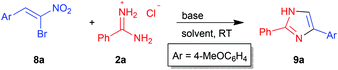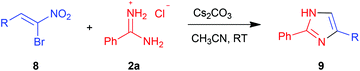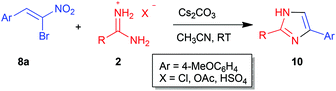Open Access Article
Open Access ArticleImidazoles from nitroallylic acetates and α-bromonitroalkenes with amidines: synthesis and trypanocidal activity studies†
Elumalai
Gopi
a,
Tarun
Kumar
a,
Rubem F. S.
Menna-Barreto
b,
Wagner O.
Valença
c,
Eufrânio N.
da Silva Júnior
*c and
Irishi N. N.
Namboothiri
*a
aDepartment of Chemistry, Indian Institute of Technology Bombay, Mumbai 400 076, India. E-mail: irishi@iitb.ac.in
bOswaldo Cruz Institute, FIOCRUZ, Rio de Janeiro, RJ 21045-900, Brazil
cInstitute of Exact Sciences, Department of Chemistry, Federal University of Minas Gerais, Belo Horizonte, MG 31270-901, Brazil. E-mail: eufranio@ufmg.br
First published on 11th August 2015
Abstract
Cascade reactions of amidines with nitroallylic acetates and α-bromonitroalkenes provide potentially bioactive imidazoles in good to excellent yields in most cases. While 2,4-disubstituted imidazol-5-yl acetates are formed in the first case, 2,4-disubstituted imidazoles, bearing no substituent at position 5, are the products in the second case. These two series of imidazoles, viz. 2,4,5-trisubstituted and 2,4-disubstituted, were screened for their activity against the protozoan parasite Trypanosoma cruzi which is responsible for Chagas disease. As many as three compounds were as active as the standard benznidazole and two others were 2–3-fold more active highlighting the potential of substituted imidazoles, easily accessible from nitroalkenes, as possible anti-parasitic agents.
Introduction
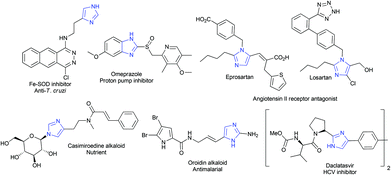
Several imidazole containing compounds exhibit activity against Trypanosoma cruzi, a parasite that causes Chagas disease.1 Synthesis and detailed evaluation of such anti-parasitic activity of imidazoles, including studies on their mechanism of action, have been reported in the recent literature.2 Other biological properties of imidazoles and their applications for the treatment of various diseases have also been well documented (Fig. 1).3 The presence of imidazoles in bioactive compounds, including natural products,4 for instance, marine alkaloids,5 have received considerable attention. Potential applications of imidazoles in coordination chemistry6 and as precursors to ionic liquids7 and stable carbenes8 are also noteworthy.Many new multi-component9 and metal-mediated10–12 approaches have appeared in the literature for the synthesis of imidazoles. However, the three component reaction of 1,2-dicarbonyl compound, aldehyde and ammonia,13 and the reaction of (α-halo)ketones or diketones with formamide/amines14 or amidines15 are the classical ones. Reactions of amidines with acetylenes11 and nitroalkenes12 also lead to substituted imidazoles.
In essence, functionalized and fused imidazoles are attractive targets for synthetic chemists due to their diverse applications in chemistry and biology.16
From another perspective, the reactivity of conjugated nitroalkenes as substrates in reactions as diverse as Michael addition, Diels–Alder reaction, 1,3-dipolar cycloaddition and Morita–Baylis–Hillman reaction has been amply demonstrated.17 In particular, the Morita–Baylis–Hillman (MBH)18 reaction of nitroalkenes has emerged as a convenient means of synthesizing α-functionalized nitroalkenes which could in turn serve as excellent substrates for the synthesis of complex molecules.19–22 Several carbocycles20 and heterocycles21,22 have been synthesized exploiting the 1,2 or 1,3-bi-electrophilic character of the nitroallylic acetates through a cascade SN2 or SN2′ reaction of a binucleophile followed by an intramolecular Michael addition.
Among nitroalkenes, α-bromonitroalkenes, by virtue of their 1,2-bielectrophilic character, are capable of taking part in cascade reactions with various binucleophiles. Their reaction with 1,3-dicarbonyl compounds,23 enamines,24 and other miscellaneous binucleophiles25 provided various functionalized heterocycles such as furans, pyrroles, pyrazoles, and triazoles among others. However, to our knowledge, there is no report on the reaction of α-bromonitroalkenes with amidines for the synthesis of functionalized imidazoles.
This work describes the full version of our studies on the synthesis of imidazoles by treating amidines with nitroallylic acetates and α-bromonitroalkenes and studies on their trypanocidal activity.26
Results and discussion
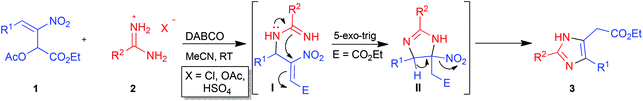
In our recent communication,26 we reported the synthesis of imidazole esters 3 from nitroallylic acetates 1 and amidines 2 through a one-pot cascade intermolecular aza-SN2′ reaction–intramolecular aza-Michael addition (Table 1). Selected imidazole esters 3 were transformed to alcohols 4, acids 5 and then to amides 6 (Scheme 1). Many of these imidazoles were screened for their activity against the protozoan parasite T. cruzi, the etiological agent of Chagas disease. In particular, the activity of imidazole esters 3a and 3b was comparable to that of the standard benznidazole. More importantly, imidazole ester 3e exhibited activity twice that of benznidazole thus prompting us to synthesize and screen more imidazoles in this series and also those with other substitution patterns.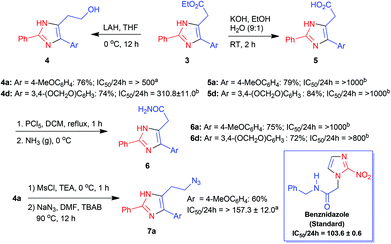 | ||
| Scheme 1 Synthesis of derivatives of selected imidazole esters 3a and 3d and their activity against T. cruzi trypomastigotes. IC50/24 h reported in μM. aNew compound. bIC50/24 h was not reported in ref. 26. | ||
| Entry | 1, R1 | 2, R2 | Time (h) | 3 | % Yielda | IC50/24 h (μM)b,c |
|---|---|---|---|---|---|---|
| a After purification by column chromatography. b Mean ± SD of at least 3 independent experiments. c Positive control: benznidazole IC50 103.6 ± 0.6. d IC50/24 h was not reported in ref. 26. e New compound. | ||||||
| 1 | 1a, 4-MeOC6H4 | C6H5 | 3 | 3a | 92 | 111.9 ± 15.4 |
| 2 | 1b, 2,4-(MeO)2C6H3 | C6H5 | 4 | 3b | 68 | 102.0 ± 10.3 |
| 3 | 1c, 3,4-(MeO)2C6H3 | C6H5 | 3 | 3c | 86 | 236.2 ± 16.4 |
| 4 | 1d, 5-Benzo[d][1,3]dioxole | C6H5 | 2 | 3d | 91 | 193.0 ± 08.6 |
| 5 | 1e, 4-MeC6H4 | C6H5 | 3 | 3e | 91 | 51.1 ± 04.3 |
| 6 | 1f, C6H5 | C6H5 | 4 | 3f | 89 | 561.7 ± 56.6 |
| 7 | 1g, 4-FC6H4 | C6H5 | 2 | 3g | 68 | 256.6 ± 21.9 |
| 8 | 1h, 4-ClC6H4 | C6H5 | 0.5 | 3h | 67 | 213.9 ± 22.8d |
| 9 | 1i, 3-BrC6H4 | C6H5 | 0.5 | 3i | 65 | 187.5 ± 05.5 |
| 10 | 1j, 1-Naphthyl | C6H5 | 4 | 3j | 67 | 190.8 ± 02.3 |
| 11 | 1k, 2-Furyl | C6H5 | 7 | 3k | 74 | 1002.6 ± 76.8 |
| 12 | 1l, 2-Thienyl | C6H5 | 2 | 3l | 62 | 734.9 ± 41.8 |
| 13 |
1m, C6H5CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH CH |
C6H5 | 1.5 | 3m | 58 | 382.2 ± 46.7 |
| 14 | 1n, Cyclohexyl | C6H5 | 1 | 3n | 67 | 172.0 ± 00.8 |
| 15 | 1o, C6H5 | 4-MeC6H4 | 2 | 3o | 69 | 192.7 ± 21.6d |
| 16 | 1p, C6H5 | 4-ClC6H4 | 1.5 | 3p | 54 | 194.5 ± 14.4d |
| 17 | 1q, C6H5 | 3-ClC6H4 | 1 | 3q | 88 | 221.2 ± 28.2 |
| 18 | 1r, C6H5 | CH3 | 1 | 3r | 62 | 946.7 ± 12.2 |
| 19 | 1s, C6H5 | CH3S | 1.5 | 3s | 32 | 1402.3 ± 260 |
| 20 | 1t, 3,4-(MeO)2C6H3 | 4-ClC6H4 | 2.5 | 3t | 63 | >600e |
| 21 | 1u, 3,4-(MeO)2C6H3 | 3-ClC6H4 | 1 | 3u | 67 | >600e |
| 22 | 1v, 3,4-(MeO)2C6H3 | 4-MeC6H4 | 2.5 | 3v | 63 | >1000e |
At the outset, imidazoles 3h, 3o and 3p (Table 1, entries 8, 15 and 16) and 4d, 5a, 5d, 6a and 6d (Scheme 1) which were reported in our previous communication,26 but could not be screened earlier were later evaluated for their activity. Unfortunately, none of them yielded positive results. While 3h, 3o and 3p showed activity approximately half (IC50/24 h in the range of 190–214 μM) of that of the standard, benznidazole (IC50/24 h 103.6 μM), the other derivatives 4d, 5a, 5d, 6a and 6d did not show any activity at all (IC50/24 h > 300 μM).
In view of the above, imidazole esters 3t–v were synthesized following the general procedure reported by us earlier (Table 1, entries 20–22). Additionally, imidazole ester 3a was transformed to alcohol 4a using LAH in 76% yield and alcohol 4a, in turn, was converted to azide 7a in 60% yield (Scheme 1). Subsequently, esters 3t–v, alcohol 4a and azide 5a were subjected to trypanocidal activity studies as reported before. However, while esters 3t–v and alcohol 4a were inactive, azide 7a showed only moderate activity (IC50/24 h 157.3 μM). It is worth mentioning the potential of the clickable imidazole 7a that can be considered as an important intermediate in click chemistry reactions for the synthesis of hybrid compounds.
In the above scenario, we decided to explore the possibility of synthesizing imidazoles with different substitution patterns by our own methodology and screen them for their trypanocidal activity. Interestingly, although synthesis of imidazoles of type 9via various miscellaneous methods is known in the literature,27–36 the reaction of amidines 2 with α-bromonitroalkenes 8 has not been employed for such purposes. At the outset, amidine 2a and α-bromonitroalkene 8a were chosen as model substrates in order to establish the optimal conditions (Table 2).
| Entry | Base (equiv.) | Solvent | Time (h) | % Yielda |
|---|---|---|---|---|
| a After silica gel column chromatography. b No reaction. | ||||
| 1 | Cs2CO3 (1) | THF | 24 | —b |
| 2 | Cs2CO3 (2) | THF | 18 | 55 |
| 3 | Cs2CO3 (3) | THF | 12 | 85 |
| 4 | K2CO3 (3) | THF | 12 | 80 |
| 5 | DMAP (3) | THF | 12 | 56 |
| 6 | DABCO (3) | THF | 12 | 61 |
| 7 | NEt3 (3) | THF | 12 | 49 |
| 8 | Cs 2 CO 3 (3) | CH 3 CN | 3 | 93 |
| 9 | Cs2CO3 (3) | Toluene | 5 | 75 |
There was no appreciable conversion when 1 equiv. of Cs2CO3 was employed as a base in THF at room temperature even after 24 h (entry 1). However, upon increasing the loading of Cs2CO3 to 2 and 3 equiv., there was a dramatic rise in the product yields to 55% and 85% as well as an improvement in the reaction rate to 18 h and 12 h, respectively (entries 2 and 3). While K2CO3 also gave comparable yields (80%) of the product 9a (entry 4), amine bases such as DMAP, DABCO and Et3N were less effective (entries 5–7) under otherwise identical conditions. Finally, changing solvent to CH3CN enabled us to improve the yield further to 93% and considerably reduce the reaction time to 3 h (entry 8) though the yield was much lower (75%) in a hydrocarbon solvent such as toluene (entry 9).
The above optimized conditions were successfully employed for the synthesis of a variety of 2,5-disubstituted imidazoles 9 and 10 (Tables 3 and 4). Initially, benzamidine 2a was treated with bromonitroalkenes 8 bearing various substituents at the β-position to afford imidazoles 9 (Table 3). In particular, bromonitroalkenes bearing electron rich aryl groups at the β-position 8a–c and 8e provided the products 9a–c and 9e, respectively, in excellent yield (>90%, entries 1–3 and 5). The only exception among nitroalkenes bearing an electron rich aryl group was 8f which afforded the product 9f in a slightly lower yield (84%) and a longer reaction time (6 h, entry 6). The yields of imidazoles derived from nitroalkenes bearing parent phenyl 8d, electron deficient aryls 8g–j and a fused aryl 8m at the β-position were in the range of 74–88% (entries 4, 7–10 and 13). Heteroaryl substituted bromonitroalkenes 8k–l also delivered the corresponding imidazoles 9k–l in good to high yield (71–85%, entries 11 and 12). While bromonitrodiene 8n afforded the product 9n in moderate yield (67%, entry 14), β-alkylated nitroalkenes 8o and p were better substrates and provided the desired imidazoles 9o–p in 75–85% yield (entries 15 and 16).
| Entry | 8 | R | Time (h) | 9 | % Yielda | IC50/24 h (μM)b,c |
|---|---|---|---|---|---|---|
| a After silica gel column chromatography. b Mean ± SD of at least 3 independent experiments. c Positive control: benznidazole IC50 103.6 ± 0.6. | ||||||
| 1 | 8a | 4-MeOC6H4 | 3.0 | 9a | 93 | 177.2 ± 20.7 |
| 2 | 8b | 3,4-(MeO)2C6H3 | 3.0 | 9b | 92 | 372.5 ± 29.9 |
| 3 | 8c | 5-Benzo[d][1,3]dioxole | 3.0 | 9c | 90 | 194.5 ± 14.2 |
| 4 | 8d | C6H5 | 3.0 | 9d | 88 | 377.2 ± 37.6 |
| 5 | 8e | 4-MeC6H4 | 3.0 | 9e | 92 | 240.6 ± 32.9 |
| 6 | 8f | 4-MeSC6H4 | 6.0 | 9f | 84 | 256.2 ± 9.8 |
| 7 | 8g | 4-ClC6H4 | 4.5 | 9g | 86 | 332.2 ± 41.9 |
| 8 | 8h | 4-FC6H4 | 4.5 | 9h | 76 | 329.8 ± 33.1 |
| 9 | 8i | 3-BrC6H4 | 4.0 | 9i | 74 | 339.4 ± 16.5 |
| 10 | 8j | 2-O2NC6H4 | 5.0 | 9j | 85 | 395.2 ± 5.3 |
| 11 | 8k | 2-Thienyl | 4.5 | 9k | 85 | 352.1 ± 16.5 |
| 12 | 8l | 3-Thienyl | 5.0 | 9l | 71 | 355.3 ± 3.0 |
| 13 | 8m | 1-Naphthyl | 5.5 | 9m | 81 | 35.5 ± 4.3 |
| 14 | 8n | C6H5CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH CH |
6.0 | 9n | 67 | 157.8 ± 23.6 |
| 15 | 8o | Cyclohexyl | 5.0 | 9o | 85 | 99.6 ± 5.2 |
| 16 | 8p | n-Butyl | 5.0 | 9p | 75 | 151.1 ± 3.2 |
| Entry | 2 | R | Time (h) | 10 | % Yielda | IC50/24 h (μM)b,c |
|---|---|---|---|---|---|---|
| a After silica gel column chromatography. b Mean ± SD of at least 3 independent experiments. c Positive control: benznidazole IC50 103.6 ± 0.6. d Complex mixture. | ||||||
| 1 | 2b | 4-MeC6H4 | 3.5 | 10b | 87 | 123.0 ± 13.9 |
| 2 | 2c | 4-ClC6H4 | 4.0 | 10c | 79 | 182.6 ± 16.5 |
| 3 | 2d | 3-ClC6H4 | 3.5 | 10d | 83 | 184.4 ± 7.1 |
| 4 | 2e | MeS | 5.0 | 10e | 55 | 213.8 ± 14.6 |
| 5 | 2f | NH2 | 2.0 | 10f | —d | — |
| 6 | 2g | H | 3.0 | 10g | —d | — |
Having demonstrated the wide scope of bromonitroalkenes 8 in the reaction with amidine 2a, the scope of amidines 2 was investigated by taking bromonitroalkene 8a as the representative substrate (Table 4). The reaction of 8a proceeded well with various arylamidines 2b–d to afford the products 10b–d in high yield (79–87%, entries 1–3). However, lower yield of imidazole 10e was encountered with thioamidine 2e (55%, entry 4). Guanidine 2f and formamidine 2g were not suitable substrates for the synthesis of imidazoles 10f–g as complex mixtures were isolated under our experimental conditions (entries 5 and 6).
The structure and regiochemistry of imidazoles 9 and 10 were confirmed by comparison of their spectral data with those reported in the literature. In the proposed mechanism, the free amidine I derived from the neutralization of amidinium hydrochloride 2 by a base adds to bromonitroalkene 8 in a Michael fashion to afford intermediate II. Intramolecular nucleophilic substitution of bromide in II in a 5-exo-tet fashion provides the cyclized intermediate nitroimidazoline III which then undergoes base mediated elimination of HNO2 to give the product 2,5-disubstituted imidazole 9 or 10 (Scheme 2).
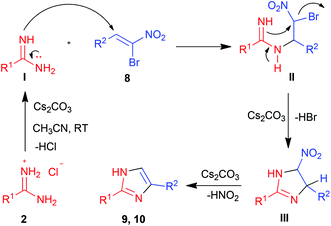 | ||
| Scheme 2 Possible mechanism for formation of imidazoles 9 and 10via [3 + 2] cycloaddition of amidines 2 with bromonitroalkenes 8. | ||
Trypanocidal activity studies
In the case of imidazoles 3–7 derived from nitroallylic acetates 1, the most active compound was imidazole ester 3e (2-fold more potent than the current drug benznidazole) followed by esters 3b and then 3a, presenting IC50/24 h values of 51.1, 102.0 and 111.9 μM, respectively. These three imidazoles possess a phenyl group at position 2 and an aryl group at position 5 bearing weakly or strongly electron donating substituent(s) at the ortho/para position(s). The presence of groups such as CH2CO2H, CH2CONH2, CH2CH2OH, and CH2CH2N3 at position 5 did not improve the activity as compared to CH2CO2Et (Scheme 1 and Table 1). This was also a motivating factor to synthesize imidazoles of types 9 and 10 wherein there is no substituent at position 5. The evaluation of the IC50 values of imidazoles 9 and 10 in Tables 3 and 4 reveal that imidazole 9m bearing a phenyl group at position 2 and a naphthyl group at position 4 is 3-fold more active (IC50/24 h = 35.5 μM, Table 3, entry 13) than the standard benznidazole. This is followed by 9o with a phenyl group at position 2 and a cyclohexyl group at position 4 (IC50/24 h = 99.6 μM, Table 3, entry 15) which is as active as benznidazole. Imidazole 10b with a p-tolyl group at position 2 and a p-anisyl group at position 4 (IC50/24 h = 123.0 μM, Table 4, entry 1) is marginally less active than benznidazole. Other analogs that show appreciable activity which is attributable to the substituents at positions 2 and 4 are 9p (Ph, n-Bu, IC50/24 h = 151.1 μM, Table 3, entry 16), 9n (Ph, styrenyl, IC50/24 h = 157.8 μM, Table 3, entry 14), 9a (Ph, p-anisyl, IC50/24 h = 177.2 μM, Table 3, entry 1), 10c (p-anisyl, p-ClC6H4, IC50/24 h = 182.6 μM, Table 4, entry 2), 10d (p-anisyl, m-ClC6H4, IC50/24 h = 184.4 μM, Table 4, entry 3) and 9c (Ph, benzo[d][1,3]dioxole, IC50/24 h 194.5 μM, Table 3, entry 3).In general, our compounds possess low molecular weight, partition coefficient and polar surface area values adhering to the Lipinski's rules. For the compounds described herein, the hydrophobicity appears sufficient for penetrating the biological membranes of the parasite, as determined by Lipinski's rule of 5 (clog![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P < 5, molecular weight ≤500, and PSA ≤ 140 Å2, number of hydrogen bond acceptors <10 and donors <5).37 Finally, a chemical informatics approach38 was used to calculate the octanol–water partition coefficient (clog
P < 5, molecular weight ≤500, and PSA ≤ 140 Å2, number of hydrogen bond acceptors <10 and donors <5).37 Finally, a chemical informatics approach38 was used to calculate the octanol–water partition coefficient (clog![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P value) and molecular polar surface area (PSA) for the most active compounds 3a, 3b, 3e, 9m, 9o and 10b, with IC50/24 h in the range of 35.5 to 123 μM. The clog
P value) and molecular polar surface area (PSA) for the most active compounds 3a, 3b, 3e, 9m, 9o and 10b, with IC50/24 h in the range of 35.5 to 123 μM. The clog![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P and PSA values were in the range of 4.16–4.87 and 28.68–73.46, respectively.
P and PSA values were in the range of 4.16–4.87 and 28.68–73.46, respectively.
Conclusions
Substituted imidazoles have been synthesized using amidines via a cascade SN2′-intramolecular aza-Michael addition–elimination with nitroallylic acetates and via a cascade Michael addition-intramolecular SN2 reaction with α-bromonitroalkenes. Imidazoles belonging to both the series have been screened against T. cruzi bloodstream trypomastigotes, an infective form of the protozoa that causes Chagas disease. While three of the imidazoles exhibited activity comparable to the effect of the standard compound benznidazole, the activity of two others was two- and three-fold that of the current drug, suggesting possible application of such imidazoles as effective anti-T. cruzi agents.Experimental section
General
The melting points recorded are uncorrected. NMR spectra (1H, 1H decoupled 13C, 13C-APT and 1H–1H COSY) were recorded with TMS as the internal standard. The coupling constants (J values) are given in Hz. High resolution mass spectra were recorded under ESI Q-TOF conditions. Amidinium salt 2a was purchased from Sigma-Aldrich and others 2b–d were prepared by following published procedures.36 The MBH alcohols and their acetates 1 were prepared by following reported procedures.39 Bromonitroalkenes 8 were prepared by following literature methods.40 Experimental data for compounds 3a–s, 4d, 5a, 5d, 6a, 6d and 7a were reported in our preliminary communication.26Trypanocidal assay
Stock solutions of the compounds were prepared in dimethyl sulfoxide (DMSO), with the final concentration of the latter in the experiments never exceeding 0.4%. Preliminary experiments showed that DMSO has no deleterious effect on the parasites when its concentration is up to 4%. T. cruzi bloodstream trypomastigotes (Y strain) were obtained at the peak of parasitaemia from infected albino mice, purified by differential centrifugation and resuspended in RPMI to a parasite concentration of 107 cells per mL in the presence of 10% of mouse blood. This suspension (100 μL) was added into the same volume of each compound previously prepared at twice the desired final concentrations for 24 h at 4 °C. Cell quantification was performed in a Neubauer chamber and the trypanocidal activity was expressed as IC50/24 h, corresponding to the concentration that leads to the lysis of 50% of the parasites. The activity of standard benznidazole was reported earlier.41 It is different from that reported by Moraes et al.42 due to different experimental conditions.General procedure for the synthesis of imidazoles from nitroallylic acetates
To a stirred solution of amidine 2 (0.24 mmol) and DABCO (61 mg, 0.5 mmol) in acetonitrile (2 mL), MBH acetate 1 (0.2 mmol) was added. After the completion of the reaction, monitored by TLC, the solvent was removed in vacuo and the crude product was purified by silica gel column chromatography by gradient elution with pet. ether/ethyl acetate (20–70%).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 400 MHz) δ 7.78 (d, J = 7.9 Hz, 2H), 7.39 (d, J = 8.7 Hz, 2H), 7.33 (t, J = 7.9 Hz, 2H), 7.23–7.27 (m, 1H), 6.87 (d, J = 8.7 Hz, 2H), 3.75–3.75 (m, 2H), 3.74 (s, 3H), 2.87 (t, J = 6.5 Hz, 2H); 13C NMR (CDCl3 + MeOH 3
1, 400 MHz) δ 7.78 (d, J = 7.9 Hz, 2H), 7.39 (d, J = 8.7 Hz, 2H), 7.33 (t, J = 7.9 Hz, 2H), 7.23–7.27 (m, 1H), 6.87 (d, J = 8.7 Hz, 2H), 3.75–3.75 (m, 2H), 3.74 (s, 3H), 2.87 (t, J = 6.5 Hz, 2H); 13C NMR (CDCl3 + MeOH 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 100 MHz) δ 158.9, 145.2, 133.0, 130.0, 129.4, 128.8 (×2), 125.4, 124.6, 114.1, 61.8, 55.3, 28.9; MS (ES+, Ar) m/z (rel. intensity) 317 (MNa+, 50), 295 (MH+, 100); HRMS (ES+, Ar) calcd for C18H19N2O2 (MH+) 295.1441, found 295.1441.
1, 100 MHz) δ 158.9, 145.2, 133.0, 130.0, 129.4, 128.8 (×2), 125.4, 124.6, 114.1, 61.8, 55.3, 28.9; MS (ES+, Ar) m/z (rel. intensity) 317 (MNa+, 50), 295 (MH+, 100); HRMS (ES+, Ar) calcd for C18H19N2O2 (MH+) 295.1441, found 295.1441.
General procedure for the synthesis of imidazoles 9 and 10 from α-bromonitroalkenes 8 and amidines 2
To a stirred solution of α-bromonitroalkene 8 (0.2 mmol) in CH3CN (3 mL), was added benzamidine hydrochloride 2 (0.2 mmol) followed by Cs2CO3 (195 mg, 0.6 mmol) at room temperature. The stirring was continued at room temperature and the completion of the reaction was monitored by TLC analysis. The crude reaction mixture was directly subjected to silica gel column chromatography by eluting with 15–50% EtOAc–pet. ether (gradient elution).Acknowledgements
INNN thanks SERB, DST India for financial assistance. EG and TK thank CSIR India for a senior research fellowship. RFSMB and ENSJ thank CNPq (PVE 401193/2014-4), FAPEMIG, FAPERJ and CAPES Brazil.Notes and references
- (a) K. C. G. de Moura, K. Salomão, R. F. S. Menna-Barreto, F. S. Emery, M. C. F. R. Pinto, A. V. Pinto and S. L. de Castro, Eur. J. Med. Chem., 2004, 39, 639 CrossRef CAS PubMed; (b) C. Neves-Pinto, A. P. Dantas, K. C. G. Moura, F. S. Emery, P. F. Polequevitch, M. C. F. R. Pinto, S. L. de Castro and A. V. Pinto, Arzneim.-Forsch., 2000, 50, 1120 Search PubMed.
- (a) A. V. Pinto, C. Neves-Pinto, M. C. F. R. Pinto, R. M. Santa Rita, C. Pezzella and S. L. de Castro, Arzneim.-Forsch., 1997, 47, 74 CAS; (b) K. C. G. de Moura, F. S. Emery, C. Neves-Pinto, M. C. F. R. Pinto, A. P. Dantas, K. Salomão, S. L. de Castro and A. V. Pinto, J. Braz. Chem. Soc., 2001, 12, 325 CrossRef CAS; (c) R. F. S. Menna-Barreto, J. R. Corrêa, A. V. Pinto, M. J. Soares and S. L. de Castro, Parasitol. Res., 2007, 101, 895 CrossRef CAS PubMed; (d) M. Sanchez-Moreno, F. Gomez-Contreras, P. Navarro, C. Marin, F. Olmo, M. J. R. Yunta, A. M. Sanz, M. J. Rosales, C. Cano and L. Campayo, J. Med. Chem., 2012, 55, 9900 CrossRef CAS PubMed.
- Selected recent reviews: (a) V. Gupta and V. Kant, Sci. Int., 2013, 1, 253 CrossRef CAS; (b) J. R. Kumar, Pharmacophore, 2010, 1, 167 CAS; (c) D. S. Zurabishvili, M. O. Lomidze, M. V. Trapaidze, S. A. Samsoniya, K. Nylund and P. Johansson, Heterocycl. Compd., 2010, 47 CAS; (d) K. M. Dawood and B. F. Abdel-Wahab, Chem. Heterocycl. Compd., 2010, 46, 255 CrossRef CAS.
- Books/monographs: (a) L. D. Quin and J. A. Tyrell, Fundamentals of Heterocyclic Chemistry: Importance in Nature and in the Synthesis of Pharmaceuticals, John Wiley & Sons, Hoboken, New Jersey, 2010 Search PubMed; (b) K. Hofmann, in The Chemistry of Heterocyclic Compounds: Imidazole and Its Derivatives, Part I, ed. A. Weissberger, Wiley Interscience, New York, 1953 Search PubMed.
- Selected recent reviews: (a) T. Imaoka, M. Iwata, T. Akimoto and K. Nagasawa, Nat. Prod. Commun., 2013, 8, 961 CAS; (b) X. Wang, Z. Ma, X. Wang, S. De, Y. Ma and C. Chen, Chem. Commun., 2014, 50, 8628 RSC; (c) H.-R. Bjoersvik and A. H. Sandtorv, Stud. Nat. Prod. Chem., 2014, 42, 33 CAS; (d) Z. Jin, Nat. Prod. Rep., 2013, 30, 869 RSC.
- Selected recent reviews: (a) P. R. Reddy and A. Shilpa, Indian J. Chem., Sect. A: Inorg., Bio-inorg., Phys., Theor. Anal. Chem., 2010, 49, 1003 Search PubMed; (b) J. Petersen, T. R. Hawkes and D. J. Lowe, J. Inorg. Biochem., 2000, 80, 161 CrossRef CAS.
- Selected recent reviews: (a) J. Dupont, R. F. de Souza and P. A. Z. Suarez, Chem. Rev., 2002, 102, 3667 CrossRef CAS PubMed; (b) N. Noujeim, L. Leclercq and A. R. Schmitzer, Curr. Org. Chem., 2010, 14, 1500 CrossRef CAS; (c) E. Ennis and S. T. Handy, Curr. Org. Synth., 2007, 4, 381 CrossRef CAS.
- Selected recent reviews: (a) R. J. Lowry, M. K. Veige, O. Clement, K. A. Abboud, I. Ghiviriga and A. S. Veige, Organometallics, 2008, 27, 5184 CrossRef CAS; (b) M. Kuriyama, Chem. Pharm. Bull., 2012, 60, 419 CrossRef CAS; (c) J. C. Garrison and W. J. Youngs, Chem. Rev., 2005, 105, 3978 CrossRef CAS PubMed.
- Selected recent articles: (a) S. Sarshar, C. Zhang, E. J. Moran, S. Krane, J. C. Rodarte, K. D. Benbatoul, R. Dixon and A. M. M. Mjalli, Bioorg. Med. Chem. Lett., 2000, 10, 2599 CrossRef CAS; (b) M. Rivara, A. R. Baheti, M. Fantini, G. Cocconcelli, C. Ghiron, C. L. Kalmar, N. Singh, E. C. Merrick, M. K. Patel and V. Zuliani, Bioorg. Med. Chem. Lett., 2008, 18, 5460 CrossRef CAS PubMed; (c) S. D. Jadhav, N. D. Kokare and S. D. Jadhav, J. Heterocycl. Chem., 2008, 45, 1461 CrossRef CAS PubMed.
- Review: (a) S. Kamijo and Y. Yamamoto, Chem. – Asian J., 2007, 2, 568 CrossRef CAS PubMed; Selected recent articles: (b) C. Kanazawa, S. Kamijo and Y. Yamamoto, J. Am. Chem. Soc., 2006, 128, 10662 CrossRef CAS PubMed; (c) T. Horneff, S. Chuprakov, N. Chernyak, V. Gevorgyan and V. V. Fokin, J. Am. Chem. Soc., 2008, 130, 14972 CrossRef CAS; (d) Y. Xiao and L. Zhang, Org. Lett., 2012, 14, 4662 CrossRef CAS PubMed; (e) S. Tong, Q. Wang, M.-X. Wang and J. Zhu, Angew. Chem., Int. Ed., 2015, 54, 1293 CrossRef CAS PubMed. Multi-component: (f) Z. Jiang, P. Lu and Y. Wang, Org. Lett., 2012, 14, 6266 CrossRef CAS PubMed.
- (a) J. Li and L. Neuville, Org. Lett., 2013, 15, 1752 CrossRef CAS PubMed; (b) Y. Wang, H. Shen and Z. Xie, Synlett, 2011, 969 Search PubMed; (c) P. Starkov, F. Rota, J. M. D'Oyley and T. D. Sheppard, Adv. Synth. Catal., 2012, 354, 3217 CrossRef CAS PubMed.
- (a) S. Mitra, A. K. Bagdi, A. Majee and A. Hajra, Tetrahedron Lett., 2013, 54, 4982 CrossRef CAS PubMed; (b) X. Liu, D. Wang and B. Chen, Tetrahedron, 2013, 69, 9417 CrossRef CAS PubMed; (c) D. Tang, P. Wu, X. Liu, Y.-X. Chen, S.-B. Guo, W.-L. Chen, J.-G. Li and B.-H. Chen, J. Org. Chem., 2013, 78, 2746 CrossRef CAS PubMed.
- (a) H. Debus, Ann. Chem. Pharm., 1858, 107, 199 CrossRef PubMed; (b) B. Radziszewski, Chem. Ber., 1882, 15, 1493 CrossRef PubMed; (c) F. R. Japp and H. H. Robinson, Chem. Ber., 1882, 15, 1268 CrossRef PubMed; (d) D. Bandyopadhyay, L. C. Smith, D. R. Garcia, R. N. Yadav and B. K. Banik, Org. Med. Chem. Lett., 2014, 4, 1 CrossRef CAS PubMed.
- (a) H. Bredereck, R. Gompper and D. Hayer, Chem. Ber., 1959, 92, 338 CrossRef CAS PubMed; (b) H. Huang, X. Ji, W. Wu and H. Jiang, Adv. Synth. Catal., 2013, 355, 170 CrossRef CAS PubMed.
- Selected recent articles: (a) R. L. Elliott, R. M. Oliver, J. A. LaFlamme, M. L. Gillaspy, M. Hammond, R. F. Hank, T. S. Maurer, D. L. Baker, P. A. DaSilva-Jardine, R. W. Stevenson, C. M. Mack and J. V. Cassella, Bioorg. Med. Chem. Lett., 2003, 13, 3593 CrossRef CAS; (b) D. Kumar, N. M. Kumar, G. Patel, S. Gupta and R. S. Varma, Tetrahedron Lett., 2011, 52, 1983 CrossRef CAS PubMed; (c) F. Bureš and J. Kulhánek, Tetrahedron: Asymmetry, 2005, 16, 1347 CrossRef PubMed; (d) T. J. Donohoe, M. A. Kabeshov, A. H. Rathi and I. E. D. Smith, Org. Biomol. Chem., 2012, 10, 1093 RSC; (e) B. Li, C. K. F. Chiu, R. F. Hank, J. Murry, J. Roth and H. Tobiassen, Org. Process Res. Dev., 2002, 6, 682 CrossRef CAS; (f) B. Li, C. K.-F. Chiu, R. F. Hank, J. Murry, J. Roth and H. Tobiassen, Org. Synth., 2005, 81, 105 CrossRef CAS; (g) M. Ueno and H. Togo, Synthesis, 2004, 2673 CAS.
- Review: C. Anshul, S. Ashu and A. K. Sharma, Pharma Chem., 2012, 4, 116 Search PubMed.
- (a) A. Z. Halimehjani, I. N. N. Namboothiri and S. E. Hooshmand, RSC Adv., 2014, 4, 31261 RSC, 48022, 51794; (b) R. Ballini, L. Barboni, G. Bosica, D. Fiorini and A. Palmieri, Pure Appl. Chem., 2006, 78, 1857 CrossRef CAS; (c) O. M. Berner, L. Tedeschi and D. Enders, Eur. J. Org. Chem., 2002, 1877 CrossRef CAS; (d) I. N. N. Namboothiri and N. Rastogi, Top. Heterocycl. Chem., 2008, 12, 1 CAS.
- Selected recent reviews: (a) Y. Wei and M. Shi, Chem. Rev., 2013, 113, 6659 CrossRef CAS PubMed; (b) D. Basavaiah and B. C. Sahu, Chimia, 2013, 67, 8 CrossRef CAS PubMed; (c) G. Guillena, D. J. Ramon and M. Yus, Catalysis, 2012, 24, 223 CAS; (d) D. Basavaiah and G. Veeraraghavaiah, Chem. Soc. Rev., 2012, 41, 68 RSC; (e) V. Singh and S. Batra, Tetrahedron, 2008, 64, 4511 CrossRef CAS PubMed.
- Review: (a) K. Kaur and I. N. N. Namboothiri, Chimia, 2012, 66, 913 CrossRef CAS PubMed; Selected articles: (b) V. Barbier, F. Couty and O. R. P. David, Eur. J. Org. Chem., 2015, 3679 CrossRef CAS PubMed; (c) B. Han, X. Xie, W. Huang, X. Li, L. Yang and C. Peng, Adv. Synth. Catal., 2014, 356, 3676 CrossRef CAS PubMed; (d) J.-Q. Zhang, J.-J. Liu, C.-L. Gu, D. Wang and L. Liu, Eur. J. Org. Chem., 2014, 5885 CrossRef CAS PubMed; (e) N. Lu, H. Wang and Y. Wang, Bull. Korean Chem. Soc., 2013, 34, 3591 CrossRef CAS; (f) X.-Y. Chen, F. Xia and S. Ye, Org. Biomol. Chem., 2013, 11, 5722 RSC; (g) N. Lu, L. Meng, D. Chen and G. Zhang, RSC Adv., 2011, 1, 1113 RSC; (h) X. Wang, Y.-F. Chen, L.-F. Niu and P.-F. Xu, Org. Lett., 2009, 11, 3310 CrossRef CAS PubMed; (i) S. Chandrasekhar, K. Mallikarjun, G. Pavankumarreddy, K. V. Rao and B. Jagadeesh, Chem. Commun., 2009, 4985 RSC; (j) S. Fioravanti, L. Pellacani and P. A. Tardella, Tetrahedron, 2009, 65, 5747 CrossRef CAS PubMed; (k) Y. Wu, A. Lu, Y. Liu, X. Yu, Y. Wang, G. Wu, H. Song, Z. Zhou and C. Tang, Tetrahedron: Asymmetry, 2010, 21, 2988 CrossRef CAS PubMed; (l) Y. Wang, S. Zhu and D. Ma, Org. Lett., 2011, 13, 1602 CrossRef CAS PubMed; (m) A. Rai and L. D. S. Yadav, Tetrahedron, 2012, 68, 2459 CrossRef CAS PubMed; (n) Z.-W. Guo, J.-W. Xie, C. Chen and W.-D. Zhu, Org. Biomol. Chem., 2012, 10, 8471 RSC; (o) G. Talavera, E. M. Reyes, J. L. Vicario and L. Carrillo, Angew. Chem., Int. Ed., 2012, 51, 4104 CrossRef CAS PubMed; (p) B.-C. Hong, D.-J. Lan, N. S. Dange, G.-H. Lee and J.-H. Liao, Eur. J. Org. Chem., 2013, 2472 CrossRef CAS PubMed; (q) J. An, L.-Q. Lu, Q.-Q. Yang, T. Wang and W.-J. Xiao, Org. Lett., 2013, 15, 542 CrossRef CAS PubMed; (r) M. Yaqub, C. Y. Yu, Y. M. Jia and Z. T. Huang, Synlett, 2008, 1357 CAS; (s) S. Takizawa, A. Horii and H. Sasai, Tetrahedron: Asymmetry, 2010, 21, 891 CrossRef CAS PubMed; (t) N. Lu, L. Meng, D. Chen and G. Zhang, RSC Adv., 2011, 1, 1113 RSC; (u) M. Bakthadoss, N. Sivakumar, A. Devaraj and D. S. Sharada, Synthesis, 2011, 2196 Search PubMed; (v) R. Kumar, T. Kumar, S. M. Mobin and I. N. N. Namboothiri, J. Org. Chem., 2013, 78, 5073 CrossRef CAS PubMed.
- (a) Terphenyls: E. Gopi and I. N. N. Namboothiri, J. Org. Chem., 2014, 79, 7468 CrossRef CAS PubMed; (b) Cyclopentenes: L. F. Yeh, S. Anwar and K. Chen, Tetrahedron, 2012, 68, 7317 CrossRef CAS PubMed; (c) Bicyclic skeletons: C.-L. Cao, Y.-Y. Zhou, J. Zhou, X.-L. Sun, Y. Tang, Y.-X. Li, G.-Y. Li and J. Sun, Chem. – Eur. J., 2009, 15, 11384 CrossRef CAS PubMed.
- (a) Imidazopyridines: D. K. Nair, S. M. Mobin and I. N. N. Namboothiri, Org. Lett., 2012, 14, 4580 CrossRef CAS PubMed; (b) Pyranonaphthaquinones: D. K. Nair, R. F. S. Menna-Barreto, E. N. da Silva Júnior, S. M. Mobin and I. N. N. Namboothiri, Chem. Commun., 2014, 50, 6973 RSC; (c) Pyrroles: D. R. Magar, Y.-J. Ke and K. Chen, Asian J. Org. Chem., 2013, 2, 330 CrossRef CAS PubMed; (d) T. Chen, N. Shao, H. Zhu, B. Zhang and H. Zou, Tetrahedron, 2013, 69, 10558 CrossRef CAS PubMed; (e) Oxa- and aza-triquinanes: J. An, L.-Q. Lu, Q.-Q. Yang, T. Wang and W.-J. Xiao, Org. Lett., 2013, 15, 542 CrossRef CAS PubMed; (f) Tetrahydropyridines: M. Yaqub, C.-Y. Yu, Y.-M. Jia and Z.-T. Huang, Synlett, 2008, 1357 CAS; (g) Different heterocyclic scaffolds: H. Zhu, N. Shao, T. Chen and H. Zou, Chem. Commun., 2013, 49, 7738 RSC.
- Furans from 1,3-dicarbonyl compounds: (a) D. K. Nair, S. M. Mobin and I. N. N. Namboothiri, Tetrahedron Lett., 2012, 53, 3349 CrossRef CAS PubMed; (b) W.-Y. Huang, Y.-C. Chen and K. Chen, Chem. – Asian J., 2012, 7, 688 CrossRef CAS PubMed; Arenofurans from arenols: (c) T. Kumar, S. M. Mobin and I. N. N. Namboothiri, Tetrahedron, 2013, 69, 4964 CrossRef CAS PubMed; (d) S. Anwar, W.-Y. Huang, C.-H. Chen, Y.-S. Cheng and K. Chen, Chem. – Eur. J., 2013, 19, 4344 CrossRef CAS PubMed.
- With 1,3-dicarbonyl to furans: (a) M. Rueping, A. Parra, U. Uria, F. O. Besselièvre and E. B. Merino, Org. Lett., 2010, 12, 5680 CrossRef CAS PubMed; (b) N. Ayyagari, D. Jose, S. M. Mobin and I. N. N. Namboothiri, Tetrahedron Lett., 2011, 52, 258 CrossRef CAS PubMed; (c) L.-P. Fan, P. Li, X.-S. Li, D.-C. Xu, M.-M. Ge, W.-D. Zhu and J.-W. Xie, J. Org. Chem., 2010, 75, 8716 CrossRef CAS PubMed; (d) J.-W. Xie, P. Li, T. Wang and F.-T. Zhou, Tetrahedron Lett., 2011, 52, 3250 CrossRef CAS PubMed.
- With enamine to pyrroles: (a) M. Rueping and A. Parra, Org. Lett., 2010, 12, 5281 CrossRef CAS PubMed; (b) C. Martín-Santos, C. Jarava-Barrera, A. Parra, F. Esteban, C. Navarro-Ranninger and J. Alemán, ChemCatChem, 2012, 4, 976 CrossRef PubMed.
- (a) With arenol to arenofurans: C. Jarava-Barrera, F. Esteban, C. Navarro-Ranninger, A. Parra and J. Aleman, Chem. Commun., 2013, 49, 2001 RSC; (b) With diazo compounds to pyrazoles: R. Muruganantham, S. M. Mobin and I. N. N. Namboothiri, Org. Lett., 2007, 9, 1125 CrossRef CAS PubMed; (c) With azide to triazoles; E. A. Sheremet, R. I. Tomanov, E. V. Trukhin and V. M. Berestovitskaya, Russ. J. Org. Chem., 2004, 40, 594 CrossRef CAS; (d) With oxindoles to spirocyclopropyl oxindoles: X. Dou and Y. Lu, Chem. – Eur. J., 2012, 18, 8315 CrossRef CAS PubMed.
- T. Kumar, D. Verma, R. F. S. Menna-Barreto, W. O. Valenca, E. N. da Silva Júnior and I. N. N. Namboothiri, Org. Biomol. Chem., 2015, 13, 1996 CAS.
- (a) V. Zuliani, G. Cocconcelli, M. Fantini, C. Ghiron and M. Rivara, J. Org. Chem., 2007, 72, 4551 CrossRef CAS PubMed; (b) J. G. Lombardino and E. H. Wiseman, J. Med. Chem., 1974, 17, 1182 CrossRef CAS.
- A. Giese, U. Bertsch, H. Kretzschmar, M. Habeck, T. Hirschberger, P. Tavan, C. Griesinger, A. Leonov, S. Ryazanov and P. Weber, PCT Int. Appl, WO 2010000372 A2, 2010 Search PubMed.
- S. Nakanishi, J. Nantaku and Y. Otsuji, Chem. Lett., 1983, 12, 341 CrossRef.
- I. Langhammer and T. Erker, Heterocycles, 2005, 65, 1975 CrossRef CAS.
- (a) B. Sezen and D. Sames, J. Am. Chem. Soc., 2003, 125, 10580 CrossRef CAS PubMed; (b) P.-F. Zhang and Z.-C. Chen, Synthesis, 2001, 2075 CrossRef CAS.
- C. Oalmann, R. B. Perni, J. S. Disch, B. Szczepankiewicz, G. Gualtieri, R. L. Casaubon and K. J. Koppetsch, PCT Int. Appl, WO 2009058348 A1, 2009 Search PubMed.
- E. V. Vlasova, A. A. Aleksandrov and M. M. El'chaninov, Russ. J. Appl. Chem., 2010, 83, 1027 CrossRef CAS.
- M. Suzuki, S. Maeda, K. Matsumoto, T. Ishizuka and Y. Iwasawa, Chem. Pharm. Bull., 1986, 34, 3111 CrossRef CAS.
- R. Kuwano, N. Kameyama and R. Ikeda, J. Am. Chem. Soc., 2011, 133, 7312 CrossRef CAS PubMed.
- (a) F. C. Schaefer and G. A. Peters, J. Org. Chem., 1961, 26, 412 CrossRef CAS; (b) R. A. Thompson, J. S. Francisco and J. B. Grutzner, Phys. Chem. Chem. Phys., 2004, 6, 756 RSC; (c) R. A. Moss, J. Terpinski, D. P. Cox, D. Z. Denney and K. K. Jespersen, J. Am. Chem. Soc., 1985, 107, 2748 CrossRef.
- A. C. Lipinski, F. Lombardo, B. W. Dominy and P. J. Feeney, Adv. Drug Delivery Rev., 1997, 23, 3 CrossRef.
- http://www.molinspiration.com/cgi-bin/properties .
- (a) MBH alcohol: I. Deb, M. Dadwal, S. M. Mobin and I. N. N. Namboothiri, Org. Lett., 2006, 8, 1201 CrossRef CAS PubMed; (b) MBH acetate: R. J. Reddy and K. Chen, Org. Lett., 2011, 13, 1458 CrossRef CAS PubMed.
- (a) W. E. Parham and J. L. Bleasdale, J. Am. Chem. Soc., 1951, 73, 4664 CrossRef CAS; For direct synthesis from aldehydes: (b) Y. Shen and B. Yang, Synth. Commun., 1993, 23, 1 CrossRef CAS PubMed; (c) A. Palmieri, S. Gabrielli and R. Ballini, Synlett, 2013, 114 CAS.
- E. N. da Silva Júnior, R. F. S. Menna-Barreto, M. do C. F. R. Pinto, R. S. F. Silva, D. V. Teixeira, M. C. B. V. de Souza, C. A. De Simone, S. L. De Castro, V. F. Ferreira and A. V. Pinto, Eur. J. Med. Chem., 2008, 43, 1774 CrossRef PubMed.
- C. B. Moraes, L. H. Freitas-Junior, M. A. Giardini, H. Kim, C. H. Franco, A. M. Araujo-Junior, S. Schenkman and E. Chatelain, Sci. Rep., 2014, 4, 4703 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: NMR spectra for all relevant compounds. See DOI: 10.1039/c5ob01444a |
| This journal is © The Royal Society of Chemistry 2015 |