Development of an efficient route toward meiogynin A-inspired dual inhibitors of Bcl-xL and Mcl-1 anti-apoptotic proteins†
Abstract
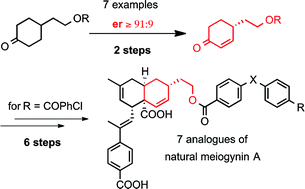
The synthesis, on a large scale, with very good yield and er via an efficient strategy, of a chiral 4-substituted 2-cyclohexenone intermediate, was a milestone in the synthesis of seven analogues of meiogynin A, a natural sesquiterpenoid dimer. These compounds were elaborated in ten linear steps. Their binding affinities for Bcl-xL and Mcl-1, two proteins of the Bcl-2 family, overexpressed in various types of cancers, were evaluated. This enabled to further SAR studies en route to the elaboration of potent dual inhibitors of anti-apoptotic proteins of the Bcl-2 family.

 Please wait while we load your content...
Please wait while we load your content...