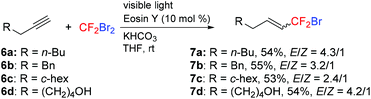Visible light-induced selective hydrobromodifluoromethylation of alkenes with dibromodifluoromethane†
Received
26th June 2015
, Accepted 13th July 2015
First published on 13th July 2015
Abstract
A visible light-induced selective hydrobromodifluoromethylation of alkenes using CF2Br2 was developed. This transformation proceeded smoothly in the presence of catalytic eosin Y at room temperature to give various hydrobromodifluoromethylated compounds with broad functional group tolerance.
Introduction
The myriad applications of fluorinated compounds have stimulated the development of novel methods for the introduction of the fluorine atom and fluorinated groups into organic molecules.1 While strategies for trifluoromethylation have been extensively developed,2 the methods for the preparation of other fluoroalkylated compounds are relatively underdeveloped despite their potential importance in many research fields. Bromodifluoromethylated compounds are well known as good candidates for the formation of halogen bonding3 and important intermediates for the preparation of valuable fluorinated compounds.4 The known methods for the preparation of these compounds were divided into indirect and direct approaches. The indirect approaches, such as bromination of gem-difluoromethylenated precursors5 and gem-difluoroalkenes6 as well as transformation from CF2Br-containing building blocks,7 require long synthetic sequences. Recently, direct approaches involving the electrophilic bromodifluoromethylating reagents have been developed by Magnier,8a Shibata,8b,c and Xiao.8d Furthermore, Hu and co-workers reported a novel formal nucleophilic bromodifluoromethylation of carbonyl compounds via bromination of in situ generated sulfinate intermediates from the Julia–Kocienski reactions of difluoromethyl 2-pyridyl sulfone.9a Very recently, Dilman accomplished the nucleophilic bromodifluoromethylation of aldehydes9b and iminium ions9c with (bromodifluoromethyl)trimethylsilane in the presence of an excess of bromide ions. Besides these methods, the addition of dibromodifluoromethane (CF2Br2) to alkenes provides convenient access to a series of bromodifluoromethylated compounds.10–13 As shown in Scheme 1, single electron transfer (SET) from a radical initiator to CF2Br2 generates the CF2Br radical, which is added to alkenes 1 to form radical intermediate A. The intermediate A may undergo different reaction processes to give compounds 2–5. The dimerization reaction of intermediate A produced compound 2.10 Bromine and hydrogen abstraction of intermediate A from CF2Br2 and a hydrogen donor gave compounds 311 and 412 respectively. In the presence of other radical trap agents such as diphenyl diselenide, intermediate A was transformed into the selenobromodifluoromethylated product 5.13 Because the atom transfer radical addition (ATRA) for the formation of product 3 is a preferred process,11 the selective formation of hydrobromodifluoromethylated compound 4 is particularly challenging.
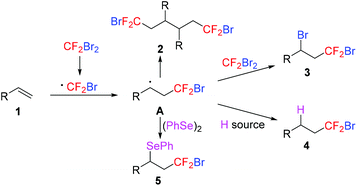 |
| | Scheme 1 The addition of CF2Br2 to alkenes. | |
To the best of our knowledge, only two reactions of the direct hydrobromodifluoromethylation of alkenes with CF2Br2 have been reported. Hu reported the hydrobromodifluoromethylation of electron-deficient alkenes initiated by a CrCl3/Fe bimetal redox system (Scheme 2a).12a Wu and co-workers disclosed that the Zn-induced addition of CF2Br2 to cyclohexene yielded the hydrobromodifluoromethylated product in low yield along with byproducts (Scheme 2b).12b Both these methods suffered from a narrow substrate scope. Recently, visible light photoredox catalysis has emerged as an efficient and eco-friendly tool in organic synthesis14 and has been applied in the fluoroalkylation of organic compounds.15,16 As part of our ongoing research on photocatalytic fluoroalkylation reactions,17 herein we disclose the selective hydrobromodifluoromethylation of alkenes with CF2Br2 through visible light photoredox catalysis (Scheme 2c).
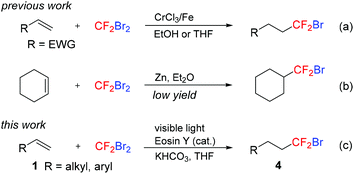 |
| | Scheme 2 Hydrobromodifluoromethylation of alkenes. | |
Results and discussion
Optimization of the reaction conditions was explored using 4-phenyl-1-butene (1a) as the substrate (Table 1). The reaction catalyzed by fac-Ir(ppy)3 in MeOH mainly led to the atom transfer radical addition (ATRA) product 3a (entry 1). When the reaction was performed in THF, a mixture of 3a and 4a was generated (entry 2). Various solvents, including toluene, CH2Cl2, Et2O, dioxane, CH3CN, DMF, and DMSO, were also investigated. However, no higher yield was gained by altering the solvent. Then different photocatalysts were screened (entries 3–6). Among them, eosin Y18 was superior to other photocatalysts, giving the desired product 4a in 54% yield (entry 6). The yield of 4a was slightly improved to 57% by increasing the amount of the photocatalyst (entry 7). The GC-MS analysis of the reaction mixture indicated that the substrate 1a was only partly converted, while the 19F NMR showed that CF2Br2 was totally consumed. Consequently, compound 4a was formed in 81% yield when another portion of CF2Br2 was added (entry 8). Finally, the addition of additives, including Et3N and KHCO3, led to a further improvement of the yield (entries 9 and 10). Control experiments showed that both the photocatalyst and visible light were indispensable for this transformation (entries 11 and 12).
Table 1 Optimization of reaction conditionsa
|

|
| Entry |
Photocat. (mol%) |
X
|
Additive |
Yield (4a/3a, %)b |
|
Reaction conditions: 1a (0.1 mmol), CF2Br2 (x equiv.), photocat., additive (0.1 mmol), THF (3.0 mL), visible light, rt, under N2, 10 h.
Yields determined by 19F NMR spectroscopy using trifluoromethylbenzene as an internal standard.
The reaction was performed in MeOH (3.0 mL).
A second portion of CF2Br2 (2 equiv.) was added after 5 h.
A second portion of CF2Br2 (2 equiv.) and additive (0.1 mmol) was added after 5 h.
No light.
|
| 1c |
fac-Ir(ppy)3 (3) |
4 |
— |
0/97 |
| 2 |
fac-Ir(ppy)3 (3) |
4 |
— |
48/46 |
| 3 |
Ru(bpy)3Cl2·6H2O (3) |
4 |
— |
10/9 |
| 4 |
Ru(bpy)3(PF6)2 (2) |
4 |
— |
15/8 |
| 5 |
Methylene blue (3) |
4 |
— |
33/8 |
| 6 |
Eosin Y (3) |
4 |
— |
54/6 |
| 7 |
Eosin Y (5) |
4 |
— |
57/Trace |
| 8d |
Eosin Y (5) |
4 + 2 |
— |
81/Trace |
| 9e |
Eosin Y (5) |
4 + 2 |
Et3N |
86/Trace |
| 10e |
Eosin Y (5) |
4 + 2 |
KHCO3 |
87/Trace |
| 11e |
— |
4 + 2 |
KHCO3 |
0/0 |
| 12e,f |
Eosin Y (5) |
4 + 2 |
KHCO3 |
0/0 |
With the optimized reaction conditions in hand (Table 1, entry 10), we next investigated the substrate scope of this photocatalytic reaction. A variety of monosubstituted and disubstituted alkenes could be transformed into the corresponding hydrobromodifluoromethylated products in moderate to excellent yields (Table 2). A wide range of functional groups were tolerated, including alkyl and allylic alcohols, aldehydes, ketones, carboxylic acids, esters, nitriles, amides, nitro groups, phosphine oxides, ethers, sulfones, and halides. Substrates bearing fluoro, chloro, and bromo substituents on the arene rings were also compatible. Heterocyclic substrates, 1p and 1q, were smoothly converted into the desired products. α,β-Unsaturated ester 1r and α,β-unsaturated sulfone 1s exhibited moderate reactivity in this transformation. It was noteworthy that the photocatalytic protocol presented herein was also easily extended to branched terminal and internal alkenes 1t–v. However, styrenes were not suitable substrates for this transformation.
Table 2 Substrate scope of photocatalytic hydrobromodifluoromethylation of alkenesa
|
Reaction conditions: 1 (0.5 mmol), CF2Br2 (3.0 mmol), eosin Y (0.025 mmol), KHCO3 (0.5 mmol), THF (15.0 mL), visible light, rt, under N2, 10 h, isolated yields.
Eosin Y (0.05 mmol).
|

|
Remarkably, this facile protocol allowed the direct hydrobromodifluoromethylation of natural product analogues, such as L-phenylalanine derivative 1w (Scheme 3). The complex compounds such as vinclozolin 1x and rotenone 1y were also examined, affording the corresponding hydrobromodifluoromethylated products 4x and 4y in moderate yields, respectively. These results showed that this photocatalytic protocol might be applicable to “late-stage hydrobromodifluoromethylation” of natural products and drugs.
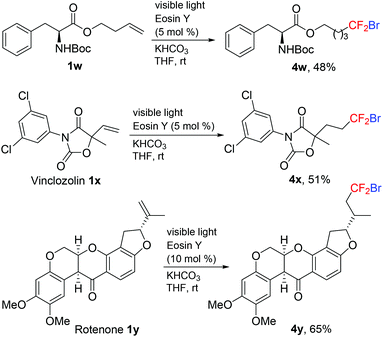 |
| | Scheme 3 Hydrobromodifluoromethylation of compounds 1w–y. | |
The hydrobromodifluoromethylation of alkynes was also successful (Scheme 4). Reactions of alkynes 6a–d with CF2Br2 in the presence of eosin Y (10 mol%) and KHCO3 under visible light irradiation provided a mixture of the E and Z alkenyl-CF2Br compounds 7a–d in moderate yields.19
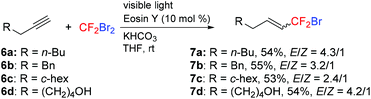 |
| | Scheme 4 Hydrobromodifluoromethylation of alkynes. | |
The bromodifluoromethylated compounds are important intermediates for the preparation of other fluorinated compounds. As shown in Scheme 5, compound 4a underwent several transformations to give products 8–12. Reduction of 4a with Zn/HCl in DMF yielded difluoromethylated product 8.20 The reaction of 4a with allyltributyltin in the presence of a catalytic amount of AIBN afforded gem-difluoromethylenated product 9.21 The gem-difluoroalkene 10 could be conveniently obtained by the elimination reaction using TBAF as the base.22 Treatment of 4a with CrCl2 generated the nucleophilic α-fluorovinylchromium intermediate,23 which subsequently reacted with HCl or PhCHO to give (Z)-fluoroalkene 11 and (Z)-β-fluoroallylic alcohol 12 respectively in high stereoselectivities.
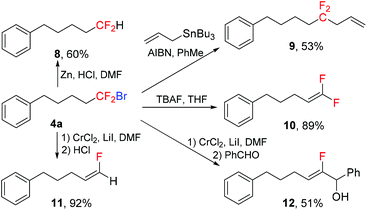 |
| | Scheme 5 Hydrobromodifluoromethylation of alkynes. | |
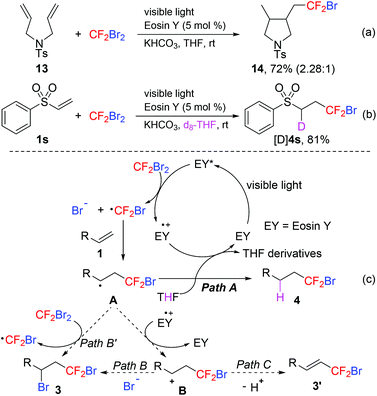
To gain insight into the reaction mechanism, a radical clock 13 was subjected to the standard reaction conditions (Scheme 6a). The cyclized bromodifluoromethylated product 14 was formed in 72% yield (2.28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr). This result revealed that the CF2Br radical was involved in this visible light-induced hydrobromodifluoromethylation of alkenes. The reaction of 1s with CF2Br2 in d8-THF exclusively gave the deuterated product [D]4s in 81% yield, which indicated that THF served as the hydrogen atom source (Scheme 6b). What is more, Stern–Volmer studies showed that CF2Br2 exhibited significant fluorescence quenching of eosin Y* (see the ESI†). This result suggested that electron transfer occurred from eosin Y* to CF2Br2 first. On the basis of these experimental results and the literature reports,18 a plausible mechanism for the hydrobromodifluoromethylation is depicted in Scheme 6c. Initially, the excitation of eosin Y with visible light produced the excited state eosin Y*. Then a single electron transfer (SET) from eosin Y* to CF2Br2 generated the CF2Br radical, which was subsequently added to alkenes 1 for the formation of radical intermediate A. Finally, intermediate A abstracted hydrogen from THF to give the desired hydrobromodifluoromethylated product 4 (Path A).15m
1 dr). This result revealed that the CF2Br radical was involved in this visible light-induced hydrobromodifluoromethylation of alkenes. The reaction of 1s with CF2Br2 in d8-THF exclusively gave the deuterated product [D]4s in 81% yield, which indicated that THF served as the hydrogen atom source (Scheme 6b). What is more, Stern–Volmer studies showed that CF2Br2 exhibited significant fluorescence quenching of eosin Y* (see the ESI†). This result suggested that electron transfer occurred from eosin Y* to CF2Br2 first. On the basis of these experimental results and the literature reports,18 a plausible mechanism for the hydrobromodifluoromethylation is depicted in Scheme 6c. Initially, the excitation of eosin Y with visible light produced the excited state eosin Y*. Then a single electron transfer (SET) from eosin Y* to CF2Br2 generated the CF2Br radical, which was subsequently added to alkenes 1 for the formation of radical intermediate A. Finally, intermediate A abstracted hydrogen from THF to give the desired hydrobromodifluoromethylated product 4 (Path A).15m
 |
| | Scheme 6 Mechanistic investigations. | |
The byproduct 3 might be formed via two different routes from intermediate A: either by oxidation to cation B followed by nucleophilic trapping (Path B) or by propagation (Path B’). From this proposed mechanism, we can explain why eosin Y is selected for this transformation. Its high reduction potential (−1.60 V vs. SCE) facilitates the generation of the CF2Br radical and its low oxidation potential (0.72 V vs. SCE) avoids the oxidation to cation B.24 Furthermore, cation B might undergo elimination of the proton to give alkenes 3′ (Path C). This process would make the reaction mixture acidic, which needs a base to neutralize the reaction system. That is why the addition of KHCO3 benefits this reaction.
Conclusions
In conclusion, we have developed a photocatalytic hydrobromodifluoromethylation of unactivated alkenes with CF2Br2 in the presence of eosin Y at room temperature. The mild reaction conditions allow the tolerance of a wide range of functional groups. This protocol could also be extended to alkyne substrates. Furthermore, the application of the bromodifluoromethylated products in organic synthesis has been demonstrated by the transformations of compound 4a into other fluorinated compounds.
Experimental
General information
1H NMR (TMS as the internal standard) and 19F NMR spectra (CFCl3 as the outside standard and low field is positive) were recorded on a 400 MHz spectrometer. 13C NMR was recorded on a 400 MHz spectrometer. Chemical shifts (δ) are reported in ppm, and coupling constants (J) are in hertz (Hz). The following abbreviations were used to explain the multiplicities: s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet. Substrates 1a–h, 1r–v, 1x, 1y, 6a–d, and 13 were purchased from commercial sources and used as received. Substrates 1i–q25 and 1w26 were prepared according to literature procedures. Unless otherwise noted, all reagents were obtained commercially and used without further purification.
General procedures for hydrobromodifluoromethylation of alkenes and alkynes
A 25 mL Schlenk flask equipped with a rubber septum and a magnetic stir bar was charged with eosin Y (16.2 mg, 0.025 mmol, 5 mol%) and substrates (0.5 mmol, 1.0 equiv.). Then a solution of CF2Br2 (420 mg, 4.0 equiv., 2.0 mmol) in THF (10 mL, 2.0 mol L−1) was added to the reaction flask by using a syringe. The flask was sealed with 3M vinyl electrical tape, and then the mixture was degassed three times by the freeze–pump–thaw procedure. The flask was placed at a distance of 2 cm from the blue LEDs (λ = 460–470 nm).27 The mixture was stirred under a nitrogen atmosphere and irradiated by blue LEDs for 5 h. After cooling in an ice-water bath, KHCO3 (50 mg, 1.0 equiv., 0.5 mmol) and the second portion of CF2Br2 (210 mg, 2.0 equiv., 1.0 mmol) in THF (5 mL, 2.0 mol L−1) were added to the reaction mixture. Then the mixture was degassed and irradiated by blue LEDs for another 5 h. After the reaction was complete, the reaction mixture was concentrated under vacuum and the crude product was purified by column chromatography on silica gel to give the product.
(3,5-Dibromo-5,5-difluoropentyl)benzene (3a).
Colorless liquid. 1H NMR (400 MHz, CDCl3) δ ppm 7.32–7.28 (m, 2H), 7.23–7.20 (m, 3H), 4.21–4.15 (m, 1H), 3.17–3.04 (m, 1H), 3.02–2.89 (m, 2H), 2.81–2.73 (m, 1H), 2.28–2.19 (m, 1H), 2.12–2.08 (m, 1H); 13C NMR (100 MHz, CDCl3) δ ppm 140.1, 128.6, 128.5, 126.4, 120.4 (t, J = 305.2 Hz), 52.7 (t, J = 21.5 Hz), 46.2 (t, J = 2.6 Hz), 39.9, 33.3; 19F NMR (376 MHz, CDCl3) δ ppm −42.2–(−43.2) (m, 2F); IR (thin film) ν 3063, 3028, 2928, 1603, 1497, 1454, 1196, 1112, 926, 748, 699, 543 cm−1; MS (EI): m/z (%) 344 ([M + 4]+, 11.0), 342 ([M + 2]+, 11.0), 340 ([M]+, 12.3), 91 (100); HRMS calculated for C11H12Br2F2: 339.9274; found: 339.9278.
(5-Bromo-5,5-difluoropentyl)benzene (4a).
Colorless liquid. 1H NMR (400 MHz, CDCl3) δ ppm 7.31 (t, J = 7.2 Hz, 2H), 7.24–7.18 (m, 3H), 2.67 (t, J = 7.0 Hz, 2H), 2.44–2.33 (m, 2H), 1.74–1.68 (m, 4H); 13C NMR (100 MHz, CDCl3) δ ppm 141.7, 128.5, 128.4, 126.0, 123.1 (t, J = 303.4 Hz), 44.2 (t, J = 21.2 Hz), 35.5, 30.3, 23.6 (t, J = 3.0 Hz); 19F NMR (376 MHz, CDCl3) δ ppm −43.4 (t, J = 13.5 Hz, 2F); IR (thin film) ν 3027, 2943, 2860, 1497, 1454, 1195, 1103, 947, 909, 747, 699 cm−1; MS (EI): m/z (%) 264 ([M + 2]+, 11.0), 262 ([M]+, 12.3), 91 (100); HRMS calculated for C11H13BrF2: 262.0169; found: 262.0173.
(3-Bromo-3,3-difluoropropyl)cyclohexane (4b).
Colorless liquid. 1H NMR (400 MHz, CDCl3) δ ppm 2.39–2.28 (m, 2H), 1.72–1.68 (m, 4H), 1.53–1.45 (m, 2H), 1.31–1.09 (m, 5H), 0.96–0.86 (m, 2H); 13C NMR (100 MHz, CDCl3) δ ppm 123.6 (t, J = 303.4 Hz), 42.0 (t, J = 20.8 Hz), 36.7, 33.0, 31.2 (t, J = 2.6 Hz), 26.4, 26.1; 19F NMR (376 MHz, CDCl3) δ ppm −43.4 (t, J = 13.7 Hz, 2F); IR (thin film) ν 2924, 2853, 1457, 1377, 923 cm−1; MS (EI): m/z (%) 240 ([M]+, 0.24), 161 (50.8), 83 (100); HRMS calculated for C9H15BrF2: 240.0325; found: 240.0319.
1,9-Dibromo-1,1-difluorononane (4c).
Colorless liquid. 1H NMR (400 MHz, CDCl3) δ ppm 3.39 (t, J = 6.8 Hz, 2H), 2.37–2.27 (m, 2H), 1.88–1.81 (m, 2H), 1.61–1.56 (m, 2H), 1.44–1.33 (m, 8H); 13C NMR (100 MHz, CDCl3) δ ppm 123.2 (t, J = 303.8 Hz), 44.2 (t, J = 21.2 Hz), 33.9, 32.7, 29.0, 28.5, 28.3, 28.0, 23.9 (t, J = 2.9 Hz); 19F NMR (376 MHz, CDCl3) δ ppm −43.4 (t, J = 13.5 Hz, 2F); IR (thin film) ν 2934, 2857, 1465, 1198, 1106, 910, 635 cm−1; MS (EI): m/z (%) 243 ([M + 2]+, 6.7), 241 (M+, 6.8), 161 (100), 119 (53.9); HRMS calculated for C9H16BrF2: 241.0403; found: 241.0400.
11-Bromo-11,11-difluoroundecan-1-ol (4d).
Colorless liquid. 1H NMR (400 MHz, CDCl3) δ ppm 3.62 (t, J = 6.6 Hz, 2H), 2.37–2.26 (m, 2H), 1.62–1.51 (m, 4H), 1.36–1.28 (m, 12H); 13C NMR (100 MHz, CDCl3) δ ppm 123.3 (t, J = 303.8 Hz), 63.0, 44.3 (t, J = 21.2 Hz), 32.8, 29.5, 29.4, 29.3, 29.2, 28.4, 25.7, 23.9 (t, J = 2.9 Hz); 19F NMR (376 MHz, CDCl3) δ ppm −43.3 (t, J = 14.3 Hz, 2F); IR (thin film) ν 3349 (w) 2928, 2856, 1466, 1198, 1086, 911 cm−1; MS (EI): m/z (%) 268 ([M − 18]+, 2.61), 133 (35.6), 69 (97.0), 55 (100); HRMS calculated for C11H19BrF2 [M − H2O]: 268.0638; found: 268.0634.
12-Bromo-12,12-difluorododecanal (4e).
Colorless liquid. 1H NMR (400 MHz, CDCl3) δ ppm 9.75 (t, J = 1.8 Hz, 1H), 2.41 (dt, J = 7.4, 2.0 Hz, 2H), 2.37–2.26 (m, 2H), 1.63–1.56 (m, 4H), 1.36–1.24 (m, 12H); 13C NMR (100 MHz, CDCl3) δ ppm 202.9, 123.3 (t, J = 303.7 Hz), 44.3 (t, J = 21.1 Hz), 43.9, 29.29, 29.26, 29.2, 29.1, 28.4, 23.9 (t, J = 2.9 Hz), 22.1; 19F NMR (376 MHz, CDCl3) δ ppm −43.3 (t, J = 13.7 Hz, 2F); IR (thin film) ν 2928, 2856, 1710, 1199, 911 cm−1; MS (EI): m/z (%) 280 ([M − 18]+, 15.4), 254 (72.7), 95 (92.0), 55 (100); HRMS calculated for C12H21BrF2O: 298.0744; found: 298.0750.
12-Bromo-12,12-difluorododecanoic acid (4f).
Colorless liquid. 1H NMR (400 MHz, CDCl3) δ ppm 2.37–2.27 (m, 4H), 1.66–1.55 (m, 4H), 1.36–1.24 (m, 12H); 13C NMR (100 MHz, CDCl3) δ ppm 179.7, 123.3 (t, J = 303.8 Hz), 44.3 (t, J = 21.2 Hz), 34.0, 29.7, 29.29, 29.27, 29.2, 29.0, 28.4, 24.6, 23.9 (t, J = 2.9 Hz); 19F NMR (376 MHz, CDCl3) δ ppm −43.3 (t, J = 14.3 Hz, 2F); IR (thin film) ν 3050, 2926, 2855, 1710, 1200, 911 cm−1; MS (EI): m/z (%) 314 ([M]+, 2.0), 254 (6.3), 73 (73.6), 60 (100); HRMS calculated for C12H21BrF2O2: 314.0693; found: 314.0692.
(4-Bromo-4,4-difluorobutyl)diphenylphosphine oxide (4g).
White solid, m.p. 80–83 °C. 1H NMR (400 MHz, CDCl3) δ ppm 7.75–7.70 (m, 4H), 7.54–7.45 (m, 6H), 2.55–2.44 (m, 2H), 2.35–2.29 (m, 2H), 2.00–1.89 (m, 2H); 13C NMR (100 MHz, CDCl3) δ ppm 132.4 (d, J = 98.5 Hz), 132.0 (d, J = 2.2 Hz), 130.7 (d, J = 8.7 Hz), 128.8 (d, J = 11.6 Hz), 122.3 (t, J = 302.2 Hz), 44.7 (td, J = 21.5, 13.1 Hz), 28.6 (d, J = 71.5 Hz), 16.8 (q, J = 2.9 Hz); 19F NMR (376 MHz, CDCl3) δ ppm −43.7 (t, J = 13.5 Hz, 2F); 31P NMR (162 MHz, CDCl3) δ ppm 31.5 (s, 1P); IR (thin film) ν 3056, 2941, 1438, 1186, 1120, 914, 718, 695, 543, 509 cm−1; MS (EI): m/z (%) 355 ([M + 2]+, 0.39), 353 ([M]+, 0.29), 293 (100), 201 (51.7); HRMS calculated for C16H16BrF2OP: 353.0118; found: 353.0101.
1-Bromo-1,1-difluoroundecan-4-ol (4h).
Colorless liquid. 1H NMR (400 MHz, CDCl3) δ ppm 3.64 (s, 1H), 2.67–2.52 (m, 1H), 2.47–2.32 (m, 1H), 1.83–1.75 (m, 1H), 1.70–1.60 (m, 1H), 1.47–1.40 (m, 4H), 1.30–1.25 (m, 9H), 0.87 (t, J = 7.0 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ ppm 123.2 (t, J = 303.4 Hz), 70.5, 40.8 (t, J = 21.5 Hz), 37.7, 31.8, 31.4 (t, J = 3.0 Hz), 29.5, 29.2, 25.6, 22.6, 14.1; 19F NMR (376 MHz, CDCl3) δ ppm −43.0–(−44.0) (m, 2F); IR (thin film) ν 3357, 2929, 2857, 1466, 1204, 1071, 988, 919 cm−1; MS (EI): m/z (%) 268 ([M − 18]+, 2.24), 169 (62.4), 167 (62.7), 129 (83.6), 69 (100); HRMS calculated for C11H19BrF2 [M − H2O]: 268.0638; found: 268.0641.
4-((6-Bromo-6,6-difluorohexyl)oxy)benzaldehyde (4i).
Colorless liquid. 1H NMR (400 MHz, CDCl3) δ ppm 9.85 (s, 1H), 7.80 (d, J = 8.8 Hz, 2H), 6.96 (d, J = 8.8 Hz, 2H), 4.03 (t, J = 6.4 Hz, 2H), 2.42–2.31 (m, 2H), 1.87–1.80 (m, 2H), 1.72–1.65 (m, 2H), 1.59–1.53 (m, 2H); 13C NMR (100 MHz, CDCl3) δ ppm 190.8, 164.0, 132.0, 129.9, 123.0 (t, J = 303.4 Hz), 114.7, 67.9, 44.1 (t, J = 21.2 Hz), 28.7, 25.0, 23.7 (t, J = 3.0 Hz); 19F NMR (376 MHz, CDCl3) δ ppm −43.5 (t, J = 13.5 Hz, 2F); IR (thin film) ν 2946, 1689, 1602, 1257, 1160, 909, 832 cm−1; MS (EI): m/z (%) 322 ([M + 2]+, 18.6), 320 ([M]+, 19.2), 193 (5.9), 121 (100); HRMS calculated for C13H15BrF2O2: 320.0223; found: 320.0222.
Ethyl 4-((6-bromo-6,6-difluorohexyl)oxy)benzoate (4j).
White solid, m.p. 38–40 °C. 1H NMR (400 MHz, CDCl3) δ ppm 7.97 (d, J = 9.2 Hz, 2H), 6.87 (d, J = 8.8 Hz, 2H), 4.32 (q, J = 7.1 Hz, 2H), 4.00 (t, J = 6.2 Hz, 2H), 2.42–2.31 (m, 2H), 1.85–1.76 (m, 2H), 1.72–1.64 (m, 2H), 1.60–1.51 (m, 2H), 1.36 (t, J = 7.2 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ ppm 166.4, 162.7, 131.6, 123.0 (t, J = 303.4 Hz), 122.9, 114.0, 67.6, 60.6, 44.2 (t, J = 21.5 Hz), 28.8, 25.1, 23.7 (t, J = 3.0 Hz), 14.4; 19F NMR (376 MHz, CDCl3) δ ppm −43.5 (t, J = 13.5 Hz, 2F); IR (thin film) ν 2945, 2872, 1712, 1606, 1277, 1253, 1168, 1103 cm−1; MS (EI): m/z (%) 366 ([M + 2]+, 19.3), 364 (M+, 18.8), 139 (84.2), 121 (100); HRMS calculated for C15H19BrF2O3: 364.0486; found: 364.0484.
1-((6-Bromo-6,6-difluorohexyl)oxy)-4-nitrobenzene (4k).
Colorless liquid. 1H NMR (400 MHz, CDCl3) δ ppm 8.17 (dd, J = 8.8, 2.0 Hz, 2H), 6.92 (dd, J = 9.6, 2.4 Hz, 2H), 4.04 (td, J = 6.2, 2.4 Hz, 2H), 4.43–2.32 (m, 2H), 1.88–1.81 (m, 2H), 1.72–1.65 (m, 2H), 1.60–1.54 (m, 2H); 13C NMR (100 MHz, CDCl3) δ ppm 164.0, 141.5, 126.0, 122.9 (t, J = 303.4 Hz), 114.4, 68.3, 44.1 (t, J = 21.2 Hz), 28.7, 25.0, 23.7 (t, J = 3.0 Hz); 19F NMR (376 MHz, CDCl3) δ ppm −43.5 (t, J = 14.3 Hz, 2F); IR (thin film) ν 3113, 2947, 1594, 1342, 1264, 1112, 910, 860, 753 cm−1; MS (EI): m/z (%) 337 (M+, 22.3), 238 (12.8), 139 (100); HRMS calculated for C12H14BrF2NO3: 337.0125; found: 337.0121.
4-((7-Bromo-7,7-difluoroheptyl)oxy)benzonitrile (4l).
White solid, m.p. 43–45 °C. 1H NMR (400 MHz, CDCl3) δ ppm 7.55 (d, J = 9.2 Hz, 2H), 6.91 (d, J = 9.2 Hz, 2H), 3.98 (t, J = 6.4 Hz, 2H), 2.39–2.28 (m, 2H), 1.84–1.77 (m, 2H), 1.67–1.60 (m, 2H), 1.51–1.41 (m, 4H); 13C NMR (100 MHz, CDCl3) δ ppm 162.4, 134.0, 123.1 (t, J = 303.4 Hz), 119.3, 115.2, 103.8, 68.1, 44.1 (t, J = 21.2 Hz), 28.7, 28.1, 25.7, 23.8 (t, J = 3.0 Hz); 19F NMR (376 MHz, CDCl3) δ ppm −43.5 (t, J = 13.7 Hz, 2F); IR (thin film) ν 2944, 2869, 2225, 1606, 1509, 1302, 1259, 1172, 835, 578 cm−1; MS (EI): m/z (%) 333 ([M + 2]+, 14.2), 331 (M+, 14.3), 238 (6.3), 119 (100); HRMS calculated for C14H16BrF2NO: 331.0383; found: 331.0378.
1-(4-((6-Bromo-6,6-difluorohexyl)oxy)phenyl)ethanone (4m).
Colorless liquid. 1H NMR (400 MHz, CDCl3) δ ppm 7.90 (dd, J = 8.8, 2.4 Hz, 2H), 6.89 (dd, J = 8.8, 2.4 Hz, 2H), 4.00 (t, J = 6.0 Hz, 2H), 2.52 (s, 3H), 2.39–2.27 (m, 2H), 1.84–1.76 (m, 2H), 1.67–1.60 (m, 2H), 1.51–1.41 (m, 4H); 13C NMR (100 MHz, CDCl3) δ ppm 196.7, 163.0, 130.6, 130.3, 123.1 (t, J = 303.4 Hz), 114.1, 67.9, 44.2 (t, J = 21.2 Hz), 28.8, 28.1, 26.3, 25.7, 23.8 (t, J = 3.0 Hz); 19F NMR (376 MHz, CDCl3) δ ppm −43.4 (t, J = 13.5 Hz, 2F); IR (thin film) ν 2943, 2869, 1677, 1601, 1256, 1172, 835, 591 cm−1; MS (EI): m/z (%) 333 ([M − CH3]+, 10.4), 269 (7.0), 121 (100); HRMS calculated for C14H16BrF2O2: 333.0302; found: 333.0305.
1-Bromo-4-((7-bromo-7,7-difluoroheptyl)oxy)-2-chlorobenzene (4n).
Colorless liquid. 1H NMR (400 MHz, CDCl3) δ ppm 7.47 (d, J = 2.4 Hz, 1H), 7.28 (dd, J = 8.8, 2.4 Hz, 1H), 6.75 (d, J = 8.8 Hz, 1H), 3.98 (t, J = 6.4 Hz, 2H), 2.40–2.29 (m, 2H), 1.86–1.79 (m, 2H), 1.68–1.60 (m, 2H), 1.56–1.43 (m, 4H); 13C NMR (100 MHz, CDCl3) δ ppm 153.9, 132.7, 130.5, 124.1, 123.1 (t, J = 303.4 Hz), 114.5, 112.4, 69.2, 44.2 (t, J = 21.2 Hz), 28.7, 28.1, 25.7, 23.8 (t, J = 2.9 Hz); 19F NMR (376 MHz, CDCl3) δ ppm −43.4 (t, J = 13.7 Hz, 2F); IR (thin film) ν 2942, 2862, 1582, 1485, 1467, 1289, 1265, 1249, 1086, 1062, 910, 802, 638 cm−1; MS (EI): m/z (%) 422 ([M + 4]+, 5.1), 420 ([M + 2]+, 7.7), 418 (M+, 3.9), 208 (100), 206 (74.2); HRMS calculated for C13H15Br2ClFO: 417.9146; found: 417.9147.
7-Bromo-7,7-difluoroheptyl-4-fluorobenzoate (4o).
Colorless liquid. 1H NMR (400 MHz, CDCl3) δ ppm 8.04–8.01 (m, 2H), 7.08 (t, J = 8.8 Hz, 2H), 4.28 (t, J = 6.4 Hz, 2H), 2.37–2.27 (m, 2H), 1.79–1.71 (m, 2H), 1.66–1.58 (m, 2H), 1.47–1.41 (m, 4H); 13C NMR (100 MHz, CDCl3) δ ppm 165.7 (d, J = 252.3 Hz), 132.1 (d, J = 8.7 Hz), 126.6 (d, J = 2.9 Hz), 123.1 (t, J = 303.4 Hz), 115.5 (d, J = 21.9 Hz), 64.9, 44.2 (t, J = 21.2 Hz), 28.5, 28.1, 25.7, 23.9 (t, J = 3.0 Hz); 19F NMR (376 MHz, CDCl3) δ ppm −43.5 (t, J = 14.3 Hz, 2F), −105.9 (m, 1F); IR (thin film) ν 2943, 2862, 1720, 1604, 1508, 1276, 1113, 930, 768, 608 cm−1; MS (EI): m/z (%) 352 (M+, 0.78), 141 (77.6), 123 (100); HRMS calculated for C14H16BrF3O2: 352.0286; found: 352.0288.
7-((6-Bromo-6,6-difluorohexyl)oxy)-4-methyl-2H-chromen-2-one (4p).
Red liquid. 1H NMR (400 MHz, CDCl3) δ ppm 7.45 (d, J = 8.8 Hz, 1H), 6.80 (d, J = 8.8 Hz, 1H), 6.74 (s, 1H), 6.08 (s, 1H), 3.99 (t, J = 6.2 Hz, 2H), 2.41–2.31 (m, 5H), 1.86–1.79 (m, 2H), 1.71–1.63 (m, 2H), 1.58–1.51 (m, 2H); 13C NMR (100 MHz, CDCl3) δ ppm 162.0, 161.3, 155.3, 152.6, 125.6, 123.0 (t, J = 303.0 Hz), 113.5, 112.5, 111.9, 101.4, 68.1, 44.1 (t, J = 21.2 Hz), 28.6, 25.0, 23.7 (t, J = 3.3 Hz), 18.6; 19F NMR (376 MHz, CDCl3) δ ppm −43.5 (t, J = 13.7 Hz, 2F); IR (thin film) ν 2946, 1728, 1614, 1200, 1147, 1071, 910, 849 cm−1; MS (EI): m/z (%) 376 ([M + 2]+, 19.9), 374 (M+, 21.7), 176 (86.1), 148 (100); HRMS calculated for C16H17BrF2O3: 374.0329; found: 374.0327.
2-(6-Bromo-6,6-difluorohexyl)isoindoline-1,3-dione (4q).
Colorless liquid. 1H NMR (400 MHz, CDCl3) δ ppm 7.82–7.80 (m, 2H), 7.70–7.67 (m, 2H), 3.67 (t, J = 7.2 Hz, 2H), 2.36–2.25 (m, 2H), 1.72–1.59 (m, 4H), 1.44–1.36 (m, 2H); 13C NMR (100 MHz, CDCl3) δ ppm 168.4, 134.0, 132.1, 123.2, 122.9 (t, J = 303.4 Hz), 44.1 (t, J = 21.2 Hz), 37.6, 28.2, 25.7, 23.5 (t, J = 3.3 Hz); 19F NMR (376 MHz, CDCl3) δ ppm −43.6 (t, J = 13.5 Hz, 2F); IR (thin film) ν 2944, 1773, 1713, 1397, 1056, 915, 720 cm−1; MS (EI): m/z (%) 347 ([M + 2]+, 13.8), 345 ([M]+, 13.6), 266 (19.1), 160 (100); HRMS calculated for C14H14BrF2NO2: 345.0176; found: 345.0178.
Benzyl 4-bromo-4,4-difluorobutanoate (4r).
Colorless liquid. 1H NMR (400 MHz, CDCl3) δ ppm 7.36 (s, 5H), 5.15 (s, 2H), 2.80–2.67 (m, 4H); 13C NMR (100 MHz, CDCl3) δ ppm 170.6, 135.4, 128.7, 128.5, 128.4, 121.6 (t, J = 303.0 Hz), 67.0, 39.5 (t, J = 22.6 Hz), 29.0 (t, J = 3.3 Hz); 19F NMR (376 MHz, CDCl3) δ ppm −45.0 (t, J = 13.0 Hz, 2F); IR (thin film) ν 3032, 2958, 1740, 1172, 1104, 920, 698 cm−1; MS (EI): m/z (%) 294 ([M + 2]+, 11.5), 292 ([M]+, 11.6), 199 (20.3), 108 (90.4), 91 (100); HRMS calculated for C11H11BrF2O2: 291.9910; found: 291.9913.
((3-Bromo-3,3-difluoropropyl)sulfonyl)benzene (4s).
White solid, m.p. 78–79 °C. 1H NMR (400 MHz, CDCl3) δ ppm 7.91 (d, J = 6.8 Hz, 2H), 7.69 (t, J = 7.6 Hz, 1H), 7.59 (t, J = 7.6 Hz, 2H), 3.35–3.31 (m, 2H), 2.85–2.75 (m, 2H); 13C NMR (100 MHz, CDCl3) δ ppm 138.2, 134.5, 129.7, 128.1, 119.7 (t, J = 303.7 Hz), 50.9 (t, J = 3.0 Hz), 37.8 (t, J = 24.5 Hz); 19F NMR (376 MHz, CDCl3) δ ppm −45.2 (t, J = 13.0 Hz, 2F); IR (thin film) ν 3053, 2992, 2915, 1448, 1311, 1291, 1146, 1097, 909, 746, 687, 530 cm−1; MS (EI): m/z (%) 298 (M+, 4.21), 219 (40.3), 77 (100); HRMS calculated for C9H9BrF2O2S: 297.9475; found: 297.9480.
4-Bromo-4,4-difluoro-2-phenylbutanoic acid (4t).
White solid, m.p. 64–66 °C. 1H NMR (400 MHz, CDCl3) δ ppm 10.9 (s, 1H), 7.38–7.30 (m, 5H), 4.01–3.98 (m, 1H), 3.48–3.35 (m, 1H), 2.84–2.73 (m, 1H); 13C NMR (100 MHz, CDCl3) δ ppm 178.1, 136.3, 129.2, 128.4, 127.8, 120.8 (t, J = 304.1 Hz), 47.0 (t, J = 21.1 Hz), 46.8; 19F NMR (376 MHz, CDCl3) δ ppm −43.4–(–44.8) (m, 2F); IR (thin film) ν 3034, 2917, 1714, 1216, 1099, 933, 697 cm−1; MS (EI): m/z (%) 280 ([M + 2]+, 63.5), 278 ([M]+, 64.4), 199 (20.3), 171 (100), 169 (92.4); HRMS calculated for C10H9BrF2O2: 277.9754; found: 277.9757.
tert-Butyl 4-(2-bromo-2,2-difluoroethyl)piperidine-1-carboxylate (4u).
Colorless liquid. 1H NMR (400 MHz, CDCl3) δ ppm 4.06 (s, 2H), 2.70 (t, J = 12.2 Hz, 2H), 2.31 (td, J = 15.2, 6.4 Hz, 2H), 1.94–1.84 (m, 1H), 1.76 (d, J = 13.2 Hz, 2H), 1.43 (s, 9H), 1.25–1.15 (m, 2H); 13C NMR (100 MHz, CDCl3) δ ppm 154.7, 122.4 (t, J = 304.9 Hz), 79.5, 50.4 (t, J = 20.5 Hz), 43.6, 32.6 (t, J = 1.9 Hz), 31.9, 28.4; 19F NMR (376 MHz, CDCl3) δ ppm −40.9–(−41.1) (m, 2F); IR (thin film) ν 2976, 2926, 1694, 1423, 1173, 965, 915 cm−1; MS (EI): m/z (%) 329 ([M + 2]+, 2.70), 327 ([M]+, 2.73), 192 (29.1), 57 (100); HRMS calculated for C12H20BrF2NO2: 327.0645; found: 327.0641.
2-(Bromodifluoromethyl)-1,4-dioxane (4v).
Colorless liquid. 1H NMR (400 MHz, CDCl3) δ ppm 4.01–3.91 (m, 3H), 3.81 (td, J = 11.4, 2.8 Hz, 1H), 3.75–3.71 (m, 1H), 3.66 (dd, J = 12.0, 2.8 Hz, 1H), 3.63–3.57 (m, 1H); 13C NMR (100 MHz, CDCl3) δ ppm 120.1 (t, J = 304.8 Hz), 78.0 (t, J = 25.2 Hz), 66.9, 66.1, 65.8 (t, J = 2.6 Hz); 19F NMR (376 MHz, CDCl3) δ ppm −56.5–(−58.6) (m, 2F); IR (thin film) ν 2975, 2921, 2866, 1726, 1453, 1121, 1048, 951, 902, 793, 698 cm−1; MS (EI): m/z (%) 218 ([M + 2]+, 25.0), 216 ([M]+, 24.7), 87 (100), 77 (51.1); HRMS calculated for C5H7BrF2O2: 215.9597; found: 215.9605.
(S)-5-Bromo-5,5-difluoropentyl-2-((tert-butoxycarbonyl)amino)-3-phenylpropanoate (4w).
Colorless liquid. 1H NMR (400 MHz, CDCl3) δ ppm 7.30–7.21 (m, 3H), 7.12 (d, J = 6.8 Hz, 2H), 4.96 (d, J = 8.4 Hz, 1H), 4.55 (q, J = 6.8 Hz, 1H), 4.13–4.03 (m, 2H), 3.05 (d, J = 6.4 Hz, 2H), 2.37–2.26 (m, 2H), 1.68–1.54 (m, 4H), 1.40 (s, 9H); 13C NMR (100 MHz, CDCl3) δ ppm 172.0, 155.1, 136.0, 129.3, 128.6, 127.1, 122.7 (t, J = 303.0 Hz), 78.0, 64.5, 54.5, 43.7 (t, J = 21.5 Hz), 38.6, 28.3, 27.3, 20.6 (t, J = 3.6 Hz); 19F NMR (376 MHz, CDCl3) δ ppm −43.7 (t, J = 13.7 Hz, 2F); IR (thin film) ν 3062, 2926, 2854, 2787, 1658, 1598, 1322, 1127, 988, 761 cm−1; MS (EI): m/z (%) 449 (M+, 0.15), 332 (38.1), 57 (100); HRMS calculated for C19H26BrF2NO4: 449.1013; found: 449.1010.
5-(3-Bromo-3,3-difluoropropyl)-3-(3,5-dichlorophenyl)-5-methyloxazolidine-2,4-dione (4x).
White solid, m.p. 119–120 °C. 1H NMR (400 MHz, CDCl3) δ ppm 7.44–7.42 (m, 3H), 2.66–2.54 (m, 1H), 2.49–2.35 (m, 1H), 2.31–2.23 (m, 2H), 1.70 (s, 3H); 13C NMR (100 MHz, CDCl3) δ ppm 172.7, 151.8, 135.7, 132.3, 129.3, 123.7, 120.9 (t, J = 303.4 Hz), 84.0, 38.2 (t, J = 23.4 Hz), 31.2 (t, J = 3.3 Hz), 22.3; 19F NMR (376 MHz, CDCl3) δ ppm −44.4–(−45.4) (m, 2F); IR (thin film) ν 3092, 2917, 1821, 1748, 1578, 1452, 1391, 1180, 923, 807 cm−1; MS (EI): m/z (%) 419 ([M + 4]+, 16.5), 417 ([M + 2]+, 39.2), 415 (M+, 25.3), 264 (100); HRMS calculated for C13H10BrCl2F2NO3: 414.9189; found: 414.9192.
(2R,6aS,12aS)-2-((R)-4-Bromo-4,4-difluorobutan-2-yl)-8,9-dimethoxy-1,2,12,12a-tetrahydrochromeno[3,4-b]furo[2,3-h]chromen-6(6aH)-one (4y).
Yellow liquid. 1H NMR (400 MHz, CDCl3) δ ppm 7.82 (d, J = 8.4 Hz, 1H), 6.74 (s, 1H), 6.46–6.43 (m, 2H), 4.92 (s, 1H), 4.85–4.58 (m, 2H), 4.17 (d, J = 12.4 Hz, 3H), 3.83 (s, 1H), 3.79 (s, 3H), 3.75 (s, 3H), 3.26–3.18 (m, 1H), 2.89–2.83 (m, 1H), 2.77–2.57 (m, 1H), 2.40–2.19 (m, 2H), 1.12–1.07 (m, 3H); 13C NMR (100 MHz, CDCl3) δ ppm 118.91, 118.89, 167.3, 167.0, 157.93, 157.90, 149.6, 147.4, 143.9, 130.10, 130.08, 122.6 (t, J = 303.5 Hz), 122.5 (t, J = 304.1 Hz), 113.42, 113.38, 112.8, 112.7, 110.4, 104.9, 104.8, 101.0, 88.1, 87.5, 72.3, 66.2, 56.3, 55.9, 46.7 (t, J = 20.8 Hz), 46.3 (t, J = 20.8 Hz), 44.6, 35.15, 35.13, 34.5, 29.8, 29.1, 15.6, 14.2; 19F NMR (376 MHz, CDCl3) δ ppm −39.3–(−43.0) (m, 2F); IR (thin film) ν 2973, 2932, 2857, 1674, 1610, 1513, 1458, 1349, 816 cm−1; MS (EI): m/z (%) 526 ([M + 2]+, 3.76), 524 (M+, 3.79), 445 (1.77), 192 (100), 177 (15.2); HRMS calculated for C24H23BrF2O6: 524.0646; found: 526.0644.
1-Bromo-1,1-difluorooct-2-ene (7a).
Colorless liquid. 1H NMR (400 MHz, CDCl3) δ ppm 6.25–6.17 (m, 1H), 5.89–5.71 (m, 1H), 2.33–2.10 (m, 2H), 1.43 (p, J = 7.3 Hz, 2H), 1.35–1.25 (m, 4H), 0.89 (t, J = 6.6 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ ppm 139.6 (t, J = 5.8 Hz, Z), 137.2 (t, J = 7.0 Hz, E), 126.7 (t, J = 23.0 Hz, E), 126.2 (t, J = 25.2 Hz, Z), 117.2 (t, J = 299.0 Hz, E), 117.0 (t, J = 300.8 Hz, Z), 31.3 (Z), 31.2 (E), 31.1 (E), 28.4 (Z), 28.1 (t, J = 1.8 Hz, Z), 27.7 (E), 22.3 (E, Z), 13.9 (E, Z); 19F NMR (376 MHz, CDCl3) δ ppm −38.4 (d, J = 10.9 Hz, 2F, Z), −43.8 (d, J = 9.4 Hz, 2F, E); IR (thin film) ν 2922, 2851, 1735, 1465, 1026 cm−1; MS (EI): m/z (%) 227 ([M + 2]+, 37.5), 225 (M+, 39.5), 145 (100), 103 (90.5); HRMS calculated for C8H12BrF2: 225.0090; found: 225.0085.
(5-Bromo-5,5-difluoropent-3-en-1-yl)benzene (7b).
Colorless liquid. 1H NMR (400 MHz, CDCl3) δ ppm 7.35–7.18 (m, 5H), 6.31–6.24 (m, 1H), 5.94–5.76 (m, 1H), 2.78 (t, J = 7.6 Hz, 2H), 2.72–2.45 (m, 2H); 13C NMR (100 MHz, CDCl3) δ ppm 140.7 (Z), 140.5 (E), 138.0 (t, J = 5.9 Hz, Z), 136.0 (t, J = 7.3 Hz, E), 128.58 (E), 128.56 (Z), 128.5 (Z), 128.4 (E), 127.4 (t, J = 23.3 Hz, E), 126.9 (t, J = 24.8 Hz, Z), 117.0 (t, J = 299.4 Hz, E), 116.8 (t, J = 300.8 Hz, Z), 34.8 (Z), 34.4 (E), 32.9 (E), 29.8 (Z); 19F NMR (376 MHz, CDCl3) δ ppm −38.8 (d, J = 12.4 Hz, 2F, Z), −44.1 (d, J = 10.9 Hz, 2F, E); IR (thin film) ν 3028, 2928, 1667, 1497, 1454, 1230, 1103, 1076, 922, 746, 698 cm−1; MS (EI): m/z (%) 262 ([M + 2]+, 1.42), 260 (M+, 1.68), 181 (22.2), 91 (100); HRMS calculated for C11H11BrF2: 262.0012; found: 262.0014.
(4-Bromo-4,4-difluorobut-2-en-1-yl)cyclohexane (7c).
Colorless liquid. 1H NMR (400 MHz, CDCl3) δ ppm 6.22–6.14 (m, 1H), 5.89–5.73 (m, 1H), 2.24–2.20 (m, 1H), 2.04–1.99 (m, 1H), 1.71–1.62 (m, 5H), 1.42–1.34 (m, 1H), 1.28–1.10 (m, 3H), 1.00–0.86 (m, 2H); 13C NMR (100 MHz, CDCl3) δ ppm 138.4 (t, J = 5.8 Hz, Z), 135.9 (t, J = 7.3 Hz, E), 127.7 (t, J = 23.0 Hz, E), 126.7 (t, J = 24.4 Hz, Z), 117.0 (t, J = 299.4 Hz, E, Z), 39.1 (E), 37.9 (Z), 37.3 (E), 35.7 (Z), 33.0 (E, Z), 26.3 (E, Z), 26.22 (Z), 26.16 (E); 19F NMR (376 MHz, CDCl3) δ ppm −38.2 (d, J = 12.4 Hz, 2F, Z), −43.8 (d, J = 9.4 Hz, 2F, E); IR (thin film) ν 2924, 2853, 1741, 1449, 1171, 922 cm−1; MS (EI): m/z (%) 173 ([M − Br]+, 11.0), 90 (49.7), 83 (100); HRMS calculated for C10H15BrF2: 252.0325; found: 252.0320.
8-Bromo-8,8-difluorooct-6-en-1-ol (7d).
Colorless liquid. 1H NMR (400 MHz, CDCl3) δ ppm 6.23–6.17 (m, 1H), 5.90–5.72 (m, 1H), 3.64 (t, J = 6.4 Hz, 2H), 2.37–2.12 (m, 2H), 1.61–1.54 (m, 3H), 1.51–1.36 (m, 4H); 13C NMR (100 MHz, CDCl3) δ ppm 139.1 (t, J = 5.9 Hz, Z), 136.8 (t, J = 7.3 Hz, E), 126.9 (t, J = 23.0 Hz, E), 126.5 (t, J = 23.7 Hz, Z), 117.1 (t, J = 299.0 Hz, E), 116.9 (t, J = 301.2 Hz, Z), 62.8 (Z), 62.7 (E), 32.4 (E), 32.2 (Z), 31.1 (E), 28.5 (Z), 28.2 (Z), 27.8 (E), 25.2 (E), 24.9 (Z); 19F NMR (376 MHz, CDCl3) δ ppm −38.5 (d, J = 12.4 Hz, 2F, Z), −43.8 (d, J = 8.3 Hz, 2F, E); IR (thin film) ν 3310 (w), 2936, 2862, 1668, 1230, 1075, 921, 737, 634 cm−1; MS (EI): m/z (%) 163 ([M − Br]+, 2.32), 145 (19.6), 103 (100); HRMS calculated for C8H11BrF2 [M − H2O]: 224.0012; found: 224.0013.
3-(2-Bromo-2,2-difluoroethyl)-4-methyl-1-tosylpyrrolidine (14).
Colorless liquid. 1H NMR (400 MHz, CDCl3) δ ppm 7.69 (d, J = 8.4 Hz, 2H), 7.32 (d, J = 8.0 Hz, 2H), 3.66–3.32 (m, 2H), 3.06–2.74 (m, 2H), 2.59–1.71 (m, 7H), 0.73 (d, J = 7.2 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ ppm 143.65, 143.61, 133.9, 133.7, 129.8, 127.5, 127.4, 121.9 (t, J = 303.7 Hz), 121.6 (t, J = 303.4 Hz), 54.4, 53.8, 53.1 (t, J = 2.2 Hz), 50.4, 46.4 (t, J = 21.9 Hz), 42.9 (t, J = 21.9 Hz), 41.40, 41.38, 38.6, 37.4 (t, J = 2.2 Hz), 35.3, 21.5, 15.6, 13.4; 19F NMR (376 MHz, CDCl3) δ ppm −42.1–(−44.8) (m, 2F); IR (thin film) ν 2959, 2929, 1598, 1346, 1222, 1094, 1051, 929, 665, 592, 548 cm−1; MS (EI): m/z (%) 383 ([M + 2]+, 31.4), 381 (M+, 31.7), 228 (97.1), 226 (100), 91 (95.8); HRMS calculated for C14H18BrF2NO2S: 381.0210; found: 381.0208.
[2-D]-((3-bromo-3,3-difluoropropyl)sulfonyl)benzene ([D]4S).
White solid, m.p. 85–87 °C. 1H NMR (400 MHz, CDCl3) δ ppm 7.91 (d, J = 6.8 Hz, 2H), 7.71–7.68 (m, 1H), 7.61–7.58 (m, 2H), 3.31–3.29 (m, 1H), 2.84–2.75 (m, 2H); 13C NMR (100 MHz, CDCl3) δ ppm 138.2, 134.5, 129.7, 128.1, 119.7 (t, J = 303.4 Hz), 50.9–50.4 (m), 37.8 (t, J = 24.1 Hz); 19F NMR (376 MHz, CDCl3) δ ppm −45.2 (t, J = 12.2 Hz, 2F); IR (thin film) ν 3059, 1448, 1308, 1254, 1088, 1021, 734, 527 cm−1; MS (EI): m/z (%) 301 ([M + 2]+, 10.2), 299 (M+, 10.2), 220 (78.7), 77 (100); HRMS calculated for C9H8DBrF2O2S: 298.9537; found: 298.9541.
(5,5-Difluoropentyl)benzene (8).
A mixture of 4a (0.2 mmol, 52.4 mg, 1.0 equiv.), activated zinc powder (1.0 mmol, 65 mg, 5.0 equiv.) and 0.1 mL HCl (2 M in water) in 2 mL DMF was stirred at 60 °C for 20 h and monitored by TLC. After the mixture was cooled to room temperature, saturated NaCl aqueous solution (10 mL) was added. Then the mixture was extracted with diethyl ether (3 × 5 mL). The combined organic extracts were dried over anhydrous MgSO4 and evaporated under reduced pressure. The crude product was purified by chromatography on silica gel (hexane) to afford compound 8 (colorless liquid, 22.3 mg, 60% yield). 1H NMR (400 MHz, CDCl3) δ ppm 7.30–7.11 (m, 5H), 5.78 (tt, J = 57.0, 4.6 Hz, 1H), 2.63 (t, J = 7.8 Hz, 2H), 1.91–1.77 (m, 2H), 1.72–1.64 (m, 2H), 1.53–1.45 (m, 2H); 13C NMR (100 MHz, CDCl3) δ ppm 142.0, 128.4, 125.9, 117.4 (t, J = 237.4 Hz), 35.7, 34.0 (t, J = 20.4 Hz), 30.9, 21.8 (t, J = 2.2 Hz); 19F NMR (376 MHz, CDCl3) δ ppm −115.8 (dt, J = 57.2, 17.7 Hz, 2F).
(5,5-Difluorooct-7-en-1-yl)benzene (9).
To a mixture of 4a (0.2 mmol, 52.4 mg, 1.0 equiv.) and allyltributyltin (1.0 mL, 9.8 mmol) in toluene (1 mL) was added catalytic amounts of AIBN several times at 90 °C under an argon atmosphere. After 2 h, saturated KF aq. and AcOEt (10 mL) was added to the reaction mixture and stirred at room temperature for 1 h. The organic layer was filtered and dried over anhydrous MgSO4, then concentrated under reduced pressure. The crude product was purified by column chromatography on silica gel (hexane) to afford the desired product 9 (colorless liquid, 24.2 mg, 53%). 1H NMR (400 MHz, CDCl3) δ ppm 7.29–7.25 (m, 2H), 7.19–7.15 (m, 3H), 5.82–5.72 (m, 1H), 5.19 (d, J = 4.0 Hz, 1H), 5.16 (d, J = 12.0 Hz, 1H), 2.61 (t, J = 7.2 Hz, 2H), 2.57 (td, J = 16.4, 7.2 Hz, 2H), 1.89–1.76 (m, 2H), 1.68–1.61 (m, 2H), 1.55–1.49 (m, 2H); 13C NMR (100 MHz, CDCl3) δ ppm 142.2, 129.8, 128.4, 128.3, 125.8, 124.2 (t, J = 239.9 Hz), 120.0, 41.2 (t, J = 25.9 Hz), 35.8 (t, J = 25.2 Hz), 35.7, 31.1, 21.8 (t, J = 4.8 Hz); 19F NMR (376 MHz, CDCl3) δ ppm −97.2 (m, J = 17.4 Hz, 2F); IR (thin film) ν 3084, 3027, 2932, 2859, 1646, 1496, 1454, 987, 925, 876, 746, 698 cm−1; MS (EI): m/z (%) 224 ([M]+, 53.7), 117 (26.7), 91 (100); HRMS calculated for C14H18F2: 224.1377; found: 224.1380.
(5,5-Difluorooct-7-en-1-yl)benzene (10).
Compound 4a (0.2 mmol, 52.4 mg, 1.0 equiv.) was added to a solution of TBAF (0.6 mmol, 1.0 equiv.) in dry THF (1 mL) at room temperature under an argon atmosphere. After 2 h, the reaction mixture was concentrated under reduced pressure. The crude product was purified by column chromatography on silica gel (hexane) to afford the desired product 10 (colorless liquid, 32.4 mg, 89%). 1H NMR (400 MHz, CDCl3) δ ppm 7.30 (t, J = 7.4 Hz, 2H), 7.25–7.17 (m, 3H), 4.16 (dtd, J = 25.6, 7.8, 2.4 Hz, 1H), 2.63 (t, J = 7.6 Hz, 2H), 2.05–2.00 (m, 2H), 1.75–1.68 (m, 2H); 13C NMR (100 MHz, CDCl3) δ ppm 156.4 (dd, J = 285.1, 282.9 Hz), 141.9, 128.42, 128.38, 125.9, 77.7 (t, J = 21.2 Hz), 35.1, 31.3 (t, J = 2.6 Hz), 21.8 (d, J = 4.4 Hz); 19F NMR (376 MHz, CDCl3) δ ppm −89.2 (d, J = 47.8 Hz, 1F), −91.5 (dd, J = 47.8, 25.6 Hz, 1F).
(Z)-(5-Fluoropent-4-en-1-yl)benzene (11).
A mixture of 4a (0.2 mmol, 52.4 mg, 1.0 equiv.), CrCl2 (1.2 mmol, 74.2 mg, 6.0 equiv.) and LiI (0.1 mmol, 13.4 mg, 0.5 equiv.) in DMF (1 mL) was stirred at room temperature under an argon atmosphere for 4 h. Then HCl solution (2 M, 1 mL) was added and the mixture was extracted with diethyl ether (3 × 5 mL). The combined organic extracts were dried over anhydrous MgSO4 and evaporated under reduced pressure. The crude product was purified by column chromatography on silica gel (hexane) to afford the desired product 11 (colorless liquid, 30.2 mg, 92%). 1H NMR (400 MHz, CDCl3) δ ppm 7.30–7.15 (m, 5H), 6.49 (ddt, J = 85.6, 4.4, 1.6 Hz, 1H), 4.77 (dtd, J = 43.2, 7.4, 4.8 Hz, 1H), 2.66 (t, J = 10.2 Hz, 2H), 2.20–2.11 (m, 2H), 1.77–1.69 (m, 2H); 13C NMR (100 MHz, CDCl3) δ ppm 147.9 (d, J = 255.2 Hz), 142.2, 128.5, 128.3, 125.8, 110.6 (d, J = 5.1 Hz), 35.4, 31.0, 22.4 (d, J = 5.1 Hz); 19F NMR (376 MHz, CDCl3) δ ppm −130.6 (dd, J = 85.7, 42.1 Hz, 1F); IR (thin film) ν 3027, 2927, 2859, 1672, 1496, 1454, 1030, 744, 699 cm−1; MS (EI): m/z (%) 164 (M+, 47.9), 117 (26.0), 91 (100); HRMS calculated for C11H13F: 164.1001; found: 164.0997.
(Z)-2-Fluoro-1,6-diphenylhex-2-en-1-ol (12).
Benzaldehyde (0.4 mmol, 42.4 mg, 2.0 equiv.) was added dropwise to a mixture of 4a (0.2 mmol, 52.4 mg, 1.0 equiv.), CrCl2 (1.2 mmol, 74.2 mg, 6.0 equiv.) and LiI (0.1 mmol, 13.4 mg, 0.5 equiv.) in DMF (1 mL) at room temperature under an argon atmosphere. The reaction mixture was stirred at room temperature for 4 h. Then H2O (10 mL) was added and the mixture was extracted with diethyl ether (3 × 5 mL). The combined organic extracts were dried over anhydrous MgSO4 and evaporated under reduced pressure. The crude product was purified by column chromatography on silica gel (hexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc = 5
EtOAc = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford the desired product 12 (colorless liquid, 27.5 mg, 51% yield). 1H NMR (400 MHz, CDCl3) δ ppm 7.44–7.25 (m, 7H), 7.20–7.16 (m, 3H), 5.20 (dd, J = 12.8, 4.0 Hz, 1H), 4.16 (dt, J = 37.2, 7.6 Hz, 1H), 2.62 (t, J = 7.6 Hz, 2H), 2.22–2.14 (m, 3H), 1.75–1.67 (m, 2H); 13C NMR (100 MHz, CDCl3) δ ppm 158.8 (d, J = 254.5 Hz), 142.2, 139.7, 128.6, 128.5, 128.3, 126.7, 125.8, 107.3 (d, J = 13.9 Hz), 72.8 (d, J = 32.1 Hz), 35.5, 30.9, 23.1 (d, J = 4.4 Hz); 19F NMR (376 MHz, CDCl3) δ ppm −122.7 (dd, J = 36.5, 12.0 Hz, 1F); IR (thin film) ν 3389 (w), 3027, 2929, 2859, 1707, 1603, 1495, 1453, 1016, 747, 699 cm−1; MS (EI): m/z (%) 270 (M+, 30.1), 107 (100), 91 (92.0); HRMS calculated for C18H19FO4: 270.1420; found: 270.1423.
1) to afford the desired product 12 (colorless liquid, 27.5 mg, 51% yield). 1H NMR (400 MHz, CDCl3) δ ppm 7.44–7.25 (m, 7H), 7.20–7.16 (m, 3H), 5.20 (dd, J = 12.8, 4.0 Hz, 1H), 4.16 (dt, J = 37.2, 7.6 Hz, 1H), 2.62 (t, J = 7.6 Hz, 2H), 2.22–2.14 (m, 3H), 1.75–1.67 (m, 2H); 13C NMR (100 MHz, CDCl3) δ ppm 158.8 (d, J = 254.5 Hz), 142.2, 139.7, 128.6, 128.5, 128.3, 126.7, 125.8, 107.3 (d, J = 13.9 Hz), 72.8 (d, J = 32.1 Hz), 35.5, 30.9, 23.1 (d, J = 4.4 Hz); 19F NMR (376 MHz, CDCl3) δ ppm −122.7 (dd, J = 36.5, 12.0 Hz, 1F); IR (thin film) ν 3389 (w), 3027, 2929, 2859, 1707, 1603, 1495, 1453, 1016, 747, 699 cm−1; MS (EI): m/z (%) 270 (M+, 30.1), 107 (100), 91 (92.0); HRMS calculated for C18H19FO4: 270.1420; found: 270.1423.
Acknowledgements
We thank the National Natural Science Foundation of China (21272036, 21332010, 21421002) and the National Basic Research Program of China (2012CB21600) for financial support.
Notes and references
-
(a) M. Cametti, B. Crousse, P. Metrangolo, R. Milani and G. Resnati, Chem. Soc. Rev., 2012, 41, 31 RSC;
(b) Y. Li, Acc. Chem. Res., 2012, 45, 723 CrossRef CAS PubMed;
(c) R. Berger, G. Resnati, P. Metrangolo, E. Weber and J. Hulliger, Chem. Soc. Rev., 2011, 40, 3496 RSC;
(d) W. K. Hagmann, J. Med. Chem., 2008, 51, 4359 CrossRef CAS PubMed;
(e) D. O'Hagan, Chem. Soc. Rev., 2008, 37, 308 RSC;
(f) S. Purser, P. R. Moore, S. Swallow and V. Gouverneur, Chem. Soc. Rev., 2008, 37, 320 RSC;
(g) K. Muller, C. Faeh and F. Diederich, Science, 2007, 317, 1881 CrossRef PubMed.
-
(a) X. Yang, T. Wu, R. J. Phipps and F. D. Toste, Chem. Rev., 2015, 115, 826 CrossRef CAS PubMed;
(b) X.-H. Xu, K. Matsuzaki and N. Shibata, Chem. Rev., 2015, 115, 731 CrossRef CAS PubMed;
(c) C. Ni, M. Hu and J. Hu, Chem. Rev., 2015, 115, 765 CrossRef CAS PubMed;
(d) X. Liu, C. Xu, M. Wang and Q. Liu, Chem. Rev., 2015, 115, 683 CrossRef CAS PubMed;
(e) B. Lantaño, M. R. Torviso, S. M. Bonesi, S. Barata-Vallejo and A. Postigo, Coord. Chem. Rev., 2015, 285, 76 CrossRef PubMed;
(f) J. Charpentier, N. Früh and A. Togni, Chem. Rev., 2015, 115, 650 CrossRef CAS PubMed;
(g) C. Alonso, E. Martínez de Marigorta, G. Rubiales and F. Palacios, Chem. Rev., 2015, 115, 1847 CrossRef CAS PubMed;
(h) N. Santschi and R. Gilmour, Angew. Chem., Int. Ed., 2014, 53, 11414 CrossRef CAS PubMed;
(i) E. Merino and C. Nevado, Chem. Soc. Rev., 2014, 43, 6598 RSC;
(j) L. Chu and F.-L. Qing, Acc. Chem. Res., 2014, 47, 1513 CrossRef CAS PubMed;
(k) D. L. Browne, Angew. Chem., Int. Ed., 2014, 53, 1482 CrossRef CAS PubMed;
(l) T. Liang, C. N. Neumann and T. Ritter, Angew. Chem., Int. Ed., 2013, 52, 8214 CrossRef CAS PubMed.
-
(a) S. Z. Zhu, C. H. Xing, W. Xu, G. F. Jin and Z. T. Li, Cryst. Growth Des., 2004, 4, 53 CrossRef CAS;
(b) P. Cardillo, E. Corradi, A. Lunghi, S. V. Meille, M. T. Messina, P. Metrangolo and G. Resnati, Tetrahedron, 2000, 56, 5535 CrossRef CAS;
(c) E. Corradi, S. V. Meille, M. T. Messina, P. Metrangolo and G. Resnati, Tetrahedron Lett., 1999, 40, 7519 CrossRef CAS;
(d) A. Farina, S. V. Meille, M. T. Messina, P. Metrangolo, G. Resnati and G. Vecchio, Angew. Chem., Int. Ed., 1999, 38, 2433 CrossRef CAS.
-
(a) Q.-Q. Min, Z. Yin, Z. Feng, W.-H. Guo and X. Zhang, J. Am. Chem. Soc., 2014, 136, 1230 CrossRef CAS PubMed;
(b) B. Gao, Y. Zhao, M. Hu, C. Ni and J. Hu, Chem. – Eur. J., 2014, 20, 7803 CrossRef CAS PubMed;
(c) S. Ou, M. Jiang and J.-T. Liu, Tetrahedron, 2013, 69, 10820 CrossRef CAS PubMed;
(d) H. Martinez, A. Rebeyrol, T. B. Nelms and W. R. Dolbier Jr., J. Fluorine Chem., 2012, 135, 167 CrossRef CAS PubMed.
-
(a) T.-P. Yang, Q. Li, J.-H. Lin and J.-C. Xiao, Chem. Commun., 2014, 50, 1077 RSC;
(b) V. V. Levin, A. A. Zemtsov, M. I. Struchkova and A. D. Dilman, Org. Lett., 2013, 15, 917 CrossRef CAS PubMed;
(c) K. M. Borys, M. D. Korzyński and Z. Ochal, Tetrahedron Lett., 2012, 53, 6606 CrossRef CAS PubMed;
(d) A. Suzuki, M. Mae, H. Amii and K. Uneyama, J. Org. Chem., 2004, 69, 5132 CrossRef CAS PubMed.
-
(a) C. Ehm, F. A. Akkerman and D. Lentz, J. Fluorine Chem., 2010, 131, 1173 CrossRef CAS PubMed;
(b) T. B. Nguyen, A. Martel, R. Dhal and G. Dujardin, Synlett, 2009, 2492 CAS;
(c) Y. Guo, K. Fujiwara, H. Amii and K. Uneyama, J. Org. Chem., 2007, 72, 8523 CrossRef CAS PubMed;
(d) S. Higashiya, W. J. Chung, D. S. Lim, S. C. Ngo, W. H. Kelly IV, P. J. Toscano and J. T. Welch, J. Org. Chem., 2004, 69, 6323 CrossRef CAS PubMed.
-
(a) M.-C. Belhomme, D. Dru, H.-Y. Xiong, D. Cahard, T. Besset, T. Poisson and X. Pannecoucke, Synthesis, 2014, 1859 Search PubMed;
(b) H. Jiang, S. Yuan, Y. Cai, W. Wan, S. Zhu and J. Hao, J. Fluorine Chem., 2012, 133, 167 CrossRef CAS PubMed;
(c) H. Jiang, L. Sun, S. Yuan, W. Lu, W. Wan, S. Zhu and J. Hao, Tetrahedron, 2012, 68, 2858 CrossRef CAS PubMed;
(d) S. N. Tverdomed, J. Kolanowski, E. Lork and G.-V. Röschenthaler, Tetrahedron, 2011, 67, 3887 CrossRef CAS PubMed.
-
(a) B. Pégot, C. Urban, P. Diter and E. Magnier, Eur. J. Org. Chem., 2013, 7800 CrossRef PubMed;
(b) G. Liu, X. Wang, X. Lu, X.-H. Xu, E. Tokunaga and N. Shibata, ChemistryOpen, 2012, 1, 227 CrossRef CAS PubMed;
(c) G. Liu, S. Mori, X. Wang, S. Noritake, E. Tokunaga and N. Shibata, New J. Chem., 2012, 36, 1769 RSC;
(d) C.-P. Zhang, H.-P. Cao, Z.-L. Wang, C.-T. Zhang, Q.-Y. Chen and J.-C. Xiao, Synlett, 2010, 1089 CAS.
-
(a) Y. Zhao, B. Gao and J. Hu, J. Am. Chem. Soc., 2012, 134, 5790 CrossRef CAS PubMed;
(b) M. D. Kosobokov, V. V. Levin, M. I. Struchkova and A. D. Dilman, Org. Lett., 2014, 16, 3784 CrossRef CAS PubMed;
(c) A. V. Tsymbal, M. D. Kosobokov, V. V. Levin, M. I. Struchkova and A. D. Dilman, J. Org. Chem., 2014, 79, 7831 CrossRef CAS PubMed.
- J. Ignatowska and W. Dmowski, J. Fluorine Chem., 2007, 128, 997 CrossRef CAS PubMed.
-
(a) C.-J. Wallentin, J. D. Nguyen, P. Finkbeiner and C. R. J. Stephenson, J. Am. Chem. Soc., 2012, 134, 8875 CrossRef CAS PubMed;
(b) X.-C. Guo and Q.-Y. Chen, J. Fluorine Chem., 1998, 88, 63 CrossRef CAS;
(c) F.-H. Wu, B.-N. Huang, L. Lu and W.-Y. Huang, J. Fluorine Chem., 1996, 80, 91 CrossRef CAS;
(d) C.-M. Hu and J. Chen, J. Fluorine Chem., 1994, 69, 79 CrossRef CAS;
(e) J. Gonzalez, C. J. Foti and S. Elsheimer, J. Org. Chem., 1991, 56, 4322 CrossRef CAS;
(f) S. Elsheimer, D. K. Slattery, M. Michael, J. Weeks and K. Topoleski, J. Org. Chem., 1989, 54, 3992 CrossRef CAS.
-
(a) C.-M. Hu and J. Chen, J. Chem. Soc., Chem. Commun., 1993, 72 RSC;
(b) S.-H. Wu, W.-Z. Liu and X.-K. Jiang, J. Org. Chem., 1994, 59, 854 CrossRef CAS.
- H. Asai and K. Uneyama, Chem. Lett., 1995, 24, 1123 CrossRef.
-
(a) D. A. Nicewicz and T. M. Nguyen, ACS Catal., 2014, 4, 355 CrossRef CAS;
(b) C. K. Prier, D. A. Rankic and D. W. C. MacMillan, Chem. Rev., 2013, 113, 5322 CrossRef CAS PubMed;
(c) J. Xuan and W.-J. Xiao, Angew. Chem., Int. Ed., 2012, 51, 6828 CrossRef CAS PubMed;
(d) J. M. R. Narayanam and C. R. J. Stephenson, Chem. Soc. Rev., 2011, 40, 102 RSC;
(e) T. P. Yoon, M. A. Ischay and J. Du, Nat. Chem., 2010, 2, 527 CrossRef CAS PubMed.
-
(a) P. Xu, A. Abdukader, K. Hu, Y. Cheng and C. Zhu, Chem. Commun., 2014, 50, 2308 RSC;
(b) J. Xie, X. Yuan, A. Abdukader, C. Zhu and J. Ma, Org. Lett., 2014, 16, 1768 CrossRef CAS PubMed;
(c) S. H. Oh, Y. R. Malpani, N. Ha, Y.-S. Jung and S. B. Han, Org. Lett., 2014, 16, 1310 CrossRef CAS PubMed;
(d) T. Koike and M. Akita, Top. Catal., 2014, 57, 967 CrossRef CAS;
(e) N. Iqbal, J. Jung, S. Park and E. J. Cho, Angew. Chem., Int. Ed., 2014, 53, 539 CrossRef CAS PubMed;
(f) G. Dagousset, A. Carboni, E. Magnier and G. Masson, Org. Lett., 2014, 16, 4340 CrossRef CAS PubMed;
(g) Y. Cheng, X. Yuan, H. Jiang, R. Wang, J. Ma, Y. Zhang and S. Yu, Adv. Synth. Catal., 2014, 356, 2859 CrossRef CAS PubMed;
(h) D. Cantillo, O. de Frutos, J. A. Rincón, C. Mateos and C. O. Kappe, Org. Lett., 2014, 16, 896 CrossRef CAS PubMed;
(i) Y. Yasu, T. Koike and M. Akita, Chem. Commun., 2013, 49, 2037 RSC;
(j) Y. Yasu, T. Koike and M. Akita, Org. Lett., 2013, 15, 2136 CrossRef CAS PubMed;
(k) D. J. Wilger, N. J. Gesmundo and D. A. Nicewicz, Chem. Sci., 2013, 4, 3160 RSC;
(l) E. Kim, S. Choi, H. Kim and E. J. Cho, Chem. – Eur. J., 2013, 19, 6209 CrossRef CAS PubMed;
(m) S. Mizuta, S. Verhoog, K. M. Engle, T. Khotavivattana, M. O'Duill, K. Wheelhouse, G. Rassias, M. Médebielle and V. Gouverneur, J. Am. Chem. Soc., 2013, 135, 2505 CrossRef CAS PubMed;
(n) Y. Yasu, T. Koike and M. Akita, Angew. Chem., Int. Ed., 2012, 51, 9567 CrossRef CAS PubMed;
(o) N. Iqbal, S. Choi, E. Ko and E. J. Cho, Tetrahedron Lett., 2012, 53, 2005 CrossRef CAS PubMed;
(p) Y. Ye and M. S. Sanford, J. Am. Chem. Soc., 2012, 134, 9034 CrossRef CAS PubMed;
(q) D. A. Nagib and D. W. C. MacMillan, Nature, 2011, 480, 224 CrossRef CAS PubMed;
(r) D. A. Nagib, M. E. Scott and D. W. C. MacMillan, J. Am. Chem. Soc., 2009, 131, 10875 CrossRef CAS PubMed.
-
(a) X.-J. Tang and W. R. Dolbier, Angew. Chem., Int. Ed., 2015, 54, 4246 CrossRef CAS PubMed;
(b) C. Yu, N. Iqbal, S. Park and E. J. Cho, Chem. Commun., 2014, 50, 12884 RSC;
(c) W. Li, X. Zhu, H. Mao, Z. Tang, Y. Cheng and C. Zhu, Chem. Commun., 2014, 50, 7521 RSC;
(d) L. Wang, X.-J. Wei, W.-L. Jia, J.-J. Zhong, L.-Z. Wu and Q. Liu, Org. Lett., 2014, 16, 5842 CrossRef CAS PubMed;
(e) X. Sun and S. Yu, Org. Lett., 2014, 16, 2938 CrossRef CAS PubMed;
(f) Y.-M. Su, Y. Hou, F. Yin, Y.-M. Xu, Y. Li, X. Zheng and X.-S. Wang, Org. Lett., 2014, 16, 2958 CrossRef CAS PubMed;
(g) J. Jung, E. Kim, Y. You and E. J. Cho, Adv. Synth. Catal., 2014, 356, 2741 CrossRef CAS PubMed;
(h) M. Rueda-Becerril, O. Mahé, M. Drouin, M. B. Majewski, J. G. West, M. O. Wolf, G. M. Sammis and J.-F. Paquin, J. Am. Chem. Soc., 2014, 136, 2637 CrossRef CAS PubMed.
-
(a) Q.-Y. Lin, X.-H. Xu and F.-L. Qing, J. Org. Chem., 2014, 79, 10434 CrossRef CAS PubMed;
(b) Q. Lin, L. Chu and F.-L. Qing, Chin. J. Chem., 2013, 31, 885 CrossRef CAS PubMed.
- D. P. Hari and B. Konig, Chem. Commun., 2014, 50, 6688 RSC.
- The ratios of E/Z isomers of compounds 7a-d were determined by the 19F NMR of isolated products. The E isomers were assigned according to a recent literature: Q.-Q. Min, Z. Yin, Z. Feng, W.-H. Guo and X. Zhang, J. Am. Chem. Soc., 2014, 136, 1230 CrossRef CAS PubMed.
- S. Peng, F.-L. Qing, Y.-Q. Li and C.-M. Hu, J. Org. Chem., 2000, 65, 694 CrossRef CAS.
- A. Suzuki, M. Mae, H. Amii and K. Uneyama, J. Org. Chem., 2004, 69, 5132 CrossRef CAS PubMed.
- P. Tarrant and E. C. Stump, J. Org. Chem., 1964, 29, 1198 CrossRef CAS.
- T. Nihei, S. Yokotani, T. Ishihara and T. Konno, Chem. Commun., 2014, 50, 1543 RSC.
- For the oxidation and reduction potentials of typical photocatalysts, see: M. Reckenthäler and A. G. Griesbeck, Adv. Synth. Catal., 2013, 355, 2727 CrossRef PubMed.
- B. H. Lipshutz, S. Ghorai and W. W. Y. Leong, J. Org. Chem., 2009, 74, 2854 CrossRef CAS PubMed.
- J. E. Enholm, T. Low, D. Cooper and I. Ghivirija, Tetrahedron, 2012, 68, 6920 CrossRef CAS PubMed.
- We would like to thank one of the referees for the comment that green irradiation should be used for exciting eosin Y. Indeed, the green LEDs (λ = 510–525 nm) were better than blue LEDs (λ = 460–470 nm) for this reaction, affording products 4 in slightly higher yields.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed experimental procedures and analytical data for all new compounds. See DOI: 10.1039/c5ob01302j |
|
| This journal is © The Royal Society of Chemistry 2015 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr). This result revealed that the CF2Br radical was involved in this visible light-induced hydrobromodifluoromethylation of alkenes. The reaction of 1s with CF2Br2 in d8-THF exclusively gave the deuterated product [D]4s in 81% yield, which indicated that THF served as the hydrogen atom source (Scheme 6b). What is more, Stern–Volmer studies showed that CF2Br2 exhibited significant fluorescence quenching of eosin Y* (see the ESI†). This result suggested that electron transfer occurred from eosin Y* to CF2Br2 first. On the basis of these experimental results and the literature reports,18 a plausible mechanism for the hydrobromodifluoromethylation is depicted in Scheme 6c. Initially, the excitation of eosin Y with visible light produced the excited state eosin Y*. Then a single electron transfer (SET) from eosin Y* to CF2Br2 generated the CF2Br radical, which was subsequently added to alkenes 1 for the formation of radical intermediate A. Finally, intermediate A abstracted hydrogen from THF to give the desired hydrobromodifluoromethylated product 4 (Path A).15m
1 dr). This result revealed that the CF2Br radical was involved in this visible light-induced hydrobromodifluoromethylation of alkenes. The reaction of 1s with CF2Br2 in d8-THF exclusively gave the deuterated product [D]4s in 81% yield, which indicated that THF served as the hydrogen atom source (Scheme 6b). What is more, Stern–Volmer studies showed that CF2Br2 exhibited significant fluorescence quenching of eosin Y* (see the ESI†). This result suggested that electron transfer occurred from eosin Y* to CF2Br2 first. On the basis of these experimental results and the literature reports,18 a plausible mechanism for the hydrobromodifluoromethylation is depicted in Scheme 6c. Initially, the excitation of eosin Y with visible light produced the excited state eosin Y*. Then a single electron transfer (SET) from eosin Y* to CF2Br2 generated the CF2Br radical, which was subsequently added to alkenes 1 for the formation of radical intermediate A. Finally, intermediate A abstracted hydrogen from THF to give the desired hydrobromodifluoromethylated product 4 (Path A).15m![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc = 5
EtOAc = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford the desired product 12 (colorless liquid, 27.5 mg, 51% yield). 1H NMR (400 MHz, CDCl3) δ ppm 7.44–7.25 (m, 7H), 7.20–7.16 (m, 3H), 5.20 (dd, J = 12.8, 4.0 Hz, 1H), 4.16 (dt, J = 37.2, 7.6 Hz, 1H), 2.62 (t, J = 7.6 Hz, 2H), 2.22–2.14 (m, 3H), 1.75–1.67 (m, 2H); 13C NMR (100 MHz, CDCl3) δ ppm 158.8 (d, J = 254.5 Hz), 142.2, 139.7, 128.6, 128.5, 128.3, 126.7, 125.8, 107.3 (d, J = 13.9 Hz), 72.8 (d, J = 32.1 Hz), 35.5, 30.9, 23.1 (d, J = 4.4 Hz); 19F NMR (376 MHz, CDCl3) δ ppm −122.7 (dd, J = 36.5, 12.0 Hz, 1F); IR (thin film) ν 3389 (w), 3027, 2929, 2859, 1707, 1603, 1495, 1453, 1016, 747, 699 cm−1; MS (EI): m/z (%) 270 (M+, 30.1), 107 (100), 91 (92.0); HRMS calculated for C18H19FO4: 270.1420; found: 270.1423.
1) to afford the desired product 12 (colorless liquid, 27.5 mg, 51% yield). 1H NMR (400 MHz, CDCl3) δ ppm 7.44–7.25 (m, 7H), 7.20–7.16 (m, 3H), 5.20 (dd, J = 12.8, 4.0 Hz, 1H), 4.16 (dt, J = 37.2, 7.6 Hz, 1H), 2.62 (t, J = 7.6 Hz, 2H), 2.22–2.14 (m, 3H), 1.75–1.67 (m, 2H); 13C NMR (100 MHz, CDCl3) δ ppm 158.8 (d, J = 254.5 Hz), 142.2, 139.7, 128.6, 128.5, 128.3, 126.7, 125.8, 107.3 (d, J = 13.9 Hz), 72.8 (d, J = 32.1 Hz), 35.5, 30.9, 23.1 (d, J = 4.4 Hz); 19F NMR (376 MHz, CDCl3) δ ppm −122.7 (dd, J = 36.5, 12.0 Hz, 1F); IR (thin film) ν 3389 (w), 3027, 2929, 2859, 1707, 1603, 1495, 1453, 1016, 747, 699 cm−1; MS (EI): m/z (%) 270 (M+, 30.1), 107 (100), 91 (92.0); HRMS calculated for C18H19FO4: 270.1420; found: 270.1423.