Highly selective, sensitive and fluorescent sensing of dimeric G-quadruplexes by a dimeric berberine†
Chun-Qiong
Zhou
,
Jian-Wei
Yang
,
Cheng
Dong
,
Yong-Min
Wang
,
Bin
Sun
,
Jin-Xiang
Chen
,
Ya-Shi
Xu
and
Wen-Hua
Chen
*
Guangdong Provincial Key Laboratory of New Drug Screening, School of Pharmaceutical Sciences, Southern Medical University, Guangzhou 510515, P. R. China. E-mail: whchen@smu.edu.cn
First published on 9th November 2015
Abstract
This paper describes the highly selective, sensitive and topology-specific fluorescent sensing of dimeric G-quadruplexes by a polyether-tethered dimeric berberine 1. Compound 1 displays high selectivity for dimeric G-quadruplexes over monomeric ones, and can be lit up by dimeric G-quadruplexes, in particular by the one linked with one TTA subunit. In addition, it shows no effect on the topology or thermal stability of the G-quadruplexes.
Introduction
Development of highly selective, sensitive and fluorescent probes for nucleic acids, in particular of high-order structures is of profound importance in a wide range of fields.1 In humans, telomeric DNA consists of a duplex region having hundreds of TTAGGG repeats that end in a short G-rich single-stranded protrusion, and tends to fold into unique, highly stable four-stranded helices known as G-quadruplexes. G-quadruplexes are ubiquitous in many crucial genomic regions and have attracted extensive attention because of their biological significance and potential applications.2 To date, major interest in G-quadruplex sensing has been focused on the development of rapid and simple approaches for the selective and sensitive detection of single-stranded G-quadruplexes.3–14 It is widely recognized that an ideal fluorescent probe should be able to detect G-quadruplexes without affecting the topology or thermal stability of the targeted G-quadruplexes.8–11 However, few such fluorescent probes have been reported to date.On the other hand, the telomeric terminal overhang has ca. 200 nucleotides. Although most of them are prone to form monomeric G-quadruplexes, a small number of G-rich sequences tend to form more-complex, higher-order G-quadruplex structures consisting of consecutive G-quadruplex units connected by TTA subunits.15–18 In addition, there are some diseases, such as ALS and Fragile X syndromes that are associated with expansion repeats of G-rich sequences capable of forming multimeric G-quadruplexes.19–22 Thus, highly selective probes for dimeric and/or multimeric G-quadruplexes may help to better understand the structures and functions of G-quadruplexes and provide useful guidance for the rational design of G-quadruplex DNA-targeting anticancer drugs.23,24 To date, several fluorescent probes have been reported to be able to discriminate G-quadruplexes from single- and double-stranded nucleic acids.25–30
Protoberberines, a class of naturally occurring isoquinoline alkaloids, are known as DNA binders. They exhibit potent binding affinities toward ds-DNA,31 G-qudruplexes,32–37 triplexes38 and RNAs.39,40 The structure–activity relationship study has indicated that modification of protoberberines with side chains, in particular having terminal amino groups, leads to a significant increase in the binding ability.31 However, this modification also affects the topology and/or thermal stability of the targeted G-quadruplexes. This is not desirable from the standpoint of fluorescent probes for G-quadruplexes. Recently Shao et al. reported that the epiberberine alkaloid could be used to fluorescently detect human telomeric G-quadruplex multimers.41 However, there is no evidence that protoberberine derivatives are able to discriminate dimeric G-quadruplexes from monomeric ones.
Herein we describe the highly selective and sensitive fluorescent sensing of dimeric G-quadruplexes by a polyether-tethered dimeric berberine 1 without side chains (Scheme 1). Specifically, we report that compound 1 exhibits high selectivity and sensitivity for dimeric G-quadruplexes of varying lengths over G-quadruplex monomers, without affecting the topological or thermal stability of the G-quadruplexes.
Results and discussion
Synthesis of compound 1
Compound 1 was synthesized in an 87% yield from the reaction of berberrubine 2 with triethylene glycol ditosylate 3. Compound 1 was fully characterized on the basis of NMR (1H and 13C) and MS (LR and HR) data (see the Experimental section). Compounds 2 and 3 were prepared according to reported protocols.42Spectrofluorimetric titrations
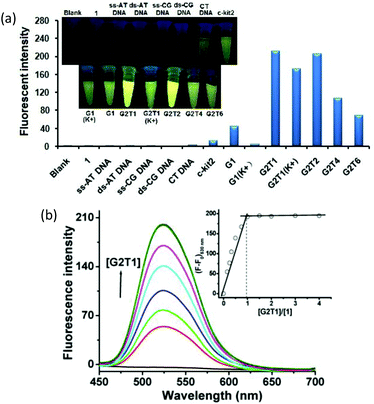
Firstly, we screened the affinity of compound 1 toward a wide range of DNA sequences, including ss-AT DNA, ds-AT DNA, ss-CG DNA, ds-CG DNA, CT DNA, monomeric G-quadruplexes (c-kit2 (parallel), G1 (K+, hybrid-type), G1 (anti-parallel), anti-parallel dimeric G-quadruplexes tethered with one, two, four and six TTA subunits, accordingly named G2T1, G2T2, G2T4 and G2T6, respectively, and hybrid-type G2T1 (K+). Fig. 1a shows the fluorescence change that compound 1 undergoes upon the addition of these DNA sequences. It can be seen that compound 1 was essentially non-fluorescent, and the addition of ss-DNA, ds-DNA and CT DNA induced negligible changes in the fluorescence intensity. Addition of monomeric G-quadruplexes, G1 (anti-parallel), c-kit2 (parallel) and G1 (K+) (hybrid-type), led to an apparent increase in the fluorescence intensity. Remarkable enhancement was observed with dimeric G-quadruplexes. In particular, dimeric G-quadruplexes G2T1, G2T1(K+) and G2T2 caused up to 212-, 171- and 205-fold fluorescence enhancement with a strong new emission peak at 530 nm, respectively, and dramatic fluorescent colour change from dark to yellow (Fig. 1a). This suggests that compound 1 exhibited a fluorescent “turn-on” response toward dimeric G-quadruplexes. It should be noted that the fluorescence enhancement varied with the length of the linkers of the G-quadruplex dimers.To quantitatively characterize the DNA-binding affinity of compound 1, we carried out photofluorimetric titrations with these DNA sequences. The data indicate that ss-DNA, ds-DNA, CT DNA, c-kit2 and G1 (K+) induced no concentration-dependent changes in the fluorescence intensity (Fig. S6†), whereas the fluorescence intensity of compound 1 increased with the concentration of G1 and dimeric G-quadruplexes (Fig. 1b and S7–12†). The results obtained from both the spectrofluorimetric titration and Job's plot (Fig. 1b, inset) illustrated a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding mode between compound 1 and these DNA sequences. Analyses of the relationship between the fluorescence intensity of compound 1 and the concentrations of these G-quadruplexes afforded binding constants in the range from 2.8 × 105 M−1 to 2.4 × 107 M−1 (Table 1 and Fig. S7–12†).31,43 Thus, compound 1 exhibited comparable binding affinity with G2T1 (K+) and G2T1, ca. 71–87-fold higher than that with G1. In addition, it exhibited 6-, 12- and 44-fold higher binding affinity toward G2T1 than toward G2T2, G2T4 and G2T6, respectively (Table 1).
1 binding mode between compound 1 and these DNA sequences. Analyses of the relationship between the fluorescence intensity of compound 1 and the concentrations of these G-quadruplexes afforded binding constants in the range from 2.8 × 105 M−1 to 2.4 × 107 M−1 (Table 1 and Fig. S7–12†).31,43 Thus, compound 1 exhibited comparable binding affinity with G2T1 (K+) and G2T1, ca. 71–87-fold higher than that with G1. In addition, it exhibited 6-, 12- and 44-fold higher binding affinity toward G2T1 than toward G2T2, G2T4 and G2T6, respectively (Table 1).
| DNA | ε | Φ F | RBa | K a | RAb |
|---|---|---|---|---|---|
| a Brightness (= molar extinction coefficient × ΦF/1000) is reported relative to compound 1 alone. b RA denotes the relative affinity. | |||||
| None | 6.38 × 104 | 0.002 | 1.0 | ||
| ss-AT DNA | 5.23 × 104 | 0.012 | 4.9 | ||
| ds-AT DNA | 5.12 × 104 | 0.013 | 5.2 | ||
| ss-CG DNA | 5.07 × 104 | 0.010 | 4.0 | ||
| ds-CG DNA | 4.98 × 104 | 0.012 | 4.7 | ||
| CT DNA | 4.87 × 104 | 0.005 | 1.9 | ||
| c-kit2 | 4.35 × 104 | 0.029 | 9.9 | ||
| G1 | 3.59 × 104 | 0.133 | 37 | (2.8 ± 0.2) × 105 | 1.0 |
| G1 (K+) | 4.66 × 104 | 0.069 | 25 | ||
| G2T1 | 2.01 × 104 | 0.447 | 70 | (2.4 ± 0.7) × 107 | 85.7 |
| G2T1 (K+) | 2.88 × 104 | 0.133 | 30 | (2.0 ± 0.5) × 107 | 71.4 |
| G2T2 | 2.76 × 104 | 0.325 | 70 | (4.4 ± 0.5) × 106 | 15.7 |
| G2T4 | 4.05 × 104 | 0.164 | 52 | (2.0 ± 0.2) × 106 | 7.1 |
| G2T6 | 4.32 × 104 | 0.111 | 38 | (5.5 ± 0.3) × 105 | 2.0 |
To further investigate the sensing properties of compound 1, we measured its photochemical properties in the presence of the above-mentioned DNA sequences (Tables 1 and S1†). It can be seen that the quantum yield of compound 1 was unaffected in the presence of ss-DNA, ds-DNA and CT DNA, whereas it greatly increased in the presence of G-quadruplexes. For example, the quantum yield of compound 1 for G2T1 was 0.447, ca. 1.4, 2.7, 3.4 and 4.0-fold higher than those for G2T2, G2T4, G2T1 (K+) and G2T6, respectively; 3.4, 6.5 and 15.4-fold higher than those for G1, G1 (K+) and c-kit2, respectively; and 34–90-fold higher than those for ss- and ds-DNA. These results suggest that compound 1 showed higher sensitivity toward G2T1 than the other dimeric and monomeric G-quadruplexes, which is in good agreement with the results obtained from spectrofluorimetric titrations.
Native gel electrophoresis
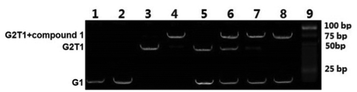
The high selectivity of compound 1 for G2T1 over G1 was further verified by native gel electrophoresis (GE) experiments.15 As shown in Fig. 2, G1 exhibited no new band in the presence of compound 1, indicating that no stable complex was formed. In contrast, addition of compound 1 to G2T1 led to a new band, indicative of the formation of a stable complex. Then we conducted the GE experiments by incubating compound 1 with a mixture of G1 and G2T1. Under the assay conditions, G1 and G2T1 formed their corresponding intramolecular monomeric and dimeric G-quadruplexes, without interfering with each other (lane 5). After addition of compound 1 to the mixture of G1 and G2T1, a new band corresponding to the compound 1–G2T1 complex appeared (lanes 6–8). These results suggest that compound 1 can selectively bind with G2T1 over G1, in full agreement with the results obtained from the spectrofluorimetric titration.Detection limit
An ideal sensor should have a low detection limit and high sensitivity. Therefore we measured the detection limit of compound 1 toward G2T1 and G1 by means of spectrofluorimetric titrations. Under the assay conditions, the detection limit for G2T1 was calculated to be 0.65 nM (Fig. S13†) based on S/N = 3,44ca. 10-fold lower than that of compound 1 toward G1 (6.0 nM, Fig. S14†). These data underline that compound 1 shows higher detection sensitivity toward G2T1 than G1.CD measurements
It is well accepted that an ideal G-quadruplex fluorescent probe should not affect the topology or thermal stability of G-quadruplexes.8–11 Therefore, we measured the circular dichroism (CD) spectra of G1 and G2T1 in the presence of compound 1. It can be seen from Fig. 3 that G1 and G2T1 exhibited two characteristic positive peaks at 245 and 295 nm and a negative peak at 260 nm. Obviously, compound 1 had a negligible impact on the characteristic peaks of both G-quadruplexes, in particular G2T1. In addition, CD-melting experiments revealed that compound 1 had a negligible effect on the stability of G2T1 (ΔTm = −0.5 ± 0.1 °C) (Table S3†). These results suggest that compound 1 can exercise its topology-specific sensing function without affecting the topological or thermal stability of G2T1.8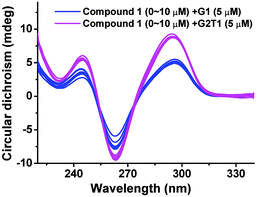 | ||
| Fig. 3 CD spectra of G1 and G2T1 (5 μM) in the presence of compound 1 of varying concentrations from 0 to 10 μM, in 10 mM Tris-HCl buffer (100 mM NaCl, pH 7.2). | ||
Binding mode and fluorescent mechanism
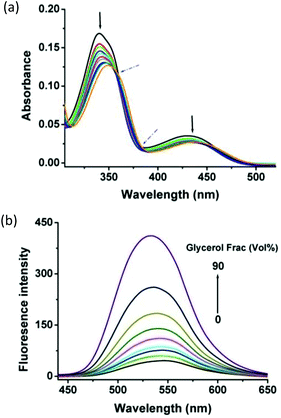
The binding of berberine and its derivatives to G-quadruplexes has been extensively studied by various analytical methods.32–36,41,45–47 The widely accepted binding mode is that berberine subunits interact with G-quadruplexes in an intercalative mode. To gain further insight into this binding mode, we conducted spectrophotometric titrations of compound 1 with G2T1 (Fig. 4a). Two well-resolved isosbestic points (at 360 nm and 385 nm) reveal the existence of one exclusive binding mode, whereas remarkable hypochromic and bathochromic changes are indicative of an intercalative binding mode, in agreement with the previous report on the binding of berberine derivatives to G-quadruplex structures.45–47 Specifically, the berberine subunits intercalate into G-quadruplexes through π–π stacking interactions with base pairs.32–36,41 Moreover, the results obtained from photofluorimetric titrations indicate that compound 1 interacted with G2T1 in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry (Fig. 1b). Thus, we deduce that the two berberine subunits of compound 1 might simultaneously interact with the two G-quartets of G2T1. This is supported by the binding constants (Ka's) of compound 1 with dimeric G-quadruplexes tethered with linkers of varying lengths from 1 to 6 TTAs (Table 1). The finding that the binding constants (Ka's) of compound 1 decreased with the length of the linkers, suggests that a short TTA linker would favour its binding and the long TTA linkers would hamper the binding because of the increasing special distance between the two G-quadruplex units.
1 stoichiometry (Fig. 1b). Thus, we deduce that the two berberine subunits of compound 1 might simultaneously interact with the two G-quartets of G2T1. This is supported by the binding constants (Ka's) of compound 1 with dimeric G-quadruplexes tethered with linkers of varying lengths from 1 to 6 TTAs (Table 1). The finding that the binding constants (Ka's) of compound 1 decreased with the length of the linkers, suggests that a short TTA linker would favour its binding and the long TTA linkers would hamper the binding because of the increasing special distance between the two G-quadruplex units.
In addition, the “light up” of compound 1 by G2T1 is a likely consequence of the conformational changes in the excited state. Specifically, compound 1 could bind into the adjacent planes of dimeric G-quadruplexes probably through π–π stacking interactions, which may hamper the rotation of the berberine plane and produce the fluorescence.27 This hypothesis was supported by the finding that compound 1 exhibited significant emission that increased with the glycerol content of aqueous glycerol solution, but exhibited negligible emission in most solvents of low viscosity (Fig. 4b, Tables 2 and S2†).8,10
Conclusions
We have successfully developed a dimeric berberine-based sensitive and fluorescent probe for dimeric G-quadruplexes, in particular for the one with one TTA linker. This compound exhibits highly sensitive, selective and fluorescent detection of dimeric G-quadruplexes without affecting the topological or thermal stability. We have also briefly discussed the binding mode and lighting mechanism between compound 1 and dimeric G-quadruplex G2T1. The high selectivity for dimeric G-quadruplexes over monomeric G-quadruplexes makes compound 1 exploitable as a potential diagnostic tool for the diseases that are associated with expansion repeats of G-rich sequences capable of forming dimeric and/or multimeric G-quadruplexes. Current efforts are aimed at synthesizing more highly specific berberine-based probes for dimeric and/or multimeric G-quadruplexes, with a view toward the discovery of novel chemotherapeutic agents for diseases.Experimental
Generals
1H and 13C NMR spectra were recorded in DMSO-d6 or CDCl3 on a Varian Mercury 400 spectrometer. ESI-MS and HR-ESI-MS spectra were recorded on Waters UPLC/Quattro Premier XE and Agilent 6460 Triple Quadrupole mass spectrometers, respectively. UV spectra were recorded on a UV-2450 spectrophotometer (Shimadzu, Japan) using a 1 cm path length quartz cuvette. Fluorescence spectra were recorded on a Shimadzu RF-5301PC spectrofluorometer. Polyacrylamide gel electrophoresis (GE) was carried out on a DYY-8C electrophoresis apparatus and DYCZ-24EN electrophoresis capillary (Beijing Liuyi Instrument Factory, Beijing, China). Native GE was analyzed with an Alpha Hp 3400 fluorescent and visible light digitized image analyzer. CD studies and CD-melting experiments were performed on a Chirascan circular dichroism spectrophotometer (Applied Photophysics, UK).The oligonucleotides used in this study were purchased from Shanghai Sangon Biological Engineering Technology & Services (Shanghai, China). Calf thymus (CT) DNA was purchased from GBCBIO Technologies Inc. The oligonucleotides c-kit2, G1 (K+) and G2T1 (K+) were dissolved in 10 mM Tris-HCl buffer (100 mM KCl, pH 7.2) at 25 °C. All the other oligonucleotides were dissolved in 10 mM Tris-HCl buffer (100 mM NaCl, pH 7.2) at 25 °C. The concentrations of the oligonucleotides were determined by measuring the absorbance at 260 nm after melting For the formation of G-quadruplexes, the oligonucleotides were annealed in the relevant buffers at 95 °C for 5 min before cooling to room temperature overnight. All the other reagents and solvents were purchased from commercial sources and were of analytical grade. Solvents were dried according to standard procedures (Table 3).
| DNA | Oligonucleotide sequence (from 5′ to 3′) | Structurec |
|---|---|---|
| a Prepared in 10 mM Tris-HCl buffer (100 mM NaCl, pH 7.2) unless otherwise specified. b Prepared in 10 mM Tris-HCl buffer (100 mM KCl, pH 7.2). c G-quadruplex structures were confirmed by CD spectra (Fig. S5). | ||
| ss-AT | CGCGATATCGCG | Single stranded |
| ds-AT | ss-AT DNA + ss-AT DNA | Double stranded |
| ss-CG | CGCGCGCGCGCG | Single stranded |
| ds-CG | ss-CG DNA + ss-CG DNA | Double stranded |
| CT DNA | Double stranded | |
| c-kit2(K+)b | G3 CG3(CG)2(AG3)2G | Parallel G4 monomer |
| G1 | A(G3TTA)3G3 | Anti-parallel G4 monomer |
| G1(K+)b | A(G3TTA)3G3 | Hybrid type G4 monomer |
| G2T1 | A(GGGTTA)7G3 | Anti-parallel G4 dimer |
| G2T1(K+)b | A(GGGTTA)7G3 | Hybrid type G4 dimer |
| G2T2 | A(GGGTTA)4(TTAGGG)4 | Anti-parallel G4 dimer |
| G2T4 | A(GGGTTA)4(TTA)2(TTAGGG)4 | Anti-parallel G4 dimer |
| G2T6 | A(GGGTTA)4(TTA)4(TTAGGG)4 | Anti-parallel G4 dimer |
Synthesis of compound 1
To a solution of berberrubine 2 (310 mg, 0.96 mmol) in MeCN (20 mL) was added triethylene glycol ditosylate 3 (200 mg, 0.44 mmol). The resulting mixture was refluxed for 84 h, and then concentrated under reduced pressure. The obtained residue was subjected to anion exchange into chloride form, and subsequently purified by chromatography on a reverse-phase column, eluted with a gradient of methanol in water (0–10%), to give compound 1 (290 mg, 87%) as a yellow powder having m.p. 163.4–164.8 °C; 1H NMR (400 MHz, DMSO-d6) δ 9.71 (s, 2H), 8.89 (s, 2H), 8.11 (d, J = 9.2 Hz, 2H), 7.93 (d, J = 9.2 Hz, 2H), 7.68 (s, 2H), 7.05 (s, 2H), 6.14 (s, 4H), 4.94 (t, J = 5.6 Hz, 4H), 4.32 (t, J = 4.0 Hz, 4H), 3.95 (s, 6H), 3.81 (t, J = 4.4 Hz, 4H), 3.67 (s, 4H), 3.20 (t, J = 6.2 Hz, 4H); 13C NMR (100 MHz, DMSO) δ 150.7, 150.1, 148.0, 145.7, 142.8, 137.7, 133.2, 130.9, 126.7, 123.9, 122.0, 120.7, 120.5, 108.7, 105.7, 102.5, 73.6, 70.1, 69.8, 57.3, 55.8, 26.7; ESI-MS: m/z 379.6 ([M − 2Cl]2+) and HRMS for C44H42N2O10 ([M − 2Cl]2+) Calcd: 379.1420, found: 379.1414.Spectrophotometric titration
All the fluorescence spectra were recorded at λex/λem = 355/530 nm with ex/em = 3/3 nm. Photofluorimetric titrations were conducted by keeping the concentration of compound 1 constant, while gradually increasing the concentrations of each DNA sequences. The stoichiometric ratios between compound 1 and DNA were obtained by plotting (F − F0) at 530 nm against the [DNA]/[1] ratios varying from 0 to 4.0. The binding constants (Ka's) were obtained by analyzing the fluorescence intensity as a function of the concentrations of the added DNA.31,43Determination of quantum yields
The quantum yield values of compound 1 in the presence of different DNAs, in a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 were measured by using the protocol described in the literature.8 Thus, the absorption and emission spectra of compound 1 were recorded in ethanol. They showed absorption and emission maxima at 355 nm and 530 nm, respectively. Quinine sulfate was chosen as a standard because its absorption and emission spectra largely overlap those of compound 1 in ethanol. Fluorescence quantum yield ΦX of compound 1 was calculated by the equation ΦX = ΦS × [AbsS/AbsX] × [AFX/AFS] × [NX/NS]2. ΦS (= 0.550) is the reported quantum yield of quinine sulfate in ethanol; AbsS and AbsX are the absorbance at the excitation wavelengths of quinine sulfate and compound 1, respectively; AFS and AFX are the areas under the emission spectra of quinine sulfate and compound 1, respectively; and NS and NX are the refractive indexes of the solvents used for quinine sulfate and compound 1, respectively. Under our assay conditions, AbsS (λex = 313 nm) and AFS are 0.011 and 17
1 were measured by using the protocol described in the literature.8 Thus, the absorption and emission spectra of compound 1 were recorded in ethanol. They showed absorption and emission maxima at 355 nm and 530 nm, respectively. Quinine sulfate was chosen as a standard because its absorption and emission spectra largely overlap those of compound 1 in ethanol. Fluorescence quantum yield ΦX of compound 1 was calculated by the equation ΦX = ΦS × [AbsS/AbsX] × [AFX/AFS] × [NX/NS]2. ΦS (= 0.550) is the reported quantum yield of quinine sulfate in ethanol; AbsS and AbsX are the absorbance at the excitation wavelengths of quinine sulfate and compound 1, respectively; AFS and AFX are the areas under the emission spectra of quinine sulfate and compound 1, respectively; and NS and NX are the refractive indexes of the solvents used for quinine sulfate and compound 1, respectively. Under our assay conditions, AbsS (λex = 313 nm) and AFS are 0.011 and 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 795.6, respectively. The AFX value of compound 1 (0.5 μM) was measured at λex/λem = 355/530 nm and ex/em = 5/5 nm.
795.6, respectively. The AFX value of compound 1 (0.5 μM) was measured at λex/λem = 355/530 nm and ex/em = 5/5 nm.
Gel electrophoresis assay
Native gel electrophoresis was carried out on acrylamide gel (15%), run at 4 °C in 1× TBE buffer (10 mM NaCl, pH 8.3) and was silver stained. DNA was heated at 95 °C for 5 min in the presence of 100 mM NaCl, gradually cooled from 95 °C to room temperature and incubated at 4 °C overnight.14 The final loading sample was prepared by mixing compound 1 (50 μM) with the annealed DNA samples, followed by incubation at 4 °C for 3 hours.Detection limit
The detection limit was calculated according to the equation: detection limit = 3σbi/m, wherein σbi is the standard deviation of blank measurements and m is the slope of the straightline between the fluorescence intensity and the concentration of G1 or G2T1.43 To determine the S/N ratio, the emission intensity of compound 1 (0.5 μM) in the absence of G1 or G2T1 was measured 10 times and the standard deviation of blank measurements was determined. Under the present conditions, a good linear relationship between the fluorescence intensity and the concentration of G1 or G2T1 was obtained in the range of 0–0.3 μM.Measurements of circular dichroism (CD) spectra and CD-melting curves
CD spectra were recorded in the wavelength range of 200–340 nm using a quartz cuvette with 1.0 nm path length. The scanning speed was 100 nm min−1, and the response time was 2 s. The oligonucleotide (5 μM) was titrated with compound 1 in a molar ratio of 0.2–5. Each spectrum was recorded thrice at 25 °C. Thermal melting was monitored at 295 nm/265 nm at a heating rate of 1 °C min−1. The melting temperature (Tm) was determined from the melting profiles using a two-state transition model implemented in Kaleida Graph.8Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (no. 21402085) and Guangdong Provincial Department of Science and Technology of China (no. 2012B050100007 and 2014A050503042).Notes and references
- D. L. Ma, H.-Z. He, K.-H. Leung, H.-J. Zhong, S.-H. Chan and C.-H. Leung, Chem. Soc. Rev., 2013, 42, 3427 RSC.
- (a) J. T. Davis, Angew. Chem., Int. Ed., 2004, 43, 668 CrossRef CAS PubMed; (b) T. S. Dexheimer, D. Sun and L. H. Hurley, J. Am. Chem. Soc., 2006, 128, 5404 CrossRef CAS PubMed.
- G. Biff, D. Tannahill, J. McCafferty and S. Balasubramanian, Nat. Chem., 2013, 5, 182 CrossRef PubMed.
- G. Biff, M. Di Antonio, D. Tannahill and S. Balasubramanian, Nat. Chem., 2014, 6, 75 CrossRef PubMed.
- N. H. A. Karim, O. Mendoza, A. Shivalingam, A. J. Thormpson, S. Ghosh, M. K. Kuimova and R. Vilar, RSC Adv., 2014, 4, 3355 RSC.
- J. Mohanty, N. Barooah, V. Dhamodharan, S. Harikrishna, P. I. Pradeepkumar and A. C. Bhasikuttan, J. Am. Chem. Soc., 2013, 135, 367 CrossRef CAS PubMed.
- H.-Z. He, K.-H. Leung, W. Wang, D. S.-H. Chan, C.-H. Leung and D.-L. Ma, Chem. Commun., 2014, 50, 5313 RSC.
- S.-B. Chen, W.-B. Wu, M.-H. Hu, T.-M. Ou, L.-Q. Gu, J.-H. Tan and Z.-S. Huang, Chem. Commun., 2014, 50, 12173 RSC.
- B. Jin, X. Zhang, W. Zheng, X. Liu, C. Qi, F. Wang and D. Shangguan, Anal. Chem., 2014, 86, 943 CrossRef CAS PubMed.
- M. Nikan, M. Di Antonio, K. Abecassis, K. McLuckie and S. Balasubramanian, Angew. Chem., Int. Ed., 2013, 52, 1428 CrossRef CAS PubMed.
- G.-L. Liao, X. Chen, L.-N. Ji and H. Chao, Chem. Commun., 2012, 48, 10781 RSC.
- A. Henderson, Y. Wu, Y. C. Huang, E. A. Chavez, J. Platt, F. B. Johnson, R. M. Brosh Jr., D. Sen and P. M. Lansdrop, Nucleic Acids Res., 2014, 42, 860 CrossRef CAS PubMed.
- A. Laguerre, L. Stefan, M. Larrouy, D. Genest, J. Novotna, M. Pirrotta and D. Monchavd, J. Am. Chem. Soc., 2014, 136, 12406 CrossRef CAS PubMed.
- A. Laguerre, K. Hukezalie, P. Winckler, F. Katranji, G. Chanteloup, M. Pirrotta, J.-M. Perrier-Corner, J. M. Y. Wong and D. Monchaud, J. Am. Chem. Soc., 2015, 137, 8521 CrossRef CAS PubMed.
- C. Q. Zhao, L. Wu, J. S. Ren, Y. Xu and X. G. Qu, J. Am. Chem. Soc., 2013, 135, 18786 CrossRef CAS PubMed.
- K. I. Shinohara, Y. Sannohe, S. Kaieda, K. I. Tanaka, H. Osuga, H. Tahara, Y. Xu, T. Kawase, T. Bando and H. Sugiyama, J. Am. Chem. Soc., 2010, 132, 3778 CrossRef CAS PubMed.
- J. A. Punnoose, Y. Cui, D. Koirala, P. M. YangYangyuoru, C. Ghimire, P. Shrestha and H. Mao, J. Am. Chem. Soc., 2014, 136, 18062 CrossRef PubMed.
- J. D. Schonhoft, R. Bajracharya, S. Dhakal, Z. Yu, H. Mao and S. Basu, Nucleic Acids Res., 2009, 37, 3310 CrossRef CAS PubMed.
- K. Reddy, B. Zamiri, S. Y. R. Stanley, R. B. Macgregor Jr. and C. E. Pearson, J. Biol. Chem., 2013, 288, 9860 CrossRef CAS PubMed.
- B. Zamiri, K. Reddy, R. B. Marcgegor Jr. and C. E. Pearson, J. Biol. Chem., 2014, 289, 4653 CrossRef CAS PubMed.
- J. Brčić and J. Plavec, Nucleic Acids Res., 2015, 43, 8590 CrossRef PubMed.
- B. Zamiri, M. Mirceta, K. Bomsztyk, R. B. Macgregor Jr. and C. E. Pearson, Nucleic Acids Res., 2015 DOI:10.1093/nar/gkv1008.
- (a) J. Dai, M. Carver, L. H. Hurley and D. Yang, J. Am. Chem. Soc., 2011, 133, 17673 CrossRef CAS PubMed; (b) H. Z. He, D. S. Chan, C. H. Leung and D. L. Ma, Nucleic Acids Res., 2013, 41, 4345 CrossRef CAS PubMed.
- (a) X. X. Huang, L. N. Zhu, B. Wu, Y. F. Huo, N. N. Duan and D. M. Kong, Nucleic Acids Res., 2014, 42, 8719 CrossRef CAS PubMed; (b) M. Nikan, M. D. Antonio, K. Abecassis, K. McLuckie and S. Balasubramanian, Angew. Chem., Int. Ed., 2013, 52, 1428 CrossRef CAS PubMed.
- A. Renaud de la Faverie, A. Guédin, A. Bedrat, L. A. Yatsunyk and J. L. Mergny, Nucleic Acids Res., 2014, 42, e65 CrossRef CAS PubMed.
- P. Yang, A. De Cian, M. P. Teulade-Fichou, J. L. Mergny and D. Monchaud, Angew. Chem., Int. Ed., 2009, 48, 2188 CrossRef CAS PubMed.
- Y.-J. Lu, S.-C. Yan, F.-Y. Chan, L. Zou, W.-H. Chung, W.-L. Wong, B. Qiu, N. Sun, P.-H. Chan, Z.-S. Huang, L.-Q. Gu and K.-Y. Wong, Chem. Commun., 2011, 47, 4971 RSC.
- J. Mohanty, N. Barooah, V. Dhamodharan, S. Harikrishna, P. I. Pradeepkumar and A. C. Bhasikuttan, J. Am. Chem. Soc., 2013, 135, 367 CrossRef CAS PubMed.
- Q. Zhang, Y.-C. Liu, D.-M. Kong and D.-S. Guo, Chem. – Eur. J., 2015, 21, 13253 CrossRef CAS PubMed.
- A. R. D. Cousins, D. Ritson, P. Sharma, M. F. G. Stevens, J. E. Moses and M. S. Searle, Chem. Commun., 2014, 50, 15202 RSC.
- (a) Y.-M. Wang, C.-Q. Zhou, J.-X. Chen, Y.-L. Lin, W. Zeng, B.-C. Kuang, W.-L. Fu and W.-H. Chen, MedChemComm., 2013, 4, 1400 RSC; (b) J.-X. Chen, W.-E. Lin, M.-Z. Chen, C.-Q. Zhou, Y.-L. Lin, M. Chen, Z.-H. Jiang and W.-H. Chen, Bioorg. Med. Chem. Lett., 2012, 22, 7056 CrossRef CAS PubMed.
- Y. Ma, T.-M. Ou, J.-H. Tan, J.-Q. Hou, S.-L. Huang, L.-Q. Gu and Z.-H. Huang, Eur. J. Med. Chem., 2011, 46, 1906 CrossRef CAS PubMed.
- S.-R. Liao, C.-X. Zhou, W.-B. Wu, T.-M. Ou, J.-H. Tan, D. Li, L.-Q. Gu and Z.-S. Huang, Eur. J. Med. Chem., 2012, 53, 52 CrossRef CAS PubMed.
- Y. Ma, T.-M. Ou, J.-Q. Hou, Y.-J. Lu, J.-H. Tan, L.-Q. Gu and Z.-H. Huang, Bioorg. Med. Chem., 2008, 16, 7582 CrossRef CAS PubMed.
- Y. Ma, T.-M. Ou, J.-H. Tan, J.-Q. Hou, S.-L. Huang, L.-Q. Gu and Z.-H. Huang, Bioorg. Med. Chem. Lett., 2009, 19, 3414 CrossRef CAS PubMed.
- W.-J. Zhang, T.-M. Ou, Y.-J. Lu, Y.-Y. Huang, W.-B. Wu, Z.-S. Huang, J.-L. Zhou, K.-Y. Wong and L.-Q. Gu, Bioorg. Med. Chem., 2007, 15, 5493 CrossRef CAS PubMed.
- C. Bazzicalupi, M. Ferraroni, A. R. Bilia, F. Scheggi and P. Gratteri, Nucleic Acids Res., 2013, 41, 632 CrossRef CAS PubMed.
- R. Sinha, I. Saha and G. S. Kumar, Chem. Biodiversity, 2011, 8, 1512 CAS.
- Ö. P. Çetinkol and N. V. Hud, Nucleic Acids Res., 2008, 37, 611 CrossRef PubMed.
- M. M. Islam and G. S. Kumar, J. Mol. Struct., 2008, 875, 382 CrossRef CAS.
- L. H. Zhang, H. Liu, Y. Shao, C. Lin, H. Jia, G. Chen, D. Z. Yang and Y. Wang, Anal. Chem., 2015, 87, 730 CrossRef CAS PubMed.
- M. Ogawa, M. Nagashima, H. Sogawa, S. Kuwata and T. Takata, Org. Lett., 2015, 17, 1664 CrossRef CAS PubMed.
- (a) J.-W. Yang, Y.-L. Lin, C. Dong, C.-Q. Zhou, J.-X. Chen, B. Wang, Z.-Z. Zhou, B. Sun and W.-H. Chen, Eur. J. Med. Chem., 2014, 87, 168 CrossRef CAS PubMed; (b) C.-Q. Zhou, Y.-L. Lin, J.-W. Yang, J.-X. Chen and W.-H. Chen, Eur. J. Med. Chem., 2013, 66, 508 CrossRef CAS PubMed; (c) C.-Q. Zhou, J.-W. Yang, C. Dong, Y.-L. Lin, J.-X. Chen and W.-H. Chen, Inorg. Chim. Acta, 2015, 429, 168 CrossRef CAS.
- C. Dong, C.-Q. Zhou, J.-W. Yang, T.-C. Liao, J.-X. Chen, C.-X. Yin and W.-H. Chen, RSC Adv., 2015, 5, 32990 RSC.
- K. Bhadra and G. S. Kumar, Biochim. Biophys. Acta, 2011, 1810, 485 CrossRef CAS PubMed.
- S. Ghosh, S. K. Pradhan, A. Kar, S. Chowdhury and D. Dasgupta, Biochim. Biophys. Acta, 2013, 1830, 4189 CrossRef CAS PubMed.
- Md. M. Islam and G. S. Kumar, J. Mol. Struct., 2008, 875, 382 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Characterization of compound 1 and measurement of its binding properties. See DOI: 10.1039/c5ob01723h |
| This journal is © The Royal Society of Chemistry 2016 |