Open Access Article
Open Access ArticleEuropium-engineered iron oxide nanocubes with high T1 and T2 contrast abilities for MRI in living subjects†
Lijiao
Yang
a,
Zijian
Zhou
a,
Hanyu
Liu
a,
Changqiang
Wu
b,
Hui
Zhang
a,
Guoming
Huang
a,
Hua
Ai
b and
Jinhao
Gao
*a
aState Key Laboratory of Physical Chemistry of Solid Surfaces, The Key Laboratory for Chemical Biology of Fujian Province and Department of Chemical Biology, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen 361005, China. E-mail: jhgao@xmu.edu.cn; Fax: +86-592-2189959; Tel: +86-592-2180278
bNational Engineering Research Center for Biomaterials, Sichuan University, Chengdu 610064, China
First published on 16th March 2015
Abstract
Magnetic resonance imaging (MRI) contrast agents with both positive (T1) and negative (T2) contrast abilities are needed in clinical diagnosis for fault-free accurate detection of lesions. We report a facile synthesis of europium-engineered iron oxide (EuIO) nanocubes as T1 and T2 contrast agents for MRI in living subjects. The Eu(III) oxide-embedded iron oxide nanoparticles significantly increase the T1 relaxivity with an enhanced positive contrast effect. EuIO nanocubes with 14 nm in diameter showed a high r1 value of 36.8 mM−1 s−1 with respect to total metal ions (Fe + Eu), which is about 3 times higher than that of Fe3O4 nanoparticles with similar size. Moreover, both r1 and r2 values of EuIO nanocubes can be tuned by varying their sizes and Eu doping ratios. After citrate coating, EuIO nanocubes can provide enhanced T1 and T2 contrast effects in small animals, particularly in the cardiac and liver regions. This work may provide an insightful strategy to design MRI contrast agents with both positive and negative contrast abilities for biomedical applications.
Introduction
Magnetic resonance imaging (MRI) has been widely used in the clinic owing to the noninvasive character and high spatial resolution in soft issue.1–3 Magnetic nanomaterials are employed as contrast agents to improve the sensitivity and reliability of MRI by accelerating the proton relaxation of the nearby water molecules.4,5 As a result, they are able to enhance the contrast of the region of interest from the background under external magnetic fields. There are two types of contrast agents, T1 positive contrast agents (e.g., Gd chelates, paramagnetic Gd2O3 and MnO nanomaterials),6–9 and T2 negative contrast agents (e.g., superparamagnetic Fe3O4, ZnFe2O4 and Fe5C2 nanomaterials).10–15 However, these single modal MRI contrast agents have high risk of pseudo-positive signals in diagnosing lesions due to the intrinsic background from tissues in their vicinity. For example, T1-weighted MRI with bright signals may confuse with neighboring adipose tissues, whereas T2-weighted MRI showing darker signals is not favorable for distinguishing tissues in some occasions.16,17 Therefore, MRI contrast agents with both positive (T1) and negative (T2) contrast abilities are needed in clinical diagnosis for fault-free accurate detection of diseases. Ultrasmall iron oxide (IO) nanoparticles can display T1–T2 dual-modal MRI behavior because of strong surface spin-canting effect and low magnetization.18,19 However, they usually show low relaxivities and worse still low stability in biological media,20 which hinders the further applications as efficient T1- and T2-weighted MRI contrast agents.Paramagnetic metals (e.g., Mn2+) promise highly efficient T1 contrast due to the presence of unpaired electrons.21–23 The doping of paramagnetic metals into iron oxide nanoparticles can achieve enhanced T1 contrast and even tunable T1–T2 dual-modal contrasts in contrast-enhanced MRI applications.24,25 There are also other strategies to construct dual modal MR imaging, such as combining T1 and T2 contrast agents together via hybrid heterostructures and designing a “magnetically decoupled” core–shell structure.26,27 On the other hand, lanthanide (Ln) ions, such as Gd3+ with seven unpaired electrons, were employed to enhance the contrast abilities of iron oxide nanoparticles through the embedding strategy, which promises enhanced T1 and T2 contrast efficiency for sensitive and accurate MR imaging and disease diagnosis.28–32 Therefore, Eu(III) ions with six unpaired electrons may hold great potential to regulate the MRI contrast ability of iron oxide nanoparticles. Herein, we synthesized europium-engineered iron oxide (EuIO) nanocubes as novel MRI contrast agents and investigated the impact of Eu(III) ions on both T1 and T2 contrast abilities of iron oxide nanoparticles. The T1 and T2 relaxivities of EuIO nanocubes are size- and composition-dependent. EuIO nanocubes with larger size have higher r1 and r2 values. A high ratio of Eu embedding can increase r1 values while reducing the r2 values. EuIO nanocubes showed good biocompatibility and relatively long blood circulation time after coating with sodium citrate molecules. More importantly, the EuIO nanocubes can serve as enhanced T1 and T2 MRI contrast agents in living subjects using a clinically available 3.0 T MRI scanner, which may open up a new avenue to design high-performance MRI contrast agents for imaging and diagnosis applications.
Experimental section
Preparation of metal–oleate complex
A metal–oleate complex was prepared by reacting sodium oleate and metal chlorides following a typical modified method from the literature.33 Briefly, 0.811 g of ferric chloride (5 mmol) and 4.567 g of sodium oleate (15 mmol) were dissolved in the solvent mixed with 20 mL ethanol, 15 mL distilled water and 30 mL hexane. The resulting mixture was heated to 70 °C and stirred for four hours. After cooling to room temperature, the upper layer containing iron–oleate was washed with distilled water three times. The resulting red-brownish iron–oleate complex in a waxy solid form was obtained after evaporating hexane. The europium–oleate complex (white powder) was produced in a similar way but using europium chlorides as precursors.Preparation of monodispersed EuIO nanocubes
Monodispersed hydrophobic EuIO nanocubes were synthesized by thermal decomposition of metal–oleate complexes. For 14 nm EuIO nanocubes with a Eu molar ratio of 10.3%, iron–oleate (0.902 g, 1 mmol), europium–oleate (0.0996 g, 0.1 mmol), oleic acid (0.176 mL, 0.55 mmol) and 1-octadecene (15 mL) were mixed in a flask. The solution was heated to 350 °C at a constant rate of 5 °C min−1 under a nitrogen atmosphere, and maintained at the temperature for 1.5 hours. After cooling the solution to room temperature, isopropanol was added to precipitate the nanoparticles. The product was washed with ethanol three times, collected by centrifugation and finally redispersed in hexane for further use. The reflux time was one hour for 10 nm EuIO nanocubes and two hours for 20 nm EuIO nanocubes. We varied the ratios of europium–oleate and iron–oleate precursors to obtain EuIO nanocubes with different Eu molar ratios.Preparation of 14 nm Fe3O4 and 14 nm Eu2O3 nanoparticles
For 14 nm monodispersed Fe3O4 nanoparticles, iron oleate (0.902 g, 1 mmol) and oleic acid (0.16 mL, 0.5 mmol) were added to a three neck bottle flask with 1-octadecene (12 mL) as the solvent. After reflux for 1.5 hours under a nitrogen atmosphere and cooling to room temperature, the nanoparticles were precipitated with isopropanol, then washed with ethanol, separated by centrifugation and redispersed in hexane for further use.For 14 nm monodispersed Eu2O3 nanoparticles, europium oleate (0.996 g, 1 mmol) was dissolved in trioctylamine (10 mL) containing oleic acid (0.16 mL), the reaction was heated to reflux temperature and maintained for one hour. After cooling to room temperature, ethanol was added to precipitate the nanoparticles, and then the product was washed with ethanol and redispersed in hexane for further use.
Preparation of sodium citrate coated EuIO nanocubes
We choose sodium citrate as a phase transfer agent because it gives a much smaller hydrodynamic radius than polymeric ligands. 4 mL of EuIO nanocube hexane solution (containing 10 mg nanocubes) was mixed with 4 mL of distilled water including 60 mg of sodium citrate, the mixture was added to 6 mL acetone and heated at 70 °C for four hours. The products were collected by centrifugation after being cooled to room temperature. The EuIO nanocubes were then purified using sterilized membrane filters (pore size of 0.22 μm) for further use. The Fe3O4 and Eu2O3 nanoparticles were also coated with sodium citrate using similar methods.Cytotoxicity assay
The cytotoxicity of the EuIO nanocubes (sodium citrate coating) was tested by the 3-(4,5-dimethylthiazol-2-y1)-2,5-diphenyltetrazolium bromide (MTT) method. SMMC-7721 or MRC-5 cells were firstly seeded into a 96-well plate with a density of 1 × 104 cells per well in RPMI 1640, and incubated in 5% CO2 at 37 °C overnight. The cells were then incubated with 14 nm EuIO nanocubes at various [Fe + Eu] concentrations (0.469, 0.938, 1.875, 3.75, 7.5, 15, 30, 60 and 120 μg mL−1) for 24 h and 48 h. Then the culture medium was removed, and each well was added 100 μL of the new culture medium containing MTT (0.5 μg mL−1) and incubated for 4 h. The OD492 value (Abs.) of each well was measured using a MultiSkan FC microplate reader immediately. Cell viability was calculated from the OD492 value of the experimental group by subtracting that of the blank group.Cell uptake study
The SMMC-7721 cells (1 × 106) were seeded in the dish for 12 h, after washing cells twice with PBS, we added 10 mL RPMI-1640 containing EuIO nanocubes and Fe3O4 nanoparticles with different concentrations (0.8, 0.4 and 0.2 mM of total metals, respectively) and incubated at 37 °C for 5 h. The cells were harvested and washed with PBS buffer three times to remove the free nanoparticles. Then we collected the cells in a 0.6 mL graduated centrifuge tube by centrifugation for MRI scanning. The MRI experiment was performed on a 0.5 T MRI scanner.Relaxivity and MRI phantom studies at 0.5 T
All experiments were performed on a 0.5 T NMI20-Analyst NMR system (Niumag Corporation, Shanghai, China). A series of EuIO nanocubes, Fe3O4 and Eu2O3 nanoparticles were prepared with different concentrations (0.4, 0.2, 0.1, 0.05 and 0.025 mM) of total metals in 1% agar-containing solution. The control sample (0 mM) was manufactured with purified water containing 1% agar. We used an inversion recovery (IR) sequence and the Carr–Purcell–Meiboom–Gill (CPMG) sequence to measure the longitudinal relaxation times (T1) and transverse relaxation times (T2). The relaxivity values r1 and r2 were calculated from the slopes of the plot of 1/T1 or 1/T2 against the total metal concentration ([Fe + Eu], [Fe] or [Eu] in mM). T1- and T2-weighted phantom images were acquired by a 2D multi-slice spin-echo (MSE) sequence with the following parameters: TR/TE = 200/2 ms (T1), TR/TE = 2000/40 ms (T2), 512 × 512 matrices, and repetition times = 4.In vivo MRI study
All the samples were filtered through sterilized membrane filters (pore size 0.22 μm) for further use. Healthy Sprague–Dawley rats weighing about 180–220 g were chosen for in vivo MRI studies. T1-weighted MR images of the heart and T2-weighted MR images of the liver were obtained on a Philips (Achieva 3.0 T) MRI scanner. The rats (for each group, n = 3) were intravenously injected 14 nm sized EuIO nanocubes with a dose of 2 mg (Eu + Fe) per kg of body weight. For T1 imaging, time-scale acquisition of images was acquired at different time points (1 min, 3 min and 5 min) post-injection with the same slices. The images were obtained using a 3D CEMRA (contrast enhanced angiography) sequence under the following parameters: TR/TE = 7/3 ms, thickness = 1 mm, 1024 × 1024 matrices, FOV = 100 × 100 mm, flip angle = 30°. For T2 imaging, the slices were further acquired at different time points (30 min, 90 min and 150 min) after the injection. The images were obtained using a fast spin-echo sequence (TSE) under the following parameters: TR/TE = 2000/66 ms, thickness = 1 mm, 144 × 144 matrices, FOV = 50 × 50 mm, flip angle = 90°. To quantify the contrast enhancement, the signal-to-noise ratio (SNR) was measured by analyzing regions of interest (ROIs) of the images, and the contrast enhancement was defined as the decrease of SNR after the injection, ΔSNR = (|SNRpost − SNRpre|)/SNRpre.Characterization
Transmission electron microscopy (TEM) and high-resolution TEM (HRTEM) images were obtained by using a JEM-2100 microscope with an accelerating voltage of 200 kV. The X-ray diffraction (XRD) patterns were acquired on a Rigaku Ultima IV system. The energy-dispersive X-ray (EDX) element mapping analysis was performed on a Tecnai F30 microscope at an accelerating voltage of 300 kV. The hysteresis loops (at 300 K) were recorded on a Quantum Design MPMS-XL-7 system. The samples ready for magnetization measurement were washed three times and then treated with plasma cleaning (PDC-32G, Harrick Plasma) to remove the surfactants. The metal concentration of the samples was detected by inductively coupled plasma atomic emission spectroscopy (ICP-AES). The dynamic light scattering (DLS) measurements were performed on a Malvern Zetasizer nano ZS instrument.Statistical analysis
Statistical analysis was performed using the Student's t-test for unpaired data and the diameter of as-synthesized nanoparticles was calculated using Image J.Results and discussion
We used iron oleate and europium oleate as precursors to prepare EuIO nanocubes in 1-octadecene solvent in the presence of oleic acid, which is different from the reported method of preparing europium doped iron oxide nanoparticles using ferric acetylacetonate and europium acetylacetonate as precursors.34 We were able to tune the size of EuIO nanoparticles by varying the reaction time. The EuIO nanocubes with the sizes of 10, 14 and 20 nm in diameter were synthesized with reflux times of 1, 1.5 and 2 hours, respectively (Fig. 1a–c). Transmission electron microscopy (TEM) images showed that they are of uniform cubic shape with narrow size distribution. After coating with sodium citrate, dynamic light scattering (DLS) analysis indicated that they are stable in water with hydrodynamic diameters of 10.0 ± 1.7, 14.0 ± 1.9, and 20.1 ± 2.4 nm, respectively (Fig. 1d–f). EuIO nanocubes with different Eu molar ratios (6.4%, 10.3% and 15.1%) were also prepared by tuning the proportions of the two precursors (ESI, Fig. S1†). High-resolution TEM (HRTEM) image of the 14 nm sized EuIO nanocubes (Fig. 2a, inset) reveals a crossed lattice spacing distance of about 2.9 Å corresponding to (220) planes of the Fe3O4 crystal. The energy-dispersive X-ray (EDX) line scanning analysis and element mapping indicate that Eu(III) ions are homogeneously distributed in iron oxide nanoparticles (Fig. 2d,e). X-ray diffraction (XRD) patterns showed mixed diffraction peaks of inverse spinel structures of magnetite (JCPDS no. 00-003-0863) and hexagonal Eu2O3 (JCPDS no. 00-019-0463) phases (Fig. 2b). The selected area electron diffraction (SAED) of EuIO nanoparticles also exhibited a mixed crystalline nature (Fig. 2c). These results are different from that of either the doped ferrites with inverse or normal spinel crystalline structures,15,35 indicating that EuIO nanocubes are composed of mixed magnetite and Eu2O3 nanoclusters. This phenomenon is probably due to the fact that the large size of Eu(III) ions (94.7 pm in radius) is unable to occupy either the tetrahedral or the octahedral interstitial sites in the spinel structure. We also used synchrotron X-ray absorption spectroscopy (XAS) to further envision the structure of EuIO nanocubes, which allows for analyzing coordination geometry, bonding environment, and the electronic structure of central atoms.36 The Fe K-edge XAS spectra of EuIO nanocubes are comparable to those of Fe3O4 nanoparticles (Fig. 3a), indicating that the inverse spinel structure of Fe3O4 was unaltered after Eu2O3 embedding. However, the peak intensity of the EuIO sample is lower than that of the Fe3O4 nanoparticles, suggesting that the embedded Eu2O3 nanoclusters may disturb the long range order of spins of iron oxide nanoparticles. The absorption peaks of the Eu LIII-edge XAS spectrum of EuIO nanocubes (Fig. 3b) are attributed to the 2p3/2/5d electron transition for the trivalent Eu LIII-edges, indicating typical Eu(III) characteristics.37–39 | ||
| Fig. 1 TEM images (a–c) and the related DLS analysis profiles (d–f) of monodispersed EuIO nanocubes with different sizes: (a, d) 10.0 ± 1.7 nm, (b, e) 14.0 ± 1.9 nm, and (c, f) 20.1 ± 2.4 nm. | ||
Standard zero-field cooling (ZFC) and field cooling (FC) measurements for EuIO nanocubes (Fig. 4a) showed the estimated blocking temperature of about 180 K, which are lower than 220 K for magnetite nanoparticles (Fig. 4b). The hysteresis loops (M–H) revealed that EuIO nanocubes are partially paramagnetic without magnetic hysteresis at 300 K, which is different from the superparamagnetic behaviors of magnetite nanoparticles (Fig. 4c,d, and insets). This phenomenon is probably attributed to the enhanced spin canting effect on the surface layer of EuIO nanocubes after Eu2O3 embedding due to the enhanced thermal agitation effect.40 The inner location of Eu2O3 clusters in iron oxide nanoparticles may disturb the local magnetic field intensity of the whole nanoparticle and finally reduce the saturated magnetization (Ms). Besides, the doping of Eu2O3 with low magnetization may also decrease the total Ms values of EuIO nanocubes.41 As a result, the Ms value of EuIO nanocubes (∼39.6 emu g−1) is lower than that of magnetite nanoparticles with a similar size (∼53.4 emu g−1) at 300 K.
To evaluate the MR contrast ability of EuIO nanocubes, we firstly measured the relaxivities of citrate coated EuIO samples with different sizes and different Eu molar ratios. Larger EuIO nanoparticles have relatively higher Ms values, which can be attributed to the loss of the predominant spin canting effect on the particle surface.42–44 Nanoparticles with higher Ms values may generate stronger local magnetic field inhomogeneities for the water proton dephasing process around the nanoparticles according to the quantum mechanical outer sphere theory.45,46 As a result, larger EuIO nanocubes have higher r1 and r2 values (Fig. S3 and Table S1†). The Eu molar ratio also plays an important role in the r1 and r2 values of EuIO nanocubes, raising the Eu molar ratio increases r1 values while reducing r2 values (Fig. S4†). The decrease of r2 values is probably due to the reduction of the Ms values after Eu embedding. The increased r1 value may be attributed to the spin order of Eu(III) which has the same orientation as the local magnetic field.31 However, when the Eu molar ratio reached 15.1%, both r1 and r2 values are diminished, which may be ascribed to the structure of Fe3O4 nanoparticles disturbed severely by a large amount of Eu2O3 nanoclusters (Table S2†).
We also prepared Fe3O4 and Eu2O3 nanoparticles with about 14 nm in diameter for comparison (Fig. S2†). EuIO nanocubes showed increased signals in T1-weighted MR images (Fig. 5a) and reduced signals in T2-weighted MR images (Fig. 5b) with increased metal concentrations. Fe3O4 nanoparticles gave reduced signals in T2-weighted MR images but there were no obvious signal changes in T1-weighted MR images. Eu2O3 nanoparticles showed no evidence of signal changes both in T1- and T2-weighted MR images. The r1 value of EuIO nanocubes is 36.79 ± 1.16 mM−1 s−1, which is much higher than that of Fe3O4 nanoparticles (12.47 ± 0.32 mM−1 s−1) and Eu2O3 nanoparticles (0.03 ± 0.01 mM−1 s−1) (Fig. 5c and S5†). The increased r1 value is probably due to the spin order of Eu(III) which has the same direction as the local magnetic field induced by the superparamagnetic iron oxide domains. Besides, the chemical exchange of the surface Eu(III) ions with the nearby water protons may enhance T1 shortening compared with iron ions. The r2 of EuIO nanocubes was 97.52 ± 2.16 mM−1 s−1, which is much higher than that of Eu2O3 nanoparticles (5.44 ± 0.12 mM−1 s−1) but slightly lower than Fe3O4 nanoparticles (116.78 ± 3.77 mM−1 s−1), which is probably due to the relatively low Ms value. It is well-known that the r2/r1 ratio is an important factor to estimate whether a contrast agent can serve as a T1 or T2 contrast agent.47 A low r2/r1 ratio is necessary for magnetic nanoparticles to show the T1 contrast enhancement effect.28,48 The 14 nm sized EuIO nanocubes showed a low r2/r1 value, together with a relatively high r2 value (Fig. 5c), further confirming that EuIO nanocubes may possess the T1–T2 dual-modal MR contrast ability.
We evaluated the cytotoxicity of EuIO nanoparticles using the tetrazolium-based colorimetric (MTT) assay. After incubating with SMMC-7721 cells or MRC-5 cells, there was scarcely any cytotoxicity even at the highest metal ion concentration (120 μg [Fe + Eu] mL−1), indicating good biocompatibility of the sodium citrate-coated EuIO nanocubes (Fig. S6†). To test the MRI contrast ability of the EuIO nanocubes in vitro, we incubated SMMC-7721 cells with EuIO nanocubes and Fe3O4 nanoparticles at different concentrations (0.2, 0.4, and 0.8 mM of total metal ions). The cellular uptake amounts of EuIO nanocubes and Fe3O4 nanoparticles are similar at equivalent total metal concentrations (Fig. S7†). As shown in Fig. 6a, cells after being incubated with EuIO nanocubes showed brighter signals in T1 imaging in comparison with Fe3O4 nanoparticle treated cells. The signal to noise ratios in T1 imaging further confirmed that the EuIO nanocubes group is much higher than the Fe3O4 nanoparticles group (Fig. 6c). The ΔSNR (ΔSNR = |SNRpost − SNRpre|/SNRpre) for cells incubated with EuIO nanocubes is 61.4 ± 5.3%, which is much larger than that of Fe3O4 nanoparticles (13.5 ± 4.5%) in the concentration of 0.8 mM (Table S3†), demonstrating that EuIO nanocubes show better T1-weighted MR contrast enhancement effect than Fe3O4 nanoparticles. In T2-weighted MR imaging, the signal intensity of cells after being incubated with EuIO nanocubes and Fe3O4 nanoparticles were both decreased along with the augment of concentrations (Fig. 6b). The signal changes in the EuIO nanocubes group are close to those in the Fe3O4 nanoparticles group (Fig. 6d and Table S4†). These results prove that EuIO nanocubes show both T1 and T2 contrast effects in vitro, indicating the potential of EuIO nanocubes as T1–T2 dual-modal contrast agents.
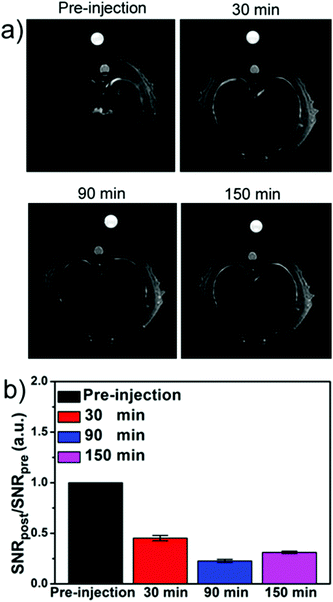
We then performed the animal experiments using healthy Sprague–Dawley rats as models for in vivo MRI studies. The T1-weighted MR images at 3 T were obtained sequentially before and after intravenous injection of EuIO nanocubes with a dose of 2 mg [Fe + Eu] per kg of body weight (Fig. 7a). The heart exhibited a significantly brighter signal at 1 min time point post-injection (p.i.) and the ΔSNR was approximately 62.2% (Fig. 7b and Table S5†), which indicates a great potential of EuIO nanocubes as excellent T1 contrast agents in vivo. Because nanoparticles are highly accumulated in the hepatic Kupffer cells of the liver due to the mononuclear phagocyte system (MPS),49,50 we then focused on the liver as the region of interest (ROI). The T2-weighted MR images at a 3 T MR scanner showed prominent T2 contrast in the rat liver region after intravenous injection of EuIO nanoparticles with a dose of 2.0 mg [Fe + Eu] per kg of body weight (Fig. 8). The analysis of MR signal changes in the liver region indicated that the maximal ΔSNR is about 77.3% at 90 min p.i. at 3 T (Fig. 8b and Table S6†). This result suggests that EuIO nanocubes have robust contrast effects for liver imaging with a diagnostic time window in two hours after intravenous administration, indicating a relatively long blood circulation time.51 The animal experiments demonstrate that EuIO nanoparticles are able to show both T1 and T2 MRI contrast effects for an in vivo study, which may provide more useful information for diagnosis with enhanced accuracy.
Conclusions
In summary, we have demonstrated that EuIO nanocubes showed both T1 and T2 contrast enhancement effects in vitro and in vivo. Moreover, we can tune T1 and T2 contrast abilities by varying their sizes and Eu doping ratios, which is helpful to better understand the effects of size and doping on r1 and r2 values and rationally design T1–T2 dual-modal MRI contrast agents. The synthesis of EuIO nanocubes is facile, highly reproducible, and convenient to scale up. The sodium citrate coated EuIO nanocubes displayed good biocompatibility and a relative long blood circulation time. These desirable characters render them as a promising candidate for T1- and T2-weighted contrast-enhanced MRI, which may provide a feasible strategy for accurate detection of lesions in future. The EuIO nanocubes may also bind with other biomolecules (e.g., antibody and peptides), thereby providing a versatile platform for targeting and self-confirmed imaging for biomedical applications.Acknowledgements
This work was supported by the National Key Basic Research Program of China (2013CB933900, 2014CB744502, and 2014CB932004), National Natural Science Foundation of China (21222106, 81370042, and 81430041), Natural Science Foundation of Fujian (2013J06005), and Fok Ying Tung Education Foundation (142012). We thank Prof. X. Guan at Tongji University and Shanghai Synchrotron Radiation Facility for XAS analysis.Notes and references
- N. Lee and T. Hyeon, Chem. Soc. Rev., 2012, 41, 2575–2589 RSC.
- C. Tassa, S. Y. Shaw and R. Weissleder, Acc. Chem. Res., 2011, 44, 842–852 CrossRef CAS PubMed.
- J. Gao, H. Gu and B. Xu, Acc. Chem. Res., 2009, 42, 1097–1107 CrossRef CAS PubMed.
- D. Ho, X. Sun and S. Sun, Acc. Chem. Res., 2011, 44, 875–882 CrossRef CAS PubMed.
- T. D. Schladt, K. Schneider, H. Schild and W. Tremel, Dalton Trans., 2011, 40, 6315–6343 RSC.
- H. Yang, Y. Zhuang, Y. Sun, A. Dai, X. Shi, D. Wu, F. Li, H. Hu and S. Yang, Biomaterials, 2011, 32, 4584–4593 CrossRef CAS PubMed.
- J. Y. Park, M. J. Baek, E. S. Choi, S. Woo, J. H. Kim, T. J. Kim, J. C. Jung, K. S. Chae, Y. Chang and G. H. Lee, ACS Nano, 2009, 3, 3663–3669 CrossRef CAS PubMed.
- H. B. Na, J. H. Lee, K. An, Y. I. Park, M. Park, I. S. Lee, D.-H. Nam, S. T. Kim, S.-H. Kim, S.-W. Kim, K.-H. Lim, K.-S. Kim, S.-O. Kim and T. Hyeon, Angew. Chem., Int. Ed., 2007, 119, 5493–5497 CrossRef.
- W. J. Rieter, K. M. L. Taylor, H. An, W. Lin and W. Lin, J. Am. Chem. Soc., 2006, 128, 9024–9025 CrossRef CAS PubMed.
- G. Huang, J. Hu, H. Zhang, Z. Zhou, X. Chi and J. Gao, Nanoscale, 2014, 6, 726–730 RSC.
- Z. Zhao, Z. Zhou, J. Bao, Z. Wang, J. Hu, X. Chi, K. Ni, R. Wang, X. Chen, Z. Chen and J. Gao, Nat. Commun., 2013, 4, 2266 Search PubMed.
- J. Gallo, N. J. Long and E. O. Aboagye, Chem. Soc. Rev., 2013, 42, 7816–7833 RSC.
- S. Cheong, P. Ferguson, K. W. Feindel, I. F. Hermans, P. T. Callaghan, C. Meyer, A. Slocombe, C.-H. Su, F.-Y. Cheng, C.-S. Yeh, B. Ingham, M. F. Toney and R. D. Tilley, Angew. Chem., Int. Ed., 2011, 123, 4292–4295 CrossRef.
- J. Yang, C.-H. Lee, H.-J. Ko, J.-S. Suh, H.-G. Yoon, K. Lee, Y.-M. Huh and S. Haam, Angew. Chem., Int. Ed., 2007, 46, 8836–8839 CrossRef CAS PubMed.
- J.-H. Lee, Y.-M. Huh, Y.-w. Jun, J.-w. Seo, J.-t. Jang, H.-T. Song, S. Kim, E.-J. Cho, H.-G. Yoon, J.-S. Suh and J. Cheon, Nat. Med., 2007, 13, 95–99 CrossRef CAS PubMed.
- B. H. Kim, N. Lee, H. Kim, K. An, Y. I. Park, Y. Choi, K. Shin, Y. Lee, S. G. Kwon, H. B. Na, J.-G. Park, T.-Y. Ahn, Y.-W. Kim, W. K. Moon, S. H. Choi and T. Hyeon, J. Am. Chem. Soc., 2011, 133, 12624–12631 CrossRef CAS PubMed.
- E. Terreno, D. D. Castelli, A. Viale and S. Aime, Chem. Rev., 2010, 110, 3019–3042 CrossRef CAS PubMed.
- C.-L. Liu, Y.-K. Peng, S.-W. Chou, W.-H. Tseng, Y.-J. Tseng, H.-C. Chen, J.-K. Hsiao and P.-T. Chou, Small, 2014, 10, 3962–3969 CrossRef CAS PubMed.
- U. I. Tromsdorf, O. T. Bruns, S. C. Salmen, U. Beisiegel and H. Weller, Nano Lett., 2009, 9, 4434–4440 CrossRef CAS PubMed.
- Z. Zhou, L. Wang, X. Chi, J. Bao, L. Yang, W. Zhao, Z. Chen, X. Wang, X. Chen and J. Gao, ACS Nano, 2013, 7, 3287–3296 CrossRef CAS PubMed.
- H. Kobayashi, M. R. Longmire, M. Ogawa and P. L. Choyke, Chem. Soc. Rev., 2011, 40, 4626–4648 RSC.
- P. Caravan, Acc. Chem. Res., 2009, 42, 851–862 CrossRef CAS PubMed.
- R. B. Lauffer, Chem. Rev., 1987, 87, 901–927 CrossRef CAS.
- G. Huang, H. Li, J. Chen, Z. Zhao, L. Yang, X. Chi, Z. Chen, X. Wang and J. Gao, Nanoscale, 2014, 6, 10404–10412 RSC.
- J. Y. Park, E. S. Choi, M. J. Baek, G. H. Lee, S. Woo and Y. Chang, Eur. J. Inorg. Chem., 2009, 17, 2477–2481 CrossRef.
- K. Cheng, M. Yang, R. Zhang, C. Qin, X. Su and Z. Cheng, ACS Nano, 2014, 8, 9884–9896 CrossRef CAS PubMed.
- J.-s. Choi, J.-H. Lee, T.-H. Shin, H.-T. Song, E. Y. Kim and J. Cheon, J. Am. Chem. Soc., 2010, 132, 11015–11017 CrossRef CAS PubMed.
- Z. Zhou, Z. Zhao, H. Zhang, Z. Wang, X. Chen, R. Wang, Z. Chen and J. Gao, ACS Nano, 2014, 8, 7976–7985 CrossRef CAS PubMed.
- W. Xu, B. A. Bony, C. R. Kim, J. S. Baeck, Y. Chang, J. E. Bae, K. S. Chae, T. J. Kim and G. H. Lee, Sci. Rep., 2013, 3, 3210 Search PubMed.
- X. Wang, Z. Zhou, Z. Wang, Y. Xue, Y. Zeng, J. Gao, L. Zhu, X. Zhang, G. Liu and X. Chen, Nanoscale, 2013, 5, 8098–8104 RSC.
- Z. Zhou, D. Huang, J. Bao, Q. Chen, G. Liu, Z. Chen, X. Chen and J. Gao, Adv. Mater., 2012, 24, 6223–6228 CrossRef CAS PubMed.
- K. Kattel, J. Y. Park, W. Xu, H. G. Kim, E. J. Lee, B. A. Bony, W. C. Heo, J. J. Lee, S. Jin, J. S. Baeck, Y. Chang, T. J. Kim, J. E. Bae, K. S. Chae and G. H. Lee, ACS Appl. Mater. Interfaces, 2011, 3, 3325–3334 CAS.
- J. Park, K. An, Y. Hwang, J.-G. Park, H.-J. Noh, J.-Y. Kim, J.-H. Park, N.-M. Hwang and T. Hyeon, Nat. Mater., 2004, 3, 891–895 CrossRef CAS PubMed.
- C. R. De Silva, S. Smith, I. Shim, J. Pyun, T. Gutu, J. Jiao and Z. Zheng, J. Am. Chem. Soc., 2009, 131, 6336–6337 CrossRef CAS PubMed.
- J.-t. Jang, H. Nah, J.-H. Lee, S. H. Moon, M. G. Kim and J. Cheon, Angew. Chem., Int. Ed., 2009, 121, 1260–1264 CrossRef.
- T. E. Westre, P. Kennepohl, J. G. DeWitt, B. Hedman, K. O. Hodgson and E. I. Solomon, J. Am. Chem. Soc., 1997, 119, 6297–6314 CrossRef CAS.
- F. A. Rabuffetti, S. P. Culver, J. S. Lee and R. L. Brutchey, Nanoscale, 2014, 6, 2909–2914 RSC.
- M. Lastusaari, H. F. Brito, S. Carlson, J. Hölsä, T. Laamanen, L. C. V. Rodrigues and E. Welter, Phys. Scr., 2014, 89, 044004 CrossRef.
- K. Takashi, H. Tetsuo, O. Koutoku, O. Susumu and O. Hitoshi, Jpn. J. Appl. Phys., 2013, 52, 042402 CrossRef.
- M. P. Morales, S. Veintemillas-Verdaguer, M. I. Montero, C. J. Serna, A. Roig, L. Casas, B. Martínez and F. Sandiumenge, Chem. Mater., 1999, 11, 3058–3064 CrossRef CAS.
- H. B. Na, I. C. Song and T. Hyeon, Adv. Mater., 2009, 21, 2133–2148 CrossRef CAS.
- E. D. Smolensky, H.-Y. E. Park, Y. Zhou, G. A. Rolla, M. Marjanska, M. Botta and V. C. Pierre, J. Mater. Chem. B, 2013, 1, 2818–2828 RSC.
- J. V. Jokerst and S. S. Gambhir, Acc. Chem. Res., 2011, 44, 1050–1060 CrossRef CAS PubMed.
- Y.-w. Jun, Y.-M. Huh, J.-s. Choi, J.-H. Lee, H.-T. Song, K. Kim, S. Yoon, K.-S. Kim, J.-S. Shin, J.-S. Suh and J. Cheon, J. Am. Chem. Soc., 2005, 127, 5732–5733 CrossRef CAS PubMed.
- Q. L. Vuong, J.-F. Berret, J. Fresnais, Y. Gossuin and O. Sandre, Adv. Healthcare Mater., 2012, 1, 502–512 CrossRef CAS PubMed.
- D. Yoo, J.-H. Lee, T.-H. Shin and J. Cheon, Acc. Chem. Res., 2011, 44, 863–874 CrossRef CAS PubMed.
- P. Caravan, J. J. Ellison, T. J. McMurry and R. B. Lauffer, Chem. Rev., 1999, 99, 2293–2352 CrossRef CAS PubMed.
- Y.-K. Peng, C.-L. Liu, H.-C. Chen, S.-W. Chou, W.-H. Tseng, Y.-J. Tseng, C.-C. Kang, J.-K. Hsiao and P.-T. Chou, J. Am. Chem. Soc., 2013, 135, 18621–18628 CrossRef CAS PubMed.
- J. Huang, L. Bu, J. Xie, K. Chen, Z. Cheng, X. Li and X. Chen, ACS Nano, 2010, 4, 7151–7160 CrossRef CAS PubMed.
- J. Gao, K. Chen, R. Xie, J. Xie, S. Lee, Z. Cheng, X. Peng and X. Chen, Small, 2010, 6, 256–261 CrossRef CAS PubMed.
- K. P. García, K. Zarschler, L. Barbaro, J. A. Barreto, W. O'Malley, L. Spiccia, H. Stephan and B. Graham, Small, 2014, 10, 2516–2529 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5nr00774g |
| This journal is © The Royal Society of Chemistry 2015 |