Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Structure of graphene oxide membranes in solvents and solutions†
Alexey
Klechikov
a,
Junchun
Yu
a,
Diana
Thomas
b,
Tiva
Sharifi
a and
Alexandr V.
Talyzin
*a
aDepartment of Physics, Umeå University, Umeå, SE-901 87, Sweden. E-mail: alexandr.talyzin@physics.umu.se
bMAX-lab, Lund University, POB 118, SE-22100 Lund, Sweden
First published on 19th August 2015
Abstract
The change of distance between individual graphene oxide sheets due to swelling is the key parameter to explain and predict permeation of multilayered graphene oxide (GO) membranes by various solvents and solutions. In situ synchrotron X-ray diffraction study shows that swelling properties of GO membranes are distinctly different compared to precursor graphite oxide powder samples. Intercalation of liquid dioxolane, acetonitrile, acetone, and chloroform into the GO membrane structure occurs with maximum one monolayer insertion (Type I), in contrast with insertion of 2–3 layers of these solvents into the graphite oxide structure. However, the structure of GO membranes expands in liquid DMSO and DMF solvents similarly to precursor graphite oxide (Type II). It can be expected that Type II solvents will permeate GO membranes significantly faster compared to Type I solvents. The membranes are found to be stable in aqueous solutions of acidic and neutral salts, but dissolve slowly in some basic solutions of certain concentrations, e.g. in NaOH, NaHCO3 and LiF. Some larger organic molecules, alkylamines and alkylammonium cations are found to intercalate and expand the lattice of GO membranes significantly, e.g. up to ∼35 Å in octadecylamine/methanol solution. Intercalation of solutes into the GO structure is one of the limiting factors for nano-filtration of certain molecules but it also allows modification of the inter-layer distance of GO membranes and tuning of their permeation properties. For example, GO membranes functionalized with alkylammonium cations are hydrophobized and they swell in non-polar solvents.
1 Introduction
Thanks to the hydrophilic structure, graphite oxides can be easily dispersed in water to give stable solutions of single-layered graphene oxide (GO).1 Simple evaporation, filtration or spin-coating procedures have been used to deposit multilayered materials from the GO solutions, e.g. thin films2–4 or free standing foils.1,5 Free standing GO foils of micrometer thickness are named either as papers,5–7 or as membranes while being essentially the same material.8–12 Unusual vapor, gas and liquid permeation properties of GO membranes have recently attracted a lot of attention.10,13–15Multilayered GO materials were already well known in the 1960s under the name “graphite oxide” membranes.16 In particular, the structure, impermeability by gases, water permeation rates and membrane potentials of the GO membranes in several salts were reported in the pioneering study by H. P. Boehm et al.1 Already these early studies of the GO membranes have demonstrated their potential for selective ion permeation, gas barrier properties, nano-filtration1 and water desalination using the reverse osmosis method.17 H. P. Boehm et al. were also the first who suggested that molecules and ions travel through a “labyrinth path” of GO interlayers.1 Therefore, the distance between individual graphene oxide sheets modified by hydration/solvation is the key parameter to explain and predict permeation of GO membranes.
It is known that GO papers and membranes show many properties similar to graphite oxides, e.g. a very similar distance between graphene oxide sheets in the dried state and the ability to swell in polar solvents.5,12 However, recent studies revealed that this similarity is only partial. For example, GO membranes show fast water permeation and decreased permeability by alcohols.8,13,18,19 It is not a trivial fact taking into account that precursor graphite oxides can be intercalated equally well by both water and ethanol with amounts corresponding to several monolayers.20,21
Our recent structural studies have shown that the GO membranes exhibit unique hydration/solvation properties, not found in precursor graphite oxides. In particular, both GO membranes and graphite oxides are hydrated very similarly in pure water, but insertion of ethanol and methanol into the GO membrane structure is hindered, being limited to one monolayer while in the graphite oxide powders up to 3–4 layers of these solvents can be inserted.22 The quantitative evaluation of selectivity in water/ethanol absorption by GO films was also performed using neutron reflectivity.19 The decreased ability of GO membranes towards intercalation with some polar solvents is the key reason to explain their selectivity in permeation of liquids. However, structural data for swelling of GO membranes in solvents other than alcohols have not been available so far.
Many recent studies have also aimed at demonstration of selective permeation of certain ions and molecules through GO membranes for nano-filtration applications.23–28 Some studies suggested simple models, considering the interlayer distance in GO membranes (“channels”) to be equal to the value observed for membranes immersed in pure water (∼12–13 Å) without assuming the effects of solutes on the GO lattice. In this case the size of hydrated ions or molecules was speculated to be the only parameter which determines the permeability of a GO membrane.11 We believe that such models are over-simplified and can be applied only to limited range of salts or molecules. It is very likely that several factors other than the size of the hydration shell can affect permeation of ions/molecules through GO membranes, first of all their chemical reactivity towards GO functional groups, the ability of solutes to intercalate and expand the GO structure, and the dependence of GO swelling on pH and concentration of dissolved molecules. Indeed, larger ions and molecules were reported to permeate through the GO membranes.1,26
Surprisingly, none of the previously published studies reported on the inter-layer distance of GO membranes measured directly in solvents and solutions tested for permeation. At the same time, precursor graphite oxides are known to exhibit easy intercalation with many large organic molecules. Significant expansion of the inter-layer distance (up to 2–6 nm) is very common for graphite oxides immersed in various solvents and solutions depending on the nature of dissolved ions/molecules and their concentration, e.g. larger alcohols and various amines.29–32 An extreme example of concentration dependence is GO swelling in basic solutions. Graphite oxide shows the cell parameter of about d(001) = 11–12 Å in 0.05–2 M NaOH solutions but dissolves completely in 0.01 M solution (d = ∞).1 The effect of dissolving in alkali solutions is usually explained by deprotonation of COOH and phenolic groups and electrostatic repulsion of negatively charged GO planes.33 Complete dissolving of graphite oxides in NaOH is regularly used to prepare solutions of less defected single-layered GO without the need for sonication.22,33 Permeation of NaOH through GO membranes was found to be faster compared to permeation of some Na salts indicating that the GO membrane structure is expanded by immersion into alkali solutions. However, the structure of GO membranes in these solutions was not characterized.23
As noted above, the properties of GO membranes are only partly similar to those of powdered graphite oxides and the results obtained for powders should not be directly applied to GO membranes. Therefore, new studies are required to verify the structural properties of GO membranes in various solvents, in ionic and molecular solutions in order to predict and understand the membrane permeation properties.
In this study we report an in situ synchrotron X-ray diffraction study of the GO membrane structure in several common organic solvents, in solutions of several molecular species and in solutions of common salts. It is found that solvation/hydration of the GO membrane structure cannot be predicted using the data obtained on graphite oxides. The inter-layer distance of GO membranes shows a rather broad variation depending on the type of solvent, chemical nature of the solutes and intercalation of dissolved species into the GO lattice. In particular, the limitations of GO membranes for nano-filtration applications are demonstrated on examples of some basic salts, amines and alkylammonium salts.
2 Experimental
GO membranes were prepared using the commercial graphite oxide powder purchased from ACS Materials. This graphite oxide was synthesized using the Hummers method and C/O = 2.47 was determined by XPS. Detailed characterization of this graphite oxide, both in the dry state and under hydration/solvation conditions was reported in our previous studies.21,34 GO membranes prepared from this precursor have also been characterized in our recent studies using a variety of methods, including X-ray diffraction in water, methanol, ethanol and water–alcohol mixtures.22The synthesis of the membranes included preparation of graphene oxide dispersion by sonication of GO powder in water for 12–16 hours, centrifugation to remove few-layered (or unexfoliated) particles and vacuum driven filtration through alumina membranes (Anodisc 25, 0.2 μm, diameter: 25 mm from Whatman GmbH). Finally, the membranes were air dried directly on alumina filters and separated from the support. The membrane sample studied here was air dried in the free standing state for 3 weeks and cut into many small pieces directly before XRD measurements. Some experiments were also repeated 6 months later with very similar results. Slow drying of GO membranes was observed in our previous study and explained by the fact that water from the inner part of the membranes needs to go through a labyrinth of interlayer spacings before it reaches the surface and evaporates.22
The structural study of GO membranes in various solvents and solutions was performed by X-ray diffraction using synchrotron radiation at MaxLab, beamline I711, Sweden. The radiation wavelength (0.99148 Å) was calibrated using a LaB6 standard. Fit2D software was used to integrate the diffraction images into diffraction patterns. Depending on the quality of data, particular broadness and peak shape, the precision of peak positions determined using the d(001) was within 0.01–0.1 Å.
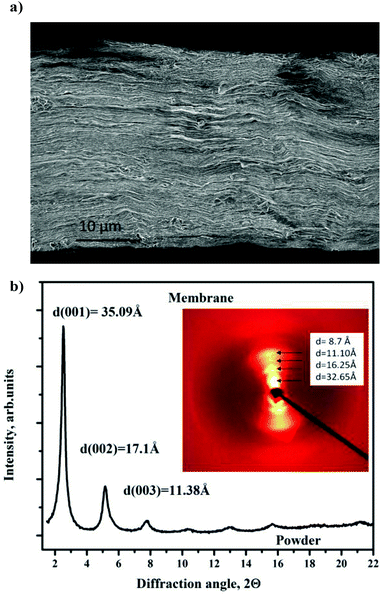
X-ray diffraction images were collected from powder samples loaded in glass capillaries using the transmission geometry. Very strong intensity of synchrotron radiation allows recording of high quality XRD images for all studied GO/solvent systems. The structure of GO membranes in the hydrated state was studied in situ using 0.5–0.7 mm wide and 1–2 mm long pieces inserted into a glass capillary, immersed in excess of the liquid and aligned relative to the beam in transmission geometry to record 2D XRD images. The same membrane sample was used for all in situ synchrotron radiation XRD experiments described here. The thickness of this sample was measured under ambient conditions using a digital micrometer with flat 5 mm diameter probes (±0.2 μm). The thickness of this membrane sample was also verified using direct Scanning Electron Microscopy (SEM) images (see Fig. 1a) to be about 3–4 μm. Note that the thickness measured under ambient conditions depends on humidity conditions.
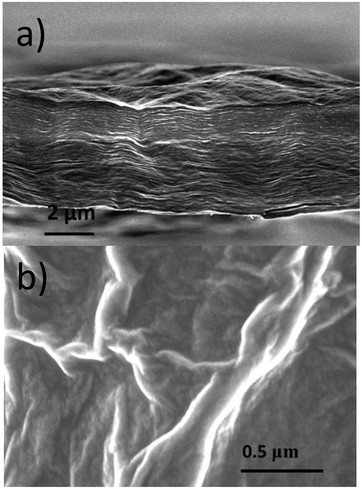 | ||
| Fig. 1 SEM images recorded from the GO membrane sample: (a) edge view showing lamellar texture with fragments of the wavy shaped membrane surface and (b) “planar” view with typical wrinkles. | ||
Using small pieces of the same membrane for most of the experiments allows to compare GO structures in various solvents independently on the possible effects of sample aging, drying conditions, variations of precipitation conditions etc. The samples were loaded into solvent immediately prior to the start of measurements. Typically loading and mounting the sample takes about 5–10 minutes, and the recording time per image is 30–180 s. Several images were usually collected from each sample to verify that the saturation state is achieved. In the case of slower swelling, new images were recorded several hours (up to 24 hours) later.
Some other but very similarly prepared membrane samples were used for experiments with intercalation of alkylammonium ions. For these experiments larger pieces of GO membranes (discs with diameter up to 25 mm) were immersed into 50 mg ml−1 solution of hexadecyltrimethylammonium chloride (HDTMA) in methanol and heated at 60 °C for 5 days according to the procedure described in ref. 31. Swelling tests of these membranes were performed on pieces with ∼1 cm2 size.
Larger pieces of membranes (∼10 × 10 mm) were also tested for swelling in several solvents (water, 1 M NaCl, toluene, ethanol, acetone, 1-methyl-2-pyrrolidinone and N,N-dimethylacetamide) using the standard diffractometer setup in the reflection mode. The inter-layer distance measured for larger area samples (∼100 mm2) were found to be very similar (within ±0.1 Å) to the values found using synchrotron radiation experiments with smaller pieces (1–5 mm2) and transmission geometry. We also tested in a similar way the membrane sample dried in air for 14 months and for several key solvents obtained qualitatively similar swelling (see the ESI†). The maximal swelling state of GO membranes was found to be not dependent on the area of studied samples within the 1–100 mm2 range.
In the later experiments membranes were characterized using a Siemens D5000 diffractometer (CuKα) in reflecting mode.
3 Results
The morphology of the typical “as-deposited” air dried membrane sample was verified using Scanning Electron Microscopy (SEM) as shown in Fig. 1 for the regions of the cut edge and “planar” surface.The edges of the membrane exhibit densely packed paper-like wavy shape lamellae. The typical thickness of these lamellae corresponds approximately to 15–30 layers of GO. The “planar” surface of the membrane is actually not flat with a network of wrinkles distributed over the whole surface. These types of shapes for the edges and surface of the membrane could be expected since the samples are prepared in water solution and then slowly dried from the fully hydrated state. A very similar lamellar texture of GO membrane samples preserved even after a prolonged reaction in the methanol solution of alkylammonium salts and drying (see Fig. 7a below).
3.1 GO membranes in polar solvents
Examples of XRD patterns recorded from solvent immersed graphite oxide powder and GO membranes at ambient temperature are shown in Fig. 2 and 3. Very different swelling of GO membranes compared to swelling of precursor graphite oxide was observed for most of the tested solvents, but some examples of similar swelling were also found.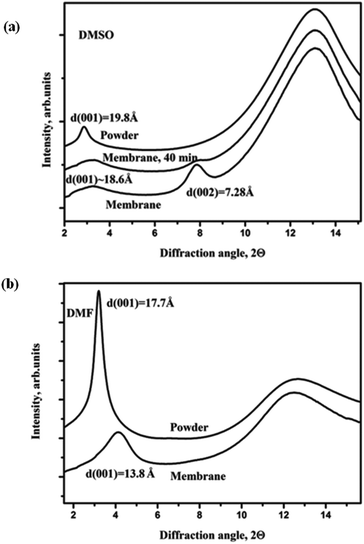 | ||
| Fig. 3 XRD patterns recorded from graphite oxide powder and GO membrane immersed in excess amounts of liquid (a) DMSO and (b) DMF at ambient temperature. | ||
Graphite oxides and GO membranes do not swell in non-polar solvents (e.g. toluene) but easily get intercalated by polar solvents. The amount of solvent inserted into the GO structure correlates with the increase of the interlayer distance and can be approximately evaluated using the change of d(001). Other reflections, those from in-plane lattice spacings are not affected by solvation and hydration.35,36 The interlayer distance of graphite oxides depends on the temperature and corresponds typically to 1–3 solvent monolayers.21
Note that d(001) of the Hummers GO structure is often observed to change gradually due to the effects of interstratification and non-uniform hydration of inter-layers on the nanometer scale (intrastratification).37 The Brodie graphite oxide is known to show step-like transitions between one and two layer solvate states upon variations of pressure or temperature.34,36,38 Therefore, the thickness of the solvent monolayer can be determined using phase transitions in Brodie graphite oxide, but for Hummers graphite oxide studied here the number of intercalated layers can be discussed only conventionally, assuming an approximate relationship between the change of the inter-layer distance and the size of solvent molecules.
As expected, the solvents which are not intercalated into the precursor powder graphite oxide structure (e.g. toluene) also do not intercalate GO membranes. For example, a GO membrane immersed in ethylene glycol showed the interlayer distance d(001) = 7.7 Å similarly to the air exposed solvent free membrane sample. The interlayer spacing of the GO membrane actually decreased slightly to 7.5 Å after forty minutes of immersion in ethylene glycol. The air exposed GO samples unavoidably absorb some moisture from air proportionally to humidity. The ethylene glycol in excess amounts extracts water from the GO membrane, the process of dehydration being slow due to diffusion of water through the labyrinth of interlayers. The value of 7.5 Å can be used with good approximation as a reference for the inter-layer distance in the solvent free state of the GO membrane.
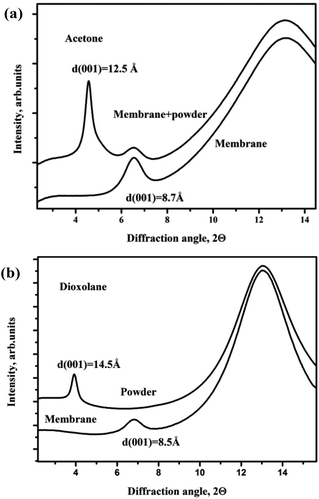
Several polar solvents were then tested for swelling with graphite oxide powder and GO membrane. Dioxolane intercalates graphite oxide powder with expansion of the interlayer distance by ∼7 Å compared to the solvent free state reaching d(001) = 14.5 Å. The expansion of the graphite oxide lattice corresponds to intercalation of at least two dioxolane layers (thickness of one dioxolane layer is ∼4.1 Å). In contrast, the solvent immersed GO membrane shows much lower d(001) = 8.5 Å, thus exhibiting strongly hindered insertion of dioxolane, Fig. 2(a). The increase of the inter-layer distance by only ∼1 Å is smaller than the size of the dioxolane molecule. Therefore, the amount of the intercalated solvent certainly does not exceed one monolayer. This type of difference between insertion of solvent into membranes and powder (named henceforth as Type I) is similar to swelling of GO in alcohols reported in our earlier studies.22
The Type I intercalation was also found for several other tested solvents. Fig. 2(b) shows XRD patterns recorded from the GO membrane and powder immersed in excess of acetone. In this experiment the diffraction image was first recorded from the membrane and then the powder was added directly to the same capillary, thus the integrated pattern shown in Fig. 1(b) allows the comparison of 001-peak positions in the same pattern.
Comparing the d(001) values for the acetone immersed powder and membrane shows that the powder is intercalated by at least two solvent layers while the membrane only by one. Note that Type I intercalation is not connected to slow kinetics, some samples were verified 12–24 hours after immersion into the solvent and did not show further increase of the interlayer distance. Type I intercalation was also found for acetonitrile (d(001) = 14.0 Å and d(001) = 9.0 Å for powder and membrane respectively), and for chloroform: d(001) = 14.6 Å for powder and d(001) = 8.8 Å for the membrane. The GO membrane sample showed also a very small change of the structure when immersed in trimethylamine (d(001) = 8.2 Å) whereas graphite oxide powder showed rather complex and inhomogeneous intercalation: three peaks with d-spacings 12.7 Å, 11.0 Å and 8.2 Å were observed.
A plausible explanation of Type I intercalation behavior could be that only a fraction of the membrane layers is intercalated with solvent while penetration into other layers is hindered. As a result, the averaged interlayer distance increases only by ∼1–1.5 Å compared to the dried state and does not exceed 9 Å even after prolonged exposure to the liquid solvent. It should also be noted that the first layer of solvent is always intercalated into the GO structure with lattice expansion smaller compared to the size of solvent molecules reflecting the presence of voids in the inhomogeneously oxidized GO structure which can be filled without causing strong expansion of the lattice.33,37
It can be anticipated that small expansion of the GO lattice typical of Type I intercalation will result in low or zero permeation of these solvents through membranes.
A different type of intercalation is observed for the GO membrane in DMSO solvent, Fig. 3(a).
The d(001) of graphite oxide powder in DMSO is 19.8 Å reflecting intercalation of several solvent layers. The membrane sample showed a peak of pristine GO (d(001) = 7.3 Å) but also the presence of strong and broad diffuse scattering with higher d-spacings in the first scan recorded a few minutes after solvent loading. The peak from the non-intercalated membrane layers became significantly lower after 40 minutes of solvent immersion and completely disappeared after ∼120 minutes. Only a rather broad and diffuse peak centered approximately at 18.8 Å is found in XRD images recorded after prolonged immersion in DMSO. Fig. 3(a) shows that the GO membrane can be intercalated by DMSO with lattice expansion similar to the one typical of powders but with much slower kinetics. Until now this type of intercalation was never observed for solvents other than water;10,22 it will be named in following discussions as Type II. The Type II intercalation was also observed for two other solvents: (a) N,N-dimethylacetamide and (b) 1-methyl-2-pyrrolidinone (Fig. 2S in the ESI†).
Swelling of the graphite oxide powder and GO membrane in dimethylformamide (DMF) (Fig. 3(b)) showed rather individual behavior. The interlayer distance of the GO membrane in DMF is d(001) = 13.8 Å. The 001 peak is rather broad and asymmetric indicating the presence of layers with even stronger lattice expansion. As shown in Fig. 3(b), the GO membrane lattice in DMF expands less compared to the GO powder (d(001) = 17.7 Å) but stronger compared to Type I intercalation (d(001) = ∼9 Å). The thickness of the DMF layer intercalated into the GO structure is known from our earlier experiments with the Brodie GO powder and is equal to ∼4.4 Å.38 Therefore, expansion of the GO membrane lattice by 7.3 Å corresponds to insertion of two DMF layers whereas the d(001) = 17.7 Å observed for the precursor GO powder corresponds to insertion of three solvent layers.
It can be concluded that intercalation of polar solvents into the GO membrane structure is not trivial and cannot be predicted from swelling properties of graphite oxide powder. The difference between Type I and Type II swelling of GO membranes should be reflected in their permeation properties. Based on our structural data we can speculate that permeation of solvents with Type I swelling should be slow or nearly absent, similarly to earlier reported permeation of alcohols. For DMSO (Type II swelling) relatively rapid permeation can be expected as it intercalates the GO membrane structure with 3 solvent layers similarly to water. The case of DMF solvent is somewhat intermediate: it can be anticipated that DMF would be permeating the GO membrane but at slower rates.
3.2 GO membranes in aqueous solutions
The d(001) of the studied GO membrane sample in pure water was found to be 12.3 Å. This value is slightly lower compared to d(001) = 12.7 Å found for pristine GO powder. Note that freshly prepared membranes are not stable and delaminate almost completely after few hours of water immersion. The membranes need to be dried in air for minimum 2–3 weeks while slow drying corresponds to gradual decrease of the inter-layer distance.22 Well dried membranes are stable in water exhibiting well defined spots from 001 reflection in XRD images but also always strong diffuse scattering at low angles which demonstrate the presence of disordered “channels” with a continuous distribution of inter-layer distances up to at least 30–40 Å.22Swelling of GO membranes and powder was verified in several solutions which can be divided into the following groups: inorganic salts (acidic and basic), molecular ionic solutions, and molecular non-electrolyte solutions. It could be anticipated that dissolved species can alter the interlayer distance of GO membranes due to several possible effects: intercalation of solutes into the GO structure, change of GO sheet charging state, chemical reaction of dissolved ions and molecules with GO functional groups.
It is well known that graphite oxides are relatively stable in diluted acidic solutions (strongly acidic conditions result in GO reduction). In contrast, graphite oxides immersed in slightly basic solutions can be dispersed on single layered graphene oxide sheets.1 Our experiments demonstrate that the same trend is observed for GO membranes as well, but the membranes are somewhat more stable against delamination in basic solutions.
Both graphite oxide powder and GO membranes showed d(001) in acidic and neutral salts close or slightly higher compared to the value 12.4 Å found for pure water. For example, the GO membrane showed the following d(001) in 1 M solutions: KBr – 13.07 Å, NaCl – 12.42 Å, NH4F – 12.2 Å and in 2 M formic acid – 12.2 Å. Similar d-spacings were also found for acidic solutions in other solvents. For example, the GO membrane in 1 M methanol solutions of phenylboronic and benzene-1,4-diboronic acids showed the inter-layer distances 12.57 Å and 12.64 Å respectively.
The notable difference was that the penetration of solutions into GO membranes is slower. Powder samples typically exhibit rapid swelling with the same value of d(001) immediately after loading with solution (after 3–4 minutes) and after prolonged immersion. The GO membranes in some salts (e.g. KBr) showed a slow increase of d(001) which seems to stabilize only after some tens of minutes.
It can be concluded that for acidic solutions the size of “channels” available for permeation of GO membrane is indeed similar to the value known for pure water. This explains why certain ion size effect was observed in permeation of several ions through GO membranes in ref. 11. Note that only acidic salts were tested for permeation in this study but the effect of the ion size on the selectivity of nano-filtration was claimed to be general for any molecules and solutions.11 This claim is not valid for basic salts and solutions as demonstrated by our experiments presented below.
It is well known that graphite oxide powder exhibits strongly different d(001) depending on the concentration of NaOH.1 NaOH solution with a concentration around or below 0.01 M is used routinely to prepare solutions of single layered graphene oxide.22 Our experiments demonstrate that the same phenomena are observed also for GO membranes. The main difference is slow kinetics of GO membrane penetration by solutions. XRD patterns recorded from GO membranes in 0.01 M NaOH solution showed the presence of a d(001) peak even after 2–3 hours of immersion, with the interlayer distance of about 13.4–13.5 Å, while for powders the diffraction peaks are not visible immediately after immersion due to delamination. However, placing a small piece of membrane in 0.01 M NaOH for 24 hours resulted in dissolving, as detected by appearance of characteristic brownish color of the solution. The same experiment was repeated with other membranes which were allowed to dry in air for 1–6 months: membranes were slowly dissolved in diluted NaOH solutions. Only the membrane sample stored in air for 2.5 years exhibited no obvious dissolving in diluted NaOH solutions, but even this sample broke into pieces after light shaking of the vial (see the ESI†).
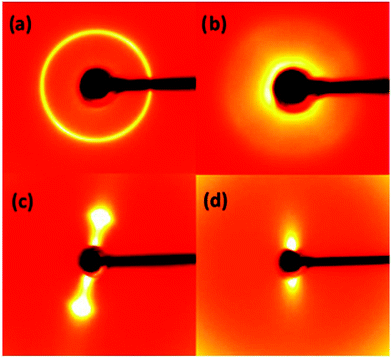
Further experiments showed that GO membranes dissolve not only in NaOH but also in other basic solutions. Fig. 4 shows XRD data recorded from precursor graphite oxide powder in NaHCO3 solutions of different concentrations. The inter-layer distance of graphite oxide powder shows very strong dependence on the concentration of salt, with d(001) which significantly exceeds the value of 12.4 Å found for GO membranes in pure water. For concentrations of NaHCO3 below 0.2 M the powder delaminated completely: the d(001) diffraction peak disappeared and only some diffuse scattering in the regions of high d-spacings could be observed. Note that the diffuse scattering is clearly visible in 2D diffraction images but can be rather difficult to identify in patterns recorded using conventional diffractometers, especially if the background subtraction is applied.11 The integrated pattern for 0.2 M solution shows diffuse scattering only as non-linear background at low angles (Fig. 4).
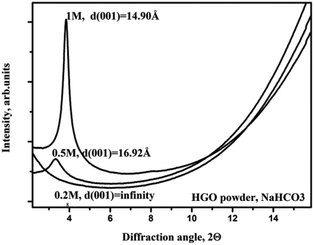 | ||
| Fig. 4 Integrated XRD patterns obtained for graphite oxide powder immersed in NaHCO3 solutions with concentrations 0.2 M, 0.5 M and 1 M. | ||
Using data shown in Fig. 4 as a reference, we verified the structure of the GO membrane in the 0.2 M NaHCO3 solution. The first pattern recorded 3–4 minutes after immersion showed d(001) = 13.4 Å, ten minutes later 13.5 Å, 105 minutes after immersion 14.0 Å, and 6 hours after immersion 14.2 Å. At this point it could be assumed that the swelling had stopped, but after 24 hours the membrane piece dissolved completely. In a control experiment, we placed a piece of the membrane into a small vial with 0.2 M NaHCO3 solution and after one day the solution obtained the characteristic brown color due to partial dissolving of GO. The membrane piece became obviously jelly-like under microscope observations and disintegrated after light shaking of the vial.
The slow delamination of the GO membrane is difficult to observe by XRD since the dissolving starts from outer layers and can proceed for many hours by removing GO sheets layer by layer while the core remains well packed. In this case, the 001 diffraction peak originated most likely from the inner part of the membrane. The reflection from the membrane core becomes weaker with time and replaced slowly by diffuse scattering from the jelly phase without a very strong shift of the peak position. However, visual analysis of the membrane piece in NaHCO3 solution leaves no doubts that slow dissolving does occur similarly to diluted NaOH solutions.
Similar experiments were also performed with sodium acetate (NaAc) which represents another example of basic salts, Fig. 5(a)–(c). Here the difference between the powder and membranes appeared to be stronger. The GO powder showed d(001) = 14.42 Å in 1 M and 0.5 M solutions, but dissolved in 0.2 M NaAc solution exhibiting no diffraction peaks immediately after solvent loading. The GO membrane in the same solution showed d(001) = 13.4 Å which did not change significantly with time. The membrane piece was observed not to dissolve even after several days of storage in the vial with 0.2 M NaAc solution. Using even smaller concentrations of NaAc also did not result in membrane dissolving. Once again, these examples demonstrate that the properties of the GO membrane cannot be described referring to data obtained on graphite oxide powder.
Results presented above indicate that the possible application of GO membranes for nano-filtration of basic salts is rather limited due to the strong concentration dependence of the inter-layer distance and complete disintegration of the membranes at certain concentrations. This problem can be demonstrated clearly on the example of LiF salt which gives basic solutions. A saturated solution of LiF was added to the powder and GO membranes which resulted in the immediate disappearance of 001 reflection for both materials. The membrane was transformed into a thick jelly-like state and diffraction images recorded 3–4 minutes after immersion exhibited only very strong diffuse scattering (Fig. 5(d)). To our knowledge, it is the first report of using LiF to prepare jelly like GO. The jelly phase is extremely soft, mechanically unstable and obviously not suitable for membrane applications.
Summarizing this section, we demonstrated that at least some basic salts and solutions are not suitable for nano-filtration using GO membranes due to the effects of delamination and strong concentration dependence of the inter-layer distance. Each salt has an individual effect on the GO membrane structure and this effect cannot be predicted from the properties of graphite oxide powders studied in the same solutions. It should be noted that the solutions of many organic molecules are basic, which will possibly limit significantly applications of GO membranes for nano-filtration.
3.3 GO membranes in alkylamines and methanol solutions of alkylammonium salts
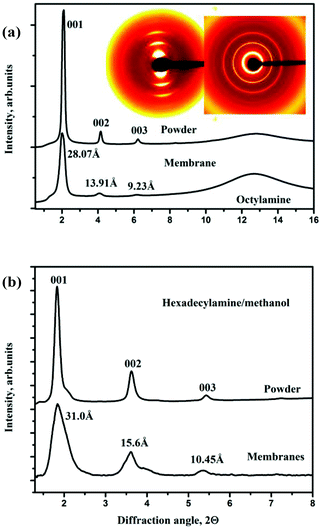
It is well known that many organic molecules are easily intercalated into the graphite oxide structure with significant expansion of the inter-layer distance. If similar intercalation occurs with the GO membrane structure as well, it will certainly affect the permeation properties rather dramatically. To demonstrate the intercalation of GO membranes by larger molecules we selected several amines both in the form of liquid solvents and solutions in alcohols. It is known that these molecules can be easily intercalated into precursor graphite oxide powders with very strong expansion of the inter-layer distance.32,39,40Hexylamine and octylamine are liquids under ambient conditions. As expected, a test on membrane samples showed that these molecules rapidly intercalated the GO structure with lattice expansion almost identical to that observed for powder samples (Fig. 6(a)). Remarkably the peaks from intercalated compounds are much stronger and sharper compared to pristine graphite oxide. Rather nice (00![[small script l]](https://www.rsc.org/images/entities/i_char_e146.gif) ) series of reflections with 8–10 peaks were observed for alkylamine-intercalated GO while for pristine GO only relatively broad and weak 001 and 002 peaks are observed.
) series of reflections with 8–10 peaks were observed for alkylamine-intercalated GO while for pristine GO only relatively broad and weak 001 and 002 peaks are observed.
Hexadecylamine and octadecylamine are solids under ambient conditions, for intercalation experiments these materials were dissolved in methanol (50 mg ml−1). Note that pure methanol shows Type I, strongly hindered intercalation,22 but this does not prevent intercalation of hexadecylamine molecules dissolved in methanol not only into powder but also into GO membrane samples with an interlayer distance of about 31 Å (Fig. 6(b)). Immersion of samples into octadecylamine/methanol solution also resulted in rapid intercalation of the GO membrane with an inter-layer distance of 34.5 Å. However, for this system also another set of (00![[small script l]](https://www.rsc.org/images/entities/i_char_e146.gif) ) peaks with d = 27.5 Å from a less intercalated structure was found, thus the similarity between membrane and powder samples is not complete.
) peaks with d = 27.5 Å from a less intercalated structure was found, thus the similarity between membrane and powder samples is not complete.
Another example of large molecule intercalation into the GO lattice was demonstrated using the solution of hexadecyltrimethylammonium chloride (HDTMA). Exposing graphite oxide powder to methanol or ethanol solutions of this salt resulted in immediate expansion of the graphene oxide lattice with an increase of inter-layer distance up to ∼35 Å. Interestingly, the alkylammonium ions are not rigidly attached to GO sheets if the reaction is performed at ambient temperature. Washing the samples with methanol completely removed the intercalated molecules and pristine GO could be recovered. Similar experiments with GO membranes showed that intercalation is also observed but becomes much slower compared to powders and the resulting material is not crystalline. Overnight soaking of the GO membrane in methanol solution of HDTMA (50–100 mg ml−1) resulted in amorphisation of the material which exhibited only broad diffuse scattering and the absence of sharp diffraction peaks.
We also tested the procedure described earlier by Dekany et al.,31 and performed the reaction with HDTMA at 60 °C both with powders and membranes. In this case, the expanded structure preserves even when methanol solution is removed, the alkylammonium ions are attached to the GO sheets. Intercalation of GO membranes and powders at 60 °C appeared to be very similar, Fig. 7. Note that the lamellar texture of the GO membrane reacted with HDTMA in methanol solution and air-dried (Fig. 7a) is very similar to the texture of pristine air dried GO membranes prepared using water solution (Fig. 1a). The expanded lattice with a set of (00![[small script l]](https://www.rsc.org/images/entities/i_char_e146.gif) ) reflections up to 8–10 order was observed for both powders and membranes (later with strong diffuse scattering); the interlayer distance is found at d(001) = 32–35 Å in good agreement with ref. 31.
) reflections up to 8–10 order was observed for both powders and membranes (later with strong diffuse scattering); the interlayer distance is found at d(001) = 32–35 Å in good agreement with ref. 31.
Moreover, in agreement with earlier studies, the structure of the GO-alkylammonium complex is found to be hydrophobic and swells in non-polar solvents. Both graphite oxide and GO membranes swell reversibly in toluene with further increase of the interlayer distance up to 46–47 Å (Fig. 8). A very strong change in the distance between GO sheets in this case can possibly originate not only from insertion of toluene but also due to the fact that the alkylammonium molecule geometry changes to more vertical. Therefore, we cannot evaluate exactly the amount of toluene inserted into the GO structure using only XRD data.
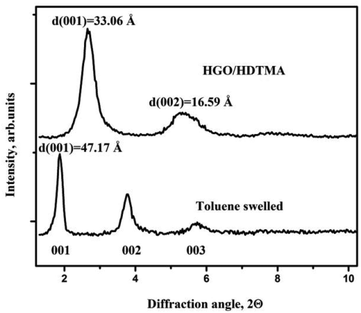 | ||
| Fig. 8 GO membrane sample intercalated with HDTMA following the procedure described in ref. 31, in the pristine and toluene swelled state (CuKα radiation). | ||
At the same time, these membranes do not show swelling in alcohols. In fact, the inter-layer distance of GO-HDTMA powder and membranes was found to decrease by ∼3 Å when immersed in methanol and ethanol. Therefore, it can be anticipated that HDTMA functionalized GO membranes will be selectively permeable by non-polar solvents such as toluene and not permeable by polar solvents like water or alcohols, which is exactly opposite to properties of pristine GO membranes.11
It can be concluded that functionalization of GO with HDTMA can possibly be used to switch membranes between permeation of polar and non-polar solvents. Direct characterization of the membrane permeation properties is out of scope of this study.
4 Discussion
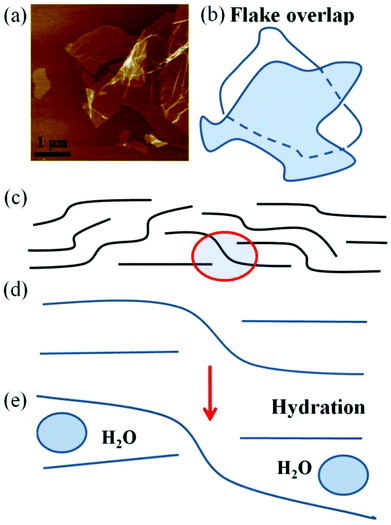
It is interesting to discuss the possible reasons for the strong difference between swelling of graphite oxide powders and GO membranes. We suggest that a simple geometrical difference is responsible for the observed effects. As found in our previous study, the dispersion of a graphite oxide precursor and deposition of a membrane do not lead to obvious changes in the chemical composition of graphene oxide sheets.22 Therefore, the reason for the difference in swelling properties should be explained by the different packing of GO flakes in the GO multilayered structure.It is known that graphite oxide grains inherit their shape from the parent graphite41 with individual graphene oxide sheets packed parallel to each other. However, this will not be the case for GO membranes. The individual GO flakes present in solution are of random irregular shape and size. Therefore, deposition of these flakes over each other into a multilayered film will unavoidably lead to overlaps and deformation of the flake shape, Fig. 9.
It is obvious that all GO flakes will be not flat and not strictly parallel to each other in GO membranes. The GO membrane model considered in several earlier studies always assumed strictly horizontal and almost parallel shape of GO flakes,1,10 whereas the real shape of GO sheets should be curved with all edges enveloped by sheets situated over and under the layers. We suggest here that the enveloped edges hinder the penetration of solvent and expansion of the GO membrane lattice due to the locking effect of the overlapped sheets illustrated schematically in Fig. 9(d) and (e). Individual GO sheets shown in this scheme cannot separate from each other without strong deformation of the shape and horizontal slip along the neighboring surfaces.
Therefore, we believe that expansion of the inter-layer distance of GO membranes under swelling conditions is likely to be controlled by the interaction of solvent molecules with overlapped edge regions of GO flakes.
It is known that dissolving of GO in basic solutions is due to deprotonation of carboxylic and phenolic groups which are terminating the GO flake edges. Therefore, the absence of GO membrane delamination in some of the studied basic solutions could be explained by the geometrical constraints (enveloping) which prevent solutions from penetration to some edges of GO flakes. If parts of the GO flake edges are not accessible for solutions, the delamination becomes more difficult.
It should be noted that the membrane samples studied here were completely immersed in solvents exposing both the cut edges and planar surface to penetration of solvent. In practical applications of membranes the permeation of solvent can be directed either across the planes11,23,24 or along the planes,27 thus using preferentially one or another path for solvent insertion. We believe that maximal separation of GO layers will be the same for both of these geometries since the entrance of solvent occurs in both cases through the interlayer space of the GO structure. It is possible that the kinetics of membrane hydration/solvation can be affected by the size of the membrane piece and how exactly the membrane was cut. However, a detailed study of the kinetics of solvent penetration into GO interlayers is out of scope of this study.
It is rather common in recent studies to cite the values of the inter-layer distance for hydrated and solvated graphite oxides when GO membranes are studied for permeation of solvents. Our experiments demonstrated that this will often lead to incorrect conclusions about the size and chemical nature of “channels” available in GO membranes for permeation of various solvents and ions. It is important to emphasize that GO sheets are essentially hydrophilic and most of their surface is covered with functional groups, evidence for the existence of interconnected hydrophobic “graphene capillaries” suggested in some studies10,11 is not available as discussed in our previous study.22 Moreover, “graphene capillaries” of 1–2 nm width are not hydrophobic due to confinement between the hydrophilic surrounding regions as shown by theoretical studies.42
It is clear that permeation properties of membranes will be strongly affected by the changes of the inter-layer distance due to the effects of swelling and intercalation of the GO structure. The size of “channels” can be similar to the one observed for GO membranes in pure water but also can be much smaller and much larger depending on the solute chemical nature. It is also interesting to mention the possible effect of flake size on geometry of overlaps. The smaller average size of flakes provides a higher total length of edges over the whole sample. On other hand, making smaller flakes should result in significant decrease of the path length for solvent diffusion between GO sheets. Therefore, the total effect of the flake size on swelling properties of GO membranes could be non-trivial.
It is also difficult to predict the size of “channels” for systems of GO with mixed polar solvents and, therefore, to evaluate the possibility of binary mixture separation using GO membranes. We demonstrated earlier that the GO membrane structure in mixed solvents (water/alcohols) shows rather non-trivial dependence on proportion between the components of the mixture.22 Pure alcohols show slow permeation through GO membranes,10 but adding water results in permeation of both solvents (e.g. propanol/water mixture).11 Therefore, using our results obtained for solutions of individual salts or solvents should not be directly used for the prediction of permeation of mixed solvents. Further studies are required to model permeation experiments with multiple ions and to explain selective adsorption/permeation of certain ions of similar sizes, observed in some recent studies.23,24
It should be noted that our experiments not only demonstrated several limiting factors for applications of GO membranes but also showed that the membranes can be tuned in many different ways using functionalization and intercalation of larger molecules. It can be expected that a large variety of membranes will be prepared in future starting from pristine GO including those suitable for permeation of polar, non-polar solvents and various solutions in these solvents. The inter-layer distance of GO membranes can obviously be tuned within a large range starting from ∼7 Å for dry membranes and up to several nanometers in the solvated or intercalated state.
In conclusion, structural characterization of GO membranes immersed in excess of several solvents and solutions was performed using in situ X-ray diffraction. The swelling properties of GO membranes in aqueous and non-aqueous solutions were compared with swelling of precursor graphite oxide. It is confirmed that GO membranes are distinctly different materials compared to precursor graphite oxide powder. The solvation of GO membranes is found to be strongly hindered in some solvents (acetone, acetonitrile, chloroform, dioxolane), the membranes exhibiting 3–6 Å smaller lattice expansion upon immersion into these liquid solvents compared to powders. For other solvents (DMSO, DMF) swelling of GO membranes is qualitatively similar to swelling of graphite oxide, except for slower kinetics. Individual approach to every system is also required for GO membranes in various types of aqueous and non-aqueous solutions. We found that GO membranes in solutions of several acidic and neutral salts exhibit a structure which only depends slightly on the nature of dissolved ions. However, using basic solutions leads to extreme swelling and delamination of the membranes. The swelling parameters of GO membranes show certain dependence on the concentration of solute (most clearly in basic solutions) and slower kinetics compared to powder. Finally we demonstrated that rather large organic molecules (liquid alkylamines and methanol solutions of alkylamines) can be intercalated into GO membranes as easily as into precursor graphite oxides. The reaction of GO membranes with alkylammonium salts was also observed for both graphite oxide and GO membranes making their structure hydrophobic and easily swelling in toluene.
Acknowledgements
This work was financially supported by the Swedish Research Council, grant no. 621-2012-3654, and by Ångpanneföreningens Forskningsstifelse. Per Hörstedt and Cheng Choo Lee are acknowledged for help with SEM imaging. Olivier Balmes is acknowledged for support at I711 beamline.Notes and references
- H. P. Boehm, A. Clauss and U. Hofmann, J. Chim. Phys. Phys.-Chim. Biol., 1961, 58, 141–147 CAS.
- N. A. Kotov, I. Dekany and J. H. Fendler, Adv. Mater., 1996, 8, 637–641 CrossRef CAS PubMed.
- N. I. Kovtyukhova, P. J. Ollivier, B. R. Martin, T. E. Mallouk, S. A. Chizhik, E. V. Buzaneva and A. D. Gorchinskiy, Chem. Mater., 1999, 11, 771–778 CrossRef CAS.
- Y. W. Zhu, W. W. Cai, R. D. Piner, A. Velamakanni and R. S. Ruoff, Appl. Phys. Lett., 2009, 95 Search PubMed.
- D. A. Dikin, S. Stankovich, E. J. Zimney, R. D. Piner, G. H. B. Dommett, G. Evmenenko, S. T. Nguyen and R. S. Ruoff, Nature, 2007, 448, 457–460 CrossRef CAS PubMed.
- N. V. Medhekar, A. Ramasubramaniam, R. S. Ruoff and V. B. Shenoy, ACS Nano, 2010, 4, 2300–2306 CrossRef CAS PubMed.
- J. M. Zhu, L. W. Zhu, Z. F. Lu, L. Gu, S. L. Cao and X. B. Cao, J. Phys. Chem. C, 2012, 116, 23075–23082 CAS.
- M. Krueger, S. Berg, D. Stone, E. Strelcov, D. A. Dikin, J. Kim, L. J. Cote, J. X. Huang and A. Kolmakov, ACS Nano, 2011, 5, 10047–10054 CrossRef CAS PubMed.
- Z. T. Luo, Y. Lu, L. A. Somers and A. T. C. Johnson, J. Am. Chem. Soc., 2009, 131, 898–899 CrossRef CAS PubMed.
- R. R. Nair, H. A. Wu, P. N. Jayaram, I. V. Grigorieva and A. K. Geim, Science, 2012, 335, 442–444 CrossRef CAS PubMed.
- R. K. Joshi, P. Carbone, F. C. Wang, V. G. Kravets, Y. Su, I. V. Grigorieva, H. A. Wu, A. K. Geim and R. R. Nair, Science, 2014, 343, 752–754 CrossRef CAS PubMed.
- C. M. Chen, Q. H. Yang, Y. G. Yang, W. Lv, Y. F. Wen, P. X. Hou, M. Z. Wang and H. M. Cheng, Adv. Mater., 2009, 21, 3007–3011 CrossRef CAS PubMed.
- R. Liu, G. Arabale, J. Kim, K. Sun, Y. Lee, C. Ryu and C. Lee, Carbon, 2014, 77, 933–938 CrossRef CAS PubMed.
- H. Li, Z. Song, X. Zhang, Y. Huang, S. Li, Y. Mao, H. J. Ploehn, Y. Bao and M. Yu, Science, 2013, 342, 95–98 CrossRef CAS PubMed.
- H. W. Kim, H. W. Yoon, S.-M. Yoon, B. K. Ahn, Y. H. Cho, H. J. Shin, H. Yang, U. Paik, S. Kwon, J.-Y. Choi and H. B. Park, Science, 2013, 342, 91–95 CrossRef CAS PubMed.
- A. Clauss and U. Hofmann, Angew. Chem., Int. Ed., 1956, 68, 522–522 CrossRef CAS PubMed.
- E. S. Bober, L. C. Flowers, P. K. Lee, D. E. Sestrich, C.-M. Wong, W. Gillam Sherman, S. Johnson and R. H. Horowitz, Research and development progress report No. 544, US Department of the Interior, reprints from the collection of the University of Michigan Library, 1970, pp. 1–113.
- Y. P. Tang, D. R. Paul and T. S. Chung, J. Membr. Sci., 2014, 458, 199–208 CrossRef CAS PubMed.
- A. Vorobiev, A. Dennison, D. Chernyshov, V. Skrypnychuk, D. Barbero and A. V. Talyzin, Nanoscale, 2014, 6, 12151–12156 RSC.
- A. V. Talyzin, S. M. Luzan, T. Szabo, D. Chernyshev and V. Dmitriev, Carbon, 2011, 49, 1894–1899 CrossRef CAS PubMed.
- S. J. You, B. Sundqvist and A. V. Talyzin, ACS Nano, 2013, 7, 1395–1399 CrossRef CAS PubMed.
- A. V. Talyzin, T. Hausmaninger, S. J. You and T. Szabo, Nanoscale, 2014, 6, 272–281 RSC.
- P. Z. Sun, M. Zhu, K. L. Wang, M. L. Zhong, J. Q. Wei, D. H. Wu, Z. P. Xu and H. W. Zhu, ACS Nano, 2013, 7, 428–437 CrossRef CAS PubMed.
- P. Z. Sun, F. Zheng, M. Zhu, Z. G. Song, K. L. Wang, M. L. Zhong, D. H. Wu, R. B. Little, Z. P. Xu and H. W. Zhu, ACS Nano, 2014, 8, 850–859 CrossRef CAS PubMed.
- K. Huang, G. P. Liu, Y. Y. Lou, Z. Y. Dong, J. Shen and W. Q. Jin, Angew. Chem., Int. Ed., 2014, 53, 6929–6932 CrossRef CAS PubMed.
- H. B. Huang, Y. Y. Mao, Y. L. Ying, Y. Liu, L. W. Sun and X. S. Peng, Chem. Commun., 2013, 49, 5963–5965 RSC.
- K. Raidongia and J. X. Huang, J. Am. Chem. Soc., 2012, 134, 16528–16531 CrossRef CAS PubMed.
- P. Z. Sun, F. Zheng, M. Zhu, K. L. Wang, M. L. Zhong, D. H. Wu and H. W. Zhu, Sci. Rep., 2014, 4 Search PubMed.
- A. R. Garcia, J. Canoruiz and D. M. C. Macewan, Nature, 1964, 203, 1063–1064 CrossRef CAS PubMed.
- F. A. Delacruz and D. M. C. Macewan, Kolloid Z. Z. Polym., 1965, 203, 36–42 CAS.
- I. Dekany, R. Kruger-Grasser and A. Weiss, Colloid Polym. Sci., 1998, 276, 570–576 CAS.
- S. Stankovich, D. A. Dikin, O. C. Compton, G. H. B. Dommett, R. S. Ruoff and S. T. Nguyen, Chem. Mater., 2010, 22, 4153–4157 CrossRef CAS.
- T. Szabo, O. Berkesi, P. Forgo, K. Josepovits, Y. Sanakis, D. Petridis and I. Dekany, Chem. Mater., 2006, 18, 2740–2749 CrossRef CAS.
- S. J. You, S. M. Luzan, T. Szabo and A. V. Talyzin, Carbon, 2013, 52, 171–180 CrossRef CAS PubMed.
- A. V. Talyzin, V. L. Solozhenko, O. O. Kurakevych, T. Szabo, I. Dekany, A. Kurnosov and V. Dmitriev, Angew. Chem., Int. Ed., 2008, 47, 8268–8271 CrossRef CAS PubMed.
- A. V. Talyzin, B. Sundqvist, T. Szabo, I. Dekany and V. Dmitriev, J. Am. Chem. Soc., 2009, 131, 18445–18449 CrossRef CAS PubMed.
- B. Rezania, N. Severin, A. V. Talyzin and J. P. Rabe, Nano Lett., 2014, 14, 3993–3998 CrossRef CAS PubMed.
- S. You, S. M. Luzan, J. Yu, B. Sundqvist and A. Talyzin, J. Phys. Chem. Lett., 2012, 3, 812–817 CrossRef CAS PubMed.
- A. B. Bourlinos, D. Gournis, D. Petridis, T. Szabo, A. Szeri and I. Dekany, Langmuir, 2003, 19, 6050–6055 CrossRef CAS.
- F. A. de la Cruz and D. M. C. McEwan, Kolloid. Z. Z. Polym., 1965, 203, 36–40 CAS.
- C. Botas, P. Alvarez, C. Blanco, R. Santamaria, M. Granda, P. Ares, F. Rodriguez-Reinoso and R. Menendez, Carbon, 2012, 50, 275–282 CrossRef CAS PubMed.
- N. Wei, X. S. Peng and Z. P. Xu, ACS Appl. Mater. Interfaces, 2014, 6, 5877–5883 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5nr04096e |
| This journal is © The Royal Society of Chemistry 2015 |