Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Discovery of novel isatin-based sulfonamides with potent and selective inhibition of the tumor-associated carbonic anhydrase isoforms IX and XII
Özlen
Güzel-Akdemir
a,
Atilla
Akdemir
*b,
Nilgün
Karalı
a and
Claudiu T.
Supuran
*c
aIstanbul University, Faculty of Pharmacy, Department of Pharmaceutical Chemistry, 34116 Beyazıt, Istanbul, Turkey
bBezmialem Vakif University, Faculty of Pharmacy, Department of Pharmacology, Vatan Caddesi, 34093, Fatih, Istanbul, Turkey. E-mail: aakdemir@bezmialem.edu.tr
cUniversita degli Studi di Firenze, NEUROFARBA Dept., Sezione di Scienze Farmaceutiche, Via Ugo Schiff 6, 50019, Sesto Fiorentino (Florence), Italy. E-mail: claudiu.supuran@unifi.it
First published on 27th April 2015
Abstract
A series of 2/3/4-[(2-oxo-1,2-dihydro-3H-indol-3-ylidene)amino]benzenesulfonamides, obtained from substituted isatins and 2-, 3- or 4-aminobenzenesulfonamide, showed low nanomolar inhibitory activity against the tumor associated carbonic anhydrase (CA, EC 4.2.1.1) isoforms IX and XII – recently validated antitumor drug targets, being much less effective as inhibitors of the off-target cytosolic isoforms CA I and II.
Introduction
Carbonic anhydrases (CAs, EC 4.2.1.1) are widespread metalloenzymes with catalytic versatility, having as substrates CO2, COS, CS2, cyanamide, carboxylic, phosphoric and thiocarboxylic esters.1–4 Although their main physiological functions are related to the inter-conversion between CO2, bicarbonate and protons, they play crucial functions in pH regulation, electrolyte secretion, biosynthetic reactions, and carcinogenesis, among others.5–8 Of the 15 isoforms described in humans (hCA I–XIV), many are drug targets for pharmacological agents such as diuretics,9 antiglaucoma drugs,10 antiepileptics,11 antiobesity12 and ultimately, antitumor drugs/cancer diagnostic agents.13 The transmembrane isoforms CA IX and XII are overexpressed in hypoxic tumors as a consequence of the HIF-1α (hypoxia inducible factor-1α) activation pathway, and their inhibition by small molecules/antibodies was recently shown to lead to significant antitumor action.13,14 Furthermore, as these enzymes are present in normal tissues in a very small concentration compared to the tumors, they can also be used for imaging hypoxic tumors.15 Recently, a sulfonamide CA inhibitor (CAI) targeting CA IX and XII entered phase I clinical trials for the treatment of advanced metastatic solid tumors.16Results and discussion
Chemistry
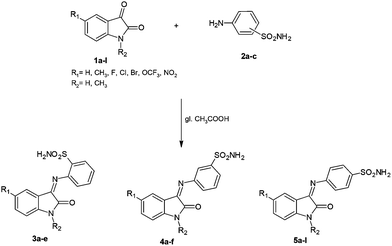
Sulfonamides (RSO2NH2) constitute the most important and investigated class of CAIs.17A large number of structurally diverse sulfonamides were investigated for their CA inhibitory properties.17,18 However the main problem with sulfonamides is their promiscuous behavior as strong inhibitors of many of the 15 CA isoforms of human (h) origin.17,18 As isoforms hCA I and II are widespread and play important physiological functions,1–3 it is of great interest to design inhibitors targeting the tumor-associated isoforms hCA IX and XII, which, at the same time, show weak affinity for the off-target isoforms hCA I and II. Some Schiff bases incorporating sulfonamide moieties were among the first types of CAIs showing selective inhibition of some CA isoforms of interest for medicinal chemistry applications,19,20 and this is the reason why we explore here these types of compounds which incorporate substituted isatin moieties (Scheme 1). Reaction of isatins with aromatic sulfonamides was in fact investigated earlier by our and other groups,21–25 and a limited number of such compounds have been reported. Here we extend the previous studies, reporting a series of 23 such derivatives which incorporate orthanilamide, metanilamide or sulfanilamide moieties, as well as isatin or N-methyl-isatins substituted with methyl, halogens, nitro or trifluoromethyloxy moieties at the heterocyclic ring. We have chosen these substitution patterns at the isatin fragment of the molecule in order to investigate the structure–activity relationship (SAR) for the inhibition of four CA isoforms (hCA I, II, IX and XII) with this class of derivatives.26
Enzyme inhibition data
hCA I was inhibited moderately by the reported compounds irrespective of the substitution pattern, with KIs ranging between 146 and 816 nM. The same was true for the cytosolic dominant isoform hCA II; for which the inhibition constants were in the range of 101–728 nM (Table 1). All KI values were in the lower nanomolar region, in a narrow range, for both tumor-associated hCA isozymes (hCA IX: 1.0–15.6 nM; hCA XII: 2.8–53.8 nM; Table 1). The KI values for the widespread hCA I/II were significantly larger (hCA I: 146–816 nM; hCA II: 101–728 nM) and thus, the new compounds showed a discrete selectivity for the tumor-associated isozymes (Table 1). The difference in KI values is relatively small for the tumor-associated isozymes (hCA IX: ∼15-fold; hCA XII: ∼19-fold) and a conclusive SAR analysis is difficult to perform. Compound 4b drew our attention since it shows very low KI values for the tumor-associated isozymes (hCA IX: 1.1 nM; hCA XII: 3.3 nM) and it shows the highest selectivity for the tumor-associated isozymes compared to the widely distributed hCA I and II (Table 1).| Compounds | K I (nM) | Selectivity ratio | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| # | R1 | R2 | I | II | IX | XII | I/IX | II/IX | I/XII | II/XII |
| 3a | H | H | 816 | 728 | 9.3 | 53.8 | 88 | 78 | 15 | 14 |
| 3b | CH3 | H | 600 | 711 | 4.2 | 49.7 | 143 | 169 | 12 | 14 |
| 3c | F | H | 652 | 428 | 1.2 | 41.3 | 543 | 357 | 16 | 10 |
| 3d | Cl | H | 778 | 652 | 5.7 | 52.5 | 136 | 114 | 15 | 12 |
| 3e | OCF3 | H | 742 | 683 | 10.3 | 53.0 | 72 | 66 | 14 | 13 |
| 4a | H | H | 579 | 618 | 8.4 | 43.9 | 69 | 74 | 13 | 14 |
| 4b | CH3 | H | 510 | 565 | 1.1 | 3.3 | 464 | 514 | 155 | 171 |
| 4c | F | H | 426 | 264 | 1.3 | 30.8 | 328 | 203 | 14 | 9 |
| 4d | Cl | H | 490 | 547 | 4.8 | 41.1 | 102 | 114 | 12 | 13 |
| 4e | OCF3 | H | 539 | 484 | 9.8 | 36.5 | 55 | 49 | 15 | 13 |
| 4f | NO2 | H | 378 | 250 | 5.7 | 3.1 | 66 | 44 | 122 | 81 |
| 5a | H | H | 422 | 523 | 6.5 | 29.3 | 65 | 80 | 14 | 18 |
| 5b | H | CH3 | 586 | 549 | 5.9 | 6.7 | 99 | 93 | 87 | 82 |
| 5c | CH3 | H | 414 | 454 | 1.0 | 18.5 | 414 | 454 | 22 | 25 |
| 5d | CH3 | CH3 | 536 | 491 | 4.1 | 39.7 | 131 | 120 | 14 | 12 |
| 5e | F | H | 375 | 468 | 4.0 | 32.5 | 94 | 117 | 12 | 14 |
| 5f | F | CH3 | 259 | 432 | 1.6 | 36.4 | 162 | 270 | 7 | 12 |
| 5g | Cl | H | 249 | 309 | 3.3 | 25.2 | 75 | 94 | 10 | 12 |
| 5h | Cl | CH3 | 368 | 462 | 4.7 | 44.6 | 78 | 98 | 8 | 10 |
| 5i | Br | CH3 | 457 | 514 | 15.6 | 37.8 | 29 | 33 | 12 | 14 |
| 5j | OCF3 | H | 295 | 236 | 3.9 | 3.8 | 76 | 61 | 78 | 62 |
| 5k | NO2 | H | 229 | 164 | 4.9 | 2.8 | 47 | 33 | 82 | 59 |
| 5l | NO2 | CH3 | 146 | 101 | 4.2 | 4.3 | 35 | 24 | 34 | 23 |
| AZ | — | — | 250 | 12 | 25.0 | 5.7 | 10 | 0 | 44 | 2 |
Molecular modelling studies
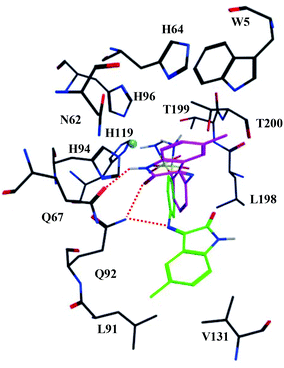
Compound 4b has one of the lowest measured KI values for hCA IX and shows the highest selectivity towards hCA IX compared to the other isozymes (Table 1). Molecular modelling studies were applied to suggest a rationale for this selectivity. Available crystal structures of hCA isozymes with sulfonamide-containing ligands such as acetazolamide bound to their active site indicate that the sulfonamide moiety is oriented in a very similar way to the Zn2+-ion of the hCA active sites. The nitrogen atom of the SO2NH− group is coordinated to the Zn2+-ion and forms a hydrogen bond with the side-chain of Thr199, whereas one of the sulfonamide oxygen atoms forms a hydrogen bond with the backbone NH of the same residue. A similar orientation and binding-interactions were enforced upon the ligands in our docking studies.Compound 5c is very similar to 4b, except for the fact that the sulfonamide is substituted on the para position instead of the meta position. This reorients the isatin fragment to form hydrogen bonds with Gln92 via the imine group between the 2-indolinone and the phenyl ring (Fig. 1). In addition, hydrophobic interactions were observed between the isatin moiety and the sidechain of Val131. The analogs with a sulfonamide in the para position (compound series 5) showed a similar docked pose. Their range of KI values is 1.0–15.6 nM and the varying substituents do not form additional interactions with the active site, as they point towards the solvent.
Experimental
Synthetic procedures
Melting points were estimated with a Buchi 540 melting point apparatus in open capillaries and are uncorrected. Elemental analyses were performed on a Thermo Finnigan Flash EA 1112 elemental analyzer. IR spectra were recorded on KBr discs, using a Perkin-Elmer Model 1600 FT-IR spectrometer. 1H-NMR, D2O-exch., HSQC and HMBC spectra were obtained on VarianUNITY INOVA 500 and Bruker Avance DPX 400 spectrophotometers using DMSO-d6.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1337, 1151 (S
O), 1337, 1151 (S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1H-NMR (DMSO-d6, 400 MHz) δ (ppm): 6.68–8.25 (10H, m, Ar–H, SO2NH2), 10.58, 10.96 (1H, 2s, indole NH). Anal. Calcd for C14H11N3O3S (301.32): C, 55.80, H, 3.68; N, 13.95; S, 10.64. Found: C, 55.51; H, 3.94; N, 13.84; S, 10.50.
O); 1H-NMR (DMSO-d6, 400 MHz) δ (ppm): 6.68–8.25 (10H, m, Ar–H, SO2NH2), 10.58, 10.96 (1H, 2s, indole NH). Anal. Calcd for C14H11N3O3S (301.32): C, 55.80, H, 3.68; N, 13.95; S, 10.64. Found: C, 55.51; H, 3.94; N, 13.84; S, 10.50.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1327, 1160 (S
O), 1327, 1160 (S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1H-NMR (DMSO-d6, 500 MHz) δ (ppm): 1.94, 2.29 (3H, 2s, 5-CH3), 6.09–7.65 (6H, m, Ar–H), 6.94, 7.03 (2H, 2s, SO2NH2), 7.80, 7.95 (1H, 2d, J = 7.81 Hz, phenyl C6-H), 10.68, 10.86 (1H, 2s, indole NH). Anal. Calcd for C15H13N3O3S (315.34): C, 57.13, H, 4.16; N, 13.33; S, 10.17. Found: C, 57.55; H, 4.44; N, 13.14; S, 9.90.
O); 1H-NMR (DMSO-d6, 500 MHz) δ (ppm): 1.94, 2.29 (3H, 2s, 5-CH3), 6.09–7.65 (6H, m, Ar–H), 6.94, 7.03 (2H, 2s, SO2NH2), 7.80, 7.95 (1H, 2d, J = 7.81 Hz, phenyl C6-H), 10.68, 10.86 (1H, 2s, indole NH). Anal. Calcd for C15H13N3O3S (315.34): C, 57.13, H, 4.16; N, 13.33; S, 10.17. Found: C, 57.55; H, 4.44; N, 13.14; S, 9.90.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1338, 1168 (S
O), 1338, 1168 (S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1H-NMR (DMSO-d6, 500 MHz) δ (ppm): 6.81 (1H, d, J = 7.32 Hz, phenyl C3–H), 6.83 (1H, t, J = 7.81 Hz, phenyl C5–H), 6.86 (1H, dd, J = 8.29, 4.39 Hz, indole C7–H), 7.15 (1H, dt, J = 9.26, 2.93 Hz, indole C6–H), 7.32–7.36 (2H, m, indole C4–H, phenyl C4–H), 7.51 (1H, dd, J = 7.81, 0.98 Hz, phenyl C6–H), 7.64, 8.36 (2H, 2s, SO2NH2, D2O exch.), 10.64 (1H, s, indole NH, D2O exch.). HSQC (DMSO-d6) δ (ppm): 111.73 (d, J = 7.66 Hz, indole C7), 114.36 (d, J = 25.87 Hz, indole C4), 117.07 (phenyl C5), 117.53 (d, J = 23.48 Hz, indole C6), 118.29 (phenyl C3), 123.42 (phenyl C6), 123.95 (phenyl C1), 130.98 (d, J = 8.15 Hz, indole C3a), 133.81 (phenyl C4), 138.10 (indole C7a), 138.11 (indole C3), 143.81 (phenyl C2), 158.50 (d, J = 237.71, indole C5), 174.00 (indole C2). Anal. Calcd for C14H10FN3O3S (319.31): C, 52.66, H, 3.16; N, 13.16; S, 10.04. Found: C, 52.52; H, 3.13; N, 13.12; S, 10.11.
O); 1H-NMR (DMSO-d6, 500 MHz) δ (ppm): 6.81 (1H, d, J = 7.32 Hz, phenyl C3–H), 6.83 (1H, t, J = 7.81 Hz, phenyl C5–H), 6.86 (1H, dd, J = 8.29, 4.39 Hz, indole C7–H), 7.15 (1H, dt, J = 9.26, 2.93 Hz, indole C6–H), 7.32–7.36 (2H, m, indole C4–H, phenyl C4–H), 7.51 (1H, dd, J = 7.81, 0.98 Hz, phenyl C6–H), 7.64, 8.36 (2H, 2s, SO2NH2, D2O exch.), 10.64 (1H, s, indole NH, D2O exch.). HSQC (DMSO-d6) δ (ppm): 111.73 (d, J = 7.66 Hz, indole C7), 114.36 (d, J = 25.87 Hz, indole C4), 117.07 (phenyl C5), 117.53 (d, J = 23.48 Hz, indole C6), 118.29 (phenyl C3), 123.42 (phenyl C6), 123.95 (phenyl C1), 130.98 (d, J = 8.15 Hz, indole C3a), 133.81 (phenyl C4), 138.10 (indole C7a), 138.11 (indole C3), 143.81 (phenyl C2), 158.50 (d, J = 237.71, indole C5), 174.00 (indole C2). Anal. Calcd for C14H10FN3O3S (319.31): C, 52.66, H, 3.16; N, 13.16; S, 10.04. Found: C, 52.52; H, 3.13; N, 13.12; S, 10.11.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1328, 1156 (S
O), 1328, 1156 (S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1H-NMR (DMSO-d6, 500 MHz) δ (ppm): 6.82 (1H, t, J = 7.81 Hz, phenyl C5-H), 6.89 (1H, d, J = 8.29 Hz, indole C7–H), 7.35 (1H, dd, J = 7.81, 1.46 Hz, phenyl C3–H), 7.36 (1H, dd, J = 8.29, 2.44 Hz, indole C6–H), 6.86, 8.41 (2H, 2s, SO2NH2, D2O exch.), 7.52 (1H, dd, J = 7.81, 2.44 Hz, phenyl C4–H), 7.54 (1H, d, J = 2.44 Hz, phenyl C6–H), 7.64 (1H, s, indole C4–H), 10.75 (1H, s, indole NH, D2O exch.). Anal. Calcd for C14H10ClN3O3S (335.76): C, 50.08, H, 3.00; N, 12.51; S, 9.55. Found: C, 49.77; H, 3.12; N, 12.35; S, 9.48.
O); 1H-NMR (DMSO-d6, 500 MHz) δ (ppm): 6.82 (1H, t, J = 7.81 Hz, phenyl C5-H), 6.89 (1H, d, J = 8.29 Hz, indole C7–H), 7.35 (1H, dd, J = 7.81, 1.46 Hz, phenyl C3–H), 7.36 (1H, dd, J = 8.29, 2.44 Hz, indole C6–H), 6.86, 8.41 (2H, 2s, SO2NH2, D2O exch.), 7.52 (1H, dd, J = 7.81, 2.44 Hz, phenyl C4–H), 7.54 (1H, d, J = 2.44 Hz, phenyl C6–H), 7.64 (1H, s, indole C4–H), 10.75 (1H, s, indole NH, D2O exch.). Anal. Calcd for C14H10ClN3O3S (335.76): C, 50.08, H, 3.00; N, 12.51; S, 9.55. Found: C, 49.77; H, 3.12; N, 12.35; S, 9.48.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1339, 1163 (S
O), 1339, 1163 (S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1H-NMR (DMSO-d6, 500 MHz) δ (ppm): 6.82 (1H, d, J = 8.29 Hz, indole C7–H), 6.86 (1H, d, J = 7.81 Hz, phenyl C3–H), 6.95 (1H, d, J = 8.29 Hz, indole C6–H), 7.32 (1H, dd, J = 8.29, 1.95 Hz, indole C4–H), 7.36 (1H, dd, J = 7.81, 1.46 Hz, phenyl C5–H), 7.52 (2H, dd, J = 7.81, 1.46 Hz, phenyl C4,6–H), 7.66, 8.46 (2H, 2s, SO2NH2), 10.81 (1H, s, indole NH). HMBC (DMSO-d6) δ (ppm): 111.86 (indole C7), 117.10 (phenyl C5), 118.41 (indole C4), 119.49 (OCF3), 120.57 (indole C6), 123.37 (phenyl C3), 124.07 (phenyl C6), 124.46 (phenyl C1), 130.99 (indole C3a), 132.69 (indole C5), 133.86 (phenyl C4), 141.09 (indole C3), 143.73 (phenyl C2), 143.85 (indole C7a), 174.05 (indole C2). Anal. Calcd for C15H10F3N3O4S (385.32): C, 46.76, H, 2.62; N, 10.91; S, 8.32. Found: C, 46.44; H, 2.84; N, 10.56; S, 8.32.
O); 1H-NMR (DMSO-d6, 500 MHz) δ (ppm): 6.82 (1H, d, J = 8.29 Hz, indole C7–H), 6.86 (1H, d, J = 7.81 Hz, phenyl C3–H), 6.95 (1H, d, J = 8.29 Hz, indole C6–H), 7.32 (1H, dd, J = 8.29, 1.95 Hz, indole C4–H), 7.36 (1H, dd, J = 7.81, 1.46 Hz, phenyl C5–H), 7.52 (2H, dd, J = 7.81, 1.46 Hz, phenyl C4,6–H), 7.66, 8.46 (2H, 2s, SO2NH2), 10.81 (1H, s, indole NH). HMBC (DMSO-d6) δ (ppm): 111.86 (indole C7), 117.10 (phenyl C5), 118.41 (indole C4), 119.49 (OCF3), 120.57 (indole C6), 123.37 (phenyl C3), 124.07 (phenyl C6), 124.46 (phenyl C1), 130.99 (indole C3a), 132.69 (indole C5), 133.86 (phenyl C4), 141.09 (indole C3), 143.73 (phenyl C2), 143.85 (indole C7a), 174.05 (indole C2). Anal. Calcd for C15H10F3N3O4S (385.32): C, 46.76, H, 2.62; N, 10.91; S, 8.32. Found: C, 46.44; H, 2.84; N, 10.56; S, 8.32.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1330, 1147 (S
O), 1330, 1147 (S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1H-NMR (DMSO-d6, 500 MHz) δ (ppm): 6.32–7.69 (8H, m, Ar–H), 7.33, 7.41 (2H, 2s, SO2NH2), 10.88, 10.98 (1H, 2s, indole NH). Anal. Calcd for C14H11N3O3S (301.32): C, 55.80, H, 3.68; N, 13.95; S, 10.64. Found: C, 55.89; H, 4.06; N, 13.64; S, 10.65.
O); 1H-NMR (DMSO-d6, 500 MHz) δ (ppm): 6.32–7.69 (8H, m, Ar–H), 7.33, 7.41 (2H, 2s, SO2NH2), 10.88, 10.98 (1H, 2s, indole NH). Anal. Calcd for C14H11N3O3S (301.32): C, 55.80, H, 3.68; N, 13.95; S, 10.64. Found: C, 55.89; H, 4.06; N, 13.64; S, 10.65.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1326, 1154 (S
O), 1326, 1154 (S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1H-NMR (DMSO-d6, 500 MHz) δ (ppm): 1.95, 2.24 (3H, 2s, 5-CH3), 6.16–7.70 (7H, m, Ar–H), 7.33, 7.43 (2H, 2s, SO2NH2), 10.77, 10.88 (1H, 2s, indole NH). Anal. Calcd for C15H13N3O3S (315.34): C, 57.13, H, 4.16; N, 13.33; S, 10.17. Found: C, 56.83; H, 4.14; N, 12.82; S, 10.01.
O); 1H-NMR (DMSO-d6, 500 MHz) δ (ppm): 1.95, 2.24 (3H, 2s, 5-CH3), 6.16–7.70 (7H, m, Ar–H), 7.33, 7.43 (2H, 2s, SO2NH2), 10.77, 10.88 (1H, 2s, indole NH). Anal. Calcd for C15H13N3O3S (315.34): C, 57.13, H, 4.16; N, 13.33; S, 10.17. Found: C, 56.83; H, 4.14; N, 12.82; S, 10.01.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1329, 1156 (S
O), 1329, 1156 (S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1H-NMR (DMSO-d6, 500 MHz) δ (ppm): 5.97–7.33 (4H, m, indole C4,6–H, phenyl C2,4–H), 6.90, 6.92 (1H, 2dd, J = 8.30, 4.39 Hz, indole C7–H), 7.34, 7.44 (2H, 2s, SO2NH2), 7.48, 7.66 (1H, 2t, J = 7.80 Hz, phenyl C5–H), 7.56, 7.71 (1H, 2d, J = 7.80 Hz, phenyl C6–H), 10.90, 11.02 (1H, 2s, indole NH). Anal. Calcd for C14H10FN3O3S (319.31): C, 52.66, H, 3.16; N, 13.16; S, 10.04. Found: C, 52.29; H, 3.58; N, 13.18; S, 10.38.
O); 1H-NMR (DMSO-d6, 500 MHz) δ (ppm): 5.97–7.33 (4H, m, indole C4,6–H, phenyl C2,4–H), 6.90, 6.92 (1H, 2dd, J = 8.30, 4.39 Hz, indole C7–H), 7.34, 7.44 (2H, 2s, SO2NH2), 7.48, 7.66 (1H, 2t, J = 7.80 Hz, phenyl C5–H), 7.56, 7.71 (1H, 2d, J = 7.80 Hz, phenyl C6–H), 10.90, 11.02 (1H, 2s, indole NH). Anal. Calcd for C14H10FN3O3S (319.31): C, 52.66, H, 3.16; N, 13.16; S, 10.04. Found: C, 52.29; H, 3.58; N, 13.18; S, 10.38.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1325, 1148 (S
O), 1325, 1148 (S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1H-NMR (DMSO-d6, 500 MHz) δ (ppm): 6.87–7.73 (7H, m, Ar–H), 7.35, 7.46 (2H, 2s, SO2NH2), 11.10, 11.12 (1H, 2s, indole NH). Anal. Calcd for C14H10ClN3O3S (335.76): C, 50.08, H, 3.00; N, 12.51; S, 9.55. Found: C, 49.96; H, 3.01; N, 12.32; S, 9.79.
O); 1H-NMR (DMSO-d6, 500 MHz) δ (ppm): 6.87–7.73 (7H, m, Ar–H), 7.35, 7.46 (2H, 2s, SO2NH2), 11.10, 11.12 (1H, 2s, indole NH). Anal. Calcd for C14H10ClN3O3S (335.76): C, 50.08, H, 3.00; N, 12.51; S, 9.55. Found: C, 49.96; H, 3.01; N, 12.32; S, 9.79.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1327, 1150 (S
O), 1327, 1150 (S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1H-NMR (DMSO-d6, 500 MHz) δ (ppm): 7.31 (2H, s, SO2NH2), 7.42–7.71 (6H, m, Ar–H), 8.13 (1H, s, indole C4–H), 10.19 (1H, s, indole NH). Anal. Calcd for C15H10F3N3O4S (385.32): C, 46.76, H, 2.62; N, 10.91; S, 8.32. Found: C, 46.97; H, 2.32; N, 10.76; S, 8.27.
O); 1H-NMR (DMSO-d6, 500 MHz) δ (ppm): 7.31 (2H, s, SO2NH2), 7.42–7.71 (6H, m, Ar–H), 8.13 (1H, s, indole C4–H), 10.19 (1H, s, indole NH). Anal. Calcd for C15H10F3N3O4S (385.32): C, 46.76, H, 2.62; N, 10.91; S, 8.32. Found: C, 46.97; H, 2.32; N, 10.76; S, 8.27.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1307, 1154 (S
O), 1307, 1154 (S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). 1H-NMR (DMSO-d6, 400 MHz) δ (ppm): 7.31, 7.42 (2H, 2s, SO2NH2), 6.97–8.65 (7H, m, Ar–H), 10.19, 11.26 (1H, 2s, indole NH). Anal. Calcd for C14H10N4O5S (346.32): C, 48.55, H, 2.91; N, 16.18; S, 9.26. Found: C, 48.74; H, 3.04; N, 16.18; S, 9.46.
O). 1H-NMR (DMSO-d6, 400 MHz) δ (ppm): 7.31, 7.42 (2H, 2s, SO2NH2), 6.97–8.65 (7H, m, Ar–H), 10.19, 11.26 (1H, 2s, indole NH). Anal. Calcd for C14H10N4O5S (346.32): C, 48.55, H, 2.91; N, 16.18; S, 9.26. Found: C, 48.74; H, 3.04; N, 16.18; S, 9.46.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1329, 1144 (S
O), 1329, 1144 (S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1H-NMR (DMSO-d6, 500 MHz) δ (ppm): 6.33, 7.60 (1H, 2d, J = 7.81 Hz, indole C7–H), 6.73, 7.06 (1H, 2t, J = 7.81 Hz, indole C6–H), 6.86, 6.89 (1H, 2d, J = 7.81 Hz, indole C4–H), 7.08, 7.15 (2H, 2d, J = 8.30 Hz, phenyl C3,5–H), 7.28, 7.35 (2H, 2s, SO2NH2), 7.35, 7.46 (1H, 2t, J = 7.81 Hz, indole C5–H), 7.73, 7.89 (2H, 2d, J = 8.30 Hz, phenyl C2,6–H), 10.89, 11.00 (1H, 2s, indole NH). Anal. Calcd for C14H11N3O3S (301.32): C, 55.80; H, 3.68; N, 13.95; S, 10.64. Found: C, 56.05; H, 4.05; N, 13.86; S, 10.53.
O); 1H-NMR (DMSO-d6, 500 MHz) δ (ppm): 6.33, 7.60 (1H, 2d, J = 7.81 Hz, indole C7–H), 6.73, 7.06 (1H, 2t, J = 7.81 Hz, indole C6–H), 6.86, 6.89 (1H, 2d, J = 7.81 Hz, indole C4–H), 7.08, 7.15 (2H, 2d, J = 8.30 Hz, phenyl C3,5–H), 7.28, 7.35 (2H, 2s, SO2NH2), 7.35, 7.46 (1H, 2t, J = 7.81 Hz, indole C5–H), 7.73, 7.89 (2H, 2d, J = 8.30 Hz, phenyl C2,6–H), 10.89, 11.00 (1H, 2s, indole NH). Anal. Calcd for C14H11N3O3S (301.32): C, 55.80; H, 3.68; N, 13.95; S, 10.64. Found: C, 56.05; H, 4.05; N, 13.86; S, 10.53.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1321, 1154 (S
O), 1321, 1154 (S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1H-NMR (DMSO-d6, 500 MHz) δ (ppm): 3.07, 3.19 (3H, 2s, 1–CH3), 6.36, 7.64 (1H, 2d, J = 7.81 Hz, indole C7–H), 6.80–7.16 (4H, m, indole C4,6–H, phenyl C3,5–H), 7.30, 7.36 (2H, 2s, SO2NH2), 7.46, 7.56 (1H, 2t, J = 7.81 Hz, indole C5–H), 7.74, 7.90 (2H, 2d, J = 8.29 Hz, phenyl C2,6–H). Anal. Calcd for C15H13N3O3S (315.34): C, 57.13, H, 4.16; N, 13.33; S, 10.17. Found: C, 57.37; H, 4.57; N, 13.20; S, 10.34.
O); 1H-NMR (DMSO-d6, 500 MHz) δ (ppm): 3.07, 3.19 (3H, 2s, 1–CH3), 6.36, 7.64 (1H, 2d, J = 7.81 Hz, indole C7–H), 6.80–7.16 (4H, m, indole C4,6–H, phenyl C3,5–H), 7.30, 7.36 (2H, 2s, SO2NH2), 7.46, 7.56 (1H, 2t, J = 7.81 Hz, indole C5–H), 7.74, 7.90 (2H, 2d, J = 8.29 Hz, phenyl C2,6–H). Anal. Calcd for C15H13N3O3S (315.34): C, 57.13, H, 4.16; N, 13.33; S, 10.17. Found: C, 57.37; H, 4.57; N, 13.20; S, 10.34.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1320, 1147 (S
O), 1320, 1147 (S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1H-NMR (DMSO-d6, 500 MHz) δ (ppm): 2.24, 2.27 (3H, 2s, 5-CH3), 5.77, 6.11 (1H, 2s, indole C4–H), 6.57, 6.79 (2H, 2d, J = 8.79 Hz, phenyl C3,5–H), 6.85, 7.36 (2H, 2s, SO2NH2, D2O exch.), 7.07, 7.29 (1H, 2d, J = 8.29 Hz, indole C6–H), 7.14, 7.28 (1H, 2d, J = 8.29 Hz, indole C7–H), 7.38, 7.43 (2H, 2d, J = 8.79 Hz, phenyl C2,6–H), 10.78, 10.89 (1H, 2s, indole NH, D2O exch.). Anal. Calcd for C15H13N3O3S (315.34): C, 57.13, H, 4.16; N, 13.33; S, 10.17. Found: C, 56.89; H, 4.14; N, 12.95; S, 10.53.
O); 1H-NMR (DMSO-d6, 500 MHz) δ (ppm): 2.24, 2.27 (3H, 2s, 5-CH3), 5.77, 6.11 (1H, 2s, indole C4–H), 6.57, 6.79 (2H, 2d, J = 8.79 Hz, phenyl C3,5–H), 6.85, 7.36 (2H, 2s, SO2NH2, D2O exch.), 7.07, 7.29 (1H, 2d, J = 8.29 Hz, indole C6–H), 7.14, 7.28 (1H, 2d, J = 8.29 Hz, indole C7–H), 7.38, 7.43 (2H, 2d, J = 8.79 Hz, phenyl C2,6–H), 10.78, 10.89 (1H, 2s, indole NH, D2O exch.). Anal. Calcd for C15H13N3O3S (315.34): C, 57.13, H, 4.16; N, 13.33; S, 10.17. Found: C, 56.89; H, 4.14; N, 12.95; S, 10.53.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1328, 1147 (S
O), 1328, 1147 (S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1H-NMR (DMSO-d6, 500 MHz) δ (ppm): 1.99, 2.31 (3H, 2s, 5-CH3), 3.04, 3.17 (3H, 2s, 1-CH3), 6.14, 7.47 (1H, 2s, indole C4–H), 6.98, 7.07 (1H, 2d, J = 8.30 Hz, indole C6–H), 7.01, 7.14 (2H, 2d, J = 8.30 Hz, phenyl C3,5–H), 7.18, 7.28 (1H, 2d, J = 8.30 Hz, indole C7–H), 7.29, 7.37 (2H, 2s, SO2NH2), 7.73, 7.89 (2H, 2d, J = 8.30 Hz, phenyl C2,6–H). Anal. Calcd for C16H15N3O3S (329.37): C, 58.34, H, 4.59; N, 12.76; S, 9.74. Found: C, 58.01; H, 4.90; N, 12.34; S, 9.65.
O); 1H-NMR (DMSO-d6, 500 MHz) δ (ppm): 1.99, 2.31 (3H, 2s, 5-CH3), 3.04, 3.17 (3H, 2s, 1-CH3), 6.14, 7.47 (1H, 2s, indole C4–H), 6.98, 7.07 (1H, 2d, J = 8.30 Hz, indole C6–H), 7.01, 7.14 (2H, 2d, J = 8.30 Hz, phenyl C3,5–H), 7.18, 7.28 (1H, 2d, J = 8.30 Hz, indole C7–H), 7.29, 7.37 (2H, 2s, SO2NH2), 7.73, 7.89 (2H, 2d, J = 8.30 Hz, phenyl C2,6–H). Anal. Calcd for C16H15N3O3S (329.37): C, 58.34, H, 4.59; N, 12.76; S, 9.74. Found: C, 58.01; H, 4.90; N, 12.34; S, 9.65.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1H-NMR (DMSO-d6, 500 MHz) δ (ppm): 6.08, 7.52 (1H, 2dd, J = 7.81, 4.32 Hz, indole C7–H), 6.95, 6.99 (1H, 2dd, J = 8.78, 2.44 Hz, indole C6–H), 7.18, 7.25 (2H, 2d, J = 8.30 Hz, phenyl C3,5–H), 7.34, 7.40 (1H, 2dd, J = 8.78, 2.44 Hz, indole C4–H), 7.37, 7.46 (2H, 2s, SO2NH2), 7.81, 7.98 (2H, 2d, J = 8.30 Hz, phenyl C2,6–H), 10.97, 11.11 (1H, 2s, indole NH). Anal. Calcd for C14H10FN3O3S (319.31): C, 52.66, H, 3.16; N, 13.16; S, 10.04. Found: C, 52.99; H, 3.17; N, 12.90; S, 9.85.
O); 1H-NMR (DMSO-d6, 500 MHz) δ (ppm): 6.08, 7.52 (1H, 2dd, J = 7.81, 4.32 Hz, indole C7–H), 6.95, 6.99 (1H, 2dd, J = 8.78, 2.44 Hz, indole C6–H), 7.18, 7.25 (2H, 2d, J = 8.30 Hz, phenyl C3,5–H), 7.34, 7.40 (1H, 2dd, J = 8.78, 2.44 Hz, indole C4–H), 7.37, 7.46 (2H, 2s, SO2NH2), 7.81, 7.98 (2H, 2d, J = 8.30 Hz, phenyl C2,6–H), 10.97, 11.11 (1H, 2s, indole NH). Anal. Calcd for C14H10FN3O3S (319.31): C, 52.66, H, 3.16; N, 13.16; S, 10.04. Found: C, 52.99; H, 3.17; N, 12.90; S, 9.85.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1322, 1155 (S
O), 1322, 1155 (S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1H-NMR (DMSO-d6, 500 MHz) δ (ppm): 3.06, 3.19 (3H, 2s, 1-CH3), 6.03–7.52 (5H, m, indole C4,6,7–H, phenyl C3,5), 7.31, 7.40 (2H, 2s, SO2NH2), 7.75, 7.92 (2H, 2d, J = 8.29 Hz, phenyl C2,6–H). Anal. Calcd for C15H12FN3O3S (333.33): C, 54.05, H, 3.63; N, 12.61; S, 9.62. Found: C, 54.06; H, 3.71; N, 12.46; S, 9.70.
O); 1H-NMR (DMSO-d6, 500 MHz) δ (ppm): 3.06, 3.19 (3H, 2s, 1-CH3), 6.03–7.52 (5H, m, indole C4,6,7–H, phenyl C3,5), 7.31, 7.40 (2H, 2s, SO2NH2), 7.75, 7.92 (2H, 2d, J = 8.29 Hz, phenyl C2,6–H). Anal. Calcd for C15H12FN3O3S (333.33): C, 54.05, H, 3.63; N, 12.61; S, 9.62. Found: C, 54.06; H, 3.71; N, 12.46; S, 9.70.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1328, 1151 (S
O), 1328, 1151 (S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1H-NMR (DMSO-d6, 500 MHz) δ (ppm): 6.28–7.50 (3H, m, indole C4,6,7–H), 7.11, 7.17 (2H, 2dd, J = 6.83, 1.95 Hz, phenyl C3,5–H), 7.29, 7.40 (2H, 2s, SO2NH2), 7.74, 7.91 (2H, 2dd, J = 6.83, 1.95 Hz, phenyl C2,6–H), 11.03, 11.14 (1H, 2s, indole NH). Anal. Calcd for C14H10ClN3O3S (335.76): C, 50.08, H, 3.00; N, 12.51; S, 9.55. Found: C, 49.96; H, 3.24; N, 12.48; S, 9.20.
O); 1H-NMR (DMSO-d6, 500 MHz) δ (ppm): 6.28–7.50 (3H, m, indole C4,6,7–H), 7.11, 7.17 (2H, 2dd, J = 6.83, 1.95 Hz, phenyl C3,5–H), 7.29, 7.40 (2H, 2s, SO2NH2), 7.74, 7.91 (2H, 2dd, J = 6.83, 1.95 Hz, phenyl C2,6–H), 11.03, 11.14 (1H, 2s, indole NH). Anal. Calcd for C14H10ClN3O3S (335.76): C, 50.08, H, 3.00; N, 12.51; S, 9.55. Found: C, 49.96; H, 3.24; N, 12.48; S, 9.20.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1332, 1157 (S
O), 1332, 1157 (S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1H-NMR (DMSO-d6, 500 MHz) δ (ppm): 3.07, 3.20 (3H, 2s, 1-CH3), 6.30–7.62 (5H, m, indole C4,6,7–H, phenyl C3,5–H), 7.30, 7.41 (2H, 2s, SO2NH2), 7.75, 7.92 (2H, 2dd, J = 8.78, 1.95 Hz, phenyl C2,6–H). Anal. Calcd for C15H12ClN3O3S (349.79): C, 51.51, H, 3.46; N, 12.01; S, 9.17. Found: C, 49.05; H, 3.93; N, 11.56; S, 9.53.
O); 1H-NMR (DMSO-d6, 500 MHz) δ (ppm): 3.07, 3.20 (3H, 2s, 1-CH3), 6.30–7.62 (5H, m, indole C4,6,7–H, phenyl C3,5–H), 7.30, 7.41 (2H, 2s, SO2NH2), 7.75, 7.92 (2H, 2dd, J = 8.78, 1.95 Hz, phenyl C2,6–H). Anal. Calcd for C15H12ClN3O3S (349.79): C, 51.51, H, 3.46; N, 12.01; S, 9.17. Found: C, 49.05; H, 3.93; N, 11.56; S, 9.53.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1331, 1156 (S
O), 1331, 1156 (S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1H-NMR (DMSO-d6, 500 MHz) δ (ppm): 3.19 (3H, s, 1-CH3), 7.11 (1H, 2dd, J = 8.78, 3.42 Hz, indole C6–H), 7.17 (1H, d, J = 8.78 Hz, indole C7–H), 7.30 (1H, s, indole C4–H), 7.41 (2H, s, SO2NH2), 7.75 (2H, d, J = 8.30 Hz, phenyl C3,5–H), 7.92 (2H, d, J = 8.30 Hz, phenyl C2,6–H). Anal. Calcd for C15H12BrN3O3S (394.24): C, 45.70, H, 3.07; N, 10.66; S, 8.13. Found: C, 45.72; H, 3.24; N, 10.96; S, 8.17.
O); 1H-NMR (DMSO-d6, 500 MHz) δ (ppm): 3.19 (3H, s, 1-CH3), 7.11 (1H, 2dd, J = 8.78, 3.42 Hz, indole C6–H), 7.17 (1H, d, J = 8.78 Hz, indole C7–H), 7.30 (1H, s, indole C4–H), 7.41 (2H, s, SO2NH2), 7.75 (2H, d, J = 8.30 Hz, phenyl C3,5–H), 7.92 (2H, d, J = 8.30 Hz, phenyl C2,6–H). Anal. Calcd for C15H12BrN3O3S (394.24): C, 45.70, H, 3.07; N, 10.66; S, 8.13. Found: C, 45.72; H, 3.24; N, 10.96; S, 8.17.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1331, 1158 (S
O), 1331, 1158 (S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1H-NMR (DMSO-d6, 400 MHz) δ (ppm): 7.19 (2H, s, SO2NH2), 7.65 (4H, s, Ar–H), 6.55–7.89 (3H, m, indole C4,6,7–H), 10.24 (1H, s, indole NH). Anal. Calcd for C15H10F3N3O4S (385.32): C, 46.76, H, 2.62; N, 10.91; S, 8.32. Found: C, 46.66; H, 2.85; N, 10.78; S, 8.50.
O); 1H-NMR (DMSO-d6, 400 MHz) δ (ppm): 7.19 (2H, s, SO2NH2), 7.65 (4H, s, Ar–H), 6.55–7.89 (3H, m, indole C4,6,7–H), 10.24 (1H, s, indole NH). Anal. Calcd for C15H10F3N3O4S (385.32): C, 46.76, H, 2.62; N, 10.91; S, 8.32. Found: C, 46.66; H, 2.85; N, 10.78; S, 8.50.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1335, 1153 (S
O), 1335, 1153 (S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1H-NMR (DMSO-d6, 400 MHz) δ (ppm): 7.32, 7.43 (2H, 2s, SO2NH2), 7.05–8.37 (7H, m, Ar–H), 11.59, 11.70 (1H, 2s, indole NH). Anal. Calcd for C14H10N4O5S (346.32): C, 48.55, H, 2.91; N, 16.18; S, 9.26. Found: C, 48.56; H, 3.28; N, 16.27; S, 9.56.
O); 1H-NMR (DMSO-d6, 400 MHz) δ (ppm): 7.32, 7.43 (2H, 2s, SO2NH2), 7.05–8.37 (7H, m, Ar–H), 11.59, 11.70 (1H, 2s, indole NH). Anal. Calcd for C14H10N4O5S (346.32): C, 48.55, H, 2.91; N, 16.18; S, 9.26. Found: C, 48.56; H, 3.28; N, 16.27; S, 9.56.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1339, 1157 (S
O), 1339, 1157 (S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1H-NMR (DMSO-d6, 500 MHz) δ (ppm): 3.16, 3.20 (3H, 2s, 1-CH3), 7.17–8.47 (9H, m, SO2NH2 and Ar–H). Anal. Calcd for C15H10N4O5S (360.34): C, 50.00, H, 3.36; N, 15.55; S, 8.90. Found: C, 50.48; H, 3.84; N, 15.53; S, 9.03.
O); 1H-NMR (DMSO-d6, 500 MHz) δ (ppm): 3.16, 3.20 (3H, 2s, 1-CH3), 7.17–8.47 (9H, m, SO2NH2 and Ar–H). Anal. Calcd for C15H10N4O5S (360.34): C, 50.00, H, 3.36; N, 15.55; S, 8.90. Found: C, 50.48; H, 3.84; N, 15.53; S, 9.03.
Enzyme inhibition assay
A stopped-flow instrument (SX.18MV-R Applied Photophysics model) was used for assaying the CA-catalyzed CO2 hydration activity.26 Inhibitor and enzyme were preincubated for 15 min for allowing the complete formation of the enzyme–inhibitor adduct. IC50 values were obtained from dose response curves working at seven different concentrations of the test compound (from 0.1 nM to 50 μM), by fitting the curves using PRISM (http://www.graphpad.com) and non-linear least squares methods, the obtained values representing the mean of at least three different determinations. The inhibition constants (KI) were derived from the IC50 values by using the Cheng–Prusoff equation, as follows: KI = IC50/(1 + [S]/Km) where [S] represents the CO2 concentration at which the measurement was carried out, and Km the concentration of the substrate at which the enzyme activity is at half maximal. All enzymes used were recombinant, produced in E. coli as reported earlier.27–30 The concentrations of enzymes used in the assay were: hCA I, 12.4 nM; hCA II, 8.7 nM; hCA IX, 9.2 nM and hCA XII, 10.8 nM.Molecular modelling studies
Conclusions
We report here a panel of 23 new sulfonamides incorporating Schiff base moieties. They were obtained by reactions of variously substituted isatins with 2-, 3- and 4-amino-benzenesulfonamides. These new derivatives were tested as inhibitors of four physiologically relevant CA isoforms, involved in crucial physiological and pathological processes: the house-keeping cytosolic hCA I and II, as well as the transmembrane, tumor-associated hCA IX and XII, validated drug targets for theranostics for the management of hypoxic tumors. The new sulfonamides were moderate–weak hCA I/II inhibitors and highly potent, low nanomolar hCA IX/XII inhibitors. By using docking studies we also explained the differential inhibition of the four CA isoforms and the structural reasons connected with the selective inhibition of the transmembrane over the cytosolic isoforms. As a sulfonamide CA IX/XII inhibitor recently entered Phase I clinical trials for the management of metastatic solid tumors, compounds of the type reported here may be useful for designing different derivatives with such properties.Acknowledgements
This project was in part financed by the Istanbul University Scientific Research Projects Department (Project Number: UDP-51361) and by an FP7 EU project (Dynano).Notes and references
- (a) C. T. Supuran, Nat. Rev. Drug Discovery, 2008, 7, 168 CrossRef CAS PubMed; (b) M. Aggarwal, B. Kondeti and R. McKenna, Expert Opin. Ther. Pat., 2013, 23, 717 CrossRef CAS PubMed; (c) J. Ivanova, J. Leitans, M. Tanc, A. Kazaks, R. Zalubovskis, C. T. Supuran and K. Tars, Chem. Commun., 2015, 51, 7108 RSC.
- (a) V. Alterio, A. Di Fiore, K. D'Ambrosio, C. T. Supuran and G. De Simone, Chem. Rev., 2012, 112, 4421 CrossRef CAS PubMed; (b) D. Neri and C. T. Supuran, Nat. Rev. Drug Discovery, 2011, 10, 767 CrossRef CAS PubMed; (c) C. Capasso and C. T. Supuran, J. Enzyme Inhib. Med. Chem., 2015, 30, 325 CrossRef CAS PubMed.
- (a) C. T. Supuran, J. Enzyme Inhib. Med. Chem., 2013, 28, 229 CrossRef PubMed; (b) F. Briganti, S. Mangani, A. Scozzafava, G. Vernaglione and C. T. Supuran, J. Biol. Inorg. Chem., 1999, 4, 528 CrossRef CAS; (c) G. De Simone and C. T. Supuran, J. Inorg. Biochem., 2012, 111, 117 CrossRef CAS PubMed; (d) K. D'Ambrosio, S. Carradori, S. M. Monti, M. Buonanno, D. Secci, D. Vullo, C. T. Supuran and G. De Simone, Chem. Commun., 2015, 51, 302 RSC.
- (a) M. Tanc, F. Carta, A. Scozzafava and C. T. Supuran, ACS Med. Chem. Lett., 2015, 6, 292 CrossRef CAS PubMed; (b) A. Innocenti, A. Scozzafava, S. Parkkila, L. Puccetti, G. De Simone and C. T. Supuran, Bioorg. Med. Chem. Lett., 2008, 18, 2267 CrossRef CAS PubMed.
- (a) C. T. Supuran, Bioorg. Med. Chem., 2013, 21, 1377 CrossRef CAS PubMed; (b) C. T. Supuran, J. Enzyme Inhib. Med. Chem., 2012, 27, 759 CrossRef CAS PubMed; (c) A. Scozzafava, L. Menabuoni, F. Mincione, G. Mincione and C. T. Supuran, Bioorg. Med. Chem. Lett., 2001, 11, 575–582 CrossRef CAS.
- G. De Simone, V. Alterio and C. T. Supuran, Expert Opin. Drug Discovery, 2013, 8, 793 CrossRef CAS PubMed.
- S. Del Prete, D. Vullo, G. M. Fisher, K. T. Andrews, S. A. Poulsen, C. Capasso and C. T. Supuran, Bioorg. Med. Chem. Lett., 2014, 24, 4389 CrossRef CAS PubMed.
- (a) P. Pan, A. B. Vermelho, G. Capaci Rodrigues, A. Scozzafava, M. E. Tolvanen, S. Parkkila, C. Capasso and C. T. Supuran, J. Med. Chem., 2013, 56, 1761 CrossRef CAS PubMed; (b) L. Syrjanen, A. B. Vermelho, I. de Almeida Rodrigues, S. Corte-Real, T. Salonen, P. Pan, D. Vullo, S. Parkkila, C. Capasso and C. T. Supuran, J. Med. Chem., 2013, 56, 7372 CrossRef CAS PubMed; (c) I. Nishimori, T. Minakuchi, K. Morimoto, S. Sano, S. Onishi, H. Takeuchi, D. Vullo, A. Scozzafava and C. T. Supuran, J. Med. Chem., 2006, 49, 2117 CrossRef CAS PubMed.
- F. Carta and C. T. Supuran, Expert Opin. Ther. Pat., 2013, 23, 681 CrossRef CAS PubMed.
- (a) E. Masini, F. Carta, A. Scozzafava and C. T. Supuran, Expert Opin. Ther. Pat., 2013, 23, 705 CrossRef CAS PubMed; (b) M. Bozdag, M. Ferraroni, F. Carta, D. Vullo, L. Lucarini, E. Orlandini, A. Rossello, E. Nuti, A. Scozzafava, E. Masini and C. T. Supuran, J. Med. Chem., 2014, 57, 9152 CrossRef CAS PubMed; (c) M. Bozdag, M. Pinard, F. Carta, E. Masini, A. Scozzafava, R. McKenna and C. T. Supuran, J. Med. Chem., 2014, 57, 9673 CrossRef CAS PubMed.
- (a) G. De Simone, A. Scozzafava and C. T. Supuran, Chem. Biol. Drug Des., 2009, 74, 317 CrossRef CAS PubMed; (b) C. Temperini, A. Innocenti, A. Scozzafava, S. Parkkila and C. T. Supuran, J. Med. Chem., 2010, 53, 850 CrossRef CAS PubMed; (c) Z. H. Chohan, A. Scozzafava and C. T. Supuran, J. Enzyme Inhib. Med. Chem., 2003, 18, 259 CrossRef CAS PubMed.
- (a) F. Carta, A. Scozzafava and C. T. Supuran, Expert Opin. Ther. Pat., 2012, 22, 747 CrossRef CAS PubMed; (b) C. T. Supuran, Expert Opin. Emerging Drugs, 2012, 17, 11 CrossRef CAS PubMed.
- (a) N. Krall, F. Pretto, W. Decurtins, G. J. L. Bernardes, C. T. Supuran and D. Neri, Angew. Chem., Int. Ed.., 2014, 53, 4231 CrossRef CAS PubMed; (b) P. C. McDonald, J. Y. Winum, C. T. Supuran and S. Dedhar, Oncotarget, 2012, 3, 84 Search PubMed; (c) F. E. Lock, P. C. McDonald, Y. Lou, I. Serrano, S. C. Chafe, C. Ostlund, S. Aparicio, J. Y. Winum, C. T. Supuran and S. Dedhar, Oncogene, 2013, 32, 5210 CrossRef CAS PubMed; (d) L. Dubois, K. Douma, C. T. Supuran, R. K. Chiu, M. A. M. J. van Zandvoort, S. Pastoreková, A. Scozzafava, B. G. Wouters and P. Lambin, Radiother. Oncol., 2007, 83, 367 CrossRef CAS PubMed.
- (a) P. Swietach, S. Wigfield, P. Cobden, C. T. Supuran, A. L. Harris and R. D. Vaughan-Jones, J. Biol. Chem., 2008, 283, 20473 CrossRef CAS PubMed; (b) V. Alterio, M. Hilvo, A. Di Fiore, C. T. Supuran, P. Pan, S. Parkkila, A. Scaloni, J. Pastorek, S. Pastorekova, C. Pedone, A. Scozzafava, S. M. Monti and G. De Simone, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 16233 CrossRef CAS PubMed.
- J. Pan, J. Lau, F. Mesak, N. Hundal, M. Pourghiasian, Z. Liu, F. Benard, S. Dedhar, C. T. Supuran and K. S. Lin, J. Enzyme Inhib. Med. Chem., 2014, 29, 249 CrossRef CAS PubMed.
- ClinicalTrails.gov: Safety Study of SLC-0111 in Subjects With Advanced Solid Tumours - Full Text View - ClinicalTrials_gov.mht.
- (a) R. McKenna and C. T. Supuran, Carbonic anhydrase inhibitors drug design, in Carbonic Anhydrase: Mechanism, regulation, Links to Disease, and Industrial Applications, ed. R. McKenna and S. Frost, Springer Verlag, Heidelberg, 2014, pp. 291–323 (Subcell. Biochem. 2014, 75, 291) Search PubMed; (b) C. T. Supuran, M. A. Ilies and A. Scozzafava, Eur. J. Med. Chem., 1998, 33, 739 CrossRef CAS; (c) A. Thiry, J. M. Dognè, B. Masereel and C. T. Supuran, Curr. Top. Med. Chem., 2007, 7, 855 CrossRef CAS.
- (a) C. Capasso and C. T. Supuran, J. Enzyme Inhib. Med. Chem., 2014, 29, 379 CAS; (b) B. P. Mahon, A. M. Hendon, J. M. Driscoll, G. M. Rankin, S. A. Poulsen, C. T. Supuran and R. McKenna, Bioorg. Med. Chem., 2015, 23, 849 CrossRef CAS PubMed.
- (a) C. T. Supuran, A. Nicolae and A. Popescu, Eur. J. Med. Chem., 1996, 31, 431 CrossRef CAS; (b) L. Puccetti, G. Fasolis, D. Vullo, Z. H. Chohan, A. Scozzafava and C. T. Supuran, Bioorg. Med. Chem. Lett., 2005, 15, 3096 CrossRef CAS PubMed.
- (a) G. Nasr, E. Petit, C. T. Supuran, J. Y. Winum and M. Barboiu, Bioorg. Med. Chem. Lett., 2009, 19, 6014 CrossRef CAS PubMed; (b) G. Nasr, E. Petit, D. Vullo, J. Y. Winum, C. T. Supuran and M. Barboiu, J. Med. Chem., 2009, 52, 4853 CrossRef CAS PubMed; (c) M. Y. Abdelrahim, M. Tanc, J. Y. Winum, C. T. Supuran and M. Barboiu, Chem. Commun., 2014, 50, 8043 RSC.
- P. Selvam, M. Chandramohan, E. De Clercq, M. Witvrouw and C. Pannecouque, Eur. J. Pharm. Sci., 2001, 14, 313 CrossRef CAS.
- M. Hassan, Z. H. Chohan, A. Scozzafava and C. T. Supuran, J. Enzyme Inhib. Med. Chem., 2004, 19, 263 CrossRef PubMed.
- Z. H. Chohan, A. U. Shaikh and M. M. Naseer, Appl. Organomet. Chem., 2006, 20, 729 CrossRef CAS PubMed.
- V. A. Muthukumar, H. C. Nagaraj, D. Bhattacherjee and S. George, Int. J. Pharm. Pharm. Sci., 2013, 5, 95 CAS.
- S. Ramachandran and V. Uma Maheswari, Int. J. Pharm. Biosci., 2011, 2, 251 CAS.
- R. G. Khalifah, J. Biol. Chem., 1971, 246, 2561 CAS.
- (a) A. Sahin, S. Isik, O. Arslan, C. T. Supuran and O. Ozensoy Guler, J. Enzyme Inhib. Med. Chem., 2015, 30, 224 CrossRef CAS PubMed; (b) Y. Akbaba, A. Akincioglu, H. Göçer, S. Göksu, I. Gülçin and C. T. Supuran, J. Enzyme Inhib. Med. Chem., 2014, 29, 35 CrossRef CAS PubMed.
- F. Liu, A. Martin-Mingot, F. Lecornué, A. Maresca, S. Thibaudeau and C. T. Supuran, J. Enzyme Inhib. Med. Chem., 2012, 27, 886 CrossRef CAS PubMed.
- (a) S. Del Prete, V. De Luca, D. Vullo, A. Scozzafava, V. Carginale, C. T. Supuran and C. Capasso, J. Enzyme Inhib. Med. Chem., 2014, 29, 532 CrossRef CAS PubMed; (b) V. De Luca, S. Del Prete, C. T. Supuran and C. Capasso, J. Enzyme Inhib. Med. Chem., 2015, 30, 277 CrossRef CAS PubMed; (c) A. Mastrolorenzo, S. Rusconi, A. Scozzafava, G. Barbaro and C. T. Supuran, Curr. Med. Chem., 2007, 14, 2734 CrossRef CAS.
- V. Alterio, M. Tanc, J. Ivanova, R. Zalubovskis, I. Vozny, S. M. Monti, A. Di Fiore, G. De Simone and C. T. Supuran, Org. Biomol. Chem., 2015, 13, 4064 CAS.
| This journal is © The Royal Society of Chemistry 2015 |