An efficient total synthesis of leukotriene B4†
Abstract
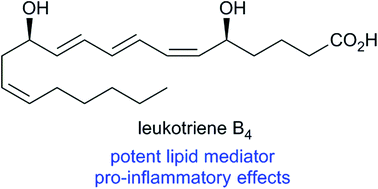
Lipid mediators have attracted great interest from scientists within the chemical, medicinal, and pharmaceutical research community. One such example is leukotriene B4 which has been the subject of many pharmacological studies. Herein, we report a convergent and stereoselective synthesis of this potent lipid mediator in 5% yield over 10 steps in the longest linear sequence from commercial starting materials. The key steps were a stereocontrolled acetate–aldol reaction with Nagao's chiral auxiliary and a Z-selective Boland reduction. All spectroscopic data were in agreement with those previously reported.

 Please wait while we load your content...
Please wait while we load your content...