, H. Kluever, L. Mabena and H. Limburg, Rapid assessment of cataract at pension pay points in South Africa, Br. J. Ophthalmol., 2007, 91, 867–868 CrossRef PubMed.
- J. Ngondi, J. F. Ole-Sempele and F. A. Onsarigo, et al., Prevalence and causes of blindness and low vision in southern Sudan, PLoS Med., 2006, 3, e477 Search PubMed.
- C. Habiyakire, G. Kabona, P. Courtright and S. Lewallen, Rapid assessment of avoidable blindness and cataract surgical services in Kilimanjaro region, Tanzania, Ophthalmic Epidemiol., 2010, 17, 90–94 CrossRef PubMed.
- P. A. Rapoza, S. K. West, S. J. Katala and H. R. Taylor, Prevalence and causes of vision loss in central Tanzania, Int. Ophthalmol., 1991, 15, 123–129 CrossRef CAS PubMed.
- B. O. Adegbehingbe and T. O. Majengbasan, Ocular health status of rural dwellers in south western Nigeria, Aust. J. Rural Health., 2007, 15, 269–272 CrossRef PubMed.
- M. Kawuma, Eye diseases and blindness in Adjumani refugee settlement camps, Uganda, East Afr. Med. J., 2000, 77, 580–582 CAS.
- S. M. Mbulaiteye, B. C. Reeves and A. Karabalinde, et al., Evaluation of E-optotypes as a screening test and the prevalence and causes of visual loss in a rural population in SW Uganda, Ophthalmic Epidemiol., 2002, 9, 251–262 CrossRef CAS PubMed.
- D. Kaimbo wa Kaimbo and L. Missotten, Eye diseases and the causes of blindness in the southwestern Equator (equatorial forest) in Zaire, data from an eye camp in three rural centers, Bull. Soc. Belge Ophthalmol., 1997, 265, 59–65 CAS.
- R. Lindfield, U. Griffiths, F. Bozzani, M. Mumba and J. Munsanje, A rapid assessment of avoidable blindness in Southern Zambia, PLoS One, 2012, 7, e38483 CAS.
- M. Khairallah, R. Kahloun and S. R. Flaxman, et al., Prevalence and causes of vision loss in North Africa and the Middle East: 1990–2010, Br. J. Ophthalmol., 2014, 98, 605–611 CrossRef PubMed.
- A. M. Mahdi, M. Rabiu and C. Gilbert, et al., Prevalence and risk factors for lens opacities in Nigeria: results of the national blindness and low vision survey, Invest. Ophthalmol. Vis. Sci., 2014, 55, 2642–2651 Search PubMed.
- O. O. Komolafe, A. O. Ashaye, B. G. Ajayi and C. O. Bekibele, Distribution pattern of lens opacity among a rural population in South Western Nigeria, Ophthalmic Epidemiol., 2009, 16, 289–295 CrossRef PubMed.
- M. Djennane, M. S. Lebbah, S. C. Roux, C. H. Djoudi, H. E. Cavalier and J. C. Souberbielle, Vitamin D status of schoolchildren in Northern Algeria, seasonal variations and determinants of vitamin D deficiency, Osteoporos. Int., 2014, 25, 1493–1502 CrossRef CAS PubMed.
- N. Amr, A. Hamid, M. Sheta and H. Elsedfy, Vitamin D status in healthy Egyptian girls, Georgian Med. News, 2012, 210, 65–71 Search PubMed.
- M. Meziane, S. Ahid and H. Azendour, et al., Results of a public awareness campaign in Morocco regarding the sun's deleterious effects, J. Eur. Acad. Dermatol. Venereol., 2010, 24, 388–394 CrossRef CAS PubMed.
- N. Abda, K. E. Rhazi and M. Obtel, et al., Determinants of self-reported sun protection practices among Moroccan population, Prev. Med., 2012, 54, 422–424 CrossRef PubMed.
- Y. Von Schirnding, N. Strauss and A. Mathee, et al., Sunscreen use and environmental awareness among beach-goers in Cape Town, South Africa, Public Health Rev., 1991/1992, 19, 209–217 Search PubMed.
- C. Guy, R. Diab and B. Martincigh, Ultraviolet radiation exposure of children and adolescents in Durban, South Africa, Photochem. Photobiol., 2003, 77, 265–270 CrossRef CAS PubMed.
- P. M. Lund and J. S. Taylor, Lack of adequate sun protection for children with oculocutaneous albinism in South Africa, BMC Public Health, 2008, 8, 225 CrossRef PubMed.
- P. M. Lund and R. Gaigher, A health intervention programme for children with albinism at a special school in South Africa, Health Educ. Res., 2002, 17, 365–372 CrossRef PubMed.
- S. R. McBride and B. J. Leppard, Attitudes and beliefs of an albino population toward sun avoidance, Arch. Dermatol., 2002, 138, 629–632 Search PubMed.
- D. P. Lookingbill, G. L. Lookingbill and B. Leppard, Actinic damage and skin cancer in albinos in northern Tanzania: findings in 164 patients enrolled in an outreach skin care program, J. Am. Acad. Dermatol., 1995, 32, 653–658 CrossRef CAS PubMed.
- C. Guinot, S. Cheffai, J. Latreille, M. A. Dhaoui, S. Youssef, K. Jaber, O. Nageotte and N. Doss, Aggravating factors for melasma: a prospective study in 197 Tunisian patients, J. Eur. Acad. Dermatol. Venereol., 2010, 24, 160–169 Search PubMed.
- A. V. Parisi, A. Willey, M. G. Kimlim and J. C. Wong, Penetration of solar eythermal UV radiation in the shade of two common Australian trees, Health Physics, 1999, 76, 682–686 CrossRef CAS PubMed.
- A. V. Parisi and D. J. Turnbull, Shade provision for UV minimisation: a review, Photochem. Photobiol., 2014, 90, 479–490 CrossRef CAS PubMed.
- A. I. Kudish, M. Harari and E. G. Evseev, The solar ultraviolet B radiation protection provided by shading devices with regard to its diffuse component, Photodermatol. Photoimmunol. Photomed., 2011, 27, 236–244 CrossRef PubMed.
- J. R. McMichael, E. Veledar and S. C. Chen, UV radiation protection by handheld umbrellas, JAMA Dermatol., 2013, 149, 757–758 CrossRef PubMed.
- W. L. Morison, Photoprotection by clothing, Dermatol. Ther., 2013, 16, 16–22 CrossRef.
- M. R. Iannacone MR, M. C. Hughes and A. C. Green, Effects of sunscreen on skin cancer and photoaging, Photodermatol. Photoimmunol. Photomed., 2014, 30, 55–61 CrossRef PubMed.
- U. Osterwalder, U. M. Sohn and B. Herzog, Global state of sunscreens, Photodermatol. Photoimmunol. Photomed., 2014, 30, 62–80 CrossRef.
- N. C. Dlova, F. T. Nevondo, E. M. Mwangi, B. Summers, J. Tsoka-Gwegweni and B. S. Martincigh, et al., Chemical analysis of in vitro UV-protection characteristics of clays traditionally used for sun protection in South Africa, Photodermatol. Photoimmunol. Photomed., 2013, 29, 164–169 CrossRef PubMed.
- N. Italia and E. A. Rehfuess, Is the Global Solar UV Index an effective instrument for promoting sun protection: a systematic review, Health Educ. Res., 2012, 27, 200–213 CrossRef PubMed.
- C. Y. Wright, M. Wilkes, J. L. du Plessis and A. I. Reeder, Self-reported skin colour and erthemal sensitivity vs. objectively measured constitutive skin colour in an African population with predominantly dark skin, Photodermatol. Photoimmunol. Photomed., 2015, 31, 315–324 CrossRef PubMed.
- S. Lewallen and P. Courtright, Blindness in Africa: present situation and future needs, Br. J. Opthalmol., 2001, 85, 897–903 CrossRef CAS.
- J. W. Kyle, J. K. Hammitt, H. W. Lim, A. C. Geller, L. H. Hall-Jordan, E. W. Maibach, E. C. De Fabo and M. C. Wagner, Economic evaluation of the US Environmental Protection Agency's SunWise program: sun protection education for young children, Pediatrics, 2008, 121, e1074–e1084 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry and Owner Societies 2016 |