Biosynthesis of 8-hydroxyquinoline-2-carboxylic acid, an iron chelator from the gut of the lepidopteran Spodoptera littoralis†
Jelena
Pesek
a,
Jiří
Svoboda
b,
Martina
Sattler
a,
Stefan
Bartram
a and
Wilhelm
Boland
*a
aDepartment of Bioorganic Chemistry, Max-Planck-Institute for Chemical Ecology, Hans-Knöll-Straße 8, 07745 Jena, Germany. E-mail: boland@ice.mpg.de; Fax: +49 (3641)571202; Tel: +49 (3641)571200
bZentiva, k.s., U Kabelovny 130, 102 37 Praha 10, Czech Republic
First published on 9th October 2014
Abstract
In the regurgitate (foregut content) of Spodoptera larvae we found high concentrations (0.5–5 mM) of 8-hydroxyquinoline-2-carboxylic acid (8-HQA). In a survey of different lepidopteran species, this compound was only detected in species belonging to the family of Noctuidae. 8-HQA was shown to derive from tryptophan metabolism. The amount of 8-HQA in the regurgitate was strongly dependent on the tryptophan content of the diet. In the insect 8-HQA is generated from tryptophan via kynurenine and 3-hydroxykynurenine. 8-HQA is produced by the larvae and not by their commensal gut bacteria. Analysis of different life stages of Spodoptera larvae revealed that 8-HQA is formed during the larval stage, probably acting as an iron chelator to control the gut microbiome.
Introduction
Quinolinic carboxylic acid derivates are widely distributed in nature. They are found in insects, bacteria, and plants. Examples are shown in Fig. 1.Methyl-8-hydroxyquinoline-2-carboxylate was isolated by H. Schildknecht from the defensive secretion of the water beetle Ilybius fenestratus.1 Xanthurenic acid (4,8-dihydroxyquinoline-2-carboxylic acid) was found in Drosophila melanogaster2,3 and several mosquito species.4,5 Elucidation of its biosynthetic pathway showed that it derives from tryptophan and is formed via 3-hydroxykynurenine5–7 (Scheme 1). As this compound has toxic effects, a conversion into xanthurenic acid has been proposed. Moreover, xanthurenic acid is known as a gametogenesis inducing factor of the malaria causing agent Plasmodium falciparum in mosquitoes.4 Quinolinic carboxylic acids also occur abundantly in bacteria. An important quorum sensing factor is the siderophore quinolobactin (8-hydroxy-4-methoxyquinoline-2-carboxylic acid)8 from Pseudomonas fluorescens. 3-Hydroxyquinoline-2-carboxylic acid (3-HQA) is a precursor of the antibiotic cinropeptin from Streptomyces griseoflavus9,10 and of thiocoralin, an antitumor compound from the marine actinomycete Micromonospora sp.11 Biosynthetic studies demonstrated that thiocoralin is derived from tryptophan via kynurenic acid and quinaldic acid intermediates (Scheme 1).11 Derivatives of 4-hydroxyquinoline-2-carboxylic acid12–15 are also found in plants, for example 6-hydroxykynurenic acid in Gingko biloba.14 The biological function in Gingko is unknown.
Spodoptera littoralis Boisduval (Lepidoptera: Noctuidae), widespread in Africa and Asia but also found in Southern Europe, is one of the most devastating pests of crops worldwide. Its host range covers over 40 families, containing at least 87 species of economic importance like cotton, corn, peanuts, vegetables, and soybean.16 The extensive use of insecticides has caused the development of resistant strains.17
During LCMS analysis of the regurgitate (foregut content) of S. littoralis larvae we observed substantial amounts of free 8-hydroxyquinoline-2-carboxylic acid (8-HQA, 0.5–5 mM).18,19 To learn more about the function of 8-HQA in the insect gut, the knowledge of the producing organism, either the insect or members of the gut microbial community, is relevant since 8-HQA is produced over the whole life span of the Spodopteran larva in high concentrations.
Here we report on the evaluation of the biosynthetic pathway of 8-HQA from tryptophan in the insect gut using antibiotics and labeled precursors such as L-tryptophan-(indole-d5) ([2H5]-Trp), 4-hydroxyquinoline-2-carboxylic-3-d acid ([2H]-kynurenic acid), 2-amino-4-(2-amino-3-hydroxyphenyl-5-d)-4-oxobutanoic-3,3-d2 acid ([2H3]-3-hydroxykynurenine), and 4,8-dihydroxyquinoline-2-carboxylic-3,5,6,7-d4 acid ([2H4]-xanthurenic acid). Our data suggest a conversion of Trp via 3-hydroxykynurenine and ring closure to give 8-HQA. Since 8-HQA is a potent siderophore, its production by the insect may substantiate its role as a control element for the gut microbiome used by the insect host.
Results and discussion
Detection of 8-HQA in the regurgitate of S. littoralis and other noctuid larvae
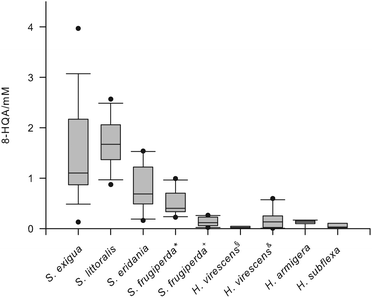
LCMS analysis of the regurgitate of S. littoralis larvae revealed an intense signal at m/z 190 [M + H]+, which could be attributed to 8-HQA. Treatment of an isolated sample with CH2N2 afforded the methylester m/z = 203 [M]+· (Fig. S1†).16 As this compound was present in high concentrations (up to 5 mmol),17 we also analyzed closely related species for the presence of 8-HQA (Table S1†). The regurgitate of three additional Spodoptera species, S. exigua, S. frugiperda (JS3C, a “corn strain” and OnaR, a “rice strain”),20 and S. eridania, three Heliothis species, H. armigera, H. subflexa, and H. virescens (JEN2 a wild type strain21 and YHD3, a strain highly resistant against Cry1-toxins from B. thuringiensis22), and Orthosia cerasi was investigated.As non-noctuid members were included larvae of Malacosoma spp., Plutella xylostella and Yponomeuta evonymellus as well as Manduca sexta and Aglais urticae. 8-HQA was detected in the regurgitate of all Spodoptera and Heliothis species (Fig. 2) and in O. cerasi, but not in M. sexta, A. urticae, Malacosoma sp., P. xylostella and Y. evonymellus. Interestingly, Spodoptera, Heliothis and O. cerasi belong to the Noctuidae family, whereas the other five species belong to other families (Sphingidae, Nymphalidae, Lasiocampidae, Plutellidae and Yponomeutidae, respectively).23
Different compartments of S. littoralis were tested for the presence of 8-HQA. The compound could not be localized in the Malpighian tubules and the fat body, but it was present in low amounts in the hemolymph, ca. 3–44 ng μl−1 (16–233 μM). The concentration in the regurgitate was substantially higher, ca. 0.1–1 μg μl−1 (0.5–5 mM). Testing different gut segments of larvae of S. littoralis revealed that the amount of 8-HQA was the highest at the end of the foregut with a steady decline via the midgut to the hindgut.17 Pupae and adults of S. littoralis do not contain 8-HQA, but in pupae an accumulation 3-hydroxykynurenin (ca. 0.18 μg mg−1) was found instead of 8-HQA.18
8-HQA is continuously synthesized from tryptophan and excreted
Since the majority of quinolinic compounds derives from Trp metabolism,24,25 first [2H5]-Trp was fed to larvae of S. littoralis. In addition, [2H5]-Trp was injected into the hemolymph. Both application modes resulted after 24 h in a signal for 8-hydroxyquinoline-2-carboxylic-5,6,7-d3 acid ([2H3]-8-HQA) in the regurgitate and in the frass, documented by m/z 193 with the same retention time as natural 8-HQA (m/z 190) (Fig. 3). Since the food also provided natural Trp, the proportion of labeled 8-HQA did not exceed 40%.Feeding larvae with defined diets, containing Trp at 0, 1.95 or 19.5 mmol g−1, demonstrated that the formation of 8-HQA in the insect is strongly dependent on the amount of ingested Trp. Larvae that fed on a normal Trp diet (1.95 mmol g−1) contained 1.69 ± 0.94 mM 8-HQA in the regurgitate and at 2.4 ± 1.03 nmol mg−1 in the excrements. Feeding on a Trp-depleted diet caused a strong decrease (0.1 ± 0.07 mM) of 8-HQA after three days, whereas ingestion of a Trp-rich diet caused an increase of 8-HQA (10.4 ± 2.2 mM) in the regurgitate. The results demonstrate that 8-HQA is produced continuously in the gut tissue from the incoming diet and is excreted with the feces. The regurgitate itself showed no biosynthetic activity. Quantitative feeding experiments with [2H5]-Trp allowed us to estimate that ca. 10% of the ingested Trp is converted into 8-HQA.
8-HQA is synthesized via 3-hydroxykynurenine
The ultimate precursor of 8-HQA is tryptophan, but details of the pathway remained to be elucidated. Two known biosynthetic pathways for quinoline carboxylic acids7,11 could principally apply for 8-HQA (Scheme 1), but none of them is compatible with the substitution pattern of 8-HQA. To obtain first information on potential intermediates, larvae were fed on a Trp-depleted diet supplemented with Trp, kynurenine, kynurenic acid, 3-hydroxykynurenine, quinaldic acid and xanthurenic acid. After two days of feeding, the concentration of 8-HQA was quantified in the regurgitate. In the presence of Trp, kynurenine and 3-hydroxykynurenine a significant increase of 8-HQA was observed compared to the Trp-depleted control. Feeding of [2H]-labeled and/or unlabeled xanthurenic acid, kynurenic acid and quinaldic acid showed no labeled 8-HQA in the regurgitate or any effect on the 8-HQA concentration, respectively. However, feeding of [2H3]-3-hydroxykynurenine (70 μg, 313 nmol) led to the formation of 8-hydroxyquinoline-2-carboxylic-3,6-d2 acid ([2H2]-8-HQA) after 3 h (Fig. 4). ESI-HR-MS: 8-HQA, [M + H]+, C10H8O3N, m/z = 190.0498, calc. 190.0498 and [2H2]-8-HQA, [M + H]+, C10H62H2O3N, m/z 192.0623, calc. 192.0624.Thus, 3-hydroxykynurenine is a precursor, whereas xanthurenic acid is not. The presence of two deuterium atoms in the product (m/z = 192, [M + H]+) allows us to reconstruct the pathway to 8-HQA as proposed in Scheme 2.
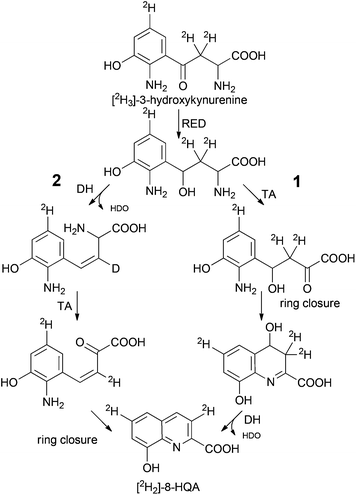 | ||
| Scheme 2 Alternative routes to 8-HQA downstream 3-hydroxykynurenine. Spontaneous or enzymatic ring closure (RED: reductase, DH: dehydratase, TA: transaminase). | ||
3-Hydroxykynurenine is most likely first reduced to an alcohol followed by regioselective transamination of the α-amino group. Ring closure between the resulting keto group and the aromatic amine yields a 4,8-dihydroxy-3,4-dihydro-quinoline-2-carboxylic acid as a potential intermediate. The final elimination of HDO generates [2H2]-8-HQA. Although the sequence of reactions has not been proven and an alternative sequence with elimination of water prior to transamination is possible, at least the occurrence of a β-diketone intermediate (transamination prior to reduction of the keto group) is unlikely as this would cause the loss of both deuterium atoms from the C4-side chain due to extensive enolization.
Reduction of 8-HQA concentration in the larval gut after inhibition of the kynurenine 3-monooxygenase
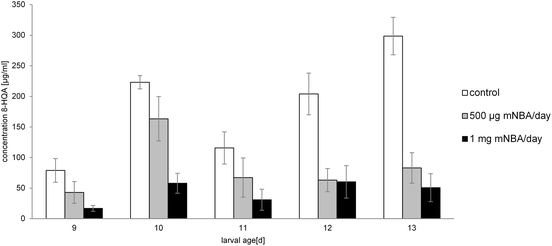
Feeding of S. littoralis larvae with m-nitrobenzoyl-alanine (mNBA), a powerful inhibitor of the kynurenine 3-monooxygenase26 (KMO converts kynurenine to 3-hydroxykynurenine), reduces the production of 8-HQA by up to 83% after 5 days (cf. Fig. 5). Toxic side effects were not observed. Moreover, inhibition of the oxidation of kynurenine directs the metabolism of Trp to the formation of quinaldic acid (HRMS: C10H8O2N m/z = 174.0543, calc. 174.0555), which was not detected in the regurgitate of the control group (cf. Scheme 1). Accordingly, also in insects the biosynthesis of quinaldic acid may proceed directly from kynurenine via similar intermediates as outlined in Scheme 2. Injection of mNBA into the hemolymph remained without effect.Although mNBA was developed as an inhibitor for the human KMO,18 the high efficiency against the insect enzyme is remarkable and points to a highly conserved biocatalyst in the tryptophan-kynurenine pathway. Inhibition of the kynurenine aminotransferase (KAT) by 3-(2-aminobenzoyl)-propionate as described by Rossi27 was not successful, but resulted in an enhanced mortality of the larvae.
8-HQA is formed by the insect and not by intestinal microorganisms
To determine the source of 8-HQA, the diet of S. littoralis was supplemented with antibiotics (erythromycin, polymyxin, tetracycline and vancomycin) and [2H5]-Trp. The broad spectrum of antibiotics guaranteed a bacteriostatic and bactericidal impact on gram-positive and gram-negative bacteria present in the larval gut. After two days, the regurgitate was collected and plated onto LB agar to count the colony-forming units (CFUs). As expected, a strong decrease (factor 104) of the CFUs was observed in the presence of antibiotics (Fig. 6a). On the other hand, the formation of 8-HQA was not affected, suggesting that the intestinal bacteria are not involved in the biosynthesis of 8-HQA (Fig. 6b).Conclusions
For the first time large quantities of 8-HQA have been found in the gut fluid of several Noctuid larvae (Fig. 2). The concentrations can be substantial (up to 5 mmol), but the biological function has as yet not been described. The biosynthesis proceeds via kynurenine and 3-hydoxykynurenin, as the production is effectively blocked by the KMO-inhibitor mNBA. Xanthurenic acid and quinaldic acid are not precursors, demonstrating that the last steps from 3-hydroxy-kynurenine towards 8-HQA are not following known bio-synthetic routes (Scheme 1) but utilize an alternative pathway (Scheme 2). As 8-HQA is a strong chelator of Fe(II)19 its function as a siderophore is feasible. Our data demonstrate that the insect itself produces continuously large amounts of 8-HQA from Trp of the ingested diet. Accordingly, the concentration of free Fe(II) in the gut should be low (stability of the complexes along the pH-gradient along the gut has to be considered).17,19 Thus, the growth of strongly Fe-dependent bacteria should be reduced or inhibited, as already shown by in vitro experiments23 using 8-HQA at typical gut concentrations (ca. 1 mmol). To which extent such Fe-mediated control of the gut microbiome is utilized by the insects remains to be established.Experimental
Insects
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 (L
8 (L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D) photoperiod. S. frugiperda, H. armigera, H. subflexa, H. virescens, and P. xylostella were kindly provided by the Department of Entomology and larvae of M. sexta were kindly provided by the Department of Biochemistry, Max-Planck-Institute for Chemical Ecology, Jena, Germany. Y. evonymellus larvae were collected in Eschweiler, Germany. Malacosoma sp. and O. cerasi were collected in Jena, Germany. A. urticae was collected in Heubach-Lautern, Germany.
D) photoperiod. S. frugiperda, H. armigera, H. subflexa, H. virescens, and P. xylostella were kindly provided by the Department of Entomology and larvae of M. sexta were kindly provided by the Department of Biochemistry, Max-Planck-Institute for Chemical Ecology, Jena, Germany. Y. evonymellus larvae were collected in Eschweiler, Germany. Malacosoma sp. and O. cerasi were collected in Jena, Germany. A. urticae was collected in Heubach-Lautern, Germany.
For incubation experiments 10–14 day old larvae of S. littoralis were reared on a chemically defined diet containing only casein as the protein source.29 The Trp-depleted diet contained hydrolyzed casein (Carl Roth), which is stated as Trp-free. This diet was supplemented with Trp at 1.95 (normal) or 19.5 mmol g−1 (rich).
Significant reduction of larval gut bacteria was achieved by feeding sub-lethal concentrations of antibiotics. A mixture of erythromycin (73 μg ml−1, Sigma-Aldrich), polymyxin (500 ng ml−1, Sigma-Aldrich), tetracycline (25 μg ml−1, Carl Roth) and vancomycin (10 μg ml−1, Sigma-Aldrich) was used. For each larva 100 μl were applied daily onto a 1.0 g chemically defined normal-Trp diet.
Inhibitor experiments were done by feeding a mNBA (synthesized according to Pellicciari20) impregnated bean diet (1 mg or 500 μg mNBA respectively per g standard bean diet) to larvae starting at a larval age of 8 days. The diet was replaced daily; control groups were fed with the same amount (1 g day−1) of mNBA free diet. Regurgitate was collected daily before replacing the diet. Regurgitate of 3 individuals per group (5 groups of treatment and control respectively) was pooled and 8-HQA was quantified by HPLC-MS.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm, the supernatant was evaporated to dryness, re-dissolved in 100 μl methanol and used for HPLC-MS analysis.
000 rpm, the supernatant was evaporated to dryness, re-dissolved in 100 μl methanol and used for HPLC-MS analysis.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm. The supernatant was evaporated to dryness, re-dissolved in 50 μl methanol and used for HPLC-MS analysis.
000 rpm. The supernatant was evaporated to dryness, re-dissolved in 50 μl methanol and used for HPLC-MS analysis.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm. The supernatant was transferred into a GC vial with a micro insert and injected without further clean-up into HPLC-MS or stored at −20 °C for further analysis.
000 rpm. The supernatant was transferred into a GC vial with a micro insert and injected without further clean-up into HPLC-MS or stored at −20 °C for further analysis.
8-HQA in biological samples was quantified in APCI+ mode (vaporizer temperature: 430 °C) using a Grom™ Sil column, ODS-3 CP, 120 Å, 3 μm, 125 × 2 mm (Grace). Gradient elution at 0.2 ml min−1: 0% B for 3 min, up to 100% in 27 min, 100% B for 15 min, 0% B for 15 min was used to separate the compounds. Kynurenine (not detected in the biological samples) served as an internal standard.
3-Hydroxykynurenine in biological samples was quantified in APCI+ mode using a Luna NH2 column 100 Å, 5 μm, 150 × 2 mm (Phenomenex). Gradient elution at 0.2 ml min−1: 100% B for 3 min, down to 0% in 27 min, 0% B for 2 min, 100% B for 13 min was used for analysis. Kynurenic acid served as the internal standard.
3-Hydroxykynurenine was not stable under these conditions and a modified method of Trachtenberg et al.32 was used. 3-Hydroxykynurenine (0.35 μmol) was dissolved in conc. deuterium chloride in deuterium oxide (340 μl) and the solution was stirred at 100 °C overnight. After removal of the solvent in vacuo, the degree of deuteration was quantified by 1H NMR.
[2H]-kynurenic acid (4-hydroxyquinoline-2-carboxylic-3-d acid). 1H NMR (500 MHz, DMSO-d6) δ ppm 6.53 (s, 0.54 H) 7.27 (ddd, J = 8.05, 6.96, 1.09 Hz, 1 H) 7.59 (ddd, J = 8.42, 6.93, 1.37 Hz, 1 H) 7.92 (d, J = 8.48 Hz, 1 H) 8.04 (dd, J = 8.13, 1.49 Hz, 1 H).
[2H4]-xanthurenic acid (4,8-dihydroxyquinoline-2-carboxylic-3,5,6,7-d4 acid). 1H NMR (500 MHz, D2O) δ ppm 6.72 (s, 0.79 H) 7.04 (d, J = 7.56 Hz, 0.39 H) 7.18 (dd, J = 8.25, 7.79 Hz, 1 H) 7.44 (d, J = 8.25 Hz, 0.53 H).
[2H3]-3-hydroxykynurenine (2-amino-4-(2-amino-3-hydroxyphenyl-5-d)-4-oxobutanoic-3,3-d2 acid). 1H NMR (500 MHz, DCl in D2O, 333 K) δ ppm 4.54 (s, 1 H) 7.36 (s, 1 H) 7.65 (s, 1 H).
NMR indicated for Kynurenic acid (DMSO-d6) a proton exchange at C3 (46%) whereas Xanthurenic acid (D2O) showed a random label that was quantified relative to H-C6 (set to 0% exchange) H-C3 (21%), H-C5 (61%) H-C7 (47%). 3-Hydroxykynurenine (DCl in D2O) was completely labeled at C5′ and C3. Hydrogen atoms at C4′ and C6′ were not affected.
Acknowledgements
We thank Angelika Berg for laboratory assistance. This work was supported and financed by the Max Planck Society and the excellence graduate school “Jena School for Microbial Communication” (JSMC).Notes and references
- H. Schildknecht, H. Birringer and D. Krauß, Z. Naturforsch., 1969, 24b, 28–47 Search PubMed.
- Y. Umebachi and K. Tsuchitani, J. Biochem., 1955, 42, 817–824 CAS.
- A. Wessing and D. Eichelberg, Z. Naturforsch., 1968, 23b, 376–386 Search PubMed.
- O. Billker, V. Lindo, M. Panico, A. E. Etienne, T. Paxton, A. Dell, M. Rogers, R. E. Sinden and H. R. Morris, Nature, 1998, 392, 289–292 CrossRef CAS PubMed.
- J. Y. Li and G. Y. Li, Insect Biochem. Mol. Biol., 1997, 27, 859–867 CrossRef CAS.
- J. Y. Li, B. T. Beerntsen and A. A. James, Insect Biochem. Mol. Biol., 1999, 29, 329–338 CrossRef CAS.
- Q. Han, B. T. Beerntsen and J. Y. Li, J. Insect Physiol., 2007, 53, 254–263 CrossRef CAS PubMed.
- D. Mossialos, J. M. Meyer, H. Budzikiewicz, U. Wolff, N. Koedam, C. Baysse, V. L. Anjaiah and P. Cornelis, Appl. Environ. Microbiol., 2000, 66, 487–492 CrossRef CAS.
- V. I. Frolova, M. M. Taig, A. D. Kuzovkov and G. S. Katrukha, Antibiotiki, 1981, 26, 323–327 CAS.
- S. Breiding-Mack and A. Zeeck, J. Antibiot., 1987, 40, 953–960 CrossRef CAS.
- F. Lombo, A. Velasco, A. Castro, F. de la Calle, A. F. Brana, J. M. Sanchez-Puelles, C. Mendez and J. A. Salas, ChemBioChem, 2006, 7, 366–376 CrossRef CAS PubMed.
- P. K. Macnicol, Biochem. J., 1968, 107, 473–479 CAS.
- J. Mendez and A. Masa, Phytochemistry, 1975, 14, 1136–1137 CrossRef CAS.
- A. Schennen and J. Holzl, Planta Med., 1986, 52, 235–236 CrossRef PubMed.
- A. N. Starratt and S. Caveney, Phytochemistry, 1996, 42, 1477–1478 CrossRef CAS.
- E. S. Brown and C. F. Dewhurst, Bull. Entomol. Res., 1975, 65, 221–262 CrossRef.
- F. Apone, A. Ruggiero, A. Tortora, A. Tito, M. R. Grimaldi, S. Arciello, D. Andrenacci, I. Di Lelio and G. Colucci, J. Insect Sci., 2014, 14, 1–16 CrossRef.
- D. Spiteller, PhD Thesis, Universität Jena, 2002 Search PubMed.
- M. Funke, PhD Thesis, Universität Jena, 2009 Search PubMed.
- A. T. Groot, M. Marr, G. Schofl, S. Lorenz, A. Svatos and D. G. Heckel, Front. Zool., 2008, 5, 20 CrossRef PubMed.
- A. Barthel, I. Kopka, H. Vogel, P. Zipfel, D. G. Heckel and A. T. Groot, Proc. R. Soc. B, 2014, 281, 20140897 CrossRef PubMed.
- L. J. Gahan, Y. Pauchet, H. Vogel and D. G. Heckel, PLoS Genet., 2010, 6, e1001248 CAS.
- J. Pesek, PhD Thesis, Universität Jena, 2010 Search PubMed.
- P. M. Dewick, Medicinal Natural Products, John Wiley & Sons, Ltd, 1st edn, 1997 Search PubMed.
- P. Nuhn, Naturstoffchemie. Mikrobielle, pflanzliche und tierische Naturstoffe, Hirtzel, Stuttgart, 2006 Search PubMed.
- R. Pellicciari, B. Natalini, G. Costantino, M. R. Mahmoud, L. Mattoli, B. M. Sadeghpour, F. Moroni, A. Chiarugi and R. Carpenedo, J. Med. Chem., 1994, 37, 647–655 CrossRef CAS.
- F. Rossi, S. Garavaglia, G. B. Giovenzana, B. Arca, J. Li and M. Rizzi, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 5711–5716 CrossRef CAS PubMed.
- M. Funke, R. Büchler, V. Mahobia, A. Schneeberg, M. Ramm and W. Boland, ChemBioChem, 2008, 9, 1953–1959 CrossRef CAS PubMed.
- Y. Pauchet, A. Muck, A. Svatos, D. G. Heckel and S. Preiss, J. Proteome Res., 2008, 7, 1629–1639 CrossRef CAS PubMed.
- A. P. Davenport and D. J. Wright, J. Econ. Entomol., 1985, 78, 1151–1153 CAS.
- V. Derdau, J. Atzrodt and W. Holla, J. Labelled. Compd. Radiopharm., 2007, 50, 295–299 CrossRef CAS.
- E. N. Trachtenberg and T. J. Whall, J. Org. Chem., 1972, 37, 1494–1499 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ob01857e |
| This journal is © The Royal Society of Chemistry 2015 |