Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Singlet oxygen luminescence kinetics in a heterogeneous environment – identification of the photosensitizer localization in small unilamellar vesicles†
S.
Hackbarth
and
B.
Röder
Humboldt-Universität zu Berlin, Institute of Physics, Photobiophysics, Newtonstr. 15, 12489 Berlin, Germany. E-mail: hacky@physik.hu-berlin.de; roeder@physik.hu-berlin.de
First published on 27th October 2014
Abstract
In vivo measurement of singlet oxygen luminescence kinetics is affected by the heterogeneity of biological samples. Even though singlet oxygen luminescence detection is technically getting easier, the analysis of signals from biological samples is still far from quantitative real time surveillance as it is aspired by the community. In this paper small unilamellar vesicles (SUVs) are used for modelling the general behaviour of heterogeneous samples. The geometry of the SUVs can be determined independently using dynamic light scattering. Therefore an accurate theoretical description of the generation, deactivation and diffusion of the singlet oxygen is possible. The theoretical model developed here perfectly fits the experimental results. Thus the location of the singlet oxygen generating a photosensitizer molecule can be exactly determined from the kinetics of the singlet oxygen luminescence. The application of the used theoretical approach thus allows for accurate quantitative measurements in SUVs.
1. Introduction
Singlet oxygen, the lowest electronically excited state of molecular oxygen may deactivate via weak, but characteristic luminescence around 1270 nm. For a long time the detection of this luminescence was quite difficult. The development of NIR-PMTs by Hamamatsu improved this situation and counting devices (multi scaler or time-correlated multi photon counting) became state of the art for such luminescence measurements as they are superior to classical analogue devices.1 Measurements in vitro and in vivo became possible,2–4 but the results obtained this way have already revealed that fitting based on the assumption of a homogeneous environment near the photosensitizer (PS) may not be sufficient to describe the observed luminescence kinetics.In a homogeneous environment the kinetics of singlet oxygen concentration (cΔ) after a delta pulse excitation at time t = 0 can be described as a double exponential curve, determined by the feeding at the speed of the photosensitizer (PS) triplet decay τT and quenching by the solvent and other quenchers therein. The natural lifetime of 1O2 is way too long to have a significant influence on the kinetics in any solution. The tricky part is that the assignment of the involved decay times to signal increase and fall may switch. It is the faster of both times that determines the increase and the slower that determines the decay:
| cΔ(t) = (cPS(0)ΦΔτT−1/(τT−1 − τΔ−1))(exp(−t/τΔ) − exp(−t/τT)), | (1) |
In contrast to former assumptions that in heterogeneous environments the luminescence follows an average kinetics, if only the areas of the different phases were small compared to the diffusion length of 1O2,2,5 recent improvements of the sensitivity of 1O2 luminescence detection proved that this is not the case.6
If the 1O2 generating PSs are not homogeneously distributed over all phases of a heterogeneous environment and the radiative rate constant differs between the phases, then the diffusion of 1O2 affects the signal kinetics. In a biological environment this effect is especially visible, if the 1O2 is generated by membrane localized PSs. On the other hand the radiative rate constant in the membrane is much bigger compared to the surrounding aqueous environment.7 On the other hand the diffusion length of 1O2 in water is quite short. Based on the generally agreed value of the diffusion coefficient of oxygen in water D = 2 × 10−5 cm2 s−1,8,9 three dimensional diffusion results in an average diffusion length of just around 200 nm. This value is based on the reported 1O2 decay time in water of 3.7 ± 0.1 μs.6 If just one dimensional diffusion applies, like in extended membranes (on the scale of 1O2 diffusion this means just about a μm2) the average diffusion length is about 120 nm. In comparison the thickness of a natural membrane of 4–12 nm10,11 has to be taken into account. Therefore in the case of membrane localized PS a non-negligible signal can be registered from inside the membrane. This applies especially in case the radiative rate constant inside the membranes is bigger compared to water.6 Diffusion of 1O2 out of the membrane changes the temporal signal shape. Described vividly, the diffusion of 1O2 out of the membrane (the area with a higher radiative rate constant) opens an additional decay channel for 1O2 from inside the membrane. Any quantitative 1O2 luminescence measurement in heterogeneous (e.g. biological) environments has to account for diffusion and different radiative rate constants. Here we report the first trial to do so. The scope of this paper is to demonstrate that for known membrane geometry evidence can be provided for such diffusion processes measuring the luminescence signal kinetics with high accuracy. Based on this knowledge it becomes possible to determine the localization of the PS relative to the membrane: whether it is in/at the lipid bilayer or not.
2. Materials and methods
The theoretical model
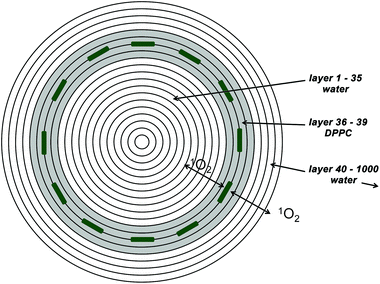
Liposomes were chosen as a model membrane system, since they are highly symmetric, having a defined thickness of the lipoid bilayer. Also their size can be determined via dynamic light scattering (DLS).The diffusion of 1O2 in this geometry can be described by a spherical symmetrical diffusion if the generation of 1O2 (starting conditions) falls under this symmetry as well. The samples that will be analyzed in this paper are loaded with 50–100 PS molecules per liposome. Therefore the estimation of a spherical symmetrical PS distribution and singlet oxygen generation seems to be appropriate. Taking a liposome concentration where the distance between two liposomes is big compared to their size the model can be limited to a single liposome. The simulation therefore consists of 1000 concentric spherical layers of 1 nm thickness (Fig. 1), where layers 36 to 39 represent the liposomal membrane (according to the measured size of the SUVs using DLS – vide infra). The diffusion constant of oxygen in water at room temperature is reported to be 2 × 10−5 cm2 s−1 and for membranes values in the literature are around 1 × 10−5 cm2 s−1.5 These values are assigned to the layers accordingly as well as singlet oxygen decay times.
Recent calculations of the free energy for translocation of 1O2 across a very similar bilayer (1-palmitoyl,2-oleoyl-sn-glycero-3-phosphocholine) report a higher solubility of 1O2 inside the bilayer compared to the aqueous phase around.12 This was also found experimentally for oxygen in another very similar bilayer (1,2-dimyristoyl-sn-glycero-3-phosphocholine) before.13
Similar partition coefficients (concentration ratio) of around 3.5 are reported in both papers and therefore the same value is used for our simulation.
If a PS is incorporated in a liposome membrane, it causes a certain deviation from the spherical shape, but since the model is spherically symmetric and thus diffusion only occurs radially the space occupied by the PS itself can be neglected. The volume increase of the layers with diameter is taken into account.
The numerical simulation for a given parameter set (PS triplet decay time and 1O2 decay time outside the membrane) is carried out with time steps of 1.25 × 10−10 s. 1O2 decay in the lipid bilayer is slow compared to the diffusion out of it and thus it has only minimal influence on the kinetics.6 It is therefore set to 14 μs as reported for the DPPC films in ref. 2.
The overall 1O2 amount in lipid layers and in water is calculated for the time interval 0–30 μs in steps of 20 ns.
The fit then is just an optimized linear combination of these two curves plus a constant background.
It is assumed that the singlet oxygen is generated in at least two layers with kinetics defined by the triplet decay time.
If a PS is really incorporated in the membrane the generation thus happens in layers 37 and 38, if the PS is attached to the membrane, the generation would happen in 4 layers: 34, 35, 39, and 40 as the oxygen can get the energy of the PS in the triplet state on both sides of the PS. In the case of water soluble PSs the singlet oxygen would be generated everywhere except in the membrane.
Due to the very fast diffusion between the layers and the small thickness of the membrane, the kinetics determined with this theoretical model are nearly indistinguishable for the PS embedded and attached to the layer. Just the amplitudes of the kinetics are minimally reduced for attached molecules, about 6% for the lipid signal and 2% for the water signal. The SNR of singlet oxygen luminescence kinetics does not allow differentiation between these cases (not shown – after scaling they appear as one line).
Hydrodynamic diameter determined by dynamic light scattering (DLS)
The size of the prepared liposomes was determined using a commercial dynamic light scattering (DLS) setup Zetasizer Nano ZS (Malvern Instruments, UK) equipped with a He–Ne-Laser (wavelength = 632.8 nm) as described elsewhere.14 The data were recorded at 25 ± 1 °C in backscattering mode at a 173° scattering angle. Each sample was measured three times for 100 s each measurement and the recorded data were averaged. Since the liposomes were filtered with a 0.22 μm syringe filter already, no further preparation was necessary. SUV concentrations were in the range of 1012–1013 particles ml−1 which was reported to be a good value15 for particles in this size range. Besides the average particle size the polydispersity index is determined during a DLS measurement. For a theoretical Gaussian size distribution it is the square of the normalized width at half height.Time-resolved singlet oxygen luminescence kinetics
Luminescence kinetics were recorded using IF filter-based optics with central wavelengths at 1250, 1275 and 1300 nm. The collection times for the measurements at 1250 and 1300 nm were adjusted to compensate the different overall transmission of the filters. This means that any emission that has a more or less linear intensity distribution in this wavelength range causes the same signal intensity using the 1270 nm filter compared to the sum of the signals of the other two filters. So by subtracting the latter two signals from the first one results in a signal nearly free of distortion from scattering or PS phosphorescence if the maxima of these disturbing signals are sufficiently far away from this wavelength range.Measurements were taken using the compact table-top 1O2 luminescence detection system TCMPC1270 of SHB Analytics, Germany with the extension for using external lasers. Excitation was done using the frequency doubled Nd3+-YAG Laser Vector from Coherent, Germany. Samples were excited with 5 mW at 532 nm, 10 ns pulses with a repetition rate of 12 kHz for 100 s when the 1270 nm filter was used.
The quality of each fit was ascertained using reduced χ2 (χ2red) in the time region of 0.3–30 μs. The lower time limit was given by the ever present short time artifact.
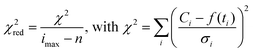 | (2) |
Triplet decay times
Flash photolysis measurements were taken using a ns-Nd3+-YAG pumped OPO (Ekspla, 420–2500 nm). Transient triplet–triplet absorption was observed, perpendicular to the excitation, using a stabilized LED (λmax = 488 nm), a band pass interference filter (488 nm) and a Si-photodiode with an integrated fast differential pre-amplifier (Elektronik Manufaktur Mahlsdorf, Germany). The setup allows the determination of triplet decay times down to 0.1 μs using a recording oscilloscope (HP5415).Liposome preparation
Liposomes were prepared using the injection method described in ref. 16. Before injecting the ethanol solution of DPPC the PSs were mixed with the lipid molecules to ensure a homogeneous distribution. The concentration of the PS in the mixture before injection was about 0.1 mM. 1,2-Dipalmitoyl-rac-glycero-3-phosphocholine hydrate (DPPC) from Sigma-Aldrich, Germany was used as purchased. Directly after preparation, the liposomal solution was filtered through a 0.22 μm syringe filter. DLS measurements and detection of singlet oxygen kinetics as well as flash photolysis were done within a time frame of 6 hours after preparation. All measurements were taken at room temperature.Photosensitizers
Pheophorbide a (Pheo) from Frontier Scientific, USA and meso-tetra(4-N-methyl-pyridyl) porphyrin (TMPyP) from Sigma-Aldrich, Germany were used as purchased.In a previous study we used Pheo extracted from Urtica urens.6,7 Analysing the purity of the extract using molecular mass spectrometry, besides Pheo with 593 Da, small amounts of pyro-Pheo have been identified with 535 Da. Since pyro-Pheo has a slightly longer triplet decay time compared to Pheo, even small contaminations disturb the analytic approach developed in this paper.
Because of that, for the actual investigation we used a commercial batch of Pheo. Its purity was checked using an Acquity-UPLC®-System equipped with a LCT Premier XE ESI-ToF mass spectrometer (positive mode). UPLC parameters: Acquity UPLC® BEH C18 column, 0.6 ml min−1, 35 °C, solvent: 0.1% formic acid and acetonitrile in water, with a concentration gradient of acetonitrile from 60% to 95% within 10 minutes.
The sample we bought from Frontier Scientific did not contain other Pheo derivatives as e.g. pyro-pheophorbide according to this test and was therefore used for the experiments reported here.
3. Results and discussion
Pheo is well known to be incorporated in membranes due to its hydrophobicity17 while TMPyP has very good water solubility3 and thus it is a good reference for PSs equally distributed all over the water phase. Because of this these two PSs were chosen to test the applicability of the new diffusion model in competition to the standard two-exponential decay model imaging whether the luminescence kinetics reflect their localization relative to the SUVs.The size of the SUVs formed after injection of DPPC mixed with Pheo as determined by DLS was astonishingly reproducible with a diameter of about 78 nm and a narrow particle distribution index (0.17–0.18). The SUVs formed in the presence of TMPyP also had a narrow distribution index (0.19–0.20) in each case but the average size was in the range 70–100 nm for different trials. However, this did not influence the results of the measured kinetics, as expected.
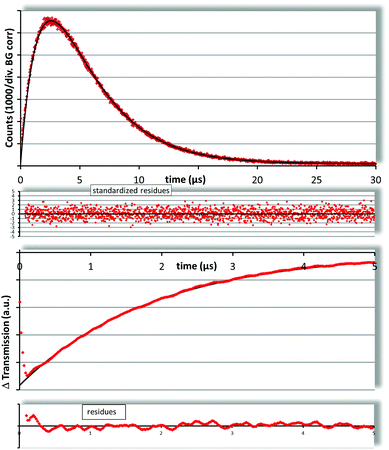
For TMPyP the results of flash photolysis and 1O2 luminescence are shown in Fig. 2. The PS triplet decay was fitted as 1.89 ± 0.20 μs assuming a mono exponential decay of the transient triplet absorption. A standard two-exponential fit of the singlet oxygen kinetics results in 1.85 ± 0.10 μs and 3.76 ± 0.10 μs. The PS triplet decay times determined with both methods match perfectly and the 1O2 decay time is just slightly longer than reported before for water but within the error range. χ2red for this fit is 0.99, which indicates a perfect fit. From the results it seems obvious that the vast majority of TMPyP is solved in the water phase of the liposomal dispersions. This result is not surprising, but it stands as a good reference for the samples with Pheo.
As mentioned above, Pheo is known to be incorporated in membranes.17
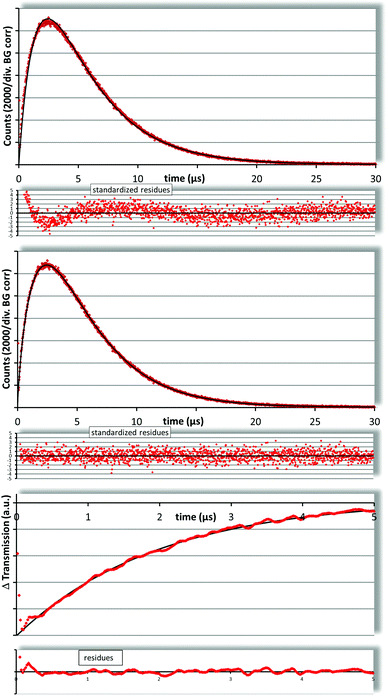
In liposomal dispersion we determined a Pheo triplet decay time of 1.98 ± 0.20 μs (Fig. 3, bottom) which is comparable to values reported before.6
In trying to fit the singlet oxygen luminescence decay curves using the standard two exponential fit, we obtained decay times of 1.5 μs and 4.2 μs. Neither does the triplet decay time coincide with the one determined by flash photolysis nor is the 1O2 decay time even near to the one in water. Due to the preparation of the liposomes with the injection method there is about 5–7% ethanol in the solution, but in test measurements of water–ethanol mixtures we found that it would require more than 30% ethanol in the solution to raise the 1O2 decay time above 4 μs (not shown). It has been reported that ethanol concentrates in the vicinity of the membranes but this effect is limited to the lipid water interface and consequently not that strong.
Finally, a look at the residua of the fit reveals that this fit does not describe the measured kinetics (Fig. 3, top).
Obviously, the commonly used two exponential model is not an appropriate description of the kinetics of 1O2 photosensitivity generated by Pheo in liposome dispersions. This proves that the 1O2 is not generated equally distributed within the sample, a fact that is known, but here for the first time can be directly derived from 1O2 kinetics.
The results shall now be fitted based on the above described model of singlet oxygen generation only in the membrane bilayer followed by diffusion.
The fitting procedure: the PS triplet (1 μs⋯3 μs) and the singlet oxygen decay time outside the membrane (3 μs⋯5 μs) is stepwise varied in steps of 0.02 μs and for each parameter set the theoretical temporal development of the amount of 1O2 inside and outside the membrane is calculated. These calculations are based on Fick's laws of diffusion (see ESI†). The fit is then a linear combination of the two curves plus a constant background since the dark counts of the device are equally distributed over time. The ratio of the amplitudes of the two curves is also the ratio of the radiative rate constants in the membrane compared to the aqueous solution around it (rate ratio). It is known that the radiative rate constant of 1O2 depends on the environment.18
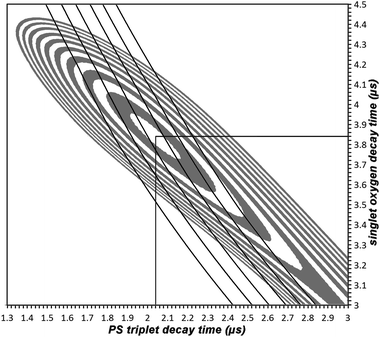
The best fit was obtained for a PS triplet decay time of 2.04 μs, a 1O2 decay time of 3.84 μs and an rate ratio of 3.25 (Fig. 3, middle). The corresponding value for χ2red is 1.07 (time region 0.3–30 μs). In Fig. 4 the best obtained χ2red are shown as a function of the parameter set. The central white area represents values below 1.1, the grey area around it goes up to 1.2 and so on. The large white field means χ2red > 2.9 and is therefore of no interest. The black lines across this plot connect the parameter sets that result in the same rate ratio. From the left to the right the lines represent rate ratios from 2 to 4.5 in steps of 0.5. The region of best fits (χ2red < 1.1) includes rate ratios from 2.9 to 3.7, so we can assume a value of 3.25 ± 0.50. This value is much smaller than the one reported earlier.6 This is mainly due a more accurate model (spherical symmetrical instead of 1D diffusion/solubility of 1O2 is taken into account, which was not performed in the past) and Pheo of a higher purity (see section 2).
The PS triplet decay time determined by our model perfectly corresponds to the result of the flash photolysis (Fig. 3, bottom) and the singlet oxygen decay time is only very slightly longer than it should be expected for the given content of ethanol in the solutions, which probably can be explained by the already mentioned affinity of ethanol to the lipid water interface.
4. Conclusions
It could be clearly shown that diffusion of 1O2 influences its luminescence kinetics in heterogeneous systems. The temporal shape of the 1O2 luminescence in the heterogeneous environment is not just an average of the involved phases, but depends strongly on the exact place of 1O2 generation and the radiative rate constants of 1O2 in different phases. For DPPC liposomes the 1O2 luminescence emanating from inside the lipid bilayer is overpronounced by a rate ratio of 3.25 ± 0.50 compared to the surrounding water. Moreover, by analyzing the 1O2 luminescence kinetics it was shown that Pheo in the liposomes generates 1O2 in or in close vicinity to the membrane bilayer. On the other hand, TMPyP is nearly homogeneously distributed in the aqueous phase of the liposome dispersion and generates 1O2 accordingly, whereby the luminescence kinetics can be described using the two exponential standard model.Knowledge about such diffusion effects and different rate ratios of 1O2 in biologically relevant environments will help to develop means for measuring 1O2 directly and quantitatively in vivo.
Acknowledgements
The authors like to thank Matthias Girod from BAM – Federal Institute for Materials Research and Testing, Berlin, Germany for his fast and kind help. He carried out the DLS measurements for us. Thanks to Sebastian Wieczorrek from the Chemical Department of Humboldt University. He checked the purity of the used Pheo with mass spectrometry. The authors gratefully acknowledge the inspirations given by the reviewers.Notes and references
- A. Jiménez-Banzo, X. Ragàs, P. Kapusta and S. Nonell, Time-resolved methods in biophysics. 7. Photon counting vs. analog time-resolved singlet oxygen phosphorescence detection, Photochem. Photobiol. Sci., 2008, 7(9), 1003 Search PubMed.
- T. Maisch, J. Baier, B. Franz, M. Maier, M. Landthaler, R.-M. Szeimies and W. Baumler, The role of singlet oxygen and oxygen concentration in photodynamic inactivation of bacteria, Proc. Natl. Acad. Sci. U. S. A., 2007, 104(17), 7223–7228 CrossRef CAS PubMed.
- M. K. Kuimova, S. W. Botchway, A. W. Parker, M. Balaz, H. A. Collins, H. L. Anderson, K. Suhling and P. R. Ogilby, Imaging intracellular viscosity of a single cell during photoinduced cell death, Nat. Chem., 2009, 1(1), 69–73 CrossRef CAS PubMed.
- M. T. Jarvi, M. J. Niedre, M. S. Patterson and B. C. Wilson, The Influence of Oxygen Depletion and Photosensitizer Triplet-state Dynamics During Photodynamic Therapy on Accurate Singlet Oxygen Luminescence Monitoring and Analysis of Treatment Dose Response, Photochem. Photobiol., 2011, 87(1), 223–234 CrossRef CAS PubMed.
- J. Baier, M. Maier, R. Engl, M. Landthaler and W. Bäumler, Time-Resolved Investigations of Singlet Oxygen Luminescence in Water, in Phosphatidylcholine, and in Aqueous Suspensions of Phosphatidylcholine or HT29 Cells, J. Phys. Chem. B, 2005, 109(7), 3041–3046 CrossRef CAS PubMed.
- S. Hackbarth, J. Schlothauer, A. Preuß and B. Röder, New insights to primary photodynamic effects – Singlet oxygen kinetics in living cells, J. Photochem. Photobiol., B: Biol., 2010, 98(3), 173–179 CrossRef CAS PubMed.
- S. Hackbarth, J. Schlothauer, A. Preuß, C. Ludwig and B. Röder, Time resolved sub-cellular singlet oxygen detection – ensemble measurements versus single cell experiments, Laser Phys. Lett., 2012, 9(6), 474–480 CrossRef CAS.
- F. C. Tse and O. C. Sandall, Diffusion Coefficients for Oxygen and Carbon Dioxide in Water at 25 °C by Unsteady State Desorption from a Quiescent Liquid, Chem. Eng. Commun., 1979, 3(3), 147–153 CrossRef CAS.
- M. Jamnongwong, K. Loubiere, N. Dietrich and G. Hébrard, Experimental study of oxygen diffusion coefficients in clean water containing salt, glucose or surfactant: Consequences on the liquid-side mass transfer coefficients, Chem. Eng. J., 2010, 165(3), 758–768 CrossRef CAS PubMed.
- R. A. Freitas, Jr., Nanomedicine, Volume I: Basic Capabilities, Landes Bioscience, Georgetown (Tex.), 1999 Search PubMed.
- K. Mitra, I. Ubarretxena-Belandia, T. Taguchi, G. Warren and D. M. Engelman, Modulation of the bilayer thickness of exocytic pathway membranes by membrane proteins rather than cholesterol, Proc. Natl. Acad. Sci. U. S. A., 2004, 101(12), 4083–4088 CrossRef CAS PubMed.
- R. M. Cordeiro, Reactive oxygen species at phospholipid bilayers: distribution, mobility and permeation, Biochim. Biophys. Acta, 2014, 1838(1 Pt B), 438–444 CrossRef CAS PubMed.
- E. Smotkin, F. T. Moy and W. Plachy, Dioxygen solubility in aqueous phosphatidylcholine dispersions, Biochim. Biophys. Acta, Biomemb., 1991, 1061, 33–38 CrossRef CAS.
- L. Böhmert, M. Girod, U. Hansen, R. Maul, P. Knappe, B. Niemann, S. M. Weidner, A. F. Thünemann and A. Lampen, Analytically monitored digestion of silver nanoparticles and their toxicity on human intestinal cells, Nanotoxicology, 2014, 8(6), 631–642 CrossRef PubMed.
- J. Panchal, J. Kotarek, E. Marszal and E. M. Topp, Analyzing Subvisible Particles in Protein Drug Products: a Comparison of Dynamic Light Scattering (DLS) and Resonant Mass Measurement (RMM), AAPS J., 2014, 16(3), 440–451 CrossRef CAS PubMed.
- A. Zeug, A. Paul and B. Röder, Observation of the phase transition in phospholipid liposomes taking advantage of the particular optical properties of octa-α-butyloxy-H 2 phthalocyanines, J. Porphyrins Phthalocyanines, 2001, 05(09), 663–667 CrossRef CAS.
- B. Röder, T. Hanke, S. Oelckers, S. Hackbarth and C. Symietz, Photophysical properties of pheophorbide a in solution and in model membrane systems, J. Porphyrins Phthalocyanines, 2000, 4(1), 37–44 CrossRef.
- T. D. Poulsen, P. R. Ogilby and K. V. Mikkelsen, Solvent Effects on the O 2 (a 1 Δ g)−O 2 (X 3 Σ g-) Radiative Transition: Comments Regarding Charge-Transfer Interactions, J. Phys. Chem. A, 1998, 102(48), 9829–9832 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Description of the mathematical formalism behind the spherical symmetrical diffusion model. See DOI: 10.1039/c4pp00229f |
| This journal is © The Royal Society of Chemistry and Owner Societies 2015 |