Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Few-layered titanium trisulfide (TiS3) field-effect transistors†
Alexey
Lipatov
a,
Peter M.
Wilson
a,
Mikhail
Shekhirev
a,
Jacob D.
Teeter
a,
Ross
Netusil
a and
Alexander
Sinitskii
*ab
aDepartment of Chemistry, University of Nebraska – Lincoln, Lincoln, NE 68588, USA. E-mail: sinitskii@unl.edu
bNebraska Center for Materials and Nanoscience, University of Nebraska – Lincoln, Lincoln, NE 68588, USA
First published on 15th June 2015
Abstract
Titanium trisulfide (TiS3) is a promising layered semiconductor material. Several-mm-long TiS3 whiskers can be conveniently grown by the direct reaction of titanium and sulfur. In this study, we exfoliated these whiskers using the adhesive tape approach and fabricated few-layered TiS3 field-effect transistors (FETs). The TiS3 FETs showed an n-type electronic transport with room-temperature field-effect mobilities of 18–24 cm2 V−1 s−1 and ON/OFF ratios up to 300. We demonstrate that TiS3 is compatible with the conventional atomic layer deposition (ALD) procedure for Al2O3. ALD of alumina on TiS3 FETs resulted in mobility increase up to 43 cm2 V−1 s−1, ON/OFF ratios up to 7000, and much improved subthreshold swing characteristics. This study shows that TiS3 is a competitive electronic material in the family of two-dimensional (2D) transition metal chalcogenides and can be considered for emerging device applications.
The recently discovered remarkable properties of graphene stimulated interest in other two-dimensional (2D) atomic crystals.1–3 Many of the actively studied 2D materials belong to the family of transition metal chalcogenides (TMCs).4–6 A large number of TMCs in bulk form have a layered structure with weak interlayer van der Waals interactions.2–6 The layers of TMCs can be exfoliated by different approaches to produce mono- and few-layered sheets that can be used for electrical and optical measurements.1–7 So far, the experimental studies have been mostly focused on TMCs with MX2 composition (M = Mo, W; X is a chalcogen), such as MoS2, MoSe2, WS2 and WSe2.4,5 However, the TMC family is very rich and contains many other layered materials with interesting properties that have received limited attention from the researchers.8,9
One such TMC material is titanium trisulfide (TiS3). In the bulk form, TiS3 has been studied for several decades.10–23 It was shown that millimeter-long whiskers of TiS3 can be synthesized by a direct reaction of metallic titanium and elemental sulfur in evacuated ampules at 500–600 °C.11,12,15,18,22–24 These whiskers were used for physical property measurements,10,12,15,17,18,22,23 which revealed that bulk TiS3 is an n-type semiconductor with an energy bandgap of about 1 eV,10,12,17,22,23 and a room-temperature Hall mobility of about 30 cm2 V−1 s−1.18
While previous studies have mostly focused on the bulk properties of TiS3, the introduction and extensive use of the micromechanical exfoliation approach1 have opened the possibility of accessing the properties of monolayered and few-layered TiS3 flakes. According to a recent theoretical study,25 in a certain crystallographic direction a monolayer of TiS3 is expected to have higher electron mobility than a single layer of MoS2. An experimental attempt to exfoliate TiS3 whiskers into few-layered nanoribbons and study their electronic and optoelectronic properties was reported by Island et al.9 TiS3 nanoribbons were shown to have high photoresponse and fast switching times, which makes nanostructured TiS3 a promising material for applications in optoelectronics and photovoltaics.9 However, the room-temperature charge carrier mobilities reported for the set of field-effect transistors (FETs) based on few-layered TiS3 nanoribbons did not exceed 2.6 cm2 V−1 s−1,9 while comparable bottom-gated FETs based on trilayered and thicker MoS2, the most studied 2D TMC material, exhibited significantly higher mobilities of 10–20 cm2 V−1 s−1,26,27 and reached up to 27 cm2 V−1 s−1 in devices with optimized contact resistances.28 Therefore, in order to demonstrate that TiS3 is a competitive TMC material for electronic applications, it is necessary to improve its field-effect mobility by at least an order of magnitude.
In this study, we report bottom-gated FETs based on few-layered TiS3 nanoribbons that have room-temperature field-effect mobilities of 18–24 cm2 V−1 s−1. Furthermore, according to theoretical predictions, charge carrier mobilities of 2D semiconductor nanostructures can be significantly improved by modifying their dielectric environment.29,30 Previously, the dielectric screening approach has been successfully applied to graphene30,31 and MoS2.32–35 Here we demonstrate the validity of this approach for mobility improvement in few-layered TiS3 FETs by coating the devices with a thin layer of a high-κ dielectric, Al2O3, which results in measured field-effect mobilities up to 43 cm2 V−1 s−1 as well as much improved subthreshold swing (S) characteristics. These results demonstrate that TiS3 is a competitive electronic material in the 2D TMC family and can be considered for emerging device applications.3,4,6
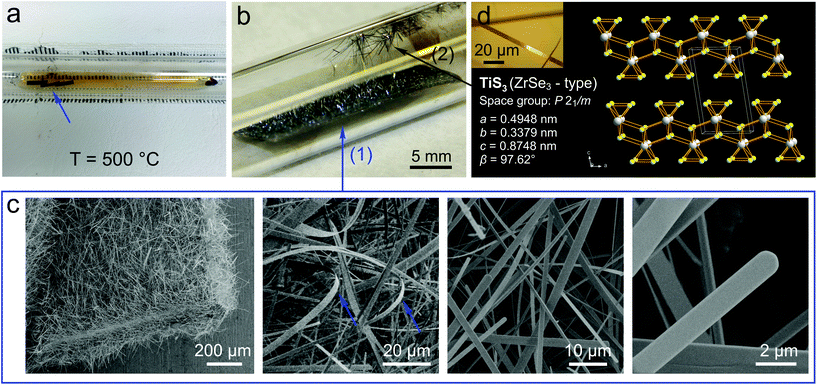
Following previous studies, we have grown TiS3 whiskers via the direct reaction of titanium and sulfur.11,12,15,18,24 In a typical synthesis, ∼0.1 g piece of a 0.25 mm thick Ti foil and ∼0.2 g of S are sealed in an evacuated (p ∼ 200 mTorr) quartz ampule. The ampule is placed in a tube furnace, where it is heated up to 500 °C and annealed for 3 days. During the reaction sulfur exists in a gaseous phase, as can be seen by the orange-brown vapor inside the ampule (Fig. 1a); three pieces of Ti foil are indicated by the blue arrow. Fig. 1b demonstrates the optical photograph of the reaction ampule after the synthesis. The arrow (1) shows one of the pieces of Ti foil; it is covered by dense forest of TiS3 whiskers that are typically 100–200 μm long. Interestingly, the whiskers grow not only on Ti foil, but also on the surface of the quartz ampule, as shown by the arrow (2) in Fig. 1b. These whiskers generally grow longer than the whiskers on Ti foil and often exceed 5 mm in length after 3 days of growth. At the end of the synthesis the ampule is moved away from the center of the furnace to create an ∼100 °C temperature gradient, such that the end of the ampule containing the pieces of Ti foil remains at ∼500 °C while the opposite end of the ampule is cooled below the boiling point of sulfur (444.7 °C). As a result, TiS3 whiskers are cleaned from unreacted sulfur, which condenses in the cold end of the ampule. One hour later the ampule is cooled down to room temperature and TiS3 whiskers are collected for materials characterization and electrical measurements.
Fig. 1c shows scanning electron microscopy (SEM) images at different magnifications of the TiS3 whiskers grown on a Ti substrate. These whiskers have a shape of thin ribbons that are typically over 100 μm long, a few μm wide and less than half a micrometer thick; these dimensions are in accord with previously reported observations for similarly prepared samples.9,22 The ribbon shape of TiS3 whiskers is illustrated in the second panel of Fig. 1c, which shows several bent whiskers (two of them are indicated by the blue arrows). Additional SEM data for TiS3 whiskers grown on a Ti substrate is provided in Fig. S1.† Based on the data presented in Fig. 1c, S1a† and similar SEM images we prepared a width distribution of TiS3 whiskers, which is shown in Fig. S1b.† The size distribution is quite broad with a maximum at w ∼ 7 μm; a substantial number of whiskers are wider than 20 μm. TiS3 whiskers were also characterized by Raman spectroscopy; the results presented in Fig. S2† are in agreement with the previously reported data.36
For the device fabrication and electrical measurements we used TiS3 whiskers grown on quartz (see the arrow (2) in Fig. 1b), which were easier to handle because of their larger size. The inset in Fig. 1d shows the optical microscopy image of one of these larger TiS3 whiskers, which had a length of about 0.6 mm and a width of about 8 μm. This whisker was studied by the single-crystal X-ray diffraction (XRD). A single crystal had a monoclinic symmetry with the space group P21/m and lattice parameters a = 0.4948(7), b = 0.3379(5), c = 0.8748(12) nm, and β = 97.62(2)°. Direct methods yielded a completely ordered atom arrangement isotypic with the structure type of ZrSe3;37 the results of XRD measurements are summarized in Tables S1 and S2 in the ESI.† The crystal structure of TiS3 can be described as a stack of 2D layers formed by one-dimensional (1D) chains of TiS3 prisms, see Fig. 1d. TiS3 can be viewed as Ti4+S2−S22−, containing both sulfide and disulfide units; these units form trigonal prisms with Ti4+ centers.
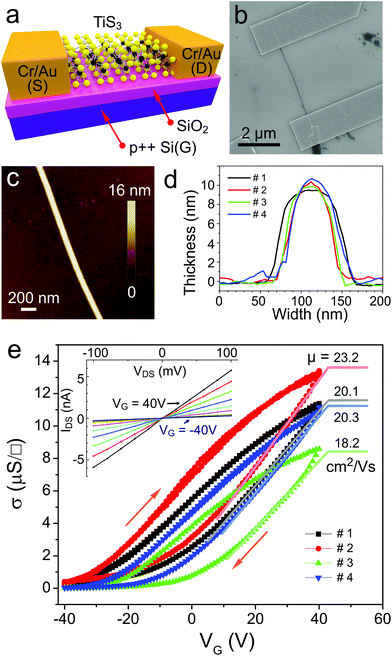
For the device fabrication, one of the TiS3 whiskers grown on a quartz surface was mechanically exfoliated using an adhesive tape1 and transferred to a p-type silicon substrate covered with a 300 nm thick layer of SiO2 (SQI). Because of the quasi-1D structure of TiS3, the whiskers not only exfoliate into 2D sheets but also break longitudinally along the b-axis, resulting in thin TiS3 nanoribbons that are much narrower than the original whiskers. Few-layered TiS3 nanoribbons with thicknesses of 9–11 nm were found on the surface of a Si/SiO2 substrate by optical microscopy. Electronic devices with few-layered TiS3 nanoribbons bridging Cr/Au (3 nm/20 nm) electrodes were fabricated by standard electron-beam lithography followed by electron-beam evaporation; the details of the device fabrication and materials characterization can be found in the ESI.† The schematic of a typical TiS3-based device with Cr/Au source (S) and drain (D) electrodes on a p++-Si/SiO2 substrate (the heavily doped p-type silicon was used as the back gate electrode, G) is shown in Fig. 2a.
Fig. 2b shows the SEM image of one of the four TiS3 FETs that were fabricated and tested in this study; SEM and AFM images of other devices are shown in Fig. S3 in the ESI.†Fig. 2c shows the AFM image of the central portion of the TiS3 nanoribbon channel of the device. The height profile measured across this TiS3 nanoribbon shows a step of ∼9.5 nm; see the black line in Fig. 2d. For comparison, we also show the height profiles measured for the other three devices (Fig. 2d) to demonstrate that all TiS3 nanoribbons measured in this work had comparable thicknesses.
Electrical measurements of TiS3 FETs were performed under vacuum (p ∼ 1 × 10−6 Torr). Prior to the measurements the devices were evacuated for ∼24 h to minimize the effect of surface adsorbates.38Fig. 2e shows that all four TiS3 devices exhibited very similar electronic behavior. For all devices the drain–source current (IDS)–drain–source voltage (VDS) dependencies at different gate voltages (VG) were linear, indicating Ohmic contacts between TiS3 nanoribbons and Cr/Au electrodes; a representative set of data for one of the TiS3 FETs is shown in the inset in Fig. 2e. This figure also shows conductivity (σ)–gate voltage (VG) dependencies for all devices, demonstrating the electronic behavior typical for FETs with n-type channels. These dependencies are also hysteretic, which is likely caused by the charge traps at the SiO2/TiS3 interface similar to SiO2/MoS2.27 From the linear regions in the σ–VG dependencies we estimate that the few-layered TiS3 FETs have field-effect mobilities of 18–23 cm2 V−1 s−1, which is comparable with the mobilities reported for few-layered MoS2 devices.26–28 The ON/OFF ratios defined as the ratios of the largest and smallest IDS values in VG dependencies were in the range of 30–300.
The conductivities presented in Fig. 2e were measured using a two-contact method and therefore include a contribution from the contact resistances. In order to evaluate the intrinsic electronic properties of exfoliated TiS3 whiskers we fabricated and tested two multiterminal devices and used them for four-point probe conductivity measurements, see Fig. S4 and S5† and associated comments in the ESI.† The field-effect mobilities measured for multiterminal TiS3 devices using a four-point probe method to exclude the contact resistance contribution were 21.1 and 24.2 cm2 V−1 s−1. These values are in a great agreement with the mobilities measured for two-terminal devices (Fig. 2e). While a more detailed investigation of contact resistance effects could be the subject of a separate study,39 our results show that contact resistances appear not to have a dominant effect on the electronic characteristics of TiS3 FETs.
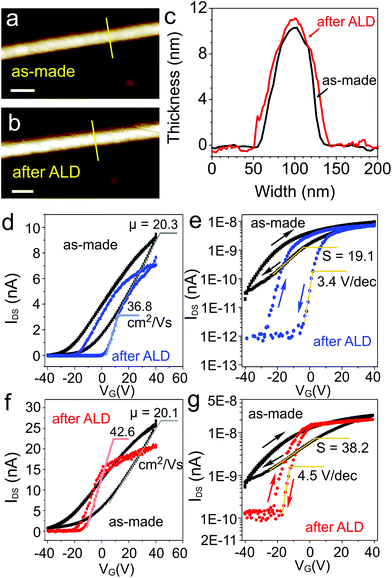
As we indicated earlier, dielectric screening is a very effective approach to improve charge carrier mobilities of 2D semiconductor nanostructures.29–35 In order to demonstrate the effectiveness of this approach for the mobility enhancement in few-layered TiS3 FETs, we used atomic layer deposition (ALD) to coat the devices with a 30 nm layer of Al2O3. Trimethylaluminum and water were used as Al2O3 precursors; additional details on ALD are given in the ESI.† Since TiS3 has not been long considered as a material for electronics, nothing has been reported so far about its compatibility with conventional ALD procedures. For example, preparation of uniform dielectric layers on graphene has proved to be a challenge,40–42 as graphene has no functional groups, which hinders the surface modification with common ALD precursors (the direct ALD of Al2O3 using the same precursors results in oxide nucleation only at the edges of graphene).40 In contrast, the standard ALD procedure for Al2O3 growth worked well for TiS3. Fig. 3a and b shows the same area of a TiS3 nanoribbon channel of one of the devices before and after ALD of Al2O3, respectively. In the process, the color of the Si/SiO2 substrate changed from purple to green. However, on the nanoscale the morphology of the TiS3 nanoribbon and the substrate barely changed, suggesting a very uniform growth of Al2O3 by ALD (Fig. 3a and b). This conclusion is further confirmed by the representative height profiles shown in Fig. 3c. Before ALD of Al2O3 the height profile measured across the TiS3 nanoribbon along the yellow line in Fig. 3a showed a step height of about 10 nm. After the growth of 30 nm of Al2O3 the height profile measured in the same place is nearly the same, suggesting that alumina layers of comparable thicknesses have grown on the TiS3 and Si/SiO2 substrate. Thus, this work demonstrates the compatibility of TiS3 with conventional ALD procedures, which is important for the future studies of TiS3 electronic devices.
Fig. 3d–g shows IDS–VG dependencies in linear and semi-logarithmic coordinates for two TiS3 FETs before and after ALD of Al2O3; other devices showed similar behavior. These IDS–VG plots show that Al2O3 deposition may have different effects on the hysteresis of electronic transport observed in alumina-coated TiS3 FETs. The Al2O3-coated device shown in Fig. 3d and e exhibits a considerable hysteresis, while in the other Al2O3-coated FET (Fig. 3f and g) the hysteresis visibly shrinks compared to the as-made TiS3 FET before Al2O3 ALD. As in the case of as-made TiS3 FETs we attribute this hysteretic behavior to interfacial charge trapping.27 While we did not investigate this effect in detail, it is interesting to note that the alumina or another high-κ dielectric layer on top of TiS3 may provide another interface for charge-trap engineering. The data presented in Fig. 3d–g suggest that with a proper optimization of the deposition procedure it may be possible to either minimize the hysteretic behavior of TiS3 FETs or create devices with large σ–VG hysteresis loops, which may be of interest, for example, for memory applications.43 The optimization of the ALD procedure using high-κ dielectrics, not limited to Al2O3, will be the subject of our future studies.
Other than the different features in the shape of IDS–VG hysteresis loops, the effect of Al2O3 deposition on the electronic properties of TiS3 devices was quite consistent. Fig. 3d–g shows that the Al2O3 deposition has an overall positive effect on the electronic properties of TiS3 FETs. For the TiS3 FET shown in Fig. 3d the dielectric screening resulted in the field-effect mobility improvement from 20.3 to 36.8 cm2 V−1 s−1, and for the other device the mobility improved from 20.1 to 42.6 cm2 V−1 s−1 (Fig. 3f). Interestingly, the Al2O3 ALD also improved the ON/OFF ratios of TiS3 FETs, which can be seen in logarithmic IDS coordinates (Fig. 3e and g). For the device shown in Fig. 3e, the ON/OFF ratio improved from 300 to 7100, and for the other device (Fig. 3g) it improved from 40 to 170. The other two Al2O3-coated TiS3 FETs exhibited ON/OFF ratios of 155 and 180. The positive effect of alumina ALD on the device characteristics of TiS3 FETs can be further illustrated by the subthreshold swing (S) change. As can be seen in Fig. 3e and g and S6,† the as-prepared TiS3 FETs had S values ranging from 19.1 to 44.3 V dec−1. After Al2O3 ALD the S values decreased to 3.4–4.8 V dec−1; the S values for all four devices before and after alumina ALD are summarized in Fig. S6c.† With these device properties TiS3 appears to be a promising electronic material that can be positively compared with other more intensively studied members of the 2D TMC family.4
In summary, we have fabricated few-layered TiS3 FETs and tested their electronic properties. The TiS3 FETs showed an n-type electronic transport with room-temperature field-effect mobilities of 18–24 cm2 V−1 s−1 and ON/OFF ratios up to 300. We also demonstrate that TiS3 is compatible with the conventional ALD procedure for Al2O3. ALD of alumina on TiS3 FETs resulted in mobility improvement up to 43 cm2 V−1 s−1 and ON/OFF ratios of up to ∼7000; S values after Al2O3 ALD decreased from 19.1–44.3 to 3.4–4.8 V dec−1. This study shows that TiS3 is a promising 2D TMC material that can be further explored in the future device studies.
Note added in proof: After the submission of this paper we became aware of the recently published study, which shows that the TiS3 growth temperature could be a promising tool to tune the material's morphology and electronic properties, possibly through the change in the concentration of sulfur vacancies.44
Acknowledgements
This work was supported by the National Science Foundation (NSF) through ECCS-1509874 with a partial support from the Nebraska Materials Research Science and Engineering Center (MRSEC) (grant no. DMR-1420645). The ALD equipment is a part of the Center for Nanohybrid Functional Materials (CNFM) facilities supported by the NSF EPSCoR (grant no. EPS-1004094). This research was performed in part in Central Facilities of the Nebraska Center for Materials and Nanoscience (NCMN), which is supported by the Nebraska Research Initiative. We thank Prof. Xiao Cheng Zeng (ref. 25) for drawing our attention to the 2D TiS3 material.Notes and references
- K. S. Novoselov, D. Jiang, F. Schedin, T. J. Booth, V. V. Khotkevich, S. V. Morozov and A. K. Geim, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 10451–10453 CrossRef CAS PubMed.
- A. K. Geim and I. V. Grigorieva, Nature, 2013, 499, 419–425 CrossRef CAS PubMed.
- S. Z. Butler, S. M. Hollen, L. Cao, Y. Cui, J. A. Gupta, H. R. Gutiérrez, T. F. Heinz, S. S. Hong, J. Huang, A. F. Ismach, E. Johnston-Halperin, M. Kuno, V. V. Plashnitsa, R. D. Robinson, R. S. Ruoff, S. Salahuddin, J. Shan, L. Shi, M. G. Spencer, M. Terrones, W. Windl and J. E. Goldberger, ACS Nano, 2013, 7, 2898–2926 CrossRef CAS PubMed.
- Q. H. Wang, K. Kalantar-Zadeh, A. Kis, J. N. Coleman and M. S. Strano, Nat. Nano., 2012, 7, 699–712 CrossRef CAS PubMed.
- M. Chhowalla, H. S. Shin, G. Eda, L.-J. Li, K. P. Loh and H. Zhang, Nat. Chem., 2013, 5, 263–275 CrossRef PubMed.
- D. Jariwala, V. K. Sangwan, L. J. Lauhon, T. J. Marks and M. C. Hersam, ACS Nano, 2014, 8, 1102–1120 CrossRef CAS PubMed.
- G. Cunningham, M. Lotya, C. S. Cucinotta, S. Sanvito, S. D. Bergin, R. Menzel, M. S. P. Shaffer and J. N. Coleman, ACS Nano, 2012, 6, 3468–3480 CrossRef CAS PubMed.
- Y. Huang, E. Sutter, J. T. Sadowski, M. Cotlet, O. L. A. Monti, D. A. Racke, M. R. Neupane, D. Wickramaratne, R. K. Lake, B. A. Parkinson and P. Sutter, ACS Nano, 2014, 8, 10743–10755 CrossRef CAS PubMed.
- J. O. Island, M. Buscema, M. Barawi, J. M. Clamagirand, J. R. Ares, C. Sánchez, I. J. Ferrer, G. A. Steele, H. S. J. van der Zant and A. Castellanos-Gomez, Adv. Opt. Mat., 2014, 2, 641–645 CrossRef CAS.
- H. G. Grimmeiss, A. Rabenau, H. Hahn and P. Ness, Z. Elektrochem., 1961, 65, 776–783 CAS.
- H. Haraldsen, E. Rost, A. Kjekshus and A. Steffens, Acta Chem. Scand., 1963, 17, 1283–1292 CrossRef.
- L. Brattas and A. Kjekshus, Acta Chem. Scand., 1972, 26, 3441–3449 CrossRef CAS.
- S. Furuseth, L. Brattas and A. Kjekshus, Acta Chem. Scand., Ser. A, 1975, 29, 623–631 CrossRef.
- D. W. Murphy and F. A. Trumbore, J. Electrochem. Soc., 1976, 123, 960–964 CrossRef CAS.
- S. Kikkawa, M. Koizumi, S. Yamanaka, Y. Onuki and S. Tanuma, Phys. Status Solidi A, 1980, 61, K55–K57 CrossRef CAS.
- P.-L. Hsieh, C. M. Jackson and G. Grüner, Solid State Commun., 1983, 46, 505–507 CrossRef CAS.
- O. Gorochov, A. Katty, N. Lenagard, C. Levyclement and D. M. Schleich, Mater. Res. Bull., 1983, 18, 111–118 CrossRef CAS.
- E. Finkman and B. Fisher, Solid State Commun., 1984, 50, 25–28 CrossRef CAS.
- I. G. Gorlova and V. Y. Pokrovskii, JETP Lett. Engl. Transl., 2009, 90, 295–298 CrossRef CAS.
- I. G. Gorlova, V. Y. Pokrovskii, S. G. Zybtsev, A. N. Titov and V. N. Timofeev, J. Exp. Theor. Phys., 2010, 111, 298–303 CrossRef CAS.
- I. G. Gorlova, S. G. Zybtsev, V. Y. Pokrovskii, N. B. Bolotina, I. A. Verin and A. N. Titov, Physica B, 2012, 407, 1707–1710 CrossRef CAS.
- I. J. Ferrer, M. D. Maciá, V. Carcelén, J. R. Ares and C. Sánchez, Energy Procedia, 2012, 22, 48–52 CrossRef CAS.
- I. J. Ferrer, J. R. Ares, J. M. Clamagirand, M. Barawi and C. Sánchez, Thin Solid Films, 2013, 535, 398–401 CrossRef CAS.
- F. Lévy and H. Berger, J. Cryst. Growth, 1983, 61, 61–68 CrossRef.
- J. Dai and X. C. Zeng, Angew. Chem., Int. Ed., 2015, 54, 7572–7576 CrossRef CAS PubMed.
- H. Wang, L. Yu, Y.-H. Lee, Y. Shi, A. Hsu, M. L. Chin, L.-J. Li, M. Dubey, J. Kong and T. Palacios, Nano Lett., 2012, 12, 4674–4680 CrossRef CAS PubMed.
- S. Ghatak, A. N. Pal and A. Ghosh, ACS Nano, 2011, 5, 7707–7712 CrossRef CAS PubMed.
- J. Kang, W. Liu and K. Banerjee, Appl. Phys. Lett., 2014, 104, 093106 CrossRef.
- D. Jena and A. Konar, Phys. Rev. Lett., 2007, 98, 136805 CrossRef PubMed.
- C. Jang, S. Adam, J. H. Chen, E. D. Williams, S. Das Sarma and M. S. Fuhrer, Phys. Rev. Lett., 2008, 101, 146805 CrossRef CAS PubMed.
- F. Chen, J. Xia, D. K. Ferry and N. Tao, Nano Lett., 2009, 9, 2571–2574 CrossRef CAS PubMed.
- B. Radisavljevic, A. Radenovic, J. Brivio, V. Giacometti and A. Kis, Nat. Nano, 2011, 6, 147–150 CrossRef CAS PubMed.
- M. S. Fuhrer and J. Hone, Nat. Nano, 2013, 8, 146–147 CrossRef CAS PubMed.
- B. Radisavljevic and A. Kis, Nat. Nano, 2013, 8, 147–148 CrossRef CAS PubMed.
- B. Radisavljevic and A. Kis, Nat. Mater., 2013, 12, 815–820 CrossRef CAS PubMed.
- D. W. Galliardt, W. R. Nieveen and R. D. Kirby, Solid State Commun., 1980, 34, 37–39 CrossRef CAS.
- S. Furuseth, L. Brattås and A. Kjekshus, Acta Chem. Scand. Ser. A, 1975, 29a, 623–631 CrossRef.
- A. Sinitskii, A. Dimiev, D. V. Kosynkin and J. M. Tour, ACS Nano, 2010, 4, 5405–5413 CrossRef CAS PubMed.
- F. N. Xia, V. Perebeinos, Y. M. Lin, Y. Q. Wu and P. Avouris, Nat. Nanotechnol., 2011, 6, 179–184 CrossRef CAS PubMed.
- X. Wang, S. M. Tabakman and H. Dai, J. Am. Chem. Soc., 2008, 130, 8152–8153 CrossRef CAS PubMed.
- J. M. P. Alaboson, Q. H. Wang, J. D. Emery, A. L. Lipson, M. J. Bedzyk, J. W. Elam, M. J. Pellin and M. C. Hersam, ACS Nano, 2011, 5, 5223–5232 CrossRef CAS PubMed.
- A. Lipatov, B. B. Wymore, A. Fursina, T. H. Vo, A. Sinitskii and J. G. Redepenning, Chem. Mater., 2015, 27, 157–165 CrossRef CAS.
- J. Yao, Z. Jin, L. Zhong, D. Natelson and J. M. Tour, ACS Nano, 2009, 3, 4122–4126 CrossRef CAS PubMed.
- J. O. Island, M. Barawi, R. Biele, A. Almazán, J. M. Clamagirand, J. R. Ares, C. Sánchez, H. S. J. van der Zant, J. V. Álvarez, R. D'Agosta, I. J. Ferrer and A. Castellanos-Gomez, Adv. Mater., 2015, 27, 2595–2601 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details (synthesis and characterization). See DOI: 10.1039/c5nr01895a |
| This journal is © The Royal Society of Chemistry 2015 |