Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Thermal stability of G-rich anti-parallel DNA triplexes upon insertion of LNA and α-L-LNA†
Tamer R.
Kosbar
ab,
Mamdouh A.
Sofan
b,
Laila
Abou-Zeid
c and
Erik B.
Pedersen
*a
aNucleic Acid Center, Department of Physics, Chemistry, and Pharmacy, University of Southern Denmark, Campusvej 55, 5230 Odense M, Denmark. E-mail: erik@sdu.dk; Fax: +45 66158780
bDepartment of Chemistry, Faculty of Science, Damietta University, 34517 New Damietta, Egypt
cDepartment of Pharmaceutical Organic Chemistry, Faculty of Pharmacy, Mansoura University, 35516 Mansoura, Egypt
First published on 24th March 2015
Abstract
G-rich anti-parallel DNA triplexes were modified with LNA or α-L-LNA in their Watson–Crick and TFO strands. The triplexes were formed by targeting a pyrimidine strand to a putative hairpin formed by Hoogsteen base pairing in order to use the UV melting method to evaluate the stability of the triplexes. Their thermal stability was reduced when the TFO strand was modified with LNA or α-L-LNA. The same trend was observed when the TFO strand and the purine Watson–Crick strand both were modified with LNA. When all triad components were modified with α-L-LNA and LNA in the middle of the triplex, the thermal melting was increased. When the pyrimidine sequence was modified with a single insertion of LNA or α-L-LNA the ΔTm increased. Moreover, increasing the number of α-L-LNA in the pyrimidine target sequence to six insertions, leads to a high increase in the thermal stability. The conformational S-type structure of α-L-LNA in anti-parallel triplexes is preferable for triplex stability.
Introduction
DNA triplexes are formed when a DNA duplex containing a polypurine tract interacts with a third strand by means of specific hydrogen bonds in the major groove of the duplex. The formation of triple helices between DNA duplexes and external DNA single strands was introduced theoretically in 1953 by Pauling and Corey1 and demonstrated experimentally by Rich and co-workers in 1957.2Depending on the orientation of the third strand, triplexes are classified into two main categories: parallel and anti-parallel oriented triplexes.3 The parallel-oriented triplexes (also named pyrimidine triplexes) are defined by three types of Hoogsteen base triads: d(T-A.T), protonated d(C-G.C+) and d(C-G.G), where the last base refers to the Hoogsteen strand. The dot and hyphen refer to Hoogsteen and Watson–Crick binding, respectively, Fig. 1. Anti-parallel triplexes (also named purine triplexes) are classified by three reverse-Hoogsteen base triads: d(T-A.T), d(T-A.A) and d(C-G.G) as shown in Fig. 1.
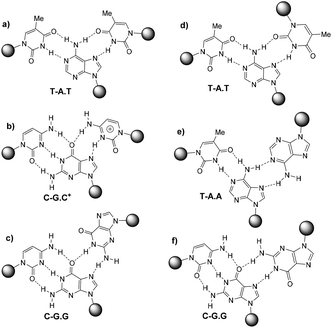 | ||
| Fig. 1 First column: Hoogsteen pairings found in parallel triplexes (a, b and c), second column: reverse Hoogsten pairings found in anti-parallel triplexes (d, e and f). | ||
Historically, guanine containing triplex forming oligonucleotides (G-rich TFOs) have been far less explored than TFOs based on pyrimidines (CT-TFOs). However, C-triplexes are unstable at neutral pH due to lacking protonation of cytosines which is required to form hydrogen bonds with the guanosines in the duplex. Therefore, it has been attempted to focus on pH-independent TFOs containing GA and/or GT nucleotides in order to design TFOs that combine DNA target-specificity with strong binding under physiological conditions.4 However this approach is hampered by the tendency of G-rich TFOs to form highly stable aggregates, mostly in the form of inter- or intramolecular G-quadruplexes, thus preventing triplex formation.5–7
Several strategies have been developed to avoid the self-aggregation of G-rich TFOs, some of them depend on formation of short duplexes on the 3′- or 5′-ends of G-rich TFOs,8 replacement of phosphodiester internucleotide bonds with positively charged phosphoramidites9 or modification of the TFO strand with twisted intercalating nucleic acid monomer (TINA) which effectively circumvents guanine-mediated self-association of G-rich anti-parallel TFOs.10 Also, 8-aminoguanine has a unique profile as a molecule showing simultaneous strong triplex-stabilization and quadruplex-destabilization.11 The introduction of 8-aminoguanine into triplex-forming oligonucleotides seems preferable compared to other modified purines like 6-thioguanine,6,12 9-deazaguanine,13 7-deazaxanthine,14 6-thio-7-deazaguanine,15 or 7-chloro-7-deazaguanine.16 Although they were found to inhibit quadruplex formation, there was no improvement on triplex stability.
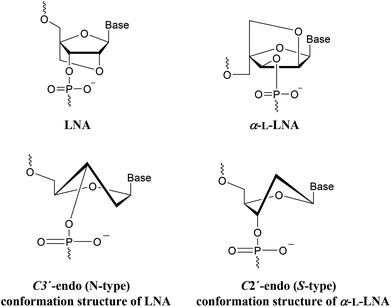
LNA (Locked Nucleic Acid) is a nucleotide modification (Fig. 2) with a 2′-O, 4′-C-methylene linked bicyclic ribonucleoside locked in an 3′-endo conformation. This preorganizes the sugar-phosphate backbone to facilitate a more efficient stacking of the nucleobases.17 α-L-LNA (α-L-ribo configured LNA, Fig. 2) is a diastereoisomer of LNA. Like LNA it improves stability against nucleases and induces high affinity towards DNA and especially RNA complements when incorporated into oligonucleotides.18,19 α-L-LNA can be described as a DNA mimic with respect to helical structure, whereas LNA is an RNA mimic.20,21
It was reported that incorporation of LNA-T or LNA-C in the homo-pyrimidine strand significantly increased the binding affinity of TFOs,22–24 but unexpectedly, a continuous stretch of 12–13 LNA-T and LNA-C monomers in the TFO decreased the affinity dramatically so that no triplex formation could be detected.24 There is also a report claiming reverse Hoogsteen hydrogen bonding with (G,A)- and (G,T)-containing LNA-TFOs to be disfavored, but without reporting the corresponding experimental data.25 In contrast to LNA-TFOs, the fully modified α-L-LNA-TFO forms a stable parallel triplex with a DNA duplex.26
Herein, G-rich triplexes were modified with LNA and α-L-LNA in the TFO and Watson–Crick strands. To the best of our knowledge this is the first report describing the incorporation of α-L-LNA into anti-parallel TFOs.
Results and discussion
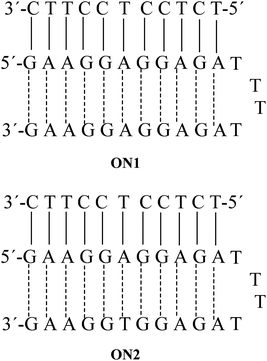
In this study, we were dealing with two hairpins that target a pyrimidine strand to form the triplexes ON1 and ON2. These two triplexes were taken from a previous anti-parallel triplex study, where the linking between the Watson–Crick polypurine and the reverse-Hoogsteen strands was done by a tetrathymidine loop,27Fig. 3.In our hands the triplexes ON1 and ON2 showed melting temperatures at 61.5 °C and 60.5 °C, respectively (Table 1). For ON1 this is close to the previously reported temperature at 62.7 °C.27 The single strands forming hairpins did not show any melting and melting was observed only when the putative hairpins were mixed with the pyrimidine target sequences. This observation is in agreement with the previous finding that reverse-Hoogsteen hairpins are not fully preorganized before binding to the third strand.27 Interestingly, the control duplexes formed by Watson–Crick 11-mer and corresponding polypurine strand ON26 and ON29 (with scrambled anti-parallel strand) melted at lower temperatures (47–48 °C, Table 2).
| Watson–Crick | Hairpin | ΔTm (°C) | ||||
|---|---|---|---|---|---|---|
| ON | Target | Watson–Crick | TFO | T m (°C) | Ref. ON1 | Ref. ON2 |
| a C = cytosine, CL = 5-methylcytosine LNA, Cα = 5-methylcytosine α-L-LNA. | ||||||
| 1 | 5′TCTCCTCCTTC3′ | 5′GAAGGAGGAGA-TTTT-AGAGGAGGAAG3′ | 61.5 | |||
| 2 | 5′TCTCCTCCTTC3′ | 5′GAAGGAGGAGA-TTTT-AGAGGTGGAAG3′ | 60.5 | |||
| 3 | 5′TCTCCTCCTTC3′ | 5′GAAGGAGGAGA-TTTT-AGAGGLAGGAAG3′ | 54.5 | −7.0 | ||
| 4 | 5′TCTCCTCCTTC3′ | 5′GAAGGAGGAGA-TTTT-AGAGGALGGAAG3′ | 56.5 | −5.0 | ||
| 5 | 5′TCTCCTCCTTC3′ | 5′GAAGGAGGAGA-TTTT-AGAGGTLGGAAG3′ | 53.5 | −7.0 | ||
| 6 | 5′TCTCCTCCTTC3′ | 5′GAAGGAGGAGA-TTTT-AGAGGTαGGAAG3′ | 55.0 | −5.5 | ||
| 7 | 5′TCTCCTCCTTC3′ | 5′GAAGGAGLGAGA-TTTT-AGAGGAGGAAG3′ | 60.5 | −1.0 | ||
| 8 | 5′TCTCCTCCTTC3′ | 5′GAAGGALGGAGA-TTTT-AGAGGAGGAAG3′ | 62.0 | 0.5 | ||
| 9 | 5′TCTCCTCCTTC3′ | 5′GAAGGALGGAGA-TTTT-AGAGGALGGAAG3′ | 58.5 | −3.0 | ||
| 10 | 5′TCTCCTCCTTC3′ | 5′GAAGGAGLGAGA-TTTT-AGAGGLAGGAAG3′ | 56.5 | −5.0 | ||
| 11 | 5′TCTCCTCCTTC3′ | 5′GAAGGALGGAGA-TTTT-AGAGGTαGGAAG3′ | 58.5 | −2.0 | ||
| 12 | 5′TCTCCTαCCTTC3′ | 5′GAAGGALGGAGA-TTTT-AGAGGTαGGAAG3′ | 64.0 | 3.5 | ||
| 13 | 5′TCTCCTLCCTTC3′ | 5′GAAGGALGGAGA-TTTT-AGAGGTαGGAAG3′ | 63.5 | 3.0 | ||
| 14 | 5′TCTCCTαCCTTC3′ | 5′GAAGGAGGAGA-TTTT-AGAGGTαGGAAG3′ | 60.0 | −0.5 | ||
| 15 | 5′TCTCCTLCCTTC3′ | 5′GAAGGAGGAGA-TTTT-AGAGGTLGGAAG3′ | 57.5 | −3.0 | ||
| 16 | 5′TCTCCTαCCTTC3′ | 5′GAAGGAGGAGA-TTTT-AGAGGTLGGAAG3′ | 59.0 | −1.5 | ||
| 17 | 5′TCTCCTLCCTTC3′ | 5′GAAGGAGGAGA-TTTT-AGAGGTGGAAG3′ | 64.0 | 3.5 | ||
| 18 | 5′TCTCCTαCCTTC3′ | 5′GAAGGAGGAGA-TTTT-AGAGGTGGAAG3′ | 64.5 | 4.0 | ||
| 19 | 5′TCTLCCTLCCTLTC3′ | 5\GAAGGAGGAGA-TTTT-AGAGGTGGAAG3′ | 69.5 | 9.0 | ||
| 20 | 5′TCTαCCTαCCTαTC3′ | 5′GAAGGAGGAGA-TTTT-AGAGGTGGAAG3′ | 72.0 | 11.5 | ||
| 21 | 5′TCTCCLTCCTTC3′ | 5′GAAGGAGGAGA-TTTT-AGAGGTGGAAG3′ | 66.0 | 5.5 | ||
| 22 | 5′TCLTCCLTCCLTTC3′ | 5′GAAGGAGGAGA-TTTT-AGAGGTGGAAG3′ | 73.5 | 13.0 | ||
| 23 | 5′TCTCCαTCCTTC3′ | 5′GAAGGAGGAGA-TTTT-AGAGGTGGAAG3′ | 66.5 | 6.0 | ||
| 24 | 5′TCαTCCαTCCαTTC3′ | 5′GAAGGAGGAGA-TTTT-AGAGGTGGAAG3′ | 76.0 | 15.5 | ||
| 25 | 5′TCαTαCCαTαCCαTαTC3′ | 5′GAAGGAGGAGA-TTTT-AGAGGTGGAAG3′ | 82.0 | 21.5 | ||
| ΔTm (°C) | |||||
|---|---|---|---|---|---|
| ON | Pyrimidine sequence | Purine sequence | T m (°C) | Ref. ON26 | Ref. ON29 |
| a C = cytosine, Cα = 5-methylcytosine α-L-LNA. | |||||
| 26 | 5′TCTCCTCCTTC3′ | 5′GAAGGAGGAGA3′ | 48.0 | ||
| 27 | 5′TCTCCTCCTTC3′ | 5′GAAGGALGGAGA3′ | 53.5 | 5.5 | |
| 28 | 5′TCTCCTCCTTC3′ | 5′GAAGGAGLGAGA3′ | 53.5 | 5.5 | |
| 29 | 5′TCTCCTCCTTC3′ | 5′GAAGGAGGAGA-TTTT-GAGAGGAAAGA3′ | 47.0 | ||
| 30 | 5′TCTCCTCCTTC3′ | 5′GAAGGALGGAGA-TTTT-GAGAGGAAAGA3′ | 51.0 | 3.0 | |
| 31 | 5′TCTCCTCCTTC3′ | 5′GAAGGAGLGAGA-TTTT-GAGAGGAAAGA3′ | 51.0 | 3.0 | |
| 32 | 5′TCTCCTαCCTTC3′ | 5′GAAGGAGGAGA3′ | 51.0 | 3.0 | |
| 33 | 5′TCTαCCTαCCTαTC3′ | 5′GAAGGAGGAGA3′ | 56.0 | 8.0 | |
| 34 | 5′TCTCCαTCCTTC3′ | 5′GAAGGAGGAGA3′ | 54.0 | 6.0 | |
| 35 | 5′TCαTCCαTCCαTTC3′ | 5′GAAGGAGGAGA3′ | 62.0 | 14.0 | |
| 36 | 5′TCαTαCCαTαCCαTαTC3′ | 5′GAAGGAGGAGA3′ | 68.0 | 20.0 | |
Firstly, we modified the TFO with adenine-LNA (AL), guanine-LNA (GL), thymine-LNA (TL) or thymine-α–L-LNA (Tα), but unfortunately, the Tm was decreased in all cases. The ΔTm was −7 °C in case of GL (ON3) and TL (ON5). In case of AL (ON4) and Tα (ON6) it was −5 °C and −5.5 °C, respectively. This confirms the previously published statement that LNA in the TFO destabilize the anti-parallel triplex.25 Also, we tried to modify the Watson–Crick part in the middle with LNA. However, the Tm increased only 0.5 °C with AL (ON8) and decreased 1 °C with GL (ON7), Table 1.
Moreover, when the TFO and Watson–Crick parts both were modified with LNA in the middle of the sequence, the Tm also decreased, but we noticed that the decrease in the Tm is much lower than in case of LNA in the TFO only. For example, when we compared the Tm values of ON3 and ON10, we observed that the Tm increased about 2 °C (from 54.5 °C to 56.5 °C, Table 1). The same trend in Tm values was observed when comparing ON4 with ON9 and ON6 with ON11.
One insertion of Tα in the pyrimidine target sequence and in the TFO part decreased the thermal stability of the anti-parallel triplex by only 0.5 °C (ON14). In contrast, using TL in the same two positions the decrease in Tm was 3 °C (ON15).
Interestingly, the stability of anti-parallel triplex increased, when all the three strands were modified with LNA. The Tm data revealed that, the difference in Tm values between triplexes ON11 with LNA in TFO and Watson–Crick parts and ON12 with LNA in all three parts is 5.5 °C (ΔTm changed from −2 °C to 3.5 °C, Table 1). We assume that, this dramatic increase in the Tm when the pyrimidine target sequence was modified with LNA, is due to increased stacking between the nucleobases in the duplex part (see molecular modeling section).
It has been reported that 2–4 insertions of twisted intercalating nucleic acid (TINA) in the TFO strand increase the stability of G-rich TFOs and prevent G-quadruplexes formation. The pyrene moiety is positioned inside the DNA duplex between base pairs during the triplex formation which increases the stacking in the duplex part.10 This means that, the stability of anti-parallel TFO depends on the stacking in Watson–Crick duplex. Based on this observation, it was found interesting to modify only the pyrimidine target sequence with LNA and therefore the triplexes ON17–ON25 were investigated. As expected, one insertion of LNA in the pyrimidine target sequence increases the Tm by 4 °C in case of ON18 with Tα and by 3.5 °C in case of ON17 with TL. When 5-methylcytosine (C) was used as base in LNA and α-L-LNA, it was found that one insertion of α-L-LNA-C (Cα) in ON23 and LNA-C (CL) in ON21 increase the melting temperature by 6 °C and 5.5 °C, respectively.
The effect seems additive since three insertions of Tα in ON20 or TL in ON19 gave higher thermal stabilities with ΔTm = 11.5 °C and 9 °C, respectively, compared to the wild type ON2 (Table 1). The same trend was observed for three modifications Cα (ON24) and CL (ON22) in the pyrimidine target sequence, ΔTm = 15.5 °C and 13 °C, respectively (Table 1), reflecting higher melting temperatures for LNAs-C than for LNAs-T.
From the results, we noticed that α-L-LNA gave higher thermal stability in all cases compared to LNA. Concerning the conformational structure of anti-parallel triplexes, it has been reported that only S-type (south-type) sugars are favorable for the anti-parallel triplex.28 On the other hand, It has been found that the conformation structure of the furanose ring in α-L-LNA is the S-type,20 and this explain that α-L-LNA is more suitable for anti-parallel TFOs. This encouraged us to increase the number of α-L-LNA in the pyrimidine target sequence to six α-L-LNA nucleobases using three Cα and three Tα (ON25) which leads to a significant increase in the thermal stability with ΔTm = 21.5 °C (Table 1).
In order to be sure that the TFO strand is contributing to the stability of the target complex, the corresponding duplexes ON26–ON36 were considered. In all cases the Tm was lower than for the corresponding triplexes. This was also the case for ON29–ON31 with a mismatched dangling TFO part (Table 2).
CD measurements
All the triplexes with or without modification of T in the pyrimidine target sequence showed a positive band at 270 nm and a negative band at 242 nm in their CD spectra. This means that the spectra of ON12–ON20 with TL and Tα are in agreement with the CD spectra of (G,A) anti-parallel triplexes,29Fig. 4a.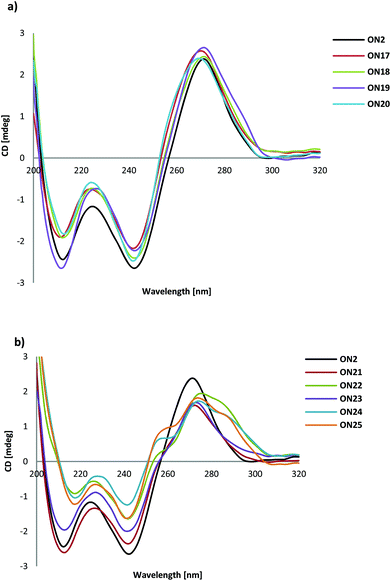 | ||
| Fig. 4 (a) Typical examples of CD spectra of ON2 and ON17–ON19 with TL and Tα. (b) CD spectra of ON2 and ON21–25 with CL and Cα. | ||
From Fig. 4b it can be seen that there is an indication of shoulders on both sides of the 270 nm band in the CD spectra of ON21 with one CL insertion in the target sequence and of ON23 with one Cα insertion in the target sequence. When the number of CL or Cα was increased, the CD spectra changed more for ON22, ON24 and ON25 and shoulders appeared clearly at 255 nm and 285 nm.
The increasing number of CL and Cα in the target sequence may change the conformation of the duplex part of the triplex. When inserted into a duplex, LNA is known to change the flanking nucleotides into an N-type sugar conformation.30 In fact, the shoulders in the CD spectra of ON21–ON25 corresponds to the main bands observed by Shibata et al. in an anti-parallel triplex with a higher G/A ratio and with 5-aminomethyl modification for one of the thymidines.31
Molecular modeling
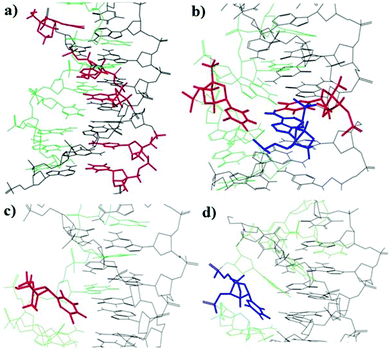
In order to understand the binding and stacking properties of the anti-parallel triplex modified with LNA and α-L-LNA, we decided to get insight into their structures via molecular modeling studies. An AMBER* force field in Macro Model 9.1, molecular modeling was used to generate representative low energy structures of the modified structures with LNA and α-L-LNA at different positions in the Watson–Crick and TFO parts of the triplexes. Patel's structure of the anti-parallel triplex d(TGGTGGT) which contains d(G-GC) and (T-AT) triads (PDB entry pdb134d)32 was used and modified with LNA and α–L-LNA.The results showed that, multi insertion of α-L-LNA (Tα and Cα) in the pyrimidine target sequence increase the stacking between the nucleobases in the three strands of the anti-parallel triplex which reflects the high thermal stability of ON25 (Fig. 5a).
In contrast, a larger distortion of the triplex structure was observed when the TFO strand was modified with TL (Fig. 5d), the LNA moiety and some other TFO's nucleobases were forced to twist out of plane of Watson–Crick base pair which is weakening the stacking interactions with the TFO nucleobases and the binding with the duplex part. The same trend was observed in case of Tα in the TFO strand (Fig. 5c). This explains the lower thermal stability of ON5 and ON6.
However, when the TFO, Watson–Crick and the target strands were modified with Tα, AL and Tα, respectively (Fig. 5b), the triplex structure was undisturbed and the Tα-nucleobase in the TFO strand hybridized nicely to the nucleobase AL in the Watson–Crick duplex, thereby explaining the increase in stability when the three strands were modified with α-L-LNA and LNA (ON12).
Conclusion
The stability of anti-parallel triplexes was increased when the pyrimidine sequence was modified with LNA (CL and TL) and α-L-LNA (Cα and Tα). Molecular model building is supportive of base stacking between the nucleobases in the duplex part. However, α-L-LNA gave higher thermal stability in all cases when compared to LNA, because the conformational S-type structure of α-L-LNA corresponding to the sugar conformation in anti-parallel triplexes is preferable for triplex stability.28 A substantial increase in thermal stability was also observed when a base triad was fully modified with LNA and α-L-LNA (Tα,AL,Tα or TL,AL,Tα) in contrast to modification in the purine strands only. When only the purine TFO strand was modified, the triplex stability was reduced. According to molecular modeling, this is due to reduced stacking in the TFO part. We think that our findings about antiparallel triplex stability could be important for developing a strategy for strand invasion by clamping a G-rich purine strand in a duplex. This corresponds to a reported strand invasion method based on formation of a parallel triplex on an A-rich sequence.33Experimental section
General
All locked nucleoside amidites were purchased from Exiqon. Unmodified oligonucleotides were purchased from Sigma on a 0.2 mmol scale; the purity was checked by ion-exchange chromatography and MALDI-TOF mass spectrometry.Oligonucleotide synthesis
Modified oligonucleotides were synthesized in 0.2 mmol scale on 500 Å CPG supports using ExpediteTM Nucleic Acid Synthesis System model 8909 from Applied Biosystems. Standard procedures were used for the coupling of commercial phosphoramidites, whereas modified phosphoramidites, LNA-A (AL), LNA-G (GL), LNA-T (TL), α-L-LNA-T (Tα), 5-methylcytosine types LNA-C (CL) and α-L-LNA-C (Cα), were coupled with 1H-tetrazole in CH3CN as an activator and an extended coupling time (15 min), the coupling efficiency was higher than 95% in all cases. ONs were de-protected and cleaved from the CPG support with 32% aq. NH3 (1 mL) and left at 55 °C for 20 h. ONs were purified as DMT-on ONs by Reversed phase-HPLC using: (1) Column: XBridge OST C18, 19 × 1000 mm, 5 μm + precolumn: XBridge 10 × 10 mm, 5 μm, the temperature of column oven was 50 °C. (2) Buffers: [Buffer A: 0.05 M TEAA (triethyl ammonium acetate) pH 7.4; Buffer B: 75% MeCN/25% Buffer A], flow: 5 mL min−1.ONs were submitted to DMT deprotection with 80% aq. AcOH (100 μL) for 30 min, followed by addition of doubly filtered H2O (100 μL), aq. AcONa (5 M, 15 μL), and aq. NaClO4 (5 M, 15 μL) before precipitation from pure acetone (1 mL). Molecular masses of all modified ONS were confirmed by MALDI-TOF analysis on a Ultraflex II TOF/TOF system from Bruker (a MALDI-LIFT system) with HPA matrix (10 mg 3-hydroxypicolinic acid, in 50 mM ammonium citrate/70% MeCN). The purity of the final ONs were found to be ∼99% when checked by ion-exchange chromatography using La-Chrom system from Merck Hitachi on Dionex DNA Pac Pa-100, 4 × 250 mm anal. column. Buffers: [Buffer A: H2O Milli-Q; Buffer B: 1 M NaClO4; Buffer C: 25 mM Tris-Cl, pH 8.0; Buffer D: MeCN], Flow: 1 mL min−1.
Thermal denaturation studies
Thermal melting measurements were performed on a Perkin-Elmer UV/VIS spectrometer Lambda 35 fitted with a PTP-6 temperature programmer. Melting experiments were performed on equimolar amounts of the appropriate oligonucleotides (target sequence and hairpin) (3 μM) in a buffer solution consisting of sodium cacodylate (10 mM), EDTA (0.1 mM), and MgCl2 (50 mM) at pH 7.2. The solutions were heated to 90 °C then cooled down slowly to room temperature, and were then kept at this temperature for 2 h. The absorbance of the formed triplexes was measured at 260 nm with a heating rate of 0.5 °C min−1. The melting temperature (Tm) was determined as the maximum of the first derivative plots of the melting curves.Circular dichroism spectra (CD)
Circular dichroism spectra were recorded on a Jasco J-600A spectropolarimeter using 1 mL quartz cuvettes with 5 mm path length. Oligonucleotides (3 μM) were dissolved in a buffer solution consisting of sodium cacodylate (10 mM), EDTA (0.1 mM), and MgCl2 (50 mM) at pH 7.2. All samples were heated for 2 minutes at 90 °C and slowly cooled to room temperature before data collection. Measurements were performed at 20 °C in the 200–350 nm wavelength range with a continuous scanning mode, 50 nm min−1 as a scanning speed, 4 s for a response and accumulation 5 times. The spectrum of the buffer solution was subtracted from the sample spectra.Molecular modeling
Molecular modeling was performed with Macro Model v9.1 from Schrödinger. All calculations were conducted with AMBER* force field and the GB/SA water model. The dynamic simulations were performed with stochastic dynamics, a SHAKE algorithm to constrain bonds to hydrogen, time step of 1.5 fs and simulation temperature of 300 K. Simulation for 0.5 ns with an equilibration time of 150 ps generated 250 structures, which all were minimized using the PRCG method with convergence threshold of 0.05 kJ mol−1. Patel's structure of the anti-parallel triplex32 was downloaded from the protein data bank (PDB entry pdb134d), followed by incorporation of the nucleobases TL, Tα, Cα and AL.Acknowledgements
The Ministry of Higher Education of Egypt is gratefully acknowledged for a predoctoral channel fellowship to Tamer Kosbar.Notes and references
- L. Pauling and R. B. Corey, Proc. Natl. Acad. Sci. U. S. A., 1953, 39, 84–97 CrossRef CAS.
- G. Felsenfeld, D. R. Davis and A. Rich, J. Am. Chem. Soc., 1957, 79, 2023–2027 CrossRef CAS.
- V. N. Soyfer and V. N. Potaman, Triple-Helical Nucleic Acids, Springer, New York, 1996 Search PubMed.
- (a) D. Praseuth, A. L. Guieysse and C. Helene, Biochim. Biophys. Acta, 1999, 1489, 181–206 CrossRef CAS PubMed; (b) M. Duca, P. Vekhoff, K. Oussedik, L. Halby and P. B. Arimondo, Nucleic Acids Res., 2008, 36, 5123–5138 CrossRef CAS PubMed; (c) M. Faria and C. Giovannangeli, J. Gene Med., 2001, 3, 299–310 CrossRef CAS PubMed.
- (a) W. M. Olivas and L. J. Maher, Biochemistry, 1995, 34, 278–284 CrossRef CAS PubMed; (b) A. J. Cheng, J. C. Wang and M. W. Van Dyke, Antisense Nucleic Acid Drug Dev., 1998, 8, 215–225 CrossRef CAS PubMed.
- W. M. Olivas and L. J. Maher, Nucleic Acids Res., 1995, 23, 1936–1941 CrossRef CAS PubMed.
- P. B. Arimondo, F. Barcelo, J. S. Sun, J. C. Maurizot, T. Garestier and C. Helene, Biochemistry, 1998, 37, 16627–16635 CrossRef CAS PubMed.
- F. Svinarchuk, D. Cherny, A. Debin, E. Delain and C. Malvy, Nucleic Acids Res., 1996, 24, 3858–3865 CrossRef CAS PubMed.
- J. M. Dagle and D. L. Weeks, Nucleic Acids Res., 1996, 24, 2143–2149 CrossRef CAS PubMed.
- O. Doluca, A. S. Boutorine and V. V. Filichev, ChemBioChem, 2011, 12, 2365–2374 CrossRef CAS PubMed.
- J. Lopez de la Osa, C. Gonzalez, R. Gargallo, M. Rueda, E. Cubero, M. Orozco, A. Avino and R. Eritja, ChemBioChem, 2006, 7, 46–48 CrossRef CAS PubMed.
- (a) N. Spackova, E. Cubero, J. Sponer and M. Orozco, J. Am. Chem. Soc., 2004, 126, 14642–14650 CrossRef CAS PubMed; (b) V. M. Marathias, M. J. Sawicki and P. H. Bolton, Nucleic Acids Res., 1999, 27, 2860–2867 CrossRef CAS PubMed; (c) T. S. Rao, R. H. Durland, D. M. Seth, M. A. Myrick, V. Bodepudi and G. R. Revankar, Biochemistry, 1995, 34, 765–772 CrossRef CAS PubMed; (d) J. E. Gee, G. R. Revankar, T. S. Rao and M. E. Hogan, Biochemistry, 1995, 34, 2042–2048 CrossRef CAS PubMed.
- T. S. Rao, A. F. Lewis, R. H. Durland and G. R. Revankar, Tetrahedron Lett., 1993, 34, 6709–6712 CrossRef CAS.
- J. F. Milligan, S. H. Krawczyk, S. Wadwani and M. D. Matteuci, Nucleic Acids Res., 1993, 21, 327–333 CrossRef CAS PubMed.
- T. S. Rao, A. F. Lewis, T. S. Hill and G. R. Revankar, Nucleosides Nucleotides, 1995, 14, 1–12 CAS.
- Y. Aubert, L. Perrouault, C. Helene, C. Giovannangeli and U. Asseline, Bioorg. Med. Chem., 2001, 9, 1617–1624 CrossRef CAS PubMed.
- (a) A. A. Koshkin, S. K. Singh, P. Nielsen, V. K. Rajwanshi, R. Kumar, M. Meldgaard, C. E. Olsen and J. Wengel, Tetrahedron, 1998, 54, 3607–3630 CrossRef CAS; (b) S. Obika, D. Nanbu, Y. Hari, J. Andoh, K. Morio, T. Doi and T. Imanishi, Tetrahedron Lett., 1998, 39, 5401–5404 CrossRef CAS; (c) B. Vester and J. Wengel, Biochemistry, 2004, 43, 13233–13241 CrossRef CAS PubMed.
- V. K. Rajwanshi, A. E. Hakansson, B. M. Dahl and J. Wengel, Chem. Commun., 1999, 1395–1396 RSC.
- M. D. Sorensen, L. Kvarno, T. Bryld, A. E. Hakansson, B. Verbeure, G. Gaubert, P. Herdewijn and J. Wengel, J. Am. Chem. Soc., 2002, 124, 2164–2176 CrossRef CAS PubMed.
- K. M. E. Nielsen, M. Petersen, A. E. Hakansson, J. Wengel and J. P. Jacobsen, Chem. – Eur. J., 2002, 8, 3001–3009 CrossRef CAS.
- M. Petersen, C. B. Nielsen, K. E. Nielsen, G. A. Jensen, K. Bondensgaard, S. K. Singh, V. K. Rajwanshi, A. A. Koshkin, B. M. Dahl, J. Wengel and J. P. Jacobsen, J. Mol. Recognit., 2000, 13, 44–53 CrossRef CAS PubMed.
- S. Obika, Y. Hari, T. Sugimoto, M. Sekiguchi and T. Imanishi, Tetrahedron Lett., 2000, 41, 8923–8927 CrossRef CAS.
- J. S. Lee, M. L. Woodsworth, L. J. P. Latimer and A. R. Morgan, Nucleic Acids Res., 1984, 12, 6603–6614 CrossRef CAS PubMed.
- S. Obika, T. Uneda, T. Sugimoto, D. Nanbu, T. Minami, T. Doi and T. Imanishi, Bioorg. Med. Chem., 2001, 9, 1001–1011 CrossRef CAS PubMed.
- E. Brunet, P. Alberti, L. Perrouault, R. Babu, J. Wengel and C. Giovannangeli, J. Biol. Chem., 2005, 280, 20076–20085 CrossRef CAS PubMed.
- A. A. Koshkin, Tetrahedron, 2006, 62, 5962–5972 CrossRef CAS.
- A. Avino, E. Cubero, C. Gonzalez, R. Eritja and M. Orozco, J. Am. Chem. Soc., 2003, 125, 16127–16138 CrossRef CAS PubMed.
- C. Dagneaux, H. Gousset, A. K. Shchyolkina1, M. Ouali, R. Letellier, J. Liquier, V. L. Florentiev and E. Taillandier, Nucleic Acids Res., 1996, 24, 4506–4512 CrossRef CAS PubMed.
- M. G. Grimau, A. Avino, R. Gargallo and R. Eritja, Chem. Biodiversity, 2005, 2, 275–285 CAS.
- K. Bondensgaard, M. Petersen, S. K. Singh, V. K. Rajwanshi, R. Kumar, J. Wengel and J. P. Jacobsen, Chem. – Eur. J., 2000, 6, 2687–2695 CrossRef CAS.
- A. Shibata, Y. Ueno, M. Iwata, H. Wakita, A. Matsuda and Y. Kitade, Bioorg. Med. Chem. Lett., 2012, 22, 2681–2683 CrossRef CAS PubMed.
- I. Radhakrishnan and D. J. Patel, Structure, 1993, 1, 135–152 CrossRef CAS PubMed.
- P. M. D. Moreno, S. Geny, Y. V. Pabon, H. Bergquist, E. M. Zaghloul, C. S. J. Rocha, I. I. Oprea, B. Bestas, S. EL Andaloussi, P. T. Jørgensen, E. B. Pedersen, K. E. Lundin, R. Zain, J. Wengel and C. I. Edvard Smith, Nucleic Acids Res., 2013, 41, 3257–3273 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ob00535c |
| This journal is © The Royal Society of Chemistry 2015 |