Kinetically-controlled growth of cubic and octahedral Rh–Pd alloy oxygen reduction electrocatalysts with high activity and durability†
Yucong
Yan‡
a,
Fangwei
Zhan‡
a,
Jingshan
Du
a,
Yingying
Jiang
a,
Chuanhong
Jin
a,
Maoshen
Fu
b,
Hui
Zhang
*a and
Deren
Yang
a
aState Key Laboratory of Silicon Materials, Department of Materials Science and Engineering, Key Laboratory of Advanced Materials and Applications for Batteries of Zhejiang Province, and Cyrus Tang Center for Sensor Materials and Applications, Zhejiang University, Hangzhou, Zhejiang 310027, P. R. China. E-mail: msezhanghui@zju.edu.cn; Fax: +86-571-87952322; Tel: +86-571-87953190
bShaanxi Materials Analysis and Research Center, School of Materials Science and Engineering, Northwestern Polytechnical University, Xi'an, Shaanxi 710072, P. R. China
First published on 3rd November 2014
Abstract
Rh is a promising candidate as an indispensible component in bimetallic catalysts due to its unique capability to resist against the aggressive corrosion from the reaction medium. However, Rh has a very strong oxygen binding ability and is generally not suitable for the oxygen reduction reaction (ORR). Here, we have demonstrated shape-controlled synthesis of Rh–Pd alloy nanocrystals with high activity and durability for ORR by retarding the reaction kinetics at an ultra-slow injection rate of metal salts using a syringe pump. Under precise control of sluggish reaction kinetics, Pd followed a preferential overgrowth along the <100> direction, whereas the growth behavior of Rh was dominant along the <111> direction. These different kinetically-controlled growth behaviors associated with Rh and Pd were essential for achieving the shape transition between the cube and the octahedron of their alloys. The Rh8Pd92 alloy octahedra exhibited the highest mass activity with a value of 0.18 mA μg−1 in terms of the equivalent Pt cost, and were two-fold higher than that of commercial Pt/C. Significantly, all Rh–Pd alloy nanocrystals were highly stable with only less than 25% loss in mass activity after 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 CV cycles in O2 saturated acid solution compared to ∼56% loss of the commercial Pt/C (E-TEK). Indeed, the mass activity of Rh8Pd92 was 3.3 times higher than that of commercial Pt/C after the accelerated stability test (ADT). This improvement in activity and durability may arise possibly from synergistic effects between the facet and the surface composition.
000 CV cycles in O2 saturated acid solution compared to ∼56% loss of the commercial Pt/C (E-TEK). Indeed, the mass activity of Rh8Pd92 was 3.3 times higher than that of commercial Pt/C after the accelerated stability test (ADT). This improvement in activity and durability may arise possibly from synergistic effects between the facet and the surface composition.
Introduction
Many catalytic reactions are known to be very sensitive to the surface structure of noble-metal nanocrystals in a catalyst.1–3 It is clear that the surface geometry of a noble-metal nanocrystal is strongly affected by its shape, which determines surface atoms at facets, edges, and corners.4–7 As such, colloidal based shape control offers a powerful and versatile means to tailor the surface of noble-metal nanocrystals as well as its reactivity and selectivity for a rich variety of catalytic reactions such as the oxygen electro-reduction reaction (ORR). For example, it is demonstrated that the extended Pt3Ni{111} surface exhibited over 8-fold more activity than its {100} surface in ORR specific activity.8 The improvement in ORR activity was also achieved in the Pt3Ni octahedral nanocrystals enclosed by the {111} facets as compared with the corresponding cubes covered by the {100} facets.9,10 This type of facet sensitivity has also received unremitting interest as an ideal platform and has been applied in various catalytic reactions over the last decade.11–13 In general, a capping agent is commonly used for shape-controlled synthesis by changing surface energy via selective adsorption and their growth rates.14,15 Thus, a capping agent could stabilize some specific facets during thermodynamically controlled crystal growth (e.g. PVP for Ag{100} and citrate for Ag{111} surface).16–21 However, the use of different capping agents to control the shape of noble-metal nanocrystals severely interferes with the results of the structure-dependent properties due to the residual adsorbates with different chemical features on the surface of such nanocrystals.22,23Recently, kinetic control has emerged as a promising approach to shape-controlled synthesis of noble-metal nanocrystals in a solution.24,25 In practice, several groups found that dramatically slowing down the growth rate of nanocrystals might substantially alter their original growth behavior, and thus control the shape for a given metal due to manipulation of its reaction kinetics.26–28 For example, the formation of the PdBr42− complex favored the formation of Pd{111} dominant shapes, including octahedron and plate, under kinetic control, although thermodynamically Br− ions could stabilize the {100} facets serving as a capping agent.29 Most recently, Xia et al. have further extended the capability of this approach to achieve nucleation and growth of Ag on one, three, and six of the equivalent {100} faces on a cubic Pd seed by manipulating the injection rate of AgNO3.30 Despite enormous success, maneuvering the shape of noble-metals by precisely controlling the reaction kinetics still remains a great challenge, especially for a system involving bimetallic alloys.
Here, we report a facile polyol approach to achieve shape and composition-controlled Rh–Pd alloy cubes and octahedra by simultaneously injecting Rh and Pd salt precursors at an extremely slow rate (e.g., 2 mL h−1) using a syringe pump. We found that the different kinetically-controlled growth behaviors associated with Rh and Pd at an extremely slow feeding rate was essential for achieving this shape transition phenomenon. Shape controlled Pd-based alloys have been studied as alternative non-Pt ORR catalysts due to their relatively less price and more abundant reserve as well as similar electronic properties to Pt.31–33 The incorporation of Rh with a unique capability to resist acid etching into Pd-based catalysts could enhance their activity and durability for ORR due to a possible synergistic effect between these two metals.34–37
Experimental section
Chemicals and materials
Sodium hexachlororhodate (Na3RhCl6, Sigma-Aldrich, 97%), sodium tetrachloropalladate (Na2PdCl4, Sigma-Aldrich, 99.998%), potassium bromide (KBr, Sinopharm Chemical Reagent Co. Ltd), poly(vinyl pyrrolidone) (PVP, MW ≈ 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, Sigma-Aldrich), ascorbic acid (AA, Sigma-Aldrich), ethylene glycol (EG), acetone, and ethanol (Sinopharm Chemical Reagent Co. Ltd) were all used as received. The type of syringe was plastic (10 mL, Hangzhou Longde Medical Apparatus Co., Ltd). All syntheses were carried out in glass vials (25 mL, Shuniu).
000, Sigma-Aldrich), ascorbic acid (AA, Sigma-Aldrich), ethylene glycol (EG), acetone, and ethanol (Sinopharm Chemical Reagent Co. Ltd) were all used as received. The type of syringe was plastic (10 mL, Hangzhou Longde Medical Apparatus Co., Ltd). All syntheses were carried out in glass vials (25 mL, Shuniu).
Synthesis of Rh–Pd cubes and octahedra
Rh–Pd cubes were synthesized by injecting a mixed EG solution of Na3RhCl6 and Na2PdCl4 into another EG solution containing PVP, AA and KBr using a syringe pump. In a standard synthesis, 6.0 mL of EG containing 111 mg PVP, 60 mg AA, and 500 mg KBr was added to a vial, and pre-heated to 110 °C in an oil bath under magnetic stirring for 30 min and then increased to 140 °C. Subsequently, 5.0 mL of EG containing 38 mg Na3RhCl6 and 30 mg Na2PdCl4 with a Rh/Pd molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was injected simultaneously into the pre-heated vial through a syringe pump at an injection rate of 2 mL h−1. The reaction was allowed to proceed for 2.5 h at 140 °C. The product was collected by centrifugation, washed three times with acetone to remove excess PVP, KBr, and AA, and re-dispersed in ethanol. In addition, Rh–Pd octahedra were synthesized by decreasing the temperature of the reaction to 120 °C with all other parameters being the same as in the standard procedure. We also systematically investigated the effects of the molar ratio (e.g., 4
1 was injected simultaneously into the pre-heated vial through a syringe pump at an injection rate of 2 mL h−1. The reaction was allowed to proceed for 2.5 h at 140 °C. The product was collected by centrifugation, washed three times with acetone to remove excess PVP, KBr, and AA, and re-dispersed in ethanol. In addition, Rh–Pd octahedra were synthesized by decreasing the temperature of the reaction to 120 °C with all other parameters being the same as in the standard procedure. We also systematically investigated the effects of the molar ratio (e.g., 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2
1, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2
1, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 4
1, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) of Na3RhCl6 and Na2PdCl4 fed into the reaction, the duration of the reaction as well as the injection rate on the final morphology of the resulting Rh–Pd alloy nanocrystals at 140 and 120 °C, respectively.
1) of Na3RhCl6 and Na2PdCl4 fed into the reaction, the duration of the reaction as well as the injection rate on the final morphology of the resulting Rh–Pd alloy nanocrystals at 140 and 120 °C, respectively.
Preparation of carbon-supported catalysts
Carbon black (Vulcan XC-72) was used as a support for making Rh–Pd catalysts (RhPd/C) according to a previous report with some minor modifications. In a standard preparation, carbon black particles were dispersed in ethanol and sonicated for 30 min. A designated amount of Rh–Pd alloy nanocrystals was added to this dispersion with a Rh–Pd/C mass ratio of 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80. This mixture was further sonicated for 10 min and stirred for 12 h. The resulting solids were precipitated out by centrifugation with ethanol.
80. This mixture was further sonicated for 10 min and stirred for 12 h. The resulting solids were precipitated out by centrifugation with ethanol.
Morphological, structural, and elemental characterization
The obtained samples were characterized by X-ray powder diffraction (XRD) using a Rigaku D/max-ga X-ray diffractometer with graphite monochromatized Cu Kα radiation (λ = 1.54![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 178 Å). Transmission electron microscopy (TEM) images of the obtained samples were obtained using a Philips CM 200 microscope operated at 160 kV. High-resolution transmission electron microscopy (HRTEM) was performed using a FEI Tecnai F30 G2 microscope operated at 300 kV. High-angle annular dark-field scanning TEM (HAADF-STEM) and Energy dispersive X-ray (EDX) mapping analyses were performed on a Cs-corrected STEM (TitanG2 80-200 ChemiSTEM equipped with a Super-X EDX detector system), operated at 200 kV using a probe with 50 pA beam current and a converge angle of 21.4 mrad. X-Ray Photoelectron Spectrometry (XPS) was performed on an ESCALAB 250Xi (Thermo, UK).
178 Å). Transmission electron microscopy (TEM) images of the obtained samples were obtained using a Philips CM 200 microscope operated at 160 kV. High-resolution transmission electron microscopy (HRTEM) was performed using a FEI Tecnai F30 G2 microscope operated at 300 kV. High-angle annular dark-field scanning TEM (HAADF-STEM) and Energy dispersive X-ray (EDX) mapping analyses were performed on a Cs-corrected STEM (TitanG2 80-200 ChemiSTEM equipped with a Super-X EDX detector system), operated at 200 kV using a probe with 50 pA beam current and a converge angle of 21.4 mrad. X-Ray Photoelectron Spectrometry (XPS) was performed on an ESCALAB 250Xi (Thermo, UK).
Electrochemical measurements
A three-electrode cell was used to measure the electrochemical performances of Rh–Pd/C catalysts including the commercial Pt/C (ETEK). A glassy-carbon rotating disk electrode (RDE) was used as the working electrode (area: ∼0.196 cm2). A 1 cm2 platinum foil and a HydroFlex hydrogen electrode were used as the counter electrode and the reference electrode, respectively. The reference electrode was placed in a separate compartment connected with the main cell via a salt bridge. The hydrogen reference electrode was calibrated before all the tests via the Hydrogen evolution reaction (HER). All potentials were referenced to the reversible hydrogen electrode (RHE). The electrolyte used for cyclic voltammetry (CV) measurements and the linear scan voltammetry (LSV) test for the oxygen reduction reaction (ORR) was 0.1 M HClO4 solution, diluted from 70% double-distilled perchloric acid (GFS Chemicals, USA) with Millipore ultrapure water (18.2 MΩ). To make catalyst ink, 5 mg of Rh–Pd/C catalysts was dispersed in 10 mL of a mixed solvent and sonicated for 10 min. The solvent contained a mixture of de-ionized water, isopropanol, and 5% Nafion 117 solution at a volumetric ratio of 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.05. 40 μL of the catalyst ink was added onto the RDE and dried under the air flow for 30 min to make the working electrode. The loading amount of Rh–Pd alloy catalysts on the RDE was determined to be ∼20 μgmetal cm−2. The electrochemical active surface area (ECSA) was determined from the CV curves, calculating the amount of charges by integrating the hydrogen desorption region after double layer correction. The CV measurement was carried out in an argon-saturated 0.1 M HClO4 solution at room temperature with a scan rate of 50 mV s−1. ORR LSV curves were measured at the rotating rate of 1600 rpm in a 0.1 M HClO4 solution, which was purged with oxygen for 30 min prior to, and during testing. The scan rate for ORR measurements was set at 10 mV s−1. Data were used without iR-drop correction. The accelerated stability test (ADT) was carried out between 0.6 V and 1.0 V at a scan rate of 100 mV s−1 for 30
0.05. 40 μL of the catalyst ink was added onto the RDE and dried under the air flow for 30 min to make the working electrode. The loading amount of Rh–Pd alloy catalysts on the RDE was determined to be ∼20 μgmetal cm−2. The electrochemical active surface area (ECSA) was determined from the CV curves, calculating the amount of charges by integrating the hydrogen desorption region after double layer correction. The CV measurement was carried out in an argon-saturated 0.1 M HClO4 solution at room temperature with a scan rate of 50 mV s−1. ORR LSV curves were measured at the rotating rate of 1600 rpm in a 0.1 M HClO4 solution, which was purged with oxygen for 30 min prior to, and during testing. The scan rate for ORR measurements was set at 10 mV s−1. Data were used without iR-drop correction. The accelerated stability test (ADT) was carried out between 0.6 V and 1.0 V at a scan rate of 100 mV s−1 for 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles in an oxygen saturated 0.1 M HClO4 solution.
000 cycles in an oxygen saturated 0.1 M HClO4 solution.
Results and discussion
This synthesis is based on a modified polyol process that involves the simultaneous injection of Na3RhCl6 and Na2PdCl4 using a syringe pump at a rate of 2 mL h−1 into ethylene glycol (EG) with ascorbic acid (AA) and Br− ions serving as reducing and capping agents, respectively. Using this strategy, Rh–Pd alloy cubes and octahedra with different compositions were successfully produced by varying the reaction temperature and molar ratios of Rh to Pd salt precursors fed into the reaction (see Fig. 1). From this schematic illustration, it is clear that the Rh-rich samples preferred to take a cubic shape, whereas the Pd-rich ones were dominated by a shape of octahedron despite different reaction temperatures (e.g., 140 and 120 °C). When the molar ratio of Rh to Pd precursors was kept at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, however the reaction temperature had a great influence on the shape of the resulting Rh–Pd alloy nanocrystals. In this case, Rh–Pd alloy cubes and octahedra were successfully produced at 140 and 120 °C, respectively. This shape evolution with the molar ratio of Rh to Pd precursors and the reaction temperature can be attributed to different growth behavior associated with Rh and Pd at an extremely slow injection rate under kinetic control.
1, however the reaction temperature had a great influence on the shape of the resulting Rh–Pd alloy nanocrystals. In this case, Rh–Pd alloy cubes and octahedra were successfully produced at 140 and 120 °C, respectively. This shape evolution with the molar ratio of Rh to Pd precursors and the reaction temperature can be attributed to different growth behavior associated with Rh and Pd at an extremely slow injection rate under kinetic control.
 | ||
| Fig. 1 Schematic illustration showing the shape evolution of Rh–Pd alloy cubes and octahedra with the molar ratio of Rh to Pd salt precursors supplied in the reaction at 140 and 120 °C, respectively. | ||
We fully characterized the morphology, structure, and composition of these Rh–Pd alloy nanocrystals using various techniques. Fig. 2 shows transmission electron microscopy (TEM), high angle annular dark field scanning transmission electron microscopy (HAADF-STEM), energy dispersive X-ray (EDX), and high-resolution TEM (HRTEM) images of the Rh–Pd alloy nanocrystals that were prepared with a Rh/Pd molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 at an injection rate of 2 mL h−1 and 140 °C (denoted as the standard procedure in ESI†). From the TEM image (Fig. 2A), most of the Rh–Pd nanocrystals (ca. 80%) exhibited a shape of cube with an edge length of about 15 nm. The magnified TEM image (inset of Fig. 2A) shows that the cube was slightly truncated at eight corners but mainly bounded by the {100} facets. The cubic shape of Rh–Pd nanocrystals was also revealed from the HAADF-STEM image (Fig. 2B). The representative HRTEM image (Fig. 2C) of an individual Rh–Pd cube recorded along the <001> zone axis (see FFT in Fig. S1†) clearly shows well-resolved, continuous fringes in the same orientation, indicating that the cube was a single crystal. The fringes with a lattice spacing of 0.196 nm can be indexed to the {200} planes of the face-centered cubic (fcc) Rh–Pd alloy. EDX mapping (Fig. 2D and S2A†) shows that both Rh and Pd were distributed evenly throughout each individual Rh–Pd cube, confirming its alloy structure. This alloy structure was also confirmed by the EDX compositional line-scan recorded through an individual cube (Fig. S2B†). In addition, the EDX quantitative analysis of hundreds of cubes (Fig. S2C†) indicates that the atomic ratio of Rh/Pd was about 46
1 at an injection rate of 2 mL h−1 and 140 °C (denoted as the standard procedure in ESI†). From the TEM image (Fig. 2A), most of the Rh–Pd nanocrystals (ca. 80%) exhibited a shape of cube with an edge length of about 15 nm. The magnified TEM image (inset of Fig. 2A) shows that the cube was slightly truncated at eight corners but mainly bounded by the {100} facets. The cubic shape of Rh–Pd nanocrystals was also revealed from the HAADF-STEM image (Fig. 2B). The representative HRTEM image (Fig. 2C) of an individual Rh–Pd cube recorded along the <001> zone axis (see FFT in Fig. S1†) clearly shows well-resolved, continuous fringes in the same orientation, indicating that the cube was a single crystal. The fringes with a lattice spacing of 0.196 nm can be indexed to the {200} planes of the face-centered cubic (fcc) Rh–Pd alloy. EDX mapping (Fig. 2D and S2A†) shows that both Rh and Pd were distributed evenly throughout each individual Rh–Pd cube, confirming its alloy structure. This alloy structure was also confirmed by the EDX compositional line-scan recorded through an individual cube (Fig. S2B†). In addition, the EDX quantitative analysis of hundreds of cubes (Fig. S2C†) indicates that the atomic ratio of Rh/Pd was about 46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 54 (denoted as Rh46Pd54), which was close to the feeding ratio (1
54 (denoted as Rh46Pd54), which was close to the feeding ratio (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) of Rh to Pd salt precursors.
1) of Rh to Pd salt precursors.
Fig. 3 shows structural and compositional analyses of the Rh–Pd alloy nanocrystals prepared using the standard procedure except for the different reaction temperature at 120 °C. As revealed by the TEM image (Fig. 3A), most of the nanocrystals (ca. 82%) were dominated by the shape of octahedron with an average edge length of 13 nm. Careful observation (inset of Fig. 3A) shows that the octahedron was also slightly truncated at six corners but mainly covered by the {111} facets. The octahedral shape of the product was also clearly visualized in a HAADF-STEM image (Fig. 3B). An HRTEM image (Fig. 3C) obtained from an individual octahedron suggests that it was a single-crystal structure with its surface enclosed by the {111} facets, which was consistent with the d-spacing lattices of 2.27 Å with an angle of 70.5°. The elemental mapping analysis in Fig. 3D demonstrates that the octahedron was a binary alloy, with both Rh and Pd homogeneously distributed throughout the nanocrystal. This demonstration was also supported by the EDX mapping analysis for multiple octahedra and line-scan (Fig. S3A and B†). As revealed from the EDX spectrum (Fig. S3C†), the Rh/Pd atomic ratio of the octahedra was about 43![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 57 (denoted as Rh43Pd57). Taken together, Rh–Pd alloy cubes covered by the {100} facets and octahedra enclosed by the {111} facets were successfully generated by simultaneously injecting Rh and Pd precursors with a molar ratio of 1
57 (denoted as Rh43Pd57). Taken together, Rh–Pd alloy cubes covered by the {100} facets and octahedra enclosed by the {111} facets were successfully generated by simultaneously injecting Rh and Pd precursors with a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 at an extremely slow rate of 2 mL h−1 performed at 140 and 120 °C, respectively.
1 at an extremely slow rate of 2 mL h−1 performed at 140 and 120 °C, respectively.
The growth behaviors associated with Rh-rich and Pd-rich crystals were systematically investigated to understand their reaction kinetics. Fig. 4 shows TEM images of Rh–Pd alloy nanocrystals prepared using the standard procedure at 140 and 120 °C, respectively, except for the variation of the Rh/Pd molar ratios from 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 2
1 to 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 1
2 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4. From these TEM micrographs, it is clear that the Rh-rich samples (Fig. 4A, B, E and F) preferred to form cubic nanocrystals, whereas the Pd-rich ones (Fig. 4C, D, G and H) were dominated by octahedra regardless of different reaction temperatures (e.g., 140 and 120 °C). The productivity of these two shapes in the samples is shown in Table S1.† We believe that this morphology variation can be attributed to the different kinetically-controlled growth behavior associated with Rh and Pd at an extremely slow injection rate under this reaction condition.29,38 Due to the difficulty in dissolving Rh, EDX instead of inductively coupled plasma mass spectrometry (ICP-MS) was employed to determine the composition of the Rh–Pd alloy nanocrystals. On the basis of EDX analysis (see Fig. S4A–D†), Rh80Pd20, Rh64Pd36, Rh30Pd70, and Rh17Pd83 alloy nanocrystals were obtained by varying the molar ratio of Rh to Pd salt precursors from 4
4. From these TEM micrographs, it is clear that the Rh-rich samples (Fig. 4A, B, E and F) preferred to form cubic nanocrystals, whereas the Pd-rich ones (Fig. 4C, D, G and H) were dominated by octahedra regardless of different reaction temperatures (e.g., 140 and 120 °C). The productivity of these two shapes in the samples is shown in Table S1.† We believe that this morphology variation can be attributed to the different kinetically-controlled growth behavior associated with Rh and Pd at an extremely slow injection rate under this reaction condition.29,38 Due to the difficulty in dissolving Rh, EDX instead of inductively coupled plasma mass spectrometry (ICP-MS) was employed to determine the composition of the Rh–Pd alloy nanocrystals. On the basis of EDX analysis (see Fig. S4A–D†), Rh80Pd20, Rh64Pd36, Rh30Pd70, and Rh17Pd83 alloy nanocrystals were obtained by varying the molar ratio of Rh to Pd salt precursors from 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 2
1 to 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, and 1
2, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 added in the synthesis at 140 °C. The final composition of Rh–Pd nanocrystals is close to the feeding ratio of metal precursors. When decreasing the reaction temperature to 120 °C (see Fig. S4E–H†), Rh–Pd alloy nanocrystals with similar compositions were produced except for the sample (labeled as Rh8Pd92) prepared using a Rh/Pd molar ratio of 1
4 added in the synthesis at 140 °C. The final composition of Rh–Pd nanocrystals is close to the feeding ratio of metal precursors. When decreasing the reaction temperature to 120 °C (see Fig. S4E–H†), Rh–Pd alloy nanocrystals with similar compositions were produced except for the sample (labeled as Rh8Pd92) prepared using a Rh/Pd molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4. In addition, XPS analysis was further used to characterize the surface composition of these five samples prepared at 120 °C since this parameter plays a more important role in determining the catalytic performance (see Table S2 and Fig. S5†). From these data, we can conclude that the surface composition of the Rh–Pd alloy nanocrystals is almost the same as the feeding ratio of metal precursors. Fig. S6† shows XRD patterns of these ten samples with different compositions prepared at 140 and 120 °C, respectively. All the samples show four well-defined diffraction peaks that originated from a single fcc lattice. The patterns gradually shift to higher positions of 2θ with the increasing amount of Rh composition, implying the formation of an alloy structure with a wide range of compositions between Rh and Pd with small lattice mismatch.
4. In addition, XPS analysis was further used to characterize the surface composition of these five samples prepared at 120 °C since this parameter plays a more important role in determining the catalytic performance (see Table S2 and Fig. S5†). From these data, we can conclude that the surface composition of the Rh–Pd alloy nanocrystals is almost the same as the feeding ratio of metal precursors. Fig. S6† shows XRD patterns of these ten samples with different compositions prepared at 140 and 120 °C, respectively. All the samples show four well-defined diffraction peaks that originated from a single fcc lattice. The patterns gradually shift to higher positions of 2θ with the increasing amount of Rh composition, implying the formation of an alloy structure with a wide range of compositions between Rh and Pd with small lattice mismatch.
In order to decipher the formation mechanism of the Rh–Pd alloy cubes and octahedra, the growth behaviors associated with pure Rh and Pd under kinetic control have been clarified separately. In our previous study, Rh nanocubes and concave nanocubes were eventually generated at different injection rates.38 This demonstration was also confirmed by the control experiments, as shown in Fig. S7.† As observed, Rh concave nanocubes were generated at a slow injection rate (2 mL h−1), while Rh conventional nanocubes were formed at a fast injection rate (2.5 mL s−1). This result indicates that the injection rate has a great influence on the growth behavior of Rh nanocrystals, especially the preferential overgrowth along the <111> direction by retarding the reaction kinetics. For a system involving Pd, retarding the reaction kinetics has a great impact on the growth behavior and thus the final shape of the nanocrystals. Fig. S8A† shows the TEM image of Pd nanocrystals prepared by injecting Na2PdCl4 into the reaction at a rate of 2 mL h−1 and 140 °C. Interestingly, a large number of Pd triangle plates, together with a small amount of Pd decahedra were mainly bounded by the {111} facets, indicating that the Pd<111> direction growth was eliminated under this reaction condition. These two shapes were confirmed from the HRTEM images (Fig. S8B and C†). When the Na2PdCl4 solution was rapidly injected into the reaction using a pipette (ca. 2.5 mL s−1), most of the Pd nanocubes enclosed by the {100} facets were eventually formed (see Fig. S8D†). In this case, the preferential adsorption of Br− ions serving as a capping agent on the Pd {100} facets led to the cubic shape, which was consistent with the conventional result under thermodynamic control.15 A similar growth behavior of Pd was also observed when the reaction was conducted at 120 °C (see Fig. S9†). Combined together, the reason why the shape evolution of Rh–Pd alloy nanocrystals from cube to octahedron with the variation of the molar ratio of the precursors from Rh rich to Pd rich can be attributed to their different growth behaviors under sluggish growth kinetics. This demonstration was further confirmed by the results that were obtained at a rapid injection rate (see Fig. S10†). When the reaction was conducted at a rapid injection rate (e.g., 0.5 mL min−1), the cubic shape of the Pd rich samples was always generated due to the selective adsorption of Br− ions on the {100} facets under thermodynamic control.
For a system involving Rh and Pd with a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, we believed that the different reduction rate between Rh and Pd salt precursors would be responsible for the different outcomes obtained at 120 and 140 °C, respectively. For this purpose, we conducted a set of experiments by separately adding Na3RhCl6 and Na2PdCl4 into the reaction and allowing it to stand for 5 min to show the difference in the reduction rate of Rh and Pd salt precursors at the aforementioned temperatures (see Fig. S11†). The color of the solution associated with Rh at 120 °C immediately turned from rose pink (the original color) to dark brown (Fig. S11A†), indicating the formation of Rh nanocrystals due to the rapid reduction of Na3RhCl6 by AA.38 For comparison, the Na2PdCl4 solution was light orange after 5 min at 120 °C owing to the [PdBr4]2− complex formation (Fig. S11B†).39 As such, Rh rich nanocrystals were initially generated in the nucleation stage due to the much more rapid reduction rate of Na3RhCl6 than the [PdBr4]2− complex at this temperature. These nanocrystals then served as seeds in the subsequent growth process. In addition, the substantially large consumption of Na3RhCl6 for the formation of Rh rich seeds enabled the growth behavior of Rh–Pd alloy nanocrystals to adopt that of Pd due to the existence of a Pd rich reaction solution, leading to the Rh–Pd alloy octahedra. This demonstration was supported by the time-dependent TEM observation (Fig. S12†). From these TEM images, a large number of nanocubes of 3–4 nm in size were formed in the initial stage (Fig. S12A†). The formation of nanocubes can probably be attributed to the preferential chemisorption of Br− ions on Rh-rich {100} rather than other facets.15 After that, these nanocubes evolved into cuboctahedra (Fig. S12B†), truncated octahedra (Fig. S12C†), and then octahedra (Fig. S12D†) by eliminating the growth along the <111> direction (i.e., the growth behavior of Pd under this condition) as the amount of the Rh and Pd precursors was slowly injected. When the reaction proceeded at 140 °C for 5 min, the color of both the Na3RhCl6 and Na2PdCl4 solution was dark brown (Fig. S11C and D†), indicating their similar reduction rate. In this case, the Rh–Pd alloy nanocubes of 5–6 nm size were also generated in the nucleation stage of the reaction due to the selective adsorption of Br− ions on the Rh-rich {100} surface (Fig. S13A†). This growth behavior should be maintained during the overall reaction due to the almost constant molar ratio of Rh and Pd precursors in the solution. As such, the cubic shape of the Rh–Pd alloy nanocrystal was also kept intact with increasing sizes (Fig. S13B–D†).
1, we believed that the different reduction rate between Rh and Pd salt precursors would be responsible for the different outcomes obtained at 120 and 140 °C, respectively. For this purpose, we conducted a set of experiments by separately adding Na3RhCl6 and Na2PdCl4 into the reaction and allowing it to stand for 5 min to show the difference in the reduction rate of Rh and Pd salt precursors at the aforementioned temperatures (see Fig. S11†). The color of the solution associated with Rh at 120 °C immediately turned from rose pink (the original color) to dark brown (Fig. S11A†), indicating the formation of Rh nanocrystals due to the rapid reduction of Na3RhCl6 by AA.38 For comparison, the Na2PdCl4 solution was light orange after 5 min at 120 °C owing to the [PdBr4]2− complex formation (Fig. S11B†).39 As such, Rh rich nanocrystals were initially generated in the nucleation stage due to the much more rapid reduction rate of Na3RhCl6 than the [PdBr4]2− complex at this temperature. These nanocrystals then served as seeds in the subsequent growth process. In addition, the substantially large consumption of Na3RhCl6 for the formation of Rh rich seeds enabled the growth behavior of Rh–Pd alloy nanocrystals to adopt that of Pd due to the existence of a Pd rich reaction solution, leading to the Rh–Pd alloy octahedra. This demonstration was supported by the time-dependent TEM observation (Fig. S12†). From these TEM images, a large number of nanocubes of 3–4 nm in size were formed in the initial stage (Fig. S12A†). The formation of nanocubes can probably be attributed to the preferential chemisorption of Br− ions on Rh-rich {100} rather than other facets.15 After that, these nanocubes evolved into cuboctahedra (Fig. S12B†), truncated octahedra (Fig. S12C†), and then octahedra (Fig. S12D†) by eliminating the growth along the <111> direction (i.e., the growth behavior of Pd under this condition) as the amount of the Rh and Pd precursors was slowly injected. When the reaction proceeded at 140 °C for 5 min, the color of both the Na3RhCl6 and Na2PdCl4 solution was dark brown (Fig. S11C and D†), indicating their similar reduction rate. In this case, the Rh–Pd alloy nanocubes of 5–6 nm size were also generated in the nucleation stage of the reaction due to the selective adsorption of Br− ions on the Rh-rich {100} surface (Fig. S13A†). This growth behavior should be maintained during the overall reaction due to the almost constant molar ratio of Rh and Pd precursors in the solution. As such, the cubic shape of the Rh–Pd alloy nanocrystal was also kept intact with increasing sizes (Fig. S13B–D†).
The ORR performances of the Rh–Pd catalysts with well defined shapes, surfaces and compositions were measured on a rotating disk electrode (RDE) in 0.1 M HClO4 solution (Fig. 5 and S14†). The ORR polarization curves of the five carbon supported Rh–Pd synthesized at 120 °C were quite different in terms of the onset potential, half wave potential and slope, because these parameters in ORR activity involved the multiple effects (e.g. compositions and facets). From Table S3,† which listed all the ORR parameters among the five samples, on the {100} facets of cube, oxygen reduction was much faster with the composition of Rh80Pd20 than that with Rh62Pd38, while the reversible trend was shown in the {111} planes of octahedron, that is the surface with higher Pd composition is more active than that with lower Pd atomic ratios in oxygen reduction. The facet dependence of ORR activity was also studied by comparing the Rh43Pd57 octahedra made at 120 °C and the Rh46Pd54 cubes synthesized at 140 °C with a similar composition (Fig. S15†). The ORR area activity of the Rh43Pd57 octahedra is 150% higher than that of the Rh46Pd54 cubes. The facet preferential ORR activity has been proven in the study of the Pt based single crystal surface, because the oxygen species adsorption on the closest packed {111} surface was weakened compared with that on {100}.8,40 Therefore, the oxygen reduction kinetics was improved. Thus, these facet and composition dependences of ORR on Rh–Pd catalysts lead to a fact that the Rh8Pd92 octahedron is the most active among the five samples (Fig. 5B and Table S3†). The mass activity of the Rh8Pd92 octahedron is 0.10 mA μgPGM−1 or 0.18 mA μgPt−1 at 0.9 V by converting the mass of Rh–Pd into Pt with an equivalent cost, which is two-fold higher than that of commercial Pt/C (see Fig. S16†). This activity of the Rh8Pd92 octahedron is almost equivalent to that of other Pd-based alloys (see Table S4†).
 | ||
| Fig. 5 (a) ORR polarization curves and (b) ORR mass activities at 0.9 V of Rh–Pd cubes and octahedra. | ||
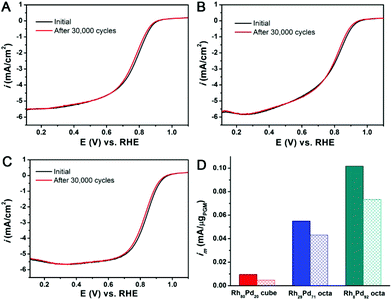
A rotating-disk-electrode (RDE)-based durability study was used to evaluate the durability of Rh–Pd catalysts by performing 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 potential cycles between 0.6 and 1.0 V in O2 saturated 0.1 M HClO4 solution. We performed 40 cycles in 0.1 M HClO4 electrolyte before the ORR test to remove the residual surfactants on the metal surface. We found that both Rh–Pd cubes and octahedra were highly stable with only less than 25% loss in mass activity after 30
000 potential cycles between 0.6 and 1.0 V in O2 saturated 0.1 M HClO4 solution. We performed 40 cycles in 0.1 M HClO4 electrolyte before the ORR test to remove the residual surfactants on the metal surface. We found that both Rh–Pd cubes and octahedra were highly stable with only less than 25% loss in mass activity after 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 CV cycles with an O2 flow (Fig. 6). However, by the comparison between Rh80Pd20, Rh29Pd71 and Rh8Pd92, there was still a slight difference among these three samples. The order of the mass activity loss is Rh80Pd20 (19.5%) < Rh29Pd71 (21.6%) < Rh8Pd92 (24.7%), which indicates that the ORR stability of Rh–Pd bimetallic catalysts could be improved with the increase of the Rh composition, although the high Rh composition might lead to the low ORR activity. The shift of the half wave potential shows the same trend and all the changes are within 7 mV (Fig. 6A–C). Indeed, Rh8Pd92 made at 120 °C shows ∼75% of the initial activity, a mass activity of 0.075 mA μgPGM−1, or 0.135 mA μgPt−1, which is 3.3 times that of commercial Pt/C after the ADT test for 30
000 CV cycles with an O2 flow (Fig. 6). However, by the comparison between Rh80Pd20, Rh29Pd71 and Rh8Pd92, there was still a slight difference among these three samples. The order of the mass activity loss is Rh80Pd20 (19.5%) < Rh29Pd71 (21.6%) < Rh8Pd92 (24.7%), which indicates that the ORR stability of Rh–Pd bimetallic catalysts could be improved with the increase of the Rh composition, although the high Rh composition might lead to the low ORR activity. The shift of the half wave potential shows the same trend and all the changes are within 7 mV (Fig. 6A–C). Indeed, Rh8Pd92 made at 120 °C shows ∼75% of the initial activity, a mass activity of 0.075 mA μgPGM−1, or 0.135 mA μgPt−1, which is 3.3 times that of commercial Pt/C after the ADT test for 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles (Fig. 6D and S16B†). Overall, Rh–Pd alloys show a substantially enhanced stability towards ORR in comparison with Pd or other Pd-based alloys (e.g., Pd–Co and Pd–Cu), which is essential for widespread applications.
000 cycles (Fig. 6D and S16B†). Overall, Rh–Pd alloys show a substantially enhanced stability towards ORR in comparison with Pd or other Pd-based alloys (e.g., Pd–Co and Pd–Cu), which is essential for widespread applications.
To further clarify the role of Rh in significantly improving the electrocatalytic stability of Rh–Pd alloy nanocrystals, Pd nanocubes of 10 nm in size were synthesized according to our previous report41 and then evaluated as electrocatalysts towards ORR (Fig. S17†). Fig. S17A and B† show CV curves and mass activities of Pd nanocubes before and after the ADT test for 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles. It is clear that Pd nanocubes show poor ORR stability with more than 82% loss in mass activity after 30
000 cycles. It is clear that Pd nanocubes show poor ORR stability with more than 82% loss in mass activity after 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles. TEM observation indicates that the morphology of the Pd nanocubes was completely destroyed after the ADT test (Fig. S17C and D†). For comparison, the octahedral morphology of Rh8Pd92 alloys almost remains intact after 30
000 cycles. TEM observation indicates that the morphology of the Pd nanocubes was completely destroyed after the ADT test (Fig. S17C and D†). For comparison, the octahedral morphology of Rh8Pd92 alloys almost remains intact after 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 CV cycles with an O2 flow (Fig. S18†). As a result, these control experiments indicate that alloying Pd with Rh is crucial to improve the stability towards ORR. It is well-known that Rh is a chemically inert metal with a high resistance to the base and acid etching.35 Our previous result also indicates that pure Rh shows the superior electrocatalytic stability towards ORR.37 Due to the relatively lower redox potential of Rh ions than Pd ions (e.g., 0.915 V for Pd2+/Pd and 0.8 V for Rh3+/Rh versus RHE),42 Rh can be considered as a sacrificial electrode in Rh–Pd alloy nanocrystals during ORR to improve the stability. In addition, a significant electron coupling between Rh and Pd might account for the observed stabilization of Pd, which was also demonstrated in other systems involving Au and Pt.43
000 CV cycles with an O2 flow (Fig. S18†). As a result, these control experiments indicate that alloying Pd with Rh is crucial to improve the stability towards ORR. It is well-known that Rh is a chemically inert metal with a high resistance to the base and acid etching.35 Our previous result also indicates that pure Rh shows the superior electrocatalytic stability towards ORR.37 Due to the relatively lower redox potential of Rh ions than Pd ions (e.g., 0.915 V for Pd2+/Pd and 0.8 V for Rh3+/Rh versus RHE),42 Rh can be considered as a sacrificial electrode in Rh–Pd alloy nanocrystals during ORR to improve the stability. In addition, a significant electron coupling between Rh and Pd might account for the observed stabilization of Pd, which was also demonstrated in other systems involving Au and Pt.43
Conclusions
We have developed a facile polyol approach to the synthesis of Rh–Pd alloy cubes and octahedra with different compositions in the presence of one capping agent (i.e., Br− ions) by extremely slowing down the injection rate of the precursors to manipulate the growth kinetics. The key to the success of this synthesis is the different growth behavior of Rh and Pd under sluggish kinetic control. This shape control was eventually achieved by varying the molar ratios of the Rh to Pd precursors and the reaction temperature. The incorporation of Rh into Pd-based nanocrystals can substantially enhance the mass activity and durability towards ORR relative to commercial Pt/C, with the Rh8Pd92 alloy octahedra being the best catalysts due to the possible synergistic effects between the facet and the composition. This work provides an effective strategy to design faceted non-Pt ORR catalysts with enhanced ORR catalytic performance, especially durability.Acknowledgements
The work on microscopy was carried out at the Center for Electron Microscopy of Zhejiang University. This work was financially supported by the National Science Foundation of China (51372222, 51222202), the National Basic Research Program of China (2014CB932500), the Program for Innovative Research Team in University of Ministry of Education of China (IRT13037) and the Fundamental Research Funds for the Central Universities (2014FZA4007, 2014XZZX003-07).Notes and references
- G. Somorjai and Y. Li, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 917–924 CrossRef CAS PubMed.
- R. Van Santen, Acc. Chem. Res., 2009, 42, 57–66 CrossRef CAS PubMed.
- M. Crespo-Quesada, A. Yarulin, M. Jin, Y. Xia and L. Kiwi-Minsker, J. Am. Chem. Soc., 2011, 133, 12787–12794 CrossRef CAS PubMed.
- A. Tao, S. Habas and P. Yang, Small, 2008, 4, 310–325 CrossRef CAS.
- J. Gu, Y. Zhang and F. Tao, Chem. Soc. Rev., 2012, 41, 8050–8065 RSC.
- K. Zhou and Y. Li, Angew. Chem., Int. Ed., 2012, 51, 602–613 CrossRef CAS PubMed.
- Z. Quan, Y. Wang and J. Fang, Acc. Chem. Res., 2013, 46, 191–202 CrossRef CAS PubMed.
- V. Stamenkovic, B. Fowler, B. Mun, G. Wang, P. Ross, C. Lucas and N. Markovic, Science, 2007, 315, 493–497 CrossRef CAS PubMed.
- J. Wu, A. Gross and H. Yang, Nano Lett., 2011, 11, 798–802 CrossRef CAS PubMed.
- J. Zhang, H. Yang, J. Fang and S. Zou, Nano Lett., 2010, 10, 638–644 CrossRef CAS PubMed.
- X. Huang, Y. Li, Y. Li, H. Zhou, X. Duan and Y. Huang, Nano Lett., 2012, 12, 4265–4270 CrossRef CAS PubMed.
- Y. Wu, S. Cai, D. Wang, W. He and Y. Li, J. Am. Chem. Soc., 2012, 134, 8975–8981 CrossRef CAS PubMed.
- A. Yin, X. Min, Y. Zhang and C. Yan, J. Am. Chem. Soc., 2011, 133, 3816–3819 CrossRef CAS PubMed.
- M. Chen, B. Wu, J. Yang and N. Zheng, Adv. Mater., 2012, 24, 862–879 CrossRef CAS PubMed.
- B. Lim, M. Jiang, J. Tao, P. Camargo, Y. Zhu and Y. Xia, Adv. Funct. Mater., 2009, 19, 189–200 CrossRef CAS.
- J. Zeng, Y. Zheng, M. Rycenga, J. Tao, Z. Li, Q. Zhang, Y. Zhu and Y. Xia, J. Am. Chem. Soc., 2010, 132, 8552–8553 CrossRef CAS PubMed.
- B. Lim, Y. Xiong and Y. Xia, Angew. Chem., Int. Ed., 2007, 46, 9279–9282 CrossRef CAS PubMed.
- X. Huang, S. Tang, X. Mu, Y. Dai, G. Chen, Z. Zhou, F. Ruan, Z. Yang and N. Zheng, Nat. Nanotechnol., 2011, 6, 28–32 CrossRef CAS PubMed.
- C. Wang, H. Daimon, Y. Lee, J. Kim and S. Sun, J. Am. Chem. Soc., 2007, 129, 6974–6975 CrossRef CAS PubMed.
- Y. Zhang, M. Grass, J. Kuhn, F. Tao, S. Habas, W. Huang, P. Yang and G. Somorjai, J. Am. Chem. Soc., 2008, 130, 5868–5869 CrossRef CAS PubMed.
- J. Wu, L. Qi, H. You, A. Gross, J. Li and H. Yang, J. Am. Chem. Soc., 2012, 134, 11880–11883 CrossRef CAS PubMed.
- J. Park, C. Aliaga, J. Renzas, H. Lee and G. Somorjai, Catal. Lett., 2009, 129, 1–6 CrossRef CAS.
- M. Crespo-Quesada, J. Andanson, A. Yarulin, B. Lim, Y. Xia and L. Kiwi-Minsker, Langmuir, 2011, 27, 7909–7916 CrossRef CAS PubMed.
- Y. Xia, Y. Xiong, B. Lim and S. Skrabalak, Angew. Chem., Int. Ed., 2009, 48, 60–103 CrossRef CAS PubMed.
- A. Biacchi and R. Schaak, ACS Nano, 2011, 5, 8089–8099 CrossRef CAS PubMed.
- H. Zhang, M. Jin and Y. Xia, Angew. Chem., Int. Ed., 2012, 51, 7656–7673 CrossRef CAS PubMed.
- J. Chen, T. Herricks and Y. Xia, Angew. Chem., Int. Ed., 2005, 44, 2589–2592 CrossRef CAS PubMed.
- C. DeSantis, A. Peverly, D. Peters and S. Skrabalak, Nano Lett., 2011, 11, 2164–2168 CrossRef CAS PubMed.
- C. Zhu, J. Zeng, P. Lu, J. Liu, Z. Gu and Y. Xia, Chem. – Eur. J., 2013, 19, 5127–5133 CrossRef CAS PubMed.
- J. Zeng, C. Zhu, J. Tao, M. Jin, H. Zhang, Z. Li, Y. Zhu and Y. Xia, Angew. Chem., Int. Ed., 2012, 51, 2354–2358 CrossRef CAS PubMed.
- E. Antolini, Energy Environ. Sci., 2009, 2, 915–931 CAS.
- B. Adams and A. Chen, Mater. Today, 2011, 14, 282–289 CrossRef CAS.
- M. Shao, J. Power Sources, 2011, 196, 2433–2444 CrossRef CAS PubMed.
- J. Handley, Platinum Met. Rev., 1989, 33, 64–72 CAS.
- S. Xie, N. Lu, Z. Xie, J. Wang, M. Kim and Y. Xia, Angew. Chem., Int. Ed., 2012, 51, 10266–10270 CrossRef CAS PubMed.
- B. Sneed, C. Kuo, C. Brodsky and C. Tsung, J. Am. Chem. Soc., 2012, 134, 18417–18426 CrossRef CAS PubMed.
- Y. Qi, J. Wu, H. Zhang, Y. Jiang, C. Jin, M. Fu, H. Yang and D. Yang, Nanoscale, 2014, 6, 7012–7018 RSC.
- H. Zhang, W. Li, M. Jin, J. Zeng, T. Yu, D. Yang and Y. Xia, Nano Lett., 2011, 11, 898–903 CrossRef CAS PubMed.
- M. Jin, H. Zhang, Z. Xie and Y. Xia, Angew. Chem., Int. Ed., 2011, 50, 7850–7854 CrossRef CAS PubMed.
- V. Stamenkovi, B. Mun, K. Mayrhofer, P. Ross, N. Markovic, J. Rossmeisl, J. Greeley and J. Nørskov, Angew. Chem., Int. Ed., 2006, 46, 2897–2901 CrossRef PubMed.
- M. Jin, H. Liu, H. Zhang, Z. Xie, J. Liu and Y. Xia, Nano Res., 2011, 4, 83–91 CrossRef CAS PubMed.
- C. Zoski, Handbook of Electrochemistry, Elsevier, Oxford, 2006, vol. 18, p. 817 Search PubMed.
- J. Zhang, K. Sasaki, E. Sutter and R. Adzic, Science, 2007, 315, 220–222 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4nr04942j |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2015 |