Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Triterpenoids
Robert A.
Hill
* and
Joseph D.
Connolly
School of Chemistry, Glasgow University, Glasgow, G12 8QQ, UK. E-mail: bob.hill@glasgow.ac.uk
First published on 24th October 2014
Abstract
Covering: 2012. Previous review: Nat. Prod. Rep., 2013, 29, 1028–1065
This review covers the isolation and structure determination of triterpenoids reported during 2012 including squalene derivatives, lanostanes, holostanes, cycloartanes, cucurbitanes, dammaranes, euphanes, tirucallanes, tetranortriterpenoids, quassinoids, lupanes, oleananes, friedelanes, ursanes, hopanes, serratanes, isomalabaricanes and saponins; 348 references are cited.
1. Introduction
There is continued interest in the anticancer activities of triterpenoids1–3 and their potential for treatment or prevention of diabetes and Alzheimer's disease.4 The oral absorption and metabolism of triterpenoid saponins has been reviewed.5 Surveys of triterpenoids from Ceriops,6 and Ilex7 species and Sapindus mukorossi,8 have appeared.2. The squalene group
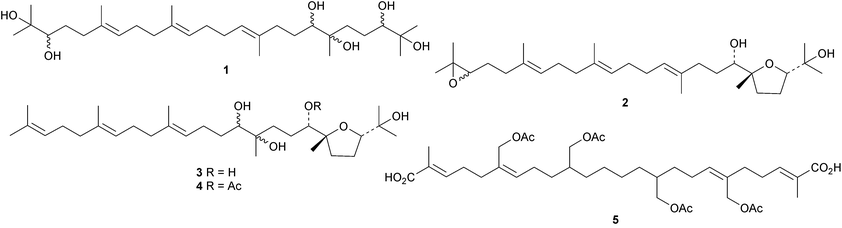
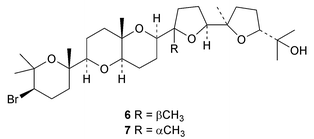
Sapelenins G 1–J 4 are further anti-inflammatory squalene derivatives from the bark of Cameroonian Entandrophragma cylindricum.9 The highly oxidised squalene derivative 5 has been isolated from Peruvian Protium subserratum.10 The structures of saiyacenols A 6 and B 7, from the red alga Laurencia viridis, support the accepted pathway for the formation of the aplysiols.11 The mechanism of triterpene biosynthesis in Botryococcus braunii has been reviewed.123. The lanostane group
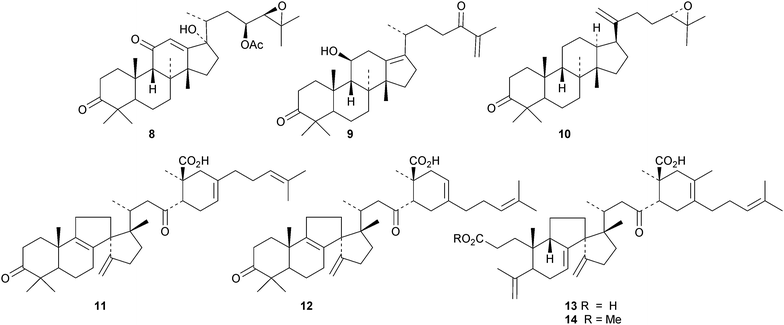
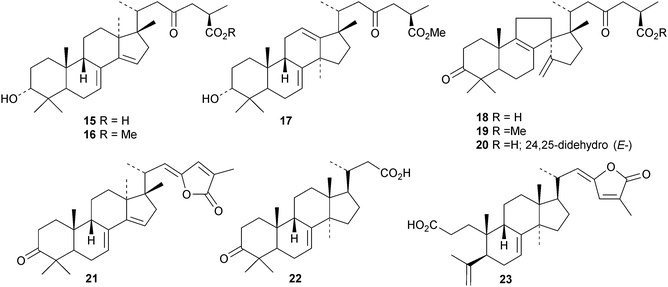
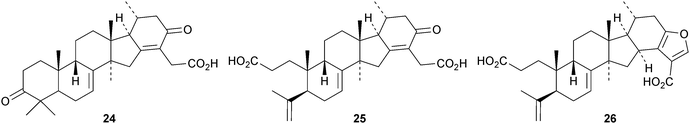
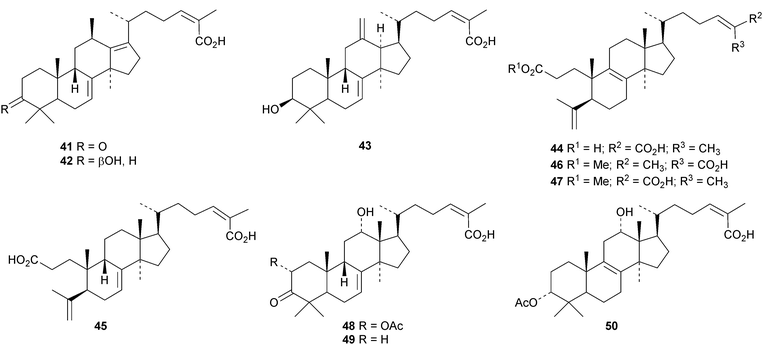
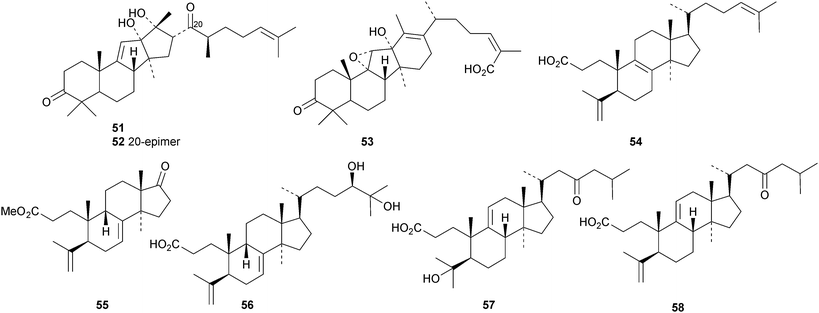
New protostanes include alisol Q acetate 8 (ref. 13) and alisol X 9 (ref. 14) from Alisma orientale and the epoxy-ketone 10 from the leaves of Aglaia odorata.15 The tetraterpenes abiestetranes A 11 and B 12, from Abies fabri,16 and abibalsamins A 13 and B 14, from the oleoresin of Abies balsamea,17 appear to have arisen by Diels–alder cycloadditions of rearranged lanostanes with the monoterpene β-myrcene. The structure of 13 was confirmed by X-ray analysis. A series of rearranged lanostanes, neoabiestrines A 15–F 20, has been reported from Abies recurvata.18 The structures of neoabiestrine A 15 were confirmed by X-ray analysis. The compounds showed some cytotoxic activity. The mariesane lactone 21 has been obtained from Abies sibirica.19 The tetranor derivative 22 and the 3,4-secolanostane 23 have been found in Abies holophylla.20Pseudoferic acids A 24, B 25 and C 26 are interesting new 16,24-cyclised lanostanes from Pseudolarix kaempferi.21 The stems of Schisandra glaucescens contain the ring A-cleaved lanostanes schiglausins A 27-H 34, together with the intact derivatives schiglausins I 35 and J 36.22 The structure of schiglausin A 27 was confirmed by X-ray analysis. Schiglausin H 34 is the methyl ester of micranoic acid A. An impressive array of rearranged, ring A-cleaved and intact lanostanes, kadpolysperins A 37–N 50, has been isolated from Kadsura polysperma.23 Kadcoccitones A 51 and B 52 are unusual rearranged lanostane derivatives from Kadsura coccinea where they occur with kadcoccitone C 53, whose structure was confirmed by X-ray analysis.24 Secococcinic acids G 54–K 58 are further constituents of Kadsura coccinea.25 Three esters 59–61 of 3-epidehydrotumulosic acid have been obtained from Wolfiporia extensa.26 Other new lanostanes include lanosta-5,15-dien-3α-ol 62 from Arctium lappa27 and inonotsuoxodiols B 63 and C 64, epoxyinonotsudiol 65 and methoxyinonotsutriol 66 from Inonotus obliquus.28
Sarasinosides N–R are 30-norlanostane glycosides from the sponge Lypastrotethya sp. with the new genins 67, 68, 69 and the 30-nor-18(13 → 14)-abeo-derivative 70.29 Scillanostaside F, with the new genin 71, and scillanostaside G, with a known tetranorlanostane genin, have been isolated from the bulbs of Scilla scilloides.30
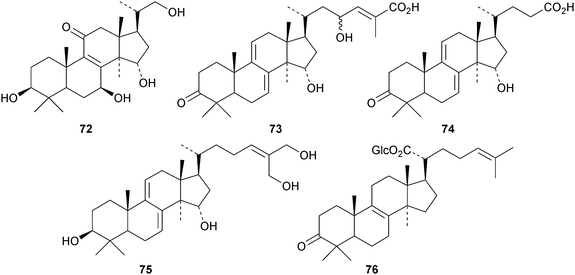
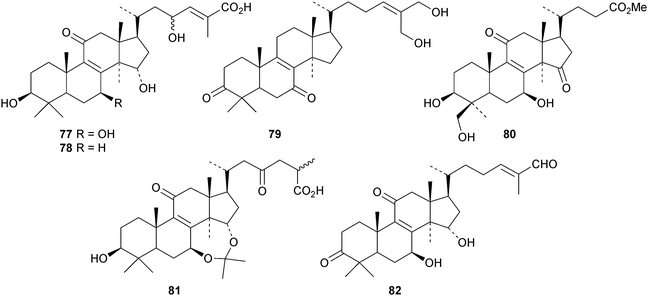
New compounds from Ganoderma sinense include the pentanor derivative ganosineniol A 72, ganoderic acids Jc73 and Jd74, ganodermatetraol 75, the glucosyl ester ganosinoside A 76 with a known genin, ganolucidic acid γa77, ganolucidate F 78, ganoderiol J 79 and methyl lucidenate Ha80.31 Ganodermacetal 81 is an acetonide from Ganoderma amboinense32 and lucialdehyde E 82 is a further compound from Ganoderma lucidum.33 Reviews have appeared on the triterpenoids of Ganoderma lucidum34,35 and lanostanes from fungi.36
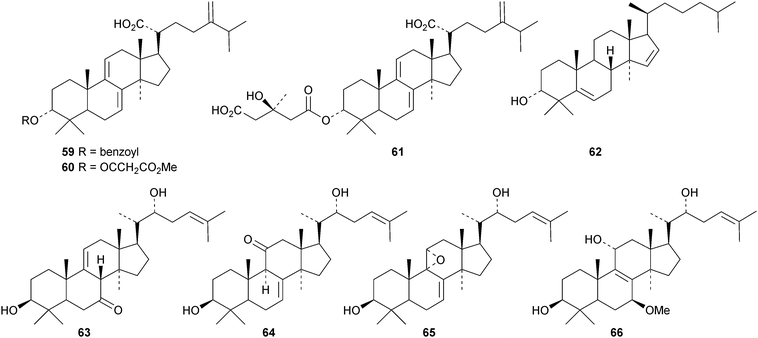
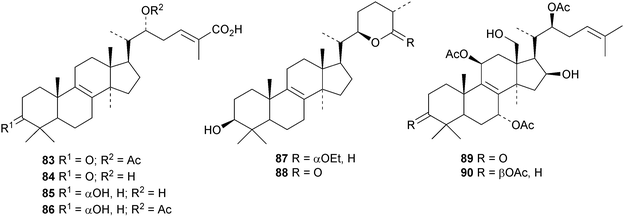
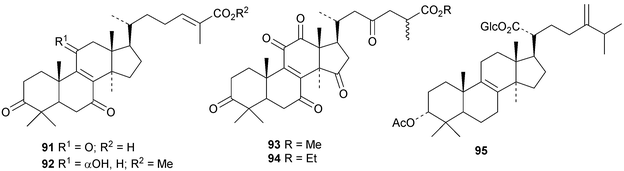
Astraodoric acids A 83–D 86 are metabolites of the mushroom Astraeus odoratus.29 The structure of astraodoric acid B 84 was confirmed by X-ray analysis. The structure of astrakurkurol 87, from the Indian edible mushroom Astaeus hygrometricus, was also confirmed by X-ray analysis.37 The corresponding lactone, astrakurkurone 88, was also obtained. Two highly acetylated lanostanes, coprinacins A 89 and B 90, have been reported from Coprinus cinereus.38 Other fungal metabolites include 91–94 from Antrodia camphorate39 and formitoside K 95, a glucoside with a new genin, from Fomitopsis nigra.40
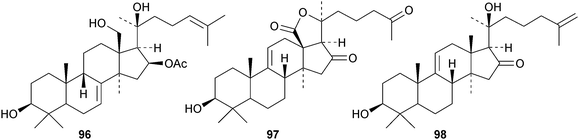
Cucumarioside A8 is a lanostane saponin with the new genin 96 from the sea cucumber Eupentacta fraudatrix.41 The sea cucumber Apostichopus japonicus is the source of several saponins, 26-nor-25-oxoholotoxin A1 with the new genin 97 and holototoxins D–G.42 Holototoxins F and G have the new genin 98 while the genins of D and E are known. Cucumariosides B1 and B243 and cucumariosides H2, H3 and H4,44 from Eupentacta fraudatrix, all have known genins. Holostane saponins and their biological activity have been reviewed.45
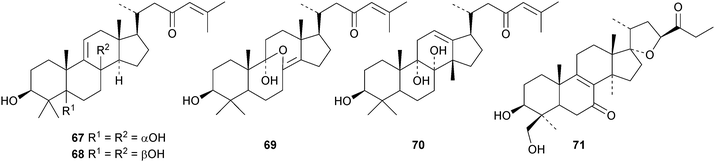
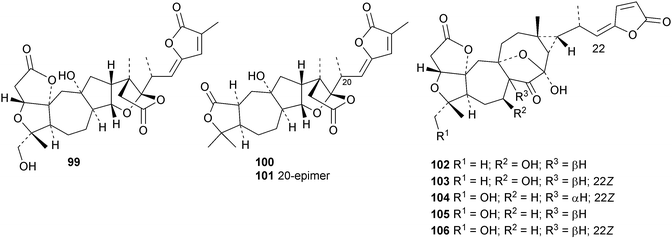
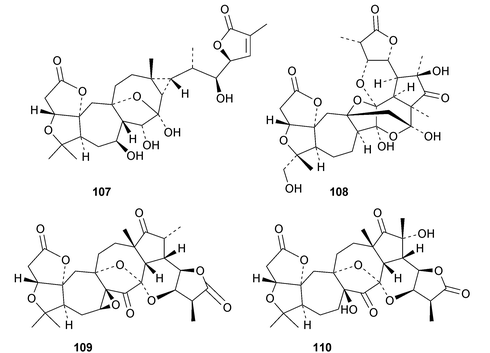
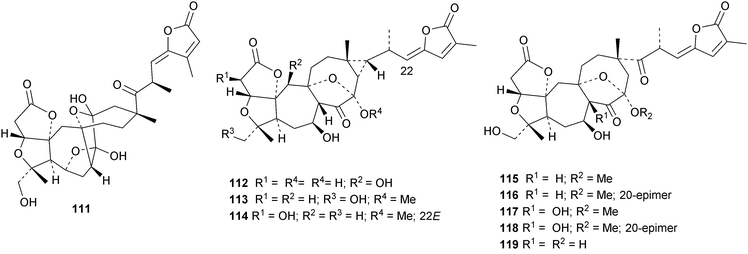
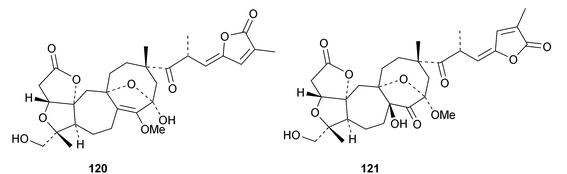
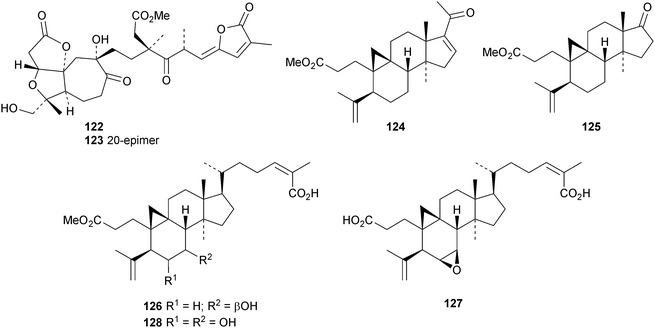
Interesting new structures continue to appear from Schisandra species. Schilancitrilactones A 99, B 100 and C 101 have been reported from Schisandra lancifolia.46 The structures of all the schilancitrilactones were confirmed by X-ray analyses. New structures from Schisandra sphenanthera include preschisanartanins E 102–J 107 and sphenadilactones D 108–F 110.47 Isoschicagenin C 111, preschisanartanins K 112–M 114, schisdilactones A 115–G 121 and schinesdilactones A 122 and B 123 constitute an impressive array of new derivatives from Schisandra chinensis.48 Schiglausins K 124–O 128 are simple ring A-cleaved cycloartanes from Schisandra glaucescens.49
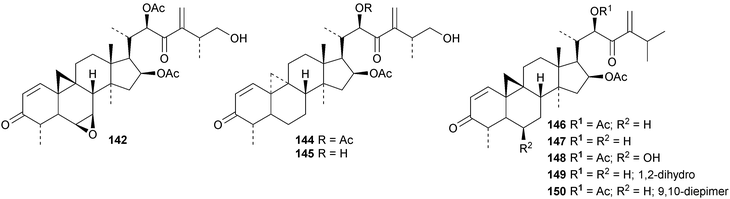
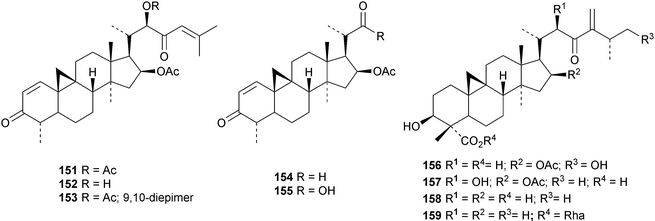
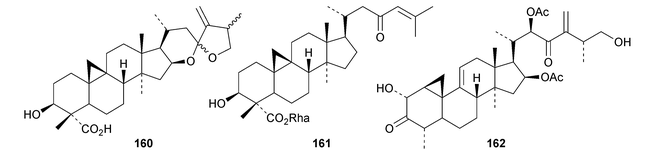
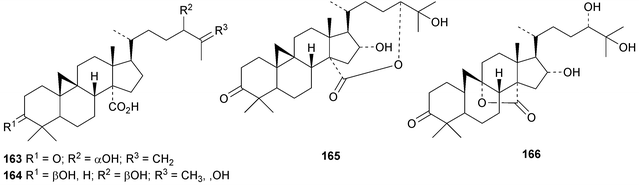
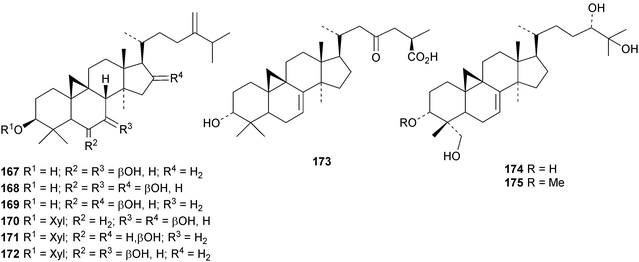
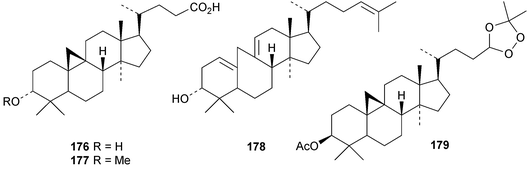
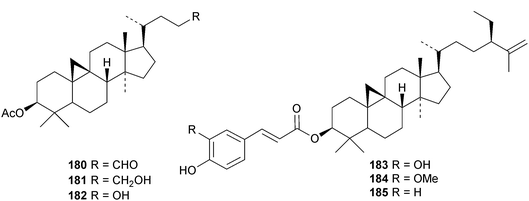
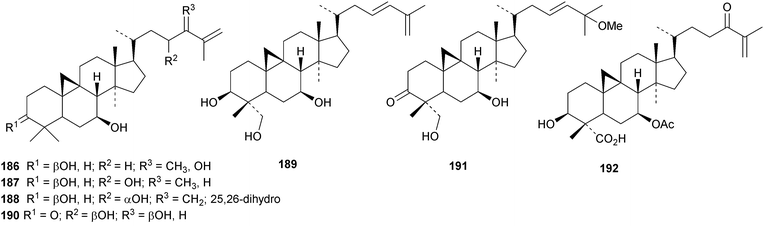
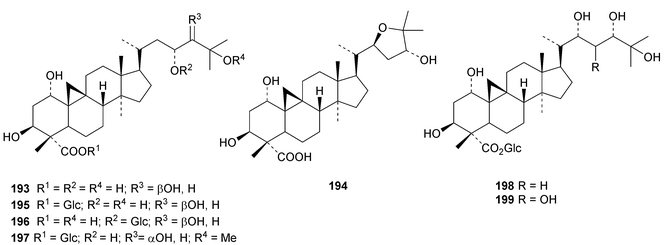
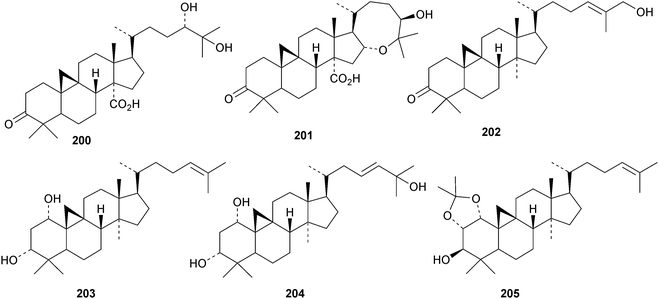
An interesting approach to the “dereplication, residual complexity and rational naming” of the Actea triterpenoids has the potential to be applied to other groups of natural products with the same inherent problems.50 Pseudolaridimers A 129 and B 130, from Pseudolarix amabilis, appear to have arisen by a Diels–Alder reaction involving a cycloartane and a labdane.51 The structure of 129 was confirmed by an X-ray analysis of the corresponding methyl ester. The cycloartanes lygodipenoids A 131 and B 132, from Lygodium japonicum, have an additional cyclopropane in their side chains.52 Thirty new cycloartane proteasome inhibitors 133–162 have been reported from Neoboutonia melleri.53 An interesting UV light-induced inversion of the configurations at C-9 and C-10 was observed in this series. Caloncobic acids A 163 and B 164 and caloncobalactones A 165 and B 166 are constituents of the leaves of Caloncoba glauca.54 Compounds 167–172 are new cycloartanes from the leaves of Homonoia riparia.55 Neoabiestrines G 173–I 175 have been reported from Abies recurvata.18 The structure of neoabiestrine H 174 was confirmed by X-ray analysis. Other new cycloartanes include the trinor derivatives 176 and 177 from Abies holophylla,20 rotundusolide C 178 from the rhizomes of Cyperus rotundus,56179–182 from the aerial parts of Atemisia lagocephala,57 three esters 183–185 of cyclomargenol from Krameria pauciflora,58 euphonerins A 186–G 192 from the leaves of Euphorbia neriifolia,59 cycloccidentalic acids A 193 and B 194 and the related saponins cycloccidentalisides I 195–V 199 from Cassia occidentalis,60 glaucartanoic acids A 200 and B 201 from the fruit of Caloncoba glauca61 and 202 from the leaves of Aglaia exima.62 Compounds 203, 204 and the acetonide 205, a likely artefact, have been obtained from the resin of Commiphora opobalsamum.63 The structures of 203 and 205 were confirmed by X-ray analyses.
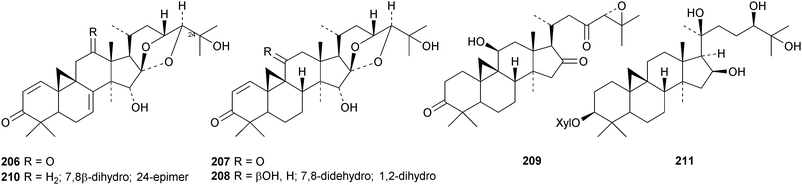
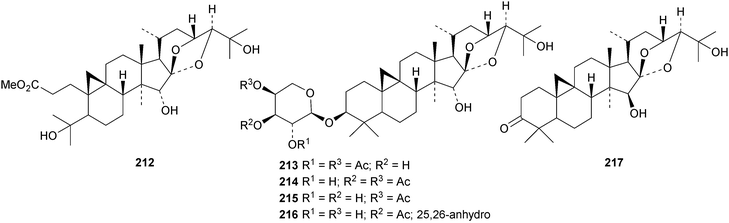
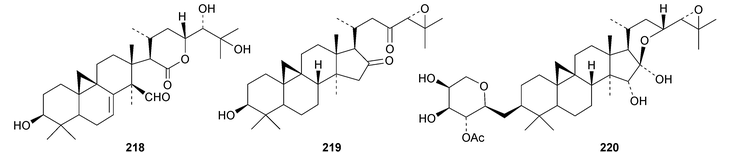
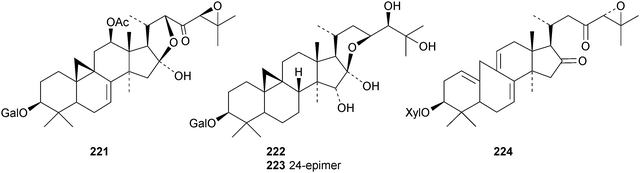
New cycloartanes continue to be isolated from Cimicifuga species. Cimicifuga foetida is the source of compounds 206–209 (ref. 70) and of 24-epi-cimigenol-3-one 210 and the xyloside foetinoside 211.64 The ring A-cleaved derivative 212, the cimigenol arabinosides 213–215, the 25-dehydrocimigenol arabinoside 216, compounds 217–219 and the shengmanol arabinoside 220 have all been isolated from the roots of Cimicifuga heracleifolia.65 Other new compounds include the galactopyranosides 221–223, all with new genins, from the roots of Cimicifuga simplex66 and isocimipodocarpaside 224 from Cimicifuga racemosa.67
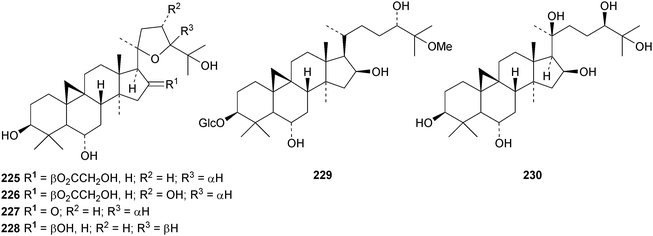
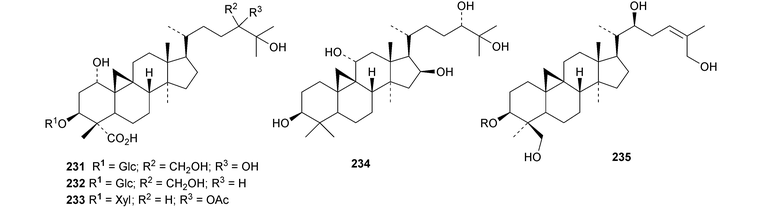
Six cycloartane saponins with the new genins 225–228 have been obtained from Astragalus angustifolius.68 Cycloquivinoside A 229 is a new saponin from Astragalus chivensis.69 The new genin cycloartane-3β,6α,16β,20S,24R,25-hexol 230 has been identified in the saponins of Astragalus stereocalyx70 and in saponins from Astragalus schottianus.71 Nervisides A 231–C 233, from Nervilia fordii, all have new genins.72 Other cycloartane saponins with new genins include curculigosaponins N and O from Curculigo orchioides with the genin 234 (ref. 73) and two saponins from Thalictrum fortune with the genins 235 and 236.74 The ring-A cleaved cycloartanes sootependial 237 and sootepenoic acid 238 have been isolated from the exudates of Gardenia sootepensis.75 Novel cycloartane saponins with known genins include cycloascidoside from Astragalus mucidus,76 cyclogaleginoside C from Astragalus galegiformis and cycloascauloside D from Astragalus caucasicus,77 hareftosides A–D from Astragalus hareftae,78 neoastragaloside I from Astragalus membranaceus79 and unnamed saponins from Astragalus erinaceus.80
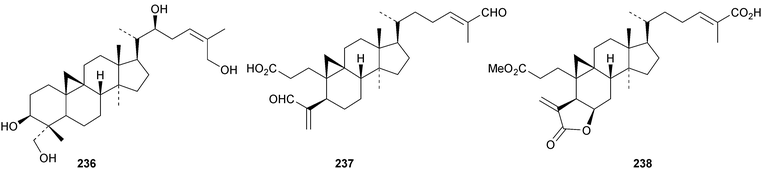
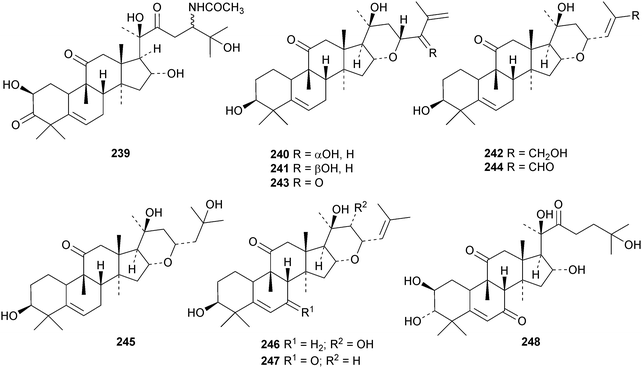
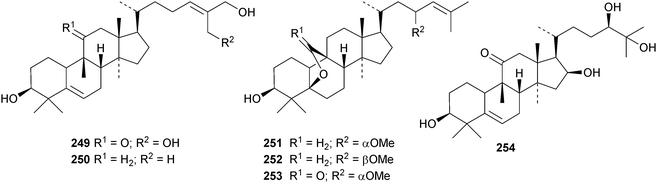
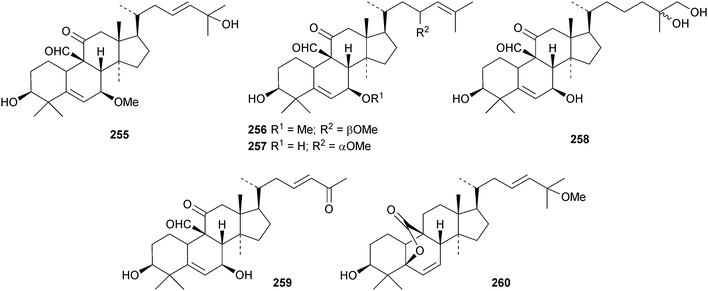
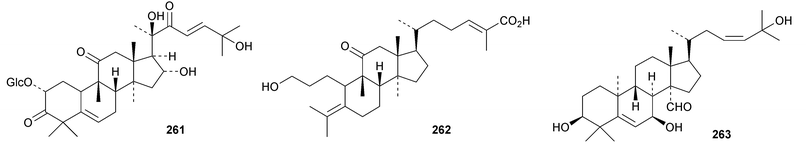
A new nitrogen-containing cucurbitane, endecaphyllacin C 239, has been reported from the tubers of Hemsleya endecaphylla.81 Eleaocarpucins A 240–H 247 are 16,23-epoxy derivatives from Eleaocarpus chinensis.82 The new cucurbitanes jinfushanencins A 248 and B 249 occur in the tubers of Hemsleya penxianensis, together with the glycosides jinfushanosides E−K.83 Jinfushanoside K has the new genin 250. Other new cucurbitanes include 251–253 from the fruit of Momordica charantia,84 10β-hydroxybryodulcosigenin 254 from Saniculiphyllum guangxiense,85 six new compounds 255–260 from the leaves of Momordica charantia86 and isoarvenin III 261 from the fruit of Trichosanthes kirilowii.87 The 3,4-seco-cucurbitane 262 is a constituent of Russula lepida and Russula amarissima.88 The unlikely 10α-methyl lanostane structure 263 has been proposed for a compound from Momordica charantia.89 Perhaps the compound is a cucurbitane! Reviews have appeared on cucurbitacins and bottle gourd toxicity,90 medicinally important plants of the Cucurbitaceae91 and the anticancer activity of the cucurbitacins.92,93
4. The dammarane group
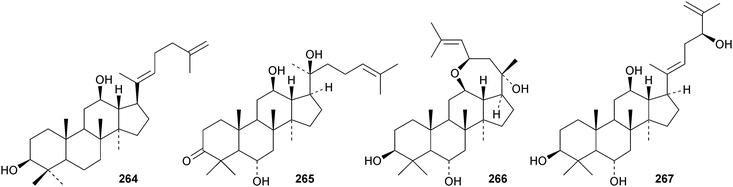
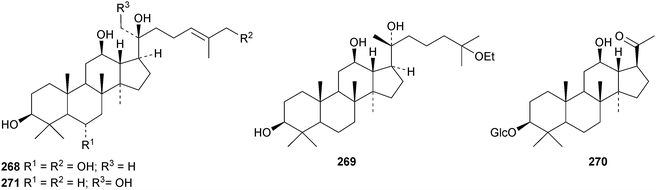
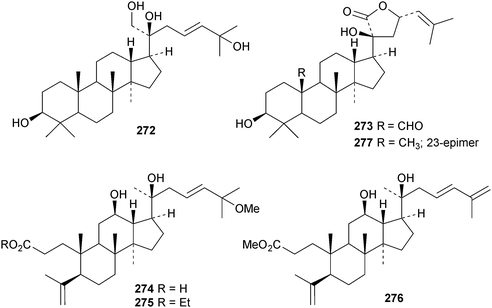
A review of ginsenoside derivatives and their antitumour activity has been published.94 New saponins from the stems and leaves of Panax ginseng include ginsenosides Rh14–Rh17 with the new genins 264 (Rh15) and 265 (Rh17)95 and ginsenosides Rh18– Rh20.96 The new genin 266 of ginsenoside Rh18 was also isolated together with the dammarane 267.96 Two new saponins of 20S-protopanaxatriol have also been found in Panax ginseng together with the dammarane 268.97 The biological activity of an artefact 269 from the acid hydrolysate of Panax ginseng has been investigated.98 The hexanordammarane saponin, ginsenoside R10270, has been isolated from the stems and leaves of Panax quinquefolium.99Saponins with the new genins 271 and 272 (ref. 100) and 273 (ref. 101) have been isolated from Gynostemma pentaphyllum. Cyclocariosides D–G, with the new genins 274 and 275, cyclocarioside H, with a known genin, and the dammarane cyclocarin A 276 are constituents of the leaves of Cyclocarya paliurus.102 Dammarane saponins with known genins have been isolated from the roots of Machilus yaoshansis.103 This paper also reports a revision the C-23 configuration of Gynostemma pentaphylla saponins as in 277.
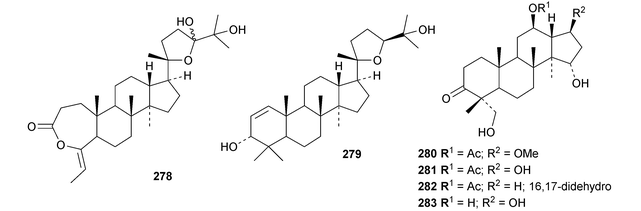
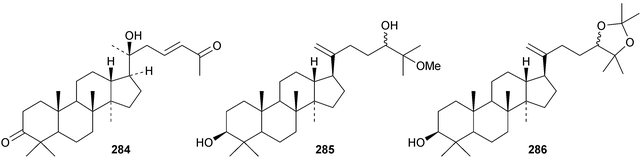
New dammaranes include the rearranged aglinone 278 and aglinin E 279 from the bark of Aglaia smithii,104 the nor-derivatives 280–283 from Dysoxylum hainanense,105 the 27-nor-derivative 284 from Dipterocarpus obtusifolius106 and the two probable artefacts 285 and 286 from the leaves of Aglaia odorata.15
Novel dammarane saponins with known genins include acerbosides A and B from Hovenia acerba,107 chikusetsusaponins FK1–FK7, FH1, FH2, FM and FT1–FT4 from Panax japonicus,108,109 gypenbiosides A and B from Gynostemma pentaphyllum,110 20R-pseudoginsenoside F11 (ref. 111) and quinquenosides Ja and Jb112 from Panax quinquefolium.
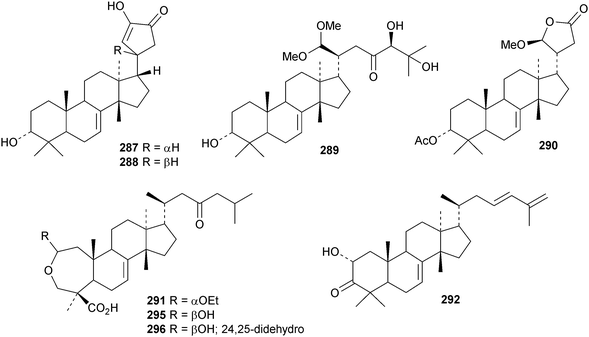
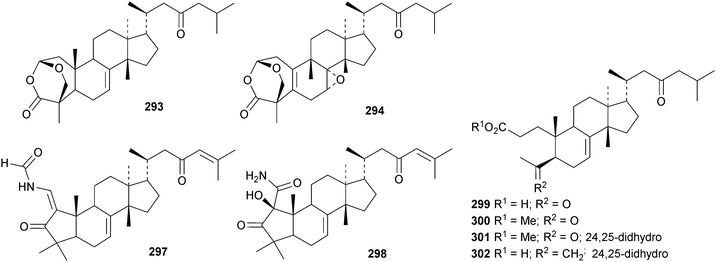
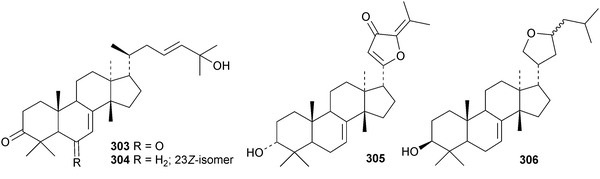
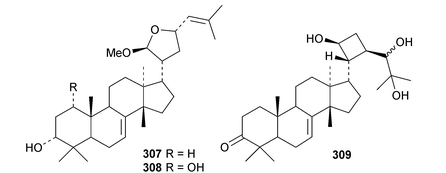
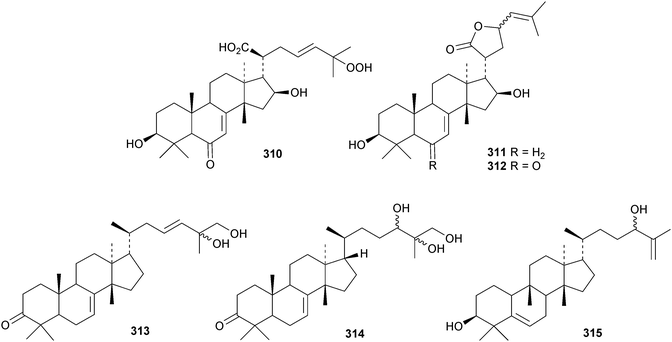
New compounds from the stem bark of Aphanamixis grandifolia include the tautomeric tirucallane cyclopentenones 287 and 288 (ref. 113) and the tirucallanes 289 and 290.114 Compounds 287 and 288 were originally named aphragranins A and B, names used by the authors for previous compounds. Their names have been changed to aphagraones A and B.115 Two further derivatives 291 and 292 were obtained from the leaves and twigs of Aphanamixis grandifolia.116 Some of these compounds clearly incorporate the extraction solvent. Aphanamgrandins A 293–J 302 constitute a series of 2,3-seco- and 3,4-seco-tirucallanes from the stems of Aphanamixis grandifolia.117 They were accompanied by the ring-A intact derivatives aphanamgrandin J 303 and the dienone 304. The structures of aphanamgramins A 293 and B 294 were confirmed by X-ray analyses. Other new tirucallanes include the 21-nor-derivative dysoxylentin A 305 from Dysoxylum lenticellatum,118 ixoroid 306 from the flowers of Ixora coccinea,119307 and 308 from the stem bark of Araliopsis synopsis,120 capulin 309 from Capuronianthus mahafalensis,121310–312 from the stem bark of Melia toosendan,122 dysohainanin F 313 from Dysoxylum hainanense123 and 24,25,26-trihydroxytirucall-7-en-3-one 314 from Salacia hainanensis.124 The 19(10 → 9)-abeotirucallane 315 has been reported from Euphorbia mellifera.125
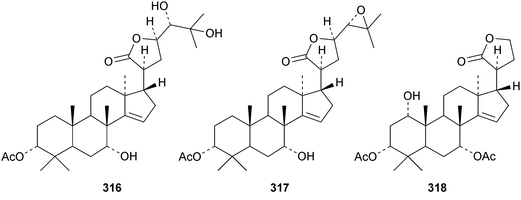
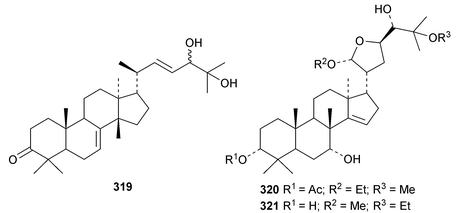
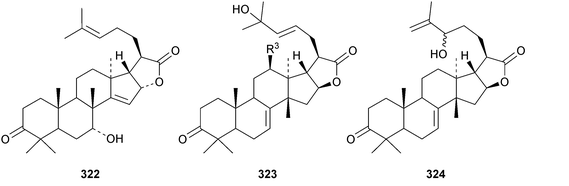
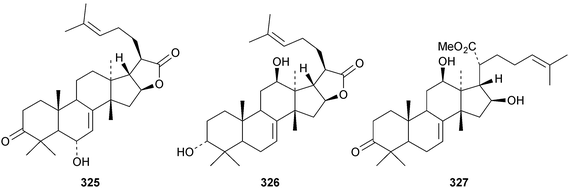
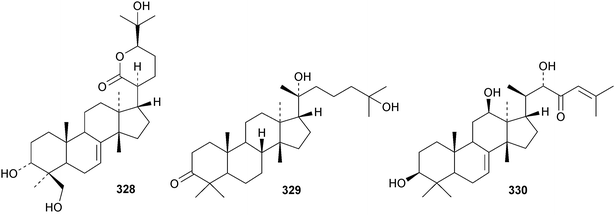
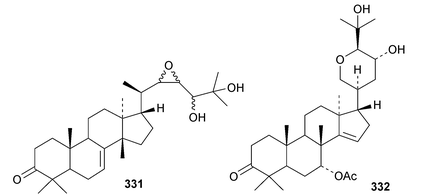
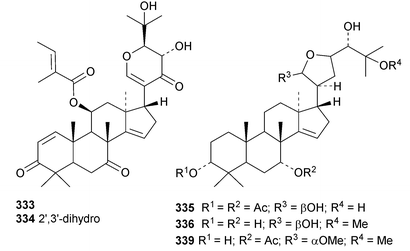
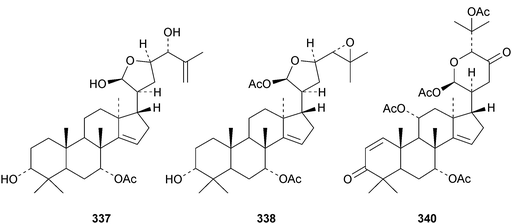
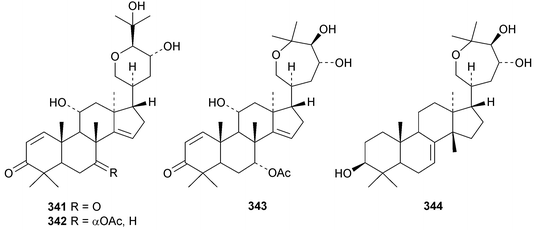
Chisiamols G 316 and H 317 (ref. 126) and chisopanins L 318–O 321 (ref. 108 and 127) are new apotirucallanes from Chisocheton paniculatus. A series of compounds from the leaves and twigs of Melia toosendan includes the apoeuphane mesendanin K 322, the euphanes mesendanins L 323–P 327, the tirucallanes mesendanins Q 328–T 331 and the apotirucallane mesendanin U 332.128 The apotirucallane 332 was also isolated from Dysoxylum hainanense and named dysohainanin E.123 Other new apotirucallanes include piscidinones A 333 and B 334 from Walsura trifoliata,129 agladorals A 335–E 339 from Aglaia odorata var. microphyllina130 and trichostemonate 340 from the roots of Walsura trichostemon.131 The structure of 340 was confirmed by X-ray analysis. Toonaciliatavarins A 341–H 348 are constituents of Toona ciliata.132
4.1 Tetranortriterpenoids
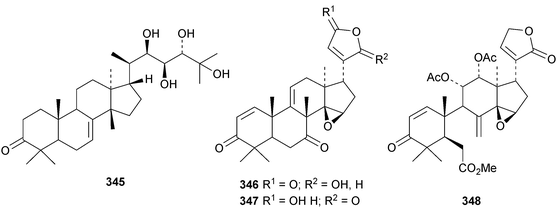
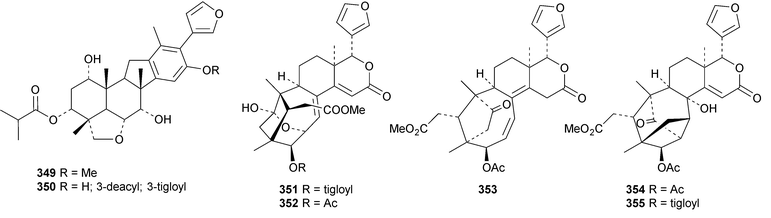
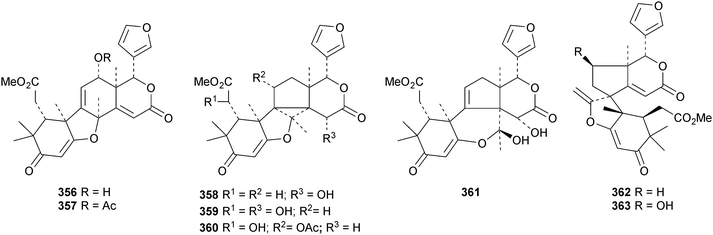
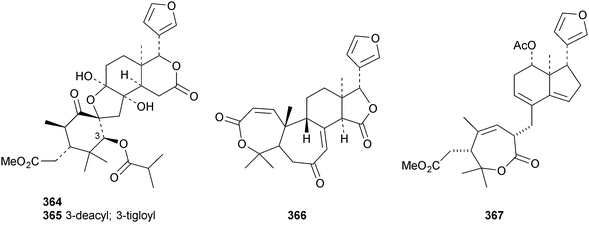
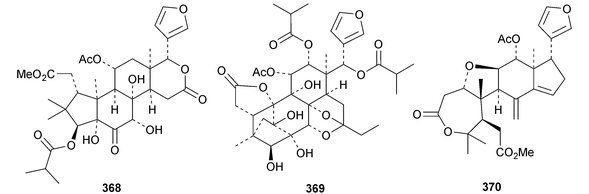
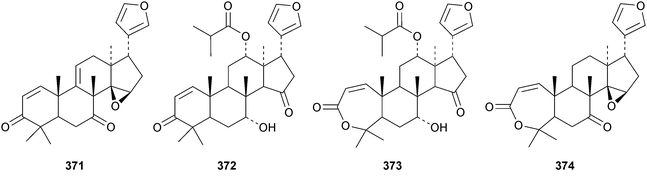
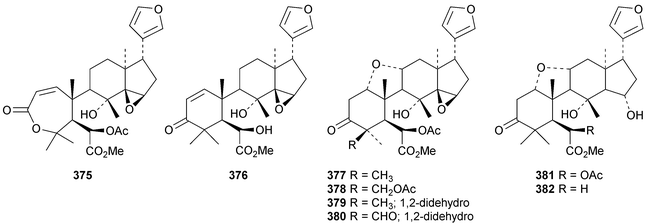
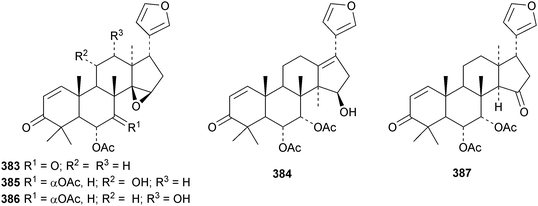
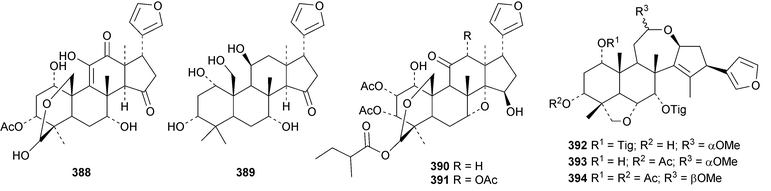
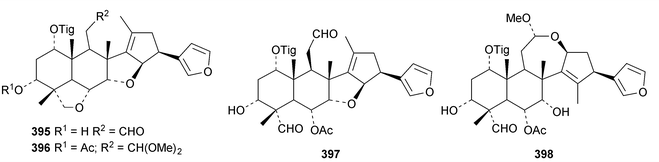
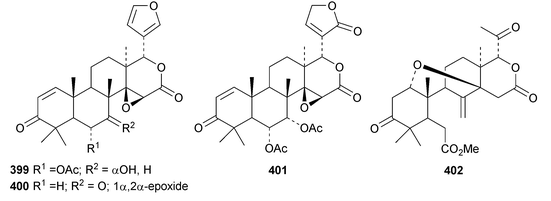
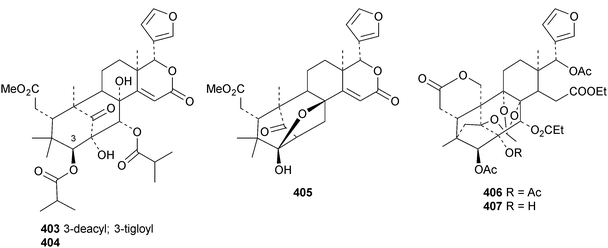
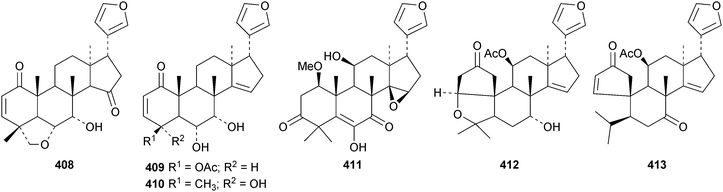
A review of the structures and biological activities of limonoids from Cipadessa species has been published.133 Walsucochinoids A 349 and B 350 are rearranged limonoids, with an aromatic ring D, from Walsura cochinchinensis.134 Andhraxylocarpins A 351, B 352, C 353 and E 354 are new skeletal types from Xylocarpus granatum.135 The structures of 349, 351 and 353 were confirmed by X-ray analyses. Andhraxylocarpin D 355 is the same as chisomicin A, published in 2011. Hortia oreadica is the source of the interesting rearranged limonoids 356–363, related to the known hortiolide A.136 The structure of hortiolide C 358 was confirmed by X-ray analysis. The configurations at C-5 and C-10 of hortiolide E 362 and 12-hydroxyhortiolide E 363 are wrongly drawn in the paper. Carapanolides A 364 and B 365 are 9,10-seco mexicanolide derivatives from the seeds of Carapa guianensis.137 Other new structural types include citriolide A 366 from the seeds of Citrus reticulate,138 aphanamixoid A 367 from Aphanamixis polystachya139 and chukrasones A 368 and B 369 from Chukrasia tabularis.140 The structure of aphanamixoid A 367 was confirmed by X-ray analysis. It was accompanied in the extract by aphanamixoid B 370.Toonayunnanins A 371–L 382 constitute a group of assorted limonoids from the leaves of Toona ciliata var. yunnanensis.141 Toonayunnanin I 378 is the same as toonaciliatin P, published in 2011. Another group of compounds, toonaciliatones B 383–F 387, has been obtained from the seeds of Toona ciliata.142 Meliatoosenins E 388, F 389, I 390, J 391, L 392–N 394 and P 395–S 398 are new compounds from the fruit of Melia toosendan.143 Meliatoosenins G, H, K and O, claimed as new compounds, have all been published previously. Other new compounds include andirolides H 399–P 407 from Carapa guianensis flowers,144 ceramicines J 408–L 410 from Chisocheton ceramicus145 and walsuranins A 411–C 413 from Walsura yunnanensis.146
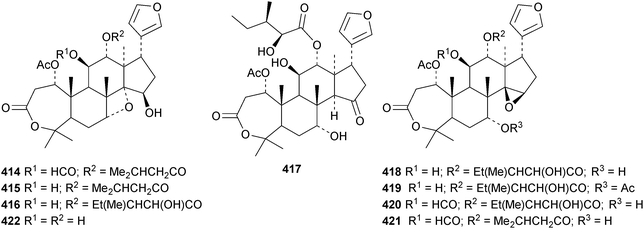
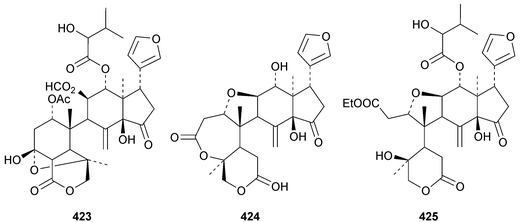
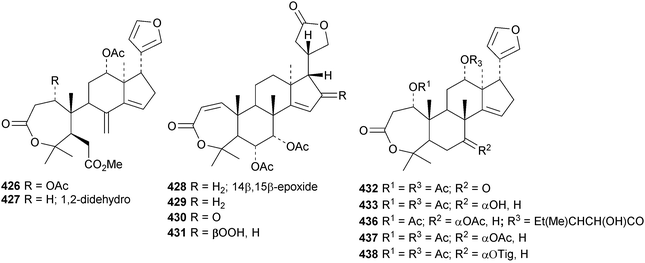
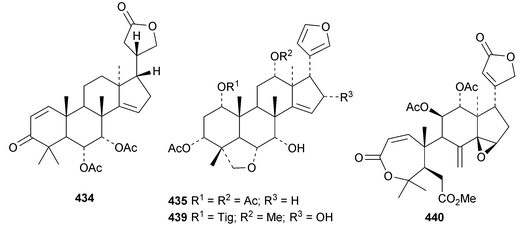
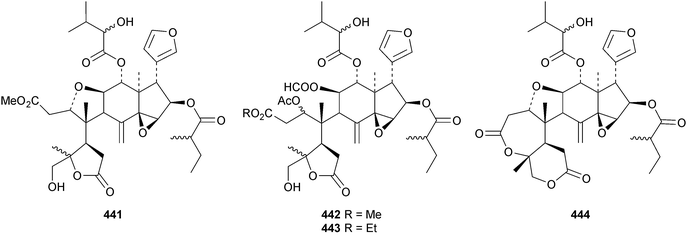
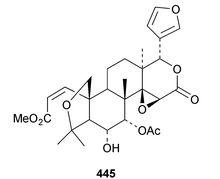
The ring A-cleaved compounds, aphanalides A 414–H 421, have been reported from the fruit of Aphanamixis polystachya.147 The structures of aphanalides A 414 and C 416 were confirmed by X-ray analyses. Aphanagranins A 422–D 425 are constituents of Aphanamixis grandifolia.148 Other species producing multiple new compounds include Munronia unifoliata with munronoids A 426–J 435 (ref. 149) and K 436–O 440 (ref. 150) and Dysoxylum hainanense with dysohainanins A 441–D 444.123 6-O-Deacetylseverinolide 445 is a constituent of Atalantia buxifolia.151
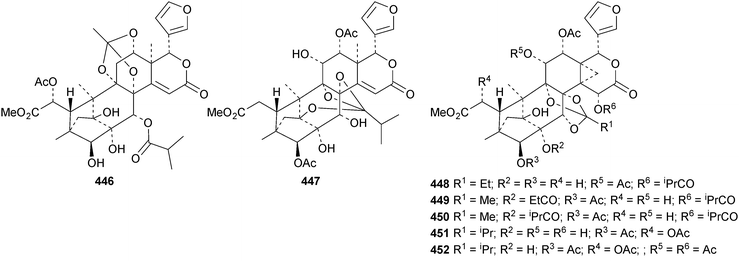
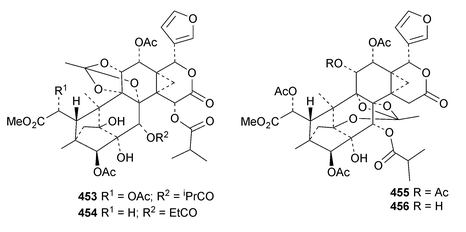
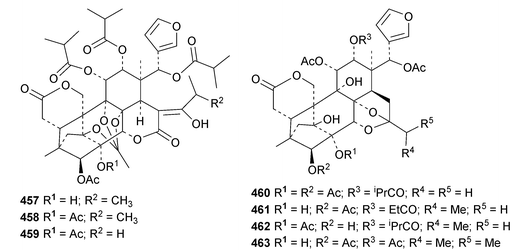
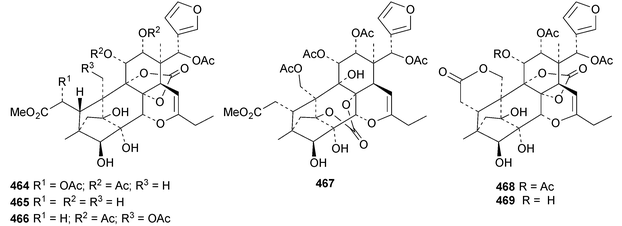
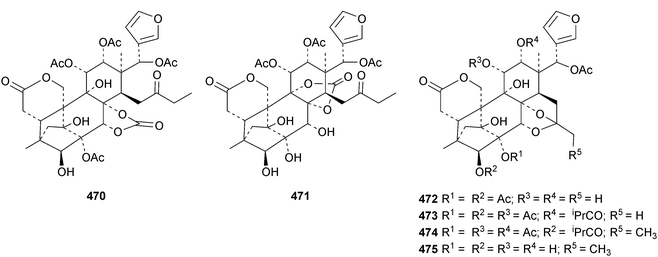
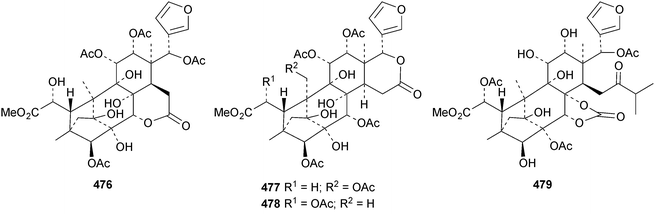
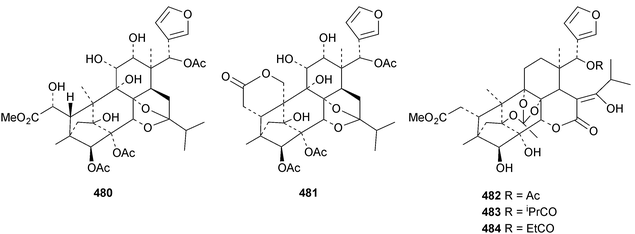
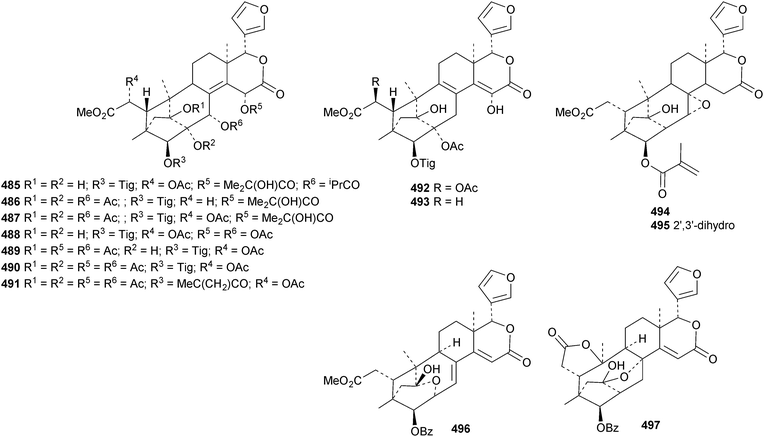
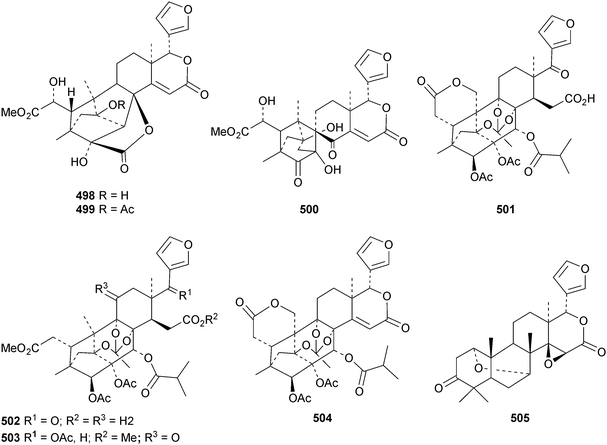
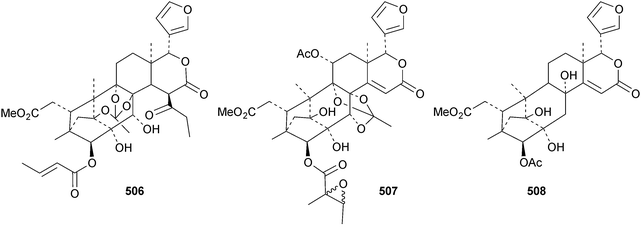
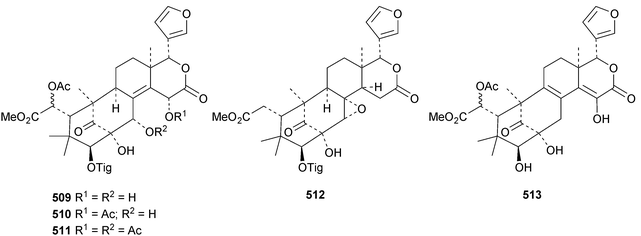
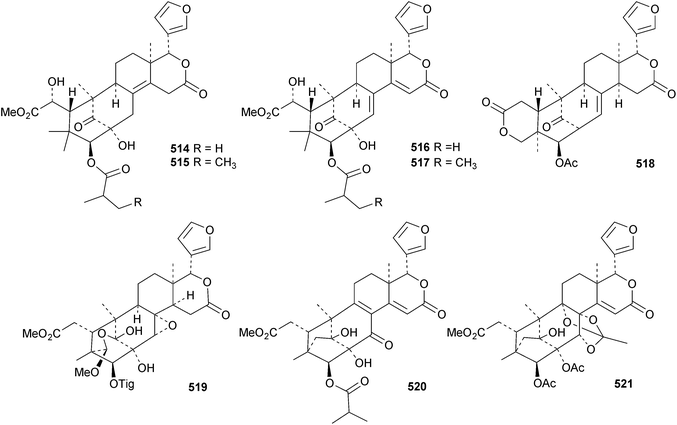
The seemingly unending flow of phragmalin/bussein derivatives and variants continues and is matched only by the proliferation of confusing trivial names. These points are well illustrated by Chukrasia tabularis and Chukrasia tabularis var. velutina, the sources of chubularisins A 446–R 463,152 chuktabrins C 464–J 471 and chuktabularins U 472–X 475,153 tabulalins G 476–I 478,154 chukvelutins D 479–F 481 (ref. 155) and R310B8 482 and velutinalides A 483 and B 484.156 Chabularisin O 460 is the same as chuktabularin V 474. The structure of chuktabrin C 464 was confirmed by X-ray analysis. Heytrijumalins A 485–I 493 have been isolated from the twigs and leaves of Heynea trijuga.157 Other new compounds in this group include hisomicines D 494 and E 495 from Chisocheton ceramicus,158 malayanines A 496 and B 497 from Chisocheton erythrocarpus,159 senegalensions A 498–C 500 from Khaya senegalensis,160 kotschyins D 501–H 505 from Pseudocedrela kotschyi,161 soymidins A 506 and B 507 from Soymida febrifuga162 and swietenin J 508 from Swietenia macrophylla.163 Five new mexicanolide derivatives, heytrijunolides A 509–E 513, have been obtained from Heynea trijuga.164 Mollucensins R 514–Y 521 are new mexicanolide and phragmalin derivatives from the seeds of Xylocarpus moluccensis.165
4.2 Quassinoids
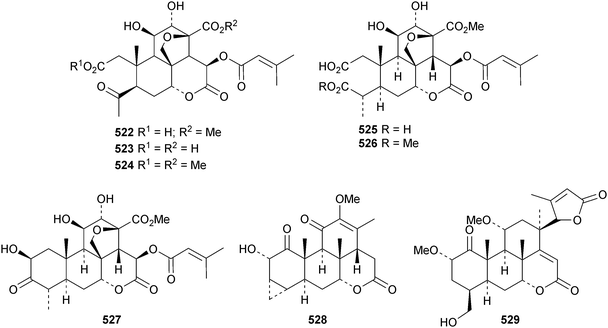
The compounds isolated from Brucea javanica and their pharmacology have been reviewed.166 Bruceanic acids E 522 and F 523, buruceanic acid E methyl ester 524, javanic acids A 525 and B 526 and javanicolide H 527 are new compounds from the seeds of Brucea javanica.167 Other new quassinoids include picrasin K 528 from Quassin amara168 and odyendanol 529 from the fruit of Odyendyea gabonensis.1695. The lupane group
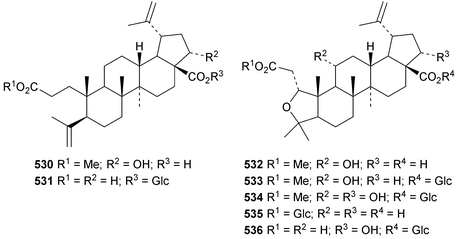
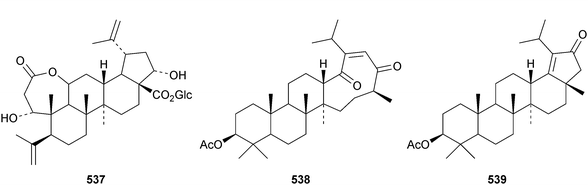
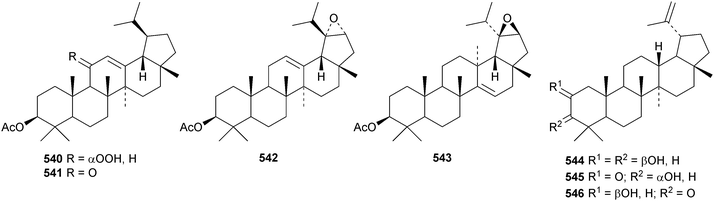
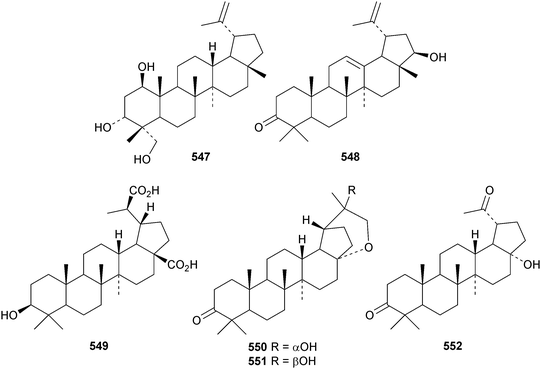
Reviews covering the sources and biological properties of betulinic acid170 and the antineoplastic effects of lupeol171 have appeared. The 3,4-secolupane derivatives acanthosessiligenins I 530 and II 531 and acanthosessiliosides A 532–F 537 have been isolated from the fruit of Acanthopanax sessiliflorus.172 The 17,18-secolupane 538 has been found in the roots of Taraxacum platycarpum together with 3β-acetoxylup-18-en-21-one 539, the neolupane derivatives 540–542 and the migrated lupane 543.173 Lup-20(29)-ene-2β,3β-diol 544 is a constituent of Salacia hainanensis124 and the related ketones 545 and 546 have be found in Fagus hayatae.174 Other simple lupane derivatives include glochitriol 547 from Glochidion lanceolarium,175 bengalensinone 548 from Ficus bengalensis,176 3β-hydroxylupane-28,29-dioic acid 549 from Aglaia duperreana177 and the norlupane derivatives 550, 551 and 552 from Dipterocarpus obtusifolius.106Two lupane saponins with the new genin 553 have been isolated from Schefflera venulosa.178 New lupane saponins with known genins include ilekudinchoside E from Ilex kudincha,179 stellatosides C, D and E, the methyl esters of stellatosides B and C and thurberoside A from Stenocereus eruca180 and unnamed saponins from Liquidambar formosana181 and Cichorium intybus.182
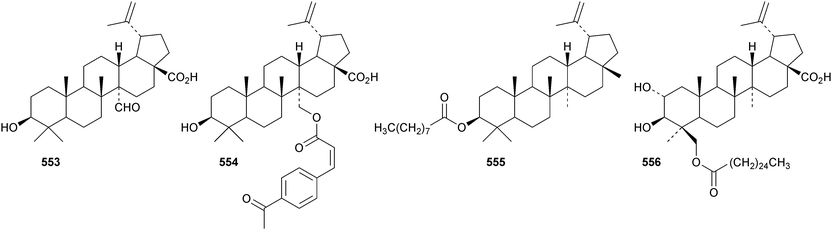
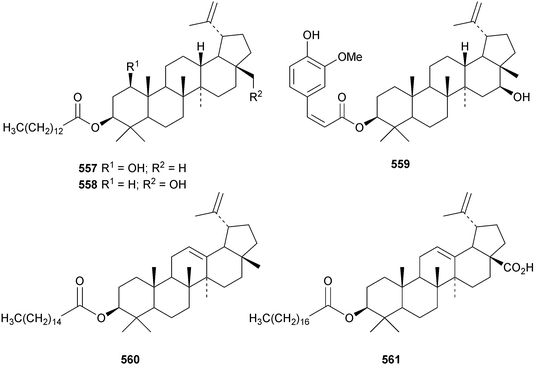
Oleanderocioic acid 554, a lupane ester with the unusual cis-4-acetylcinnamic acid, is claimed to be a constituent of Nerium oleander.183 Other new lupane esters include the nonanoyl ester of lupeol 555 from Dorstenia harmsiana,184 kurramanoic acid 556 from Nepeta clarkei,185 the myristoyl esters 557 from Glochidion wrightii186 and 558 from Sinocalamus affinis,187 the cis-feruloyl ester 559 from Panax ginseng,188 the palmitoyl ester 560 from Cichorium intybus182 and the stearoyl ester 561 from Ocimum sanctum.189
6. The oleanane group
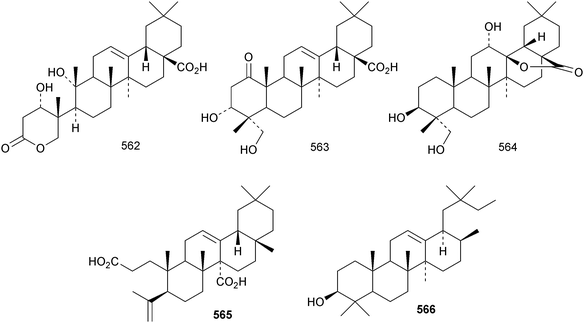
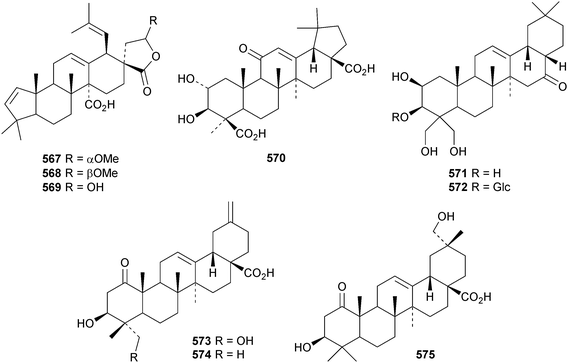
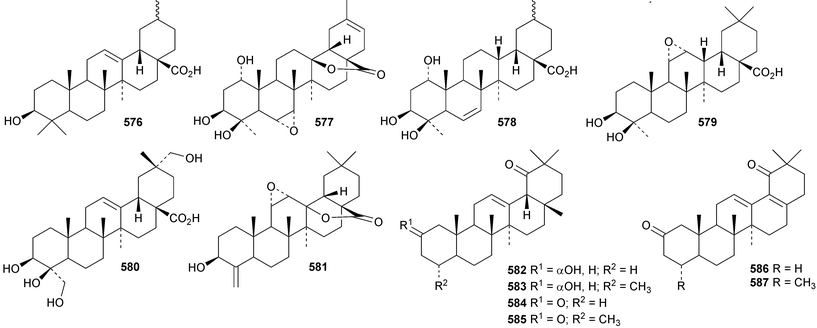
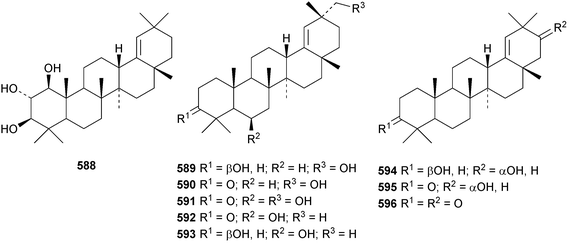
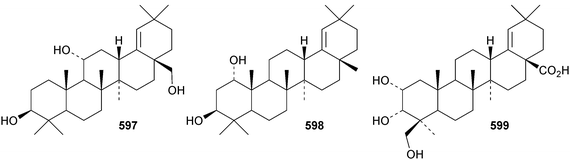
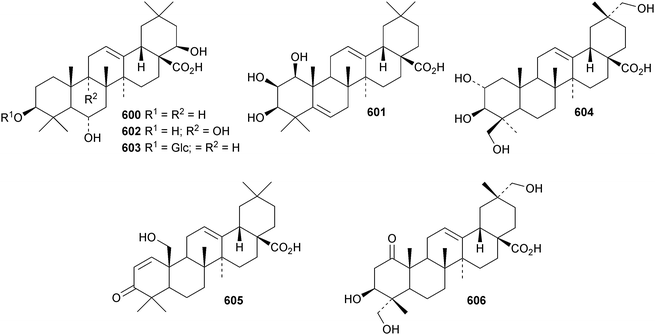
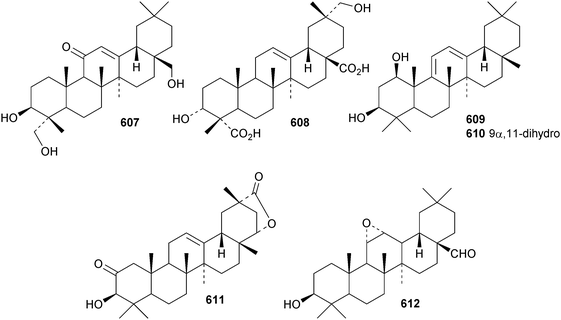
Reviews have appeared on the origins, biosynthesis and biological activity of oleanolic acid190 and the beneficial effects of arjunolic acid in type 1 diabetes.191Fagus hayatae is the source of the 1,10-secooleanane derivative 562 where it is found with 3α,23-dihydroxy-3-oxoolean-12-en-28-oic acid 563 and 3β,12α,23-trihydroxyoleanan-28,13β-olide 564.174 Sentulic acid from Sandoricum koetjape has been identified as 3,4-secooleana-4(23),12-diene-3,27-dioic acid 565.192 The 17,22-secooleanenol 566, from Pyrenacantha kaurabassana, has the unusual 18αH-configuration.193 Zizimauritic acids A 567, B 568 and C 569 are ring-A contracted 20,21-secooleanane derivatives from Ziziphus mauritiana.194 The zizimauritic acids also have the 18αH-configuration. A ring-E contracted oleanane derivative 570 has been isolated from the bark of Diospyros decandra.195 Platycodonoids A 571 and B 572 are 28-noroleanane derivatives from the roots of Platycodon grandiflorum.196 The 30-nor derivatives euscaphic acids G 573 and H 574, together with their likely precursor euscaphic acid I 575, are present in Euscaphis japonica.197 Further noroleanane derivatives include 30-noroleanolic acid 576 from Olea europea,198 the 24,30-dinor derivatives 577 and 578 and the 24-nor compounds 579 and 580 from the roots of Paeonia emodi199 and 581 from Pilea cavaleriei.200 A 4900 year old oak wood sample found in a freshwater sediment has produced six noroleanane derivatives 582–587 all lacking oxygenation at C-3.201Several olean-18-ene derivatives have been identified including olean-18-ene-1β,2α,3β-triol 588 from Salvia atropatana,202589–596 from Cassine xylocarpa, 597 from Maytenus jelskii,203 olean-18-ene-1α,3β-diol 598 from Juglans sinensis204 and 2α,3α,24-trihydroxyolean-18-en-28-oic acid 599 from Eucalyptus exserta.205 The stem bark of Terminalia arjuna is the source of oleaterminaloic acids A 600, B 601 and C 602 together with the 3-glucoside oleaterminalide 603.206 Other new simple oleanane derivatives include pseuderanic acid 604 from Pseuderanthemum carruthersii,207 punicaone 605 from Punica granatum,208 turformosinic acid 606 from Turpinia formosana,209 3β,23,28-trihydroxyolean-12-en-11-one 607 from Aster yomena,210 3α,29-dihydroxyolean-12-ene-23,28-dioic acid 608 from Schefflera farinosa,211 oleana-9(11),12-diene-1β,3β-diol 609 from Salvia xanthocheila,212 1-epi-castanopsol 610 from Simira glaziovii,213 the 29,22-olide 611 from Celastrus orbiculatus214 and the epoxy aldehyde 612 from Tetraena mongolica.215
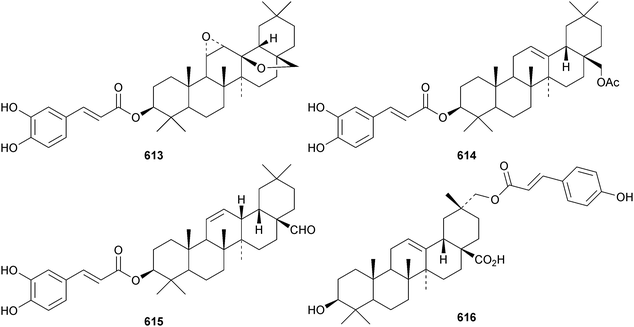
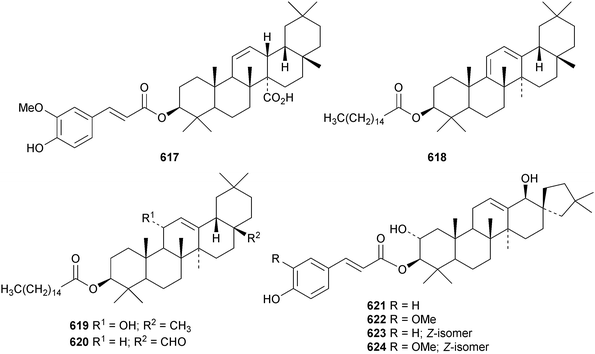
New oleanane esters include the caffeoyl derivatives 613–615, from Tetraena mongolica,215 the coumaroyl ester 616 from Rubia schumanniana,216 the ferruloyl ester 617 from Saniculiphyllum guangxiense,85 the palmitates 618 from Anemone rivularis,217619 from Barringtonia asiatica218 and 620 from Lobelia sessilifolia.219 Four esters 621–624 of the 19(18 → 17)-abeo-28-noroleanane phlomstetraol B have been isolated from Leonurus heterophyllus.220
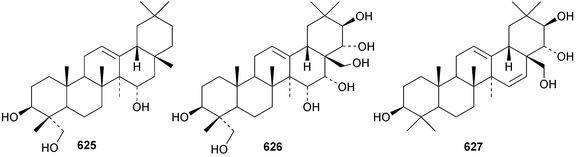
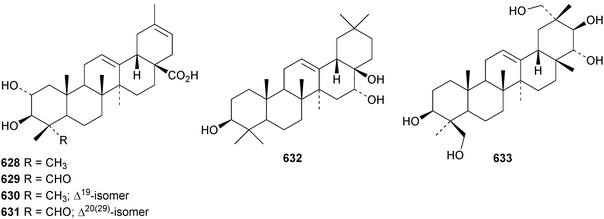
Two oleanane saponins, with the new genin olean-12-ene2β,15α,23-triol 625, have been isolated from Ammannia auriculata.221 Antoniosides E–J are oleanane saponins from Antonia ovata.222 Antioniosides E–H have new genins that are esters of olean-12-ene-3β,15α,16α,21β,22α,23,28-heptol 626. Sorbifoliasides G–J, from Xanthoceras sorbifolia, have known genins and sorbifoliaside K has the new genin oleana-12,15-diene-3β,21β,22α,28-tetrol 627.223 A variety of new 30-noroleanane genins, 628–631, are included in akemisaponins A–K from Akebia trifoliate.224 Two saponins from Camellia japonica have been assigned the names camelliosides E and F that have been used previously.225 Camellioside E has the new genin 28-norolean-12-ene-3β,16α,17β-triol 632. Four saponins have been isolated from Astragallus angustifolius including the new genin olean-12-ene-3β,21β,22α,24,29-pentol 633.68
New oleanane saponins with known genins that have been assigned trivial names are listed in Table 1.
| Trivial name | Plant species | Ref. |
|---|---|---|
| Aesculiosides G1–G16 | Aesculus glabra | 226 |
| Afrocyclamins A and B | Cyclamen africanum | 227 |
| Anemonerivulariside A | Anemone rivularis | 228 |
| Asperosaponins A–C | Dipsacus Asper | 229 |
| Besylvosides I–VI | Aegilops geniculata | 230 |
| Bigeloviis A and B | Salicornia bigelovii | 231 |
| Bodiniosides C and D | Elsholtzia bodinieri | 232 |
| Bonarienosides A–E | Hydrocotyle bonariensis | 233 |
| Bunkankasaponin F | Xanthoceras sorbifolia | 234 |
| Centelloside D | Centella asiatica | 235 |
| Chakasaponin IV | Camellia sinensis | 236 |
| Dumortierinoside A methyl ester | Isolatocereus dumortieri | 237 |
| Elmalienosides A–C | Cephalaria elmaliensis | 238 |
| Entadosides A–D | Entada phaseoloides | 239 |
| Gardenisides A–C | Gardenia jasminoides | 240 |
| Guaianin P | Guaiacum officinale | 241 |
| Gummososide A, gummososide A methyl ester | Stenocereus alamosensis | 237 |
| Hareftoside E | Astragalus hareftae | 78 |
| Hederifoliosides A–E | Cyclamen hederifolium | 242 |
| Ilexsaponins D–F | Ilex pubescens | 243 |
| Ilexsaponin D (duplicate name) | Ilex pubescens | 244 |
| Kalopanaxsaponins L and M | Kalopanax pictus | 245 |
| Lobatosides L and M | Actinostemma lobatum | 246 |
| Lonimacranthoides IV and V | Lonicera macranthoides | 247 |
| Mimengosides H and I | Buddleja lindleyana | 248 |
| Oleiferasaponin A1 | Camellia oleifera | 249 |
| Paviosides A–H | Aesculus pavia | 250 |
| Pleurosaponins A–K | Pleurospermum kamtschaticum | 251 |
| Polygalasaponins LI–LIII | Polygala japonica | 252 |
| Raddeanosides R20–R22 | Anemone raddeana | 253 |
| Sanchakasaponins A–D | Camellia japonica | 254 |
| Sanchakasaponins E–H | Camellia japonica | 255 |
| Sandrosaponin XI | Ferula hermonis | 256 |
| Senaciapittosides A and B | Pittosporum senacia | 257 |
| Silenoviscoside F | Silene viscidula | 258 |
| Sorbifoliasides A–F | Xanthoceras sorbifolia | 234 |
| Teaseedsaponins A–L | Camellia sinensis | 259 |
| Treleaseside A | Stenocereus eruca | 180 |
The sources of new oleanane saponins with known genins that have not been assigned trivial names are listed in Table 2.
| Plant species | Ref. |
|---|---|
| Abrus precatorius | 260 |
| Abuta grandifolia | 261 |
| Acanthopanax senticosus | 262 |
| Acanthophyllum gypsophiloides | 263 |
| Alhagi maurorum | 264 |
| Anemone amurensis | 265 |
| Aralia elata | 266 |
| Aralia taibaiensis | 267 |
| Ardisia gigantifolia | 268 |
| Callicarpa integerrima | 269 |
| Chenopodium foliosum | 270 |
| Clematis argentilucida | 271 and 272 |
| Cyperus rotundus | 273 |
| Dizygotheca elegantissima | 274 |
| Eragrostis tef | 275 |
| Grangea maderaspatana | 276 |
| Gymnema sylvestre | 277 |
| Gymnocladus chinensis | 278 |
| Gypsophila trichotoma | 279 |
| Kalopanax pictus | 245 |
| Kalopanax septemlobus | 280 |
| Maesa lanceolata | 281 |
| Patrinia scabiosifolia | 282 |
| Pometia pinnata | 283 |
| Salicornia europaea | 284 |
| Salicornia herbacea | 285 |
| Samanea saman | 286 |
| Sideroxylon obtusifolium | 287 |
| Tarenna grevei | 288 |
| Wedelia chinensis | 289 |
| Xanthoceras sorbifolia | 290 |
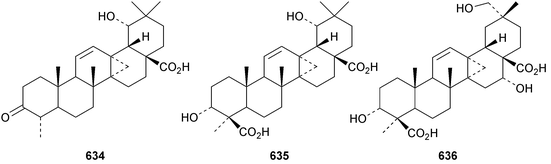
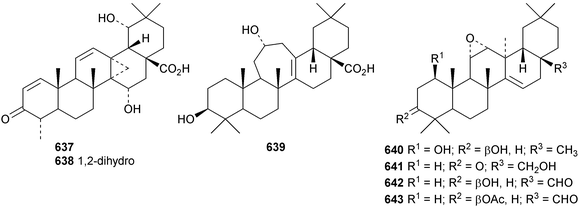
Pachanosides I1 and D1 are pachanane saponins with known genins from Isolatocereus dumortieri.237 The structures of the 13,27-cyclized oleananes donellanic acids A 634, B 635 and C 636, from Donella ubanguiensis, were all established by X-ray analyses.291 The donellanic acids are accompanied by two further 13,17-cyclized compounds that have been assigned the tentative structures 637 and 638. Ilelic acid B 639 is a rearranged oleanane with a seven-membered C-ring from Ilex latifolia.292 Four taraxerane derivatives 640–643 have been identified in Saussurea graminea.293
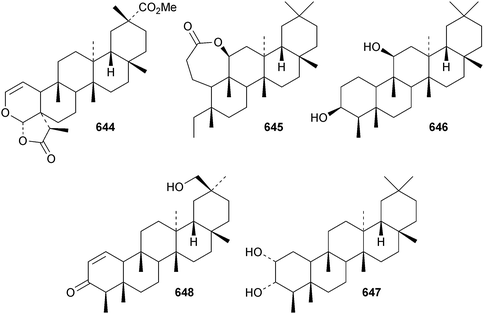
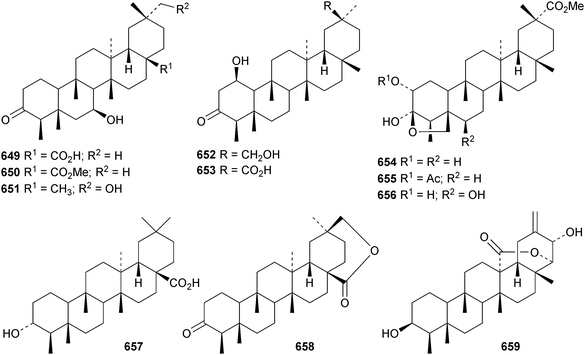
Cassinolide 644, from Cassine xylocarpa, is a 2,3-secofriedelan-3,24-olide derivative.294Maytenus robusta is the source of the 3,4-secofriedelan-3,11β-olide 645 (ref. 295) and friedelane-3β,11β-diol 646.296 Reissantiadiol, from Reisantia grahamii, has been identified as friedelane-2α,3α-diol 647.297 Other simple friedelane triterpenoids include 30-hydroxyfriedel-1-en-3-one 648 from Salacia hainanensis,124649–653 from Celastrus vulcanicola and 654–658 from Maytenus jelskii298 and glaucalactone 659 from Caloncoba glauca.61
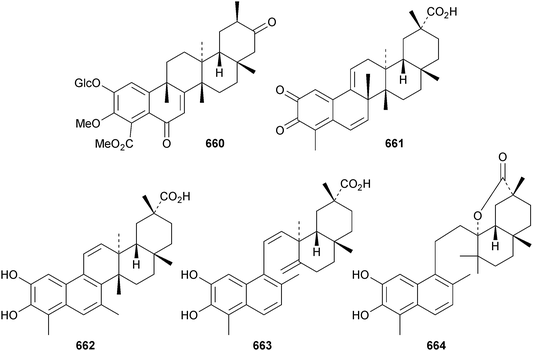
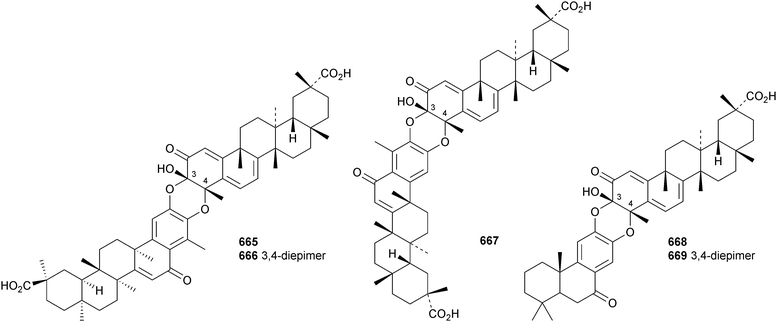
A review of the pharmacology of celastrol, a norfriedlane derivative, has been published.299 Hypoglaside A 660 is a dinorfriedelane glucoside from Tryperygium hypoglaucum.300Celastrus orbiculatus is the source of a range of norfriedelane and methyl-migrated derivatives.301 The 25(9 → 8)-abeo norfriedelane 661 and the 25(9 → 7)-abeo derivative 662 are accompanied by the 8,14-seco compounds 663 and 664. A biosynthetic pathway to these rearranged friedelane derivatives has been proposed. Also present in Celastrus orbiculatus are the “dimeric” norfriedelanes celastrolines Aα 665 and Aβ 666 and isocelastroline Aα 667, together with celastrolines Bα 668 and Bβ 669 that have a linkage to a podocarpane derivative.
7. The ursane group
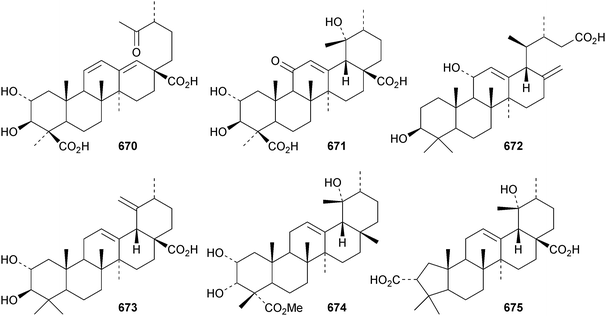
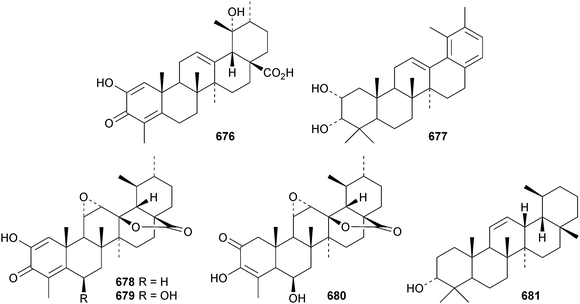
Ursane triterpenoids have shown potential as anticancer drugs.302 Reviews on the anticancer activities of ursolic acid303 and acetyl-11-keto-β-boswellic acid (AKBA)304 have been published.The 18,19-secoursane derivative 670 has been isolated from the bark of Diospyros decandra together with 671.195 The 17,22-seco derivative 672 has been found in both Salvia palaestina and Salvia syriaca.305 Euscaphic acids J 673, K 674 and L 675 have been isolated from Euscaphis japonica.197 Euscaphic acid L 675 has a contracted ring-A. Negundonorins A 676 and B 677, from Vitex negundo, are 24-nor and 28-noruranes, respectively.306 Further 24-norursanes include ulmoidol A 678 from Eucommia ulmoides307 and the related compounds 679 and 680 from Dipsacus chinensis.308 30-Norurs-11-en-3α-ol 681 has been identified in the roots of Alhagi camelorum.309
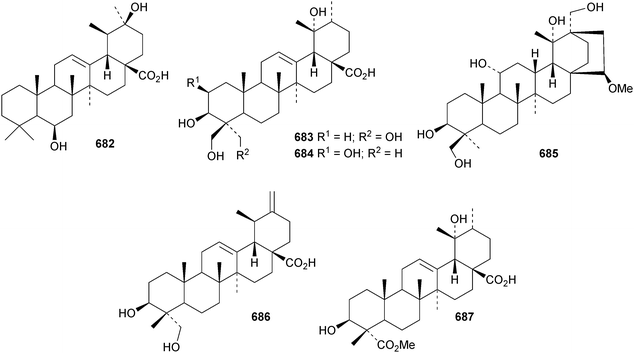
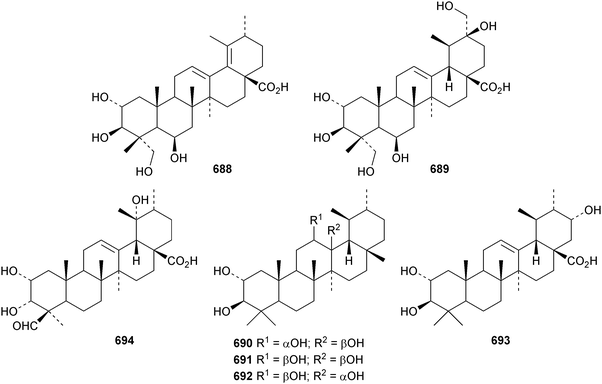
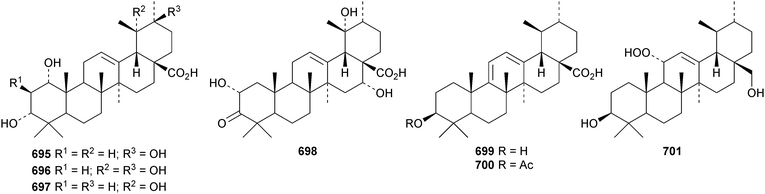
6β,20β-Dihydroxyurs-12-en-28-oic acid 682, from leaves of Psidium guajava, is unusual as it lacks oxygenation at C-3.310 The structure of 3β,19α,23,24-tetrahydroxyurs-12-en-28-oic acid 683, from Nauclea officinalis, was confirmed by X-ray analysis.311 It is accompanied by the corresponding 2β,3β,19α,24-tetrahydroxy compound 684. Further simple ursane derivatives include the acetal 685 from Juglans sinensis,204 rhododendric acid A 686 from Rhododendron brachycarpum,312 uncariursanic acid 687 from Uncaria macrophylla,313 2α,3β,6β,23-tetrahydroxyursa-12,18-dien-28-oic acid 688 from Kadsura marmorata,314 2α,3β,6β,20β,23,30-hexahydroxyurs-12-en-28-oic acid 689 and psiguanins B 690, C 691 and D 692 from Psidium guajava,315 the 2α,3β,21α-trihydroxy derivative 693 and the 24-aldehyde 694 from Berberis koreana,316 the 1α,3α-dihydroxy derivatives 695–697 from Euphorbia kansuensis,317 the 3-ketone 698 from Albizzia lebbeck,318 loxanic acid 699 and its acetate 700 from Eucalyptus loxophleba319 and tolpidiol B 701 from Tolpis proustii and Tolpis lagopoda.320
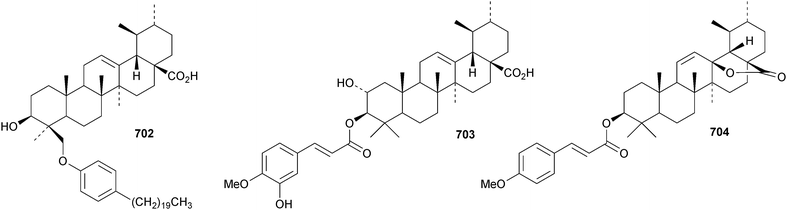
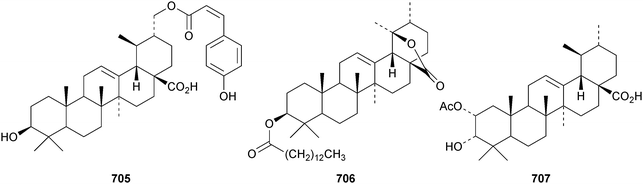
Kirmanoic acid 702, from Nepeta clarkei, is an unusual alkylphenyl ether.185 New ursane esters include the isoferuloyl ester 703 from Eucalyptus exserta,205 ehretiolide 704 from Ehretia longifolia,321 the 30-cis-coumaryl ester 705 from Rubia schumanniana,216 3β-tetradecanoyloxyurs-12-en-28,19β-olide 706 from Lysimachia clethroides322 and 3-epi-cecropic acid 707 from Dipterocarpus obtusifolius.106
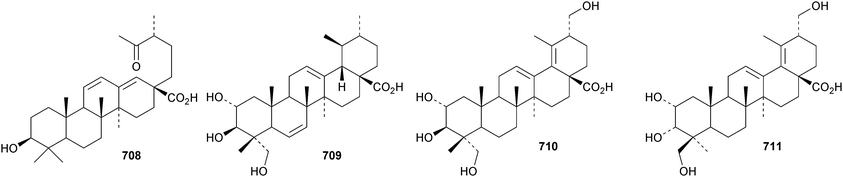
Sanguisoside A is an ursane saponin from Sanguisorba officinalis with the new 18,19-seco-ursane genin 708.323 Centelloside E from Centella asiatica has the new genin 709 (ref. 235) and the genins 710 and 711 are present in saponins from Actinidia valvata.324
Ursane saponins with known genins include asphorins A and B from Asphodelus tenuifolius,325 asprellanosides A and B from Ilex asprella,326 elasticoside from Ficus elastica,327 ilekudinchosides F and G from Ilex kudincha,328 ilemaminosides A and B from Ilex mamillata,329 ilexasosides A–H from Ilex asprella,330 phillyriside A from Stenocereus eruca,180 and unnamed saponins from Diospyros decandra,195Juglans sinensis,204Lantana camara331 and Psidium guajava.332
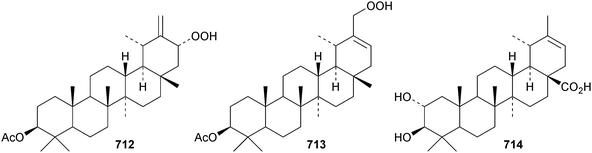
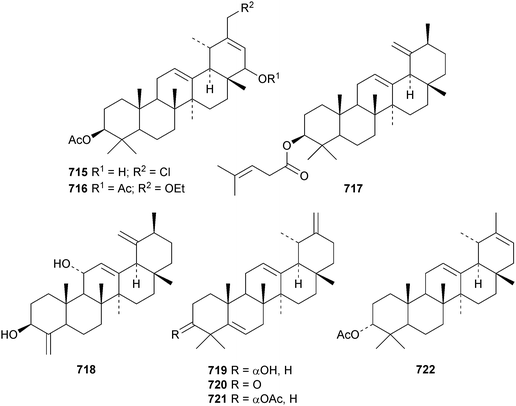
The taraxastane hydroperoxide derivatives 712 and 713 have been isolated from the roots of Taraxacum platycarpum.173 Psiguanin A, which is 2α,3β-dihydroxytaraxast-20-en-28-oic acid 714, has been found in the leaves of Psidium guajava.315 Other new taraxastane derivatives include chlorotolpidiol 715 and tolpidiol A 716 from Tolpis proustii and Tolpis lagopoda,320 pergularines A 717 and B 718 from Pergularia tomentosa333 and calotroprocerol A 719, calotroprocerone A 720 and calotroproceryl acetates A 721 and B 722 from Calotropis procera.334 Two taraxastane saponins with known genins have been found in the roots of Ilex pubescens.335
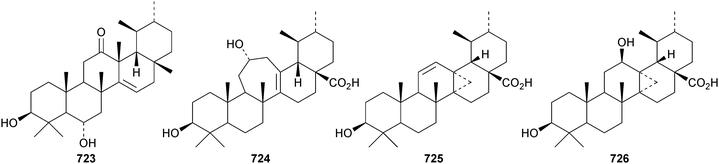
The 27(14 → 13)-abeo-urs-14-enone derivative 723, from Rubia schumanniana, has the unusual β-configuration for the migrated methyl.216 The rearranged ursanes ilelic acids A 724, C 725 and D 726 have been isolated from Ilex latifolia.292 The structure of ilelic acid D 726 was confirmed by X-ray analysis.
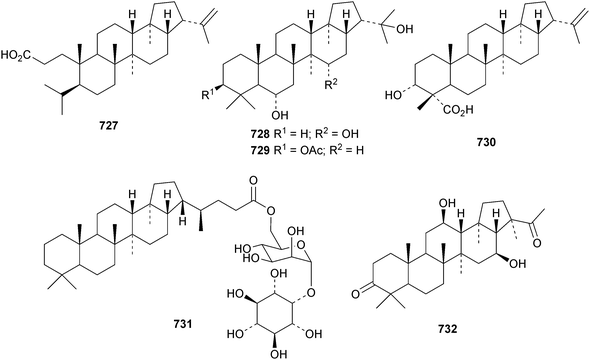
8. The hopane group
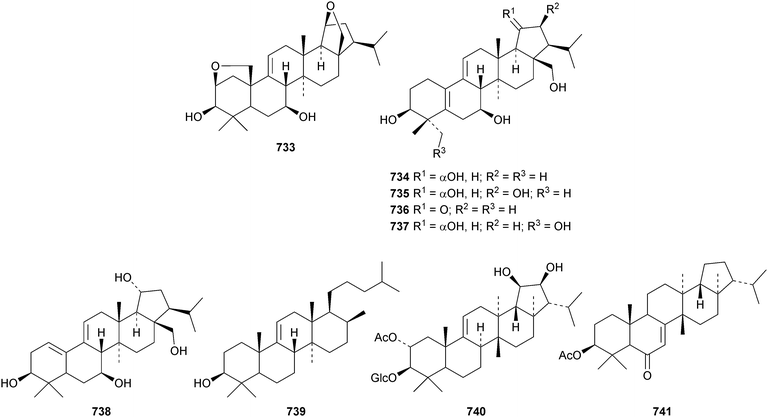
3,4-Secohop-22(29)-en-3-oic acid 727 has been isolated from Maytenus robusta.295 The scale insect pathogenic fungus Aschersonia calendulina is the source of two hopane metabolites 728 and 729.336 The leaves of Hybanthus austro-caledonicus produce 3-epiwoodwardinic acid 730.337 Plakohopanoid 731, a C32 hopanoid ester of a manosyl myoinostol, has been isolated from the sponge Plakortis cf. lita.338 The structure of plakohopanoid 731 implies that it is of bacterial origin. This is the first example of a biologically produced C32 hopanoic acid. Such acids were considered to only be geohopanoids formed by abiotic degradation of bacteriohopanoids. Oppositifolone 732, from Glinus oppositifolius, has a 29(22 → 21)-abeo-hopane skeleton and is the 3-ketone of spergulagenin A.339Pteroxygonumnol 733, from the roots of Pteroxygonum giraldii, has been identified as 2β,25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19β,28-diepoxyarborin-9(11)-ene-3β,7β-diol.340 Five 25-norarborinane derivatives 734–738 have been isolated from bamboo stems, Sinocalamus affinis.187 The structure of 734 was confirmed by X-ray analysis. Atalantia retusa is the source of the 17,21-seco-arborane derivative retusinol 739.341 Peniciside 740 is a fernane metabolite of Penicillium sp.169342 and 3β-acetoxyfern-7-en-6-one 741 is a constituent of Scorzonera latifolia.343
19β,28-diepoxyarborin-9(11)-ene-3β,7β-diol.340 Five 25-norarborinane derivatives 734–738 have been isolated from bamboo stems, Sinocalamus affinis.187 The structure of 734 was confirmed by X-ray analysis. Atalantia retusa is the source of the 17,21-seco-arborane derivative retusinol 739.341 Peniciside 740 is a fernane metabolite of Penicillium sp.169342 and 3β-acetoxyfern-7-en-6-one 741 is a constituent of Scorzonera latifolia.343
9. Miscellaneous compounds
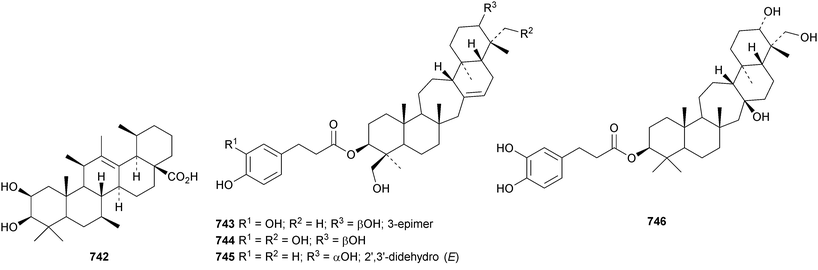
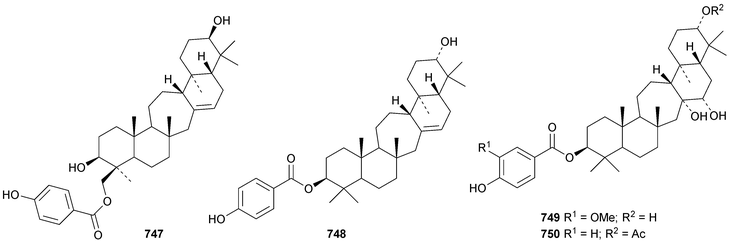
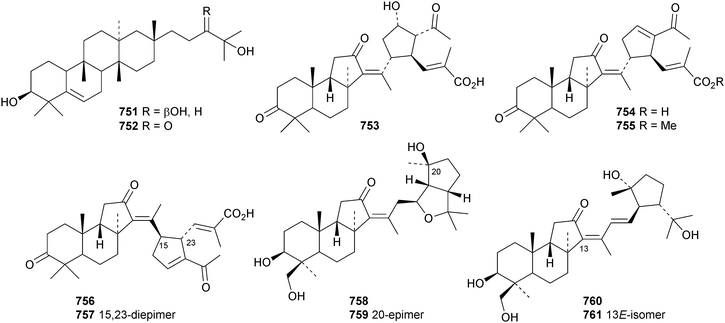
The unlikely structure 742 has been proposed for erucaoic acid from Sonchus eruca.344 The club moss Lycopodium phlegmaria is the source of the serratane esters lycophlegmariols A 743–D 746 (ref. 345) and four esters 747–750 have been found in Palhinhaea cernua (syn. Lycopodium cernuum).346 The D:B-friedobaccharane derivatives leonatriol 751 and the corresponding ketone leonatriolone 752 have been isolated from Cassine xylocarpa and Celastrus vulcanicola, respectively.294 Globostelletins J 753–S 761, from the marine sponge Rhabdastrella globostellata, are isomalabaricanes with cyclopentane side chains.347References
- J. M. R. Patlolla and C. V. Rao, Curr. Pharm. Biotechnol., 2012, 13, 147–155 CAS.
- S. H. Safe, P. L. Prather, L. K. Brents, G. Chadalapaka and I. Jutooru, Anti-Cancer Agents Med. Chem., 2012, 12, 1211–1220 CrossRef CAS.
- M. K. Shanmugam, A. H. Nguyen, A. P. Kumar, B. K. H. Tan and G. Sethi, Cancer Lett., 2012, 320, 158–170 CrossRef CAS.
- M.-C. Yin, Biomedicine, 2012, 2, 2–9 CrossRef CAS.
- J. Wang, J. Shan, L. Di, S. Wang and B. Cai, Zhongcaoyao, 2012, 43, 196–200 CAS.
- H. Wang, M.-Y. Li and J. Wu, Chem. Biodiversity, 2012, 9, 1–11 CAS.
- S. K. Kothiyal, S. C. Sati, M. S. M. Rawat, M. D. Sati, D. K. Semwal, R. B. Semwal, A. Sharma, B. Rawat and A. Kumar, Nat. Prod. J., 2012, 2, 212–224 CAS.
- A. Upadhyay and D. K. Singh, Rev. Inst. Med. Trop. Sao Paulo, 2012, 54, 273–280 CrossRef.
- S. F. Kouam, S. Kusari, M. Lamshoeft, O. K. Tatuedom and M. Spiteller, Phytochemistry, 2012, 83, 79–86 CrossRef CAS.
- J. Lokvam and P. V. A. Fine, Molecules, 2012, 17, 7451–7457 CrossRef CAS.
- F. Cen-Pacheco, F. Mollinedo, J. A. Villa-Pulgarin, M. Norte, J. J. Fernandez and A. Hernandez Daranas, Tetrahedron, 2012, 68, 7275–7279 CrossRef CAS.
- S. Okada, Kagaku to Seibutsu, 2012, 50, 93–102 CrossRef CAS.
- H.-G. Jin, Q. Jin, A. Ryun Kim, H. Choi, J. H. Lee, Y. S. Kim, D. G. Lee and E.-R. Woo, Arch. Pharmacal Res., 2012, 35, 1919–1926 CrossRef CAS.
- N. Xu, H. Zhang and X. Xie, Zhongcaoyao, 2012, 43, 841–843 CAS.
- O. Yodsaoue, J. Sonprasit, C. Karalai, C. Ponglimanont, S. Tewtrakul and S. Chantrapromma, Phytochemistry, 2012, 76, 83–91 CrossRef CAS.
- Y.-L. Li, S.-D. Zhang, H.-Z. Jin, J.-M. Tian, Y.-H. Shen, X.-W. Yang, H.-L. Li and W.-D. Zhang, Tetrahedron, 2012, 68, 7763–7767 CrossRef CAS.
- S. Lavoie, J. Legault, C. Gauthier, V. Mshvildadze, S. Mercier and A. Pichette, Org. Lett., 2012, 14, 1504–1507 CrossRef CAS.
- Y.-L. Li, Y.-X. Gao, X.-W. Yang, H.-Z. Jin, J. Ye, L. Simmons, N. Wang, A. Steinmetz and W.-D. Zhang, Phytochemistry, 2012, 81, 159–164 CrossRef CAS.
- G. R. Wang, Y. L. Li, W. D. Zhang, X. W. Yang, W. C. Liu, J. Ye, Z. J. Zhu and H. Chen, Chin. Chem. Lett., 2012, 23, 1251–1253 CrossRef CAS.
- J.-H. Xia, S.-D. Zhang, Y.-L. Li, L. Wu, Z.-J. Zhu, X.-W. Yang, H.-W. Zeng, H.-L. Li, N. Wang, A. Steinmetz and W.-D. Zhang, Phytochemistry, 2012, 74, 178–184 CrossRef CAS.
- X.-D. Wu, J. He, Y. Shen, L.-B. Dong, Z.-H. Pan, G. Xu, X. Gong, L.-D. Song, Y. Leng, Y. Li, L.-Y. Peng and Q.-S. Zhao, Tetrahedron Lett., 2012, 53, 800–803 CrossRef CAS.
- J. Zou, L.-B. Yang, J. Jiang, Y.-Y. Diao, X.-N. Li, J. Huang, J.-H. Yang, H.-L. Li, W.-L. Xiao, X. Du, S.-Z. Shang, J.-X. Pu and H.-D. Sun, Planta Med., 2012, 78, 472–479 CrossRef CAS.
- K. Dong, J.-X. Pu, X. Du, J. Su, X.-N. Li, J.-H. Yang, W. Zhao, Y. Li and H.-D. Sun, Tetrahedron, 2012, 68, 4820–4829 CrossRef CAS.
- C.-Q. Liang, Y.-M. Shi, R.-H. Luo, X.-Y. Li, Z.-H. Gao, X.-N. Li, L.-M. Yang, S.-Z. Shang, Y. Li, Y.-T. Zheng, H.-B. Zhang, W.-L. Xiao and H.-D. Sun, Org. Lett., 2012, 14, 6362–6365 CrossRef CAS.
- N. Wang, Z.-l. Li, D.-d. Song, W. Li, Y.-h. Pei, Y.-k. Jing and H.-M. Hua, Planta Med., 2012, 78, 1661–1666 CrossRef CAS.
- G. She, N. Zhu, S. Wang, Y. Liu, Y. Ba, C. Sun and R. Shi, Chem. Cent. J., 2012, 6, 39 CrossRef CAS.
- S. Jeelani and M. A. Khuroo, Nat. Prod. Res., 2012, 26, 654–658 CrossRef CAS.
- N. Handa, T. Yamada and R. Tanaka, Phytochem. Lett., 2012, 5, 480–485 CrossRef CAS.
- J.-H. Lee, J.-E. Jeon, Y.-J. Lee, H.-S. Lee, C. J. Sim, K.-B. Oh and J. Shin, J. Nat. Prod., 2012, 75, 1365–1372 CrossRef CAS.
- M. Ono, Y. Takatsu, T. Ochiai, S. Yasuda, Y. Nishida, T. Tanaka, M. Okawa, J. Kinjo, H. Yoshimitsu and T. Nohara, Chem. Pharm. Bull., 2012, 60, 1314–1319 CrossRef CAS.
- J.-Q. Liu, C.-F. Wang, Y. Li, H.-R. Luo and M.-H. Qiu, Planta Med., 2012, 78, 368–376 CrossRef CAS.
- S.-X. Yang, Z.-C. Yu, Q.-Q. Lu, W.-Q. Shi, H. Laatsch and J.-M. Gao, Phytochem. Lett., 2012, 5, 576–580 CrossRef CAS.
- B.-J. Ma, Y. Zhou, Y. Ruan, J.-C. Ma, W. Ren and C.-N. Wen, J. Antibiot., 2012, 65, 165–167 CrossRef CAS.
- Y. Li, Z. Zhu, W. Yao and R. Chen, Zhongguo Zhongyao Zazhi, 2012, 37, 165–171 CAS.
- Y. Li, Z.-M. Zhu, W.-X. Yao and R.-Y. Chen, Med. Plant, 2012, 3, 75–81 CAS.
- J.-L. Rios, I. Andujar, M.-C. Recio and R.-M. Giner, J. Nat. Prod., 2012, 75, 2016–2044 CrossRef CAS.
- T. K. Lai, G. Biswas, S. Chatterjee, A. Dutta, C. Pal, J. Banerji, N. Bhuvanesh, J. H. Reibenspies and K. Acharya, Chem. Biodiversity, 2012, 9, 1517–1524 CAS.
- Y.-Z. Liu, Y.-Y. Li, Y.-F. Sun, Z.-H. Zheng, S.-Y. Song, W.-J. Su and Y.-M. Shen, Helv. Chim. Acta, 2012, 95, 282–285 CrossRef CAS.
- H.-C. Huang, C.-C. Liaw, H.-L. Yang, Y.-C. Hseu, H.-T. Kuo, Y.-C. Tsai, S.-C. Chien, S. Amagaya, Y.-C. Chen and Y.-H. Kuo, Phytochemistry, 2012, 84, 177–183 CrossRef CAS.
- I.-K. Lee, J.-Y. Jung, J.-H. Yeom, D.-W. Ki, M.-S. Lee, W.-H. Yeo and B.-S. Yun, Mycobiology, 2012, 40, 76–78 CrossRef CAS.
- A. S. Silchenko, A. I. Kalinovsky, S. A. Avilov, P. V. Andryjashchenko, P. S. Dmitrenok, V. I. Kalinin and V. A. Stonik, Biochem. Syst. Ecol., 2012, 44, 53–60 CrossRef CAS.
- Z. Wang, H. Zhang, W. Yuan, W. Gong, H. Tang, B. Liu, K. Krohn, L. Li, Y. Yi and W. Zhang, Food Chem., 2012, 132, 295–300 CrossRef CAS.
- A. S. Silchenko, A. I. Kalinovsky, S. A. Avilov, P. V. Andryjaschenko, P. S. Dmitrenok, E. A. Martyyas and V. I. Kalinin, Nat. Prod. Commun., 2012, 7, 1157–1162 CAS.
- A. S. Silchenko, A. I. Kalinovsky, S. A. Avilov, P. V. Andryjaschenko, P. S. Dmitrenok, E. A. Yurchenko and V. I. Kalinin, Nat. Prod. Res., 2012, 26, 1765–1774 CrossRef CAS.
- S.-K. Kim and S. W. A. Himaya, Adv. Food Nutr. Res., 2012, 65, 297–319 Search PubMed.
- X. Luo, Y.-M. Shi, R.-H. Luo, S.-H. Luo, X.-N. Li, R.-R. Wang, S.-H. Li, Y.-T. Zheng, X. Du, W.-L. Xiao, J.-X. Pu and H.-D. Sun, Org. Lett., 2012, 14, 1286–1289 CrossRef CAS.
- F. He, X.-Y. Li, G.-Y. Yang, X.-N. Li, X. Luo, J. Zou, Y. Li, W.-L. Xiao and H.-D. Sun, Tetrahedron, 2012, 68, 440–446 CrossRef CAS.
- J.-R. Wang, T. Kurtan, A. Mandi and Y.-W. Guo, Eur. J. Org. Chem., 2012, 2012, 5471–5482 CrossRef CAS.
- J. Zou, J. Jiang, Y.-Y. Diao, L.-B. Yang, J. Huang, H.-L. Li, X. Du, W.-L. Xiao, J.-X. Pu and H.-D. Sun, Fitoterapia, 2012, 83, 926–931 CrossRef CAS.
- F. Qiu, A. Imai, J. B. McAlpine, D. C. Lankin, I. Burton, T. Karakach, N. R. Farnsworth, S.-N. Chen and G. F. Pauli, J. Nat. Prod., 2012, 75, 432–443 CrossRef CAS.
- B. Li, D.-Y. Kong, Y.-H. Shen, H. Yuan, R.-C. Yue, Y.-R. He, L. Lu, L. Shan, H.-L. Li, J. Ye, X.-W. Yang, J. Su, R.-H. Liu and W.-D. Zhang, Org. Lett., 2012, 14, 5432–5435 CrossRef CAS.
- Q.-H. Han, X. Liu, W.-Q. Yao, Z.-B. Cheng, T.-T. Lin, C. Song and S. Yin, Planta Med., 2012, 78, 1971–1975 CrossRef CAS.
- C. Long, J. Beck, F. Cantagrel, L. Marcourt, L. Vendier, B. David, F. Plisson, F. Derguini, I. Vandenberghe, Y. Aussagues, F. Ausseil, C. Lavaud, F. Sautel and G. Massiot, J. Nat. Prod., 2012, 75, 34–47 CrossRef CAS.
- J. D. Simo Mpetga, Y. Shen, P. Tane, S.-F. Li, H.-P. He, H. K. Wabo, M. Tene, Y. Leng and X.-J. Hao, J. Nat. Prod., 2012, 75, 599–604 CrossRef CAS.
- I. Lee, J. Kim, Y. S. Kim, N. H. Yoo, C.-S. Kim, K. Jo, J.-H. Kim, T. T. Bach and J. S. Kim, J. Nat. Prod., 2012, 75, 1312–1318 CrossRef CAS.
- J.-L. Yang and Y.-P. Shi, Planta Med., 2012, 78, 59–64 CrossRef CAS.
- L. P. Ponomarenko, A. I. Kalinovsky, E. A. Martyyas, R. V. Doudkin, P. G. Gorovoy and V. A. Stonik, Phytochem. Lett., 2012, 5, 118–122 CrossRef CAS.
- M. A. Ramirez-Cisneros, M. Y. Rios, R. Rios-Gomez and A. B. Aguilar-Guadarrama, Planta Med., 2012, 78, 1942–1948 CrossRef CAS.
- K. Toume, T. Nakazawa, T. Hoque, T. Ohtsuki, M. A. Arai, T. Koyano, T. Kowithayakorn and M. Ishibashi, Planta Med., 2012, 78, 1370–1377 CrossRef CAS.
- S.-F. Li, Y.-T. Di, R.-H. Luo, Y.-T. Zheng, Y.-H. Wang, X. Fang, Y. Zhang, L. Li, H.-P. He, S.-L. Li and X.-J. Hao, Planta Med., 2012, 78, 821–827 CrossRef CAS.
- J. D. S. Mpetga, M. Tene, H. K. Wabo, S.-F. Li, L.-M. Kong, H.-P. He, X.-J. Hao and P. Tane, Phytochem. Lett., 2012, 5, 183–187 CrossRef CAS.
- K. Awang, X.-M. Loong, K. H. Leong, U. Supratman, M. Litaudon, M. R. Mukhtar and K. Mohamad, Fitoterapia, 2012, 83, 1391–1395 CrossRef CAS.
- J.-L. Yang and Y.-P. Shi, Phytochemistry, 2012, 76, 124–132 CrossRef CAS.
- L. Lu, J.-C. Chen, Y. Li, C. Qing, Y.-Y. Wang, Y. Nian and M.-H. Qiu, Chem. Pharm. Bull., 2012, 60, 571–577 CAS.
- Y. Nian, H.-Y. Wang, J. Su, L. Zhou, G. Feng, Y. Li and M.-H. Qiu, Tetrahedron, 2012, 68, 6521–6527 CrossRef CAS.
- H. X. Kuang, Y. Su, Q. H. Wang, L. Wu, B. Y. Yang, Z. B. Wang and Y. G. Xia, Planta Med., 2012, 78, 622–625 CrossRef CAS.
- M. K. Jamróz, M. H. Jamróz, J. C. Dobrowolski, J. A. Gliński and M. Gleńsk, Spectrochim. Acta, Part A, 2012, 93, 10–18 CrossRef.
- D. Gülcemal, M. Masullo, E. Bedir, M. Festa, T. Karayildirim, O. Alankus-Caliskan and S. Piacente, Planta Med., 2012, 78, 720–729 CrossRef.
- T. K. Naubeev, K. K. Uteniyazov, M. I. Isaev, V. V. Kachala and A. S. Shashkov, Chem. Nat. Compd., 2012, 48, 810–812 CrossRef CAS.
- F. N. Yalçin, S. Piacente, A. Perrone, A. Capasso, H. Duman and I. Calis, Phytochemistry, 2012, 73, 119–126 CrossRef.
- F. Karabey, I. A. Khan and E. Bedir, Phytochem. Lett., 2012, 5, 320–324 CrossRef CAS.
- L.-B. Wei, J.-M. Chen, W.-C. Ye, X.-S. Yao and G.-X. Zhou, J. Asian Nat. Prod. Res., 2012, 14, 521–527 CrossRef CAS.
- A.-X. Zuo, Y. Shen, Z.-Y. Jiang, X.-M. Zhang, J. Zhou, J. Lue and J.-J. Chen, J. Asian Nat. Prod. Res., 2012, 14, 407–412 CrossRef CAS.
- X.-T. Zhang, S.-W. Ma, H.-Y. Jiao and Q.-W. Zhang, J. Asian Nat. Prod. Res., 2012, 14, 327–332 CrossRef.
- K. Pudhom, T. Nuanyai and K. Matsubara, Chem. Pharm. Bull., 2012, 60, 1538–1543 CAS.
- T. K. Naubeev, A. A. Zhanibekov and M. I. Isaev, Chem. Nat. Compd., 2012, 48, 813–815 CrossRef CAS.
- M. D. Alaniya and T. I. Gigoshvili, Chem. Nat. Compd., 2012, 48, 914–916 CrossRef CAS.
- I. Horo, E. Bedir, M. Masullo, S. Piacente, F. Özgökçe and Ö. Alankuş-Çalişkan, Phytochemistry, 2012, 84, 147–153 CrossRef CAS.
- M.-m. Zhang, Y.-l. Liu, Z. Chen, X.-r. Li, Q.-m. Xu and S.-l. Yang, Zhongcaoyao, 2012, 43, 1462–1470 CAS.
- T. Savran, D. Gülcemal, M. Masullo, T. Karayildirim, E. Polat, S. Piacente and Ö. Alankuş-Çalişkan, Rec. Nat. Prod., 2012, 6, 230–236 CAS.
- J.-C. Chen, Z.-Z. Xu, L.-X. Yang, X.-Y. He, C. Chen, G.-H. Zhang, Y.-T. Zheng and M.-H. Qiu, Chem. Nat. Compd., 2012, 48, 591–593 CrossRef CAS.
- L. Pan, Y. Yong, Y. Deng, D. D. Lantvit, T. N. Ninh, H. Chai, E. J. Carcache de Blanco, D. D. Soejarto, S. M. Swanson and A. D. Kinghorn, J. Nat. Prod., 2012, 75, 444–452 CrossRef CAS.
- J.-C. Chen, L. Zhou, Y.-H. Wang, R.-R. Tian, Y.-X. Yan, Y. Nian, Y. Sun, Y.-T. Zheng and M.-H. Qiu, Nat. Prod. Bioprospect., 2012, 2, 138–144 CrossRef CAS.
- Y.-W. Liao, C.-R. Chen, Y.-H. Kuo, J.-L. Hsu, W.-L. Shih, H.-L. Cheng, T.-C. Huang and C.-I. Chang, Nat. Prod. Commun., 2012, 7, 1575–1578 CAS.
- C.-A. Geng, X.-Y. Huang, L.-G. Lei, X.-M. Zhang and J.-J. Chen, Chem. Biodiversity, 2012, 9, 1508–1516 CAS.
- J. Zhang, Y. Huang, T. Kikuchi, H. Tokuda, N. Suzuki, K.-I. Inafuku, M. Miura, S. Motohashi, T. Suzuki and T. Akihisa, Chem. Biodiversity, 2012, 9, 428–440 CAS.
- X.-M. Fan, G. Chen, Y. Sha, X. Lu, M.-X. Shen, H.-M. Ma and Y.-H. Pei, J. Asian Nat. Prod. Res., 2012, 14, 528–532 CrossRef CAS.
- M. Clericuzio, C. Cassino, F. Corana and G. Vidari, Phytochemistry, 2012, 84, 154–159 CrossRef CAS.
- B. G. Panlilio, A. P. G. Macabeo, M. Knorn, P. Kohls, P. Richomme, S. F. Kouam, D. Gehle, K. Krohn, S. G. Franzblau, Q. Zhang and M. A. M. Aguinaldo, Phytochem. Lett., 2012, 5, 682–684 CrossRef CAS.
- S. L. Deore, A. Parab, B. A. Baviskar and S. S. Khadabadi, Pharm. Rev., 2012, 10, 125–127 CAS.
- K. Dhiman, A. Gupta, D. K. Sharma, N. S. Gill and A. Goyal, Asian J. Clin. Nutr., 2012, 4, 16–26 CrossRef CAS.
- X. Chen, J. Bao, J. Guo, Q. Ding, J. Lu, M. Huang and Y. Wang, Anti-Cancer Drugs, 2012, 23, 777–787 CrossRef CAS.
- J. L. Rios, I. Andujar, J. M. Escandell, R. M. Giner and M. C. Recio, Curr. Pharm. Des., 2012, 18, 1663–1676 CrossRef CAS.
- M. Cao, H.-S. Yu, X.-B. Song and B.-P. Ma, Yaoxue Xuebao, 2012, 47, 836–843 CAS.
- K.-K. Li, C.-M. Yao and X.-W. Yang, Planta Med., 2012, 78, 189–192 CrossRef CAS.
- K.-K. Li, X.-B. Yang, X.-W. Yang, J.-X. Liu and X.-J. Gong, Fitoterapia, 2012, 83, 1030–1035 CrossRef CAS.
- H.-Y. Ma, H.-Y. Gao, J. Huang, B.-H. Sun and B. Yang, J. Nat. Med., 2012, 66, 576–582 CrossRef CAS.
- W. Li, J. Y. Yin, Z. Y. Cong, Y. Liu, Y. Zhang, Y. Y. Li and Q. Meng, Chem. Nat. Compd., 2012, 48, 1017–1020 CrossRef.
- J.-P. Liu, D. Lu and P.-Y. Li, Nat. Prod. Res., 2012, 26, 744–748 CrossRef CAS.
- L. Ma, W.-J. Xiang, P. Van Khang, Y. Liang, Y. Xiao and L.-H. Hu, Planta Med., 2012, 78, 597–605 CrossRef CAS.
- L. Shi, X.-J. Meng, J.-Q. Cao and Y.-Q. Zhao, Nat. Prod. Res., 2012, 26, 1419–1422 CrossRef CAS.
- S. Li, B. Cui, Q. Liu, L. Tang, Y. Yang, X. Jin and Z. Shen, Planta Med., 2012, 78, 290–296 CrossRef CAS.
- M. Gan, M. Liu, L. Gan, S. Lin, B. Liu, Y. Zhang, J. Zi, W. Song and J. Shi, J. Nat. Prod., 2012, 75, 1373–1382 CrossRef CAS.
- D. Harneti, R. Tjokronegoro, A. Safari, U. Supratman, X.-M. Loong, M. R. Mukhtar, K. Mohamad, K. Awang and H. Hayashi, Phytochem. Lett., 2012, 5, 496–499 CrossRef CAS.
- F. Wang and Y. Guan, Fitoterapia, 2012, 83, 13–17 CrossRef CAS.
- P. Khiev, O.-K. Kwon, H.-H. Song, S.-R. Oh, K.-S. Ahn, H.-K. Lee and Y.-W. Chin, Chem. Pharm. Bull., 2012, 60, 955–961 CrossRef CAS.
- F.-F. Xu, X.-Q. Zhang, J. Zhang, B. Liu, J. Jiang, W.-J. Wang, M.-H. Gao, R.-W. Jiang and W.-C. Ye, J. Asian Nat. Prod. Res., 2012, 14, 135–140 CrossRef CAS.
- K. Yoshizaki and S. Yahara, Chem. Pharm. Bull., 2012, 60, 354–362 CrossRef CAS.
- K. Yoshizaki, M. Murakami, H. Fujino, N. Yoshida and S. Yahara, Chem. Pharm. Bull., 2012, 60, 728–735 CrossRef CAS.
- L. Shi, F. Lu, H. Zhao and Y.-Q. Zhao, J. Asian Nat. Prod. Res., 2012, 14, 856–861 CrossRef CAS.
- J.-P. Liu, F. Wang, P.-Y. Li and D. Lu, Nat. Prod. Res., 2012, 26, 731–735 CrossRef CAS.
- H. T. Nguyen and Y. Shoyama, Chem. Pharm. Bull., 2012, 60, 1329–1333 CrossRef CAS.
- J.-S. Wang, Y. Zhang, X.-B. Wang, D.-D. Wei, J. Luo, J.-G. Luo, M.-H. Yang, H.-Q. Yao, H.-B. Sun and L.-Y. Kong, Tetrahedron Lett., 2012, 53, 1705–1709 CrossRef CAS.
- Y. Zhang, J. Wang, P. Wang and L. Kong, Chin. J. Chem., 2012, 30, 1356–1360 CrossRef CAS.
- J.-S. Wang, Y. Zhang, X.-B. Wang, D.-D. Wei, J. Luo, J.-G. Luo, M.-H. Yang, H.-Q. Yao, H.-B. Sun and L.-Y. Kong, Tetrahedron Lett., 2012, 53, 4030 CrossRef CAS.
- X.-Y. Wang, G.-H. Tang, C.-M. Yuan, Y. Zhang, L. Hou, Q. Zhao, X.-J. Hao and H.-P. He, Nat. Prod. Bioprospect., 2012, 2, 222–226 CrossRef CAS.
- Q. Zeng, B. Guan, J.-J. Qin, C.-H. Wang, X.-R. Cheng, J. Ren, S.-K. Yan, H.-Z. Jin and W.-D. Zhang, Phytochemistry, 2012, 80, 148–155 CrossRef CAS.
- T. Tang, S.-G. Liao, Z. Na, Y. Li and Y.-K. Xu, Tetrahedron Lett., 2012, 53, 1183–1185 CrossRef CAS.
- M. A. Versiani, A. Ikram, S. Khalid, S. Faizi and I. A. Tahiri, Nat. Prod. Commun., 2012, 7, 831–834 CAS.
- E. N. Happi, A. T. Tcho, J. C. Sirri, J. D. Wansi, B. Neumann, H.-G. Stammler, J. Wandji and N. Sewald, Phytochem. Lett., 2012, 5, 423–426 CrossRef CAS.
- T. Fossen, P. Rasoanaivo, C. S. Manjovelo, F. H. Raharinjato, S. Yahorava, A. Yahorau and J. E. S. Wikberg, Fitoterapia, 2012, 83, 901–906 CrossRef CAS.
- Q. Zhao, Y. Song, C. Feng and H. Chen, Arch. Pharmacal Res., 2012, 35, 1903–1907 CrossRef CAS.
- W.-X. Liu, G.-H. Tang, H.-P. He, Y. Zhang, S.-L. Li and X.-J. Hao, Nat. Prod. Bioprospect., 2012, 2, 29–34 CrossRef CAS.
- J. Huang, Z.-h. Guo, P. Cheng, B.-h. Sun and H.-Y. Gao, Phytochem. Lett., 2012, 5, 432–437 CrossRef CAS.
- I. Valente, M. Reis, N. Duarte, J. Serly, J. Molnár and M.-J. U. Ferreira, J. Nat. Prod., 2012, 75, 1915–1921 CrossRef CAS.
- F. Zhang, X.-F. He, W.-B. Wu, W.-S. Chen and J.-M. Yue, Nat. Prod. Bioprospect., 2013, 2, 235–239 CrossRef CAS.
- M.-H. Yang, J.-S. Wang, J.-G. Luo, X.-B. Wang and L.-Y. Kong, Can. J. Chem., 2012, 90, 199–204 CrossRef CAS.
- S.-H. Dong, X.-F. He, L. Dong, Y. Wu and J.-M. Yue, Helv. Chim. Acta, 2012, 95, 286–300 CrossRef CAS.
- M. S. A. Rao, G. Suresh, P. Ashok Yadav, K. Rajendra Prasad, V. Lakshma Nayak, S. Ramakrishna, C. V. Rao and K. S. Babu, Tetrahedron Lett., 2012, 53, 6241–6244 CrossRef CAS.
- J. Liu, S.-P. Yang, G. Ni, Y.-C. Gu and J.-M. Yue, J. Asian Nat. Prod. Res., 2012, 14, 929–939 CrossRef CAS.
- J. Sichaem, T. Aree, S. Khumkratok, J. Jong-aramruang and S. Tip-pyang, Phytochem. Lett., 2012, 5, 665–667 CrossRef CAS.
- F. Zhang, J.-S. Wang, Y.-C. Gu and L.-Y. Kong, J. Nat. Prod., 2012, 75, 538–546 CrossRef CAS.
- A. K. R. Bandi and D.-U. Lee, Chem. Biodiversity, 2012, 9, 1403–1421 CAS.
- M.-L. Han, H. Zhang, S.-P. Yang and J.-M. Yue, Org. Lett., 2012, 14, 486–489 CrossRef CAS.
- J. Li, M.-Y. Li, T. Bruhn, D. C. G. Goetz, Q. Xiao, T. Satyanandamurty, J. Wu and G. Bringmann, Chem.–Eur. J., 2012, 18, 14342–14351 CrossRef CAS.
- V. G. P. Severino, P. A. C. Braga, M. F. d. G. F. da Silva, J. B. Fernandes, P. C. Vieira, J. E. Theodoro and J. A. Ellena, Phytochemistry, 2012, 76, 52–59 CrossRef CAS.
- T. Inoue, Y. Nagai, A. Mitooka, R. Ujike, O. Muraoka, T. Yamada and R. Tanaka, Tetrahedron Lett., 2012, 53, 6685–6688 CrossRef CAS.
- J. Liao, T. Xu, Y.-H. Liu and S.-Z. Wang, Nat. Prod. Res., 2012, 26, 756–761 CrossRef CAS.
- J.-Y. Cai, Y. Zhang, S.-H. Luo, D.-Z. Chen, G.-H. Tang, C.-M. Yuan, Y.-T. Di, S.-H. Li, X.-J. Hao and H.-P. He, Org. Lett., 2012, 14, 2524–2527 CrossRef CAS.
- H.-B. Liu, H. Zhang, P. Li, Z.-B. Gao and J.-M. Yue, Org. Lett., 2012, 14, 4438–4441 CrossRef CAS.
- J.-Q. Liu, C.-F. Wang, Y. Li, J.-C. Chen, L. Zhou and M.-H. Qiu, Phytochemistry, 2012, 76, 141–149 CrossRef CAS.
- S.-Y. Jiang, J.-Q. Liu, J.-J. Xia, Y.-X. Yan and M.-H. Qiu, Helv. Chim. Acta, 2012, 95, 301–307 CrossRef CAS.
- Y. Zhang, C.-P. Tang, C.-Q. Ke, X.-Q. Li, H. Xie and Y. Ye, Phytochemistry, 2012, 73, 106–113 CrossRef CAS.
- Y. Tanaka, A. Sakamoto, T. Inoue, T. Yamada, T. Kikuchi, T. Kajimoto, O. Muraoka, A. Sato, Y. Wataya, H.-S. Kim and R. Tanaka, Tetrahedron, 2012, 68, 3669–3677 CrossRef CAS.
- C. P. Wong, M. Shimada, A. E. Nugroho, Y. Hirasawa, T. Kaneda, A. H. A. Hadi, S. Osamu and H. Morita, J. Nat. Med., 2012, 66, 566–570 CrossRef CAS.
- L. Jiang, Chem. Nat. Compd., 2012, 48, 1013–1016 CrossRef.
- J.-S. Wang, Y. Zhang, X.-B. Wang and L.-Y. Kong, Tetrahedron, 2012, 68, 3963–3971 CrossRef CAS.
- L. Tong, Y. Zhang, H. He and X. Hao, Chin. J. Chem., 2012, 30, 1261–1264 CrossRef CAS.
- Y.-H. Ge, J.-X. Zhang, S.-Z. Mu, Y. Chen, F.-M. Yang, Y. Lu and X.-J. Hao, Tetrahedron, 2012, 68, 566–572 CrossRef CAS.
- Y.-h. Ge, K.-x. Liu, J.-x. Zhang, S.-z. Mu and X.-j. Hao, J. Agric. Food Chem., 2012, 60, 4289–4295 CrossRef CAS.
- T. Yang, Y.-B. Zeng, Z.-K. Guo, W.-J. Zuo, S.-S. Ma, S.-S. Li, W.-L. Mei and H.-F. Dai, J. Asian Nat. Prod. Res., 2012, 14, 581–585 CrossRef CAS.
- H.-B. Liu, H. Zhang, P. Li, Y. Wu, Z.-B. Gao and J.-M. Yue, Org. Biomol. Chem., 2012, 10, 1448–1458 CAS.
- J. Luo, Y. Li, J.-S. Wang, J. Lu, X.-B. Wang, J.-G. Luo and L.-Y. Kong, Chem. Pharm. Bull., 2012, 60, 195–204 CrossRef CAS.
- Y. Li, J. Luo, Q. Wang and L.-Y. Kong, Heterocycles, 2012, 85, 3035–3041 CrossRef CAS.
- J. Luo, Y. Li, J.-S. Wang, J. Lu and L.-Y. Kong, Phytochem. Lett., 2012, 5, 249–252 CrossRef CAS.
- X.-L. Chen, H.-L. Liu and Y.-W. Guo, Planta Med., 2012, 78, 286–290 CrossRef CAS.
- W. Yang, L. Kong, Y. Zhang, G. Tang, F. Zhu, S. Li, L. Guo, Y. Cheng, X. Hao and H. He, Planta Med., 2012, 78, 1676–1682 CrossRef CAS.
- I. A. Najmuldeen, A. H. A. Hadi, K. Mohamad, K. Awang, K. A. Ketuly, M. R. Mukhtar, H. Taha, N. Nordin, M. Litaudon, F. Gueritte, A. E. Nugroho and H. Morita, Heterocycles, 2012, 84, 1265–1270 CrossRef CAS.
- S.-L. Chong, K. Awang, M. T. Martin, M. R. Mokhtar, G. Chan, M. Litaudon, F. Gueritte and K. Mohamad, Tetrahedron Lett., 2012, 53, 5355–5359 CrossRef CAS.
- C.-M. Yuan, Y. Zhang, G.-H. Tang, S.-L. Li, Y.-T. Di, L. Hou, J.-Y. Cai, H.-M. Hua, H.-P. He and X.-J. Hao, Chem.–Eur. J., 2012, 7, 2024–2027 CAS.
- F. Dal Piaz, N. Malafronte, A. Romano, D. Gallotta, M. A. Belisario, G. Bifulco, M. J. Gualtieri, R. Sanogo, N. De Tommasi and C. Pisano, Phytochemistry, 2012, 75, 78–89 CrossRef CAS.
- P. A. Yadav, G. Suresh, K. R. Prasad, M. S. A. Rao and K. S. Babu, Tetrahedron Lett., 2012, 53, 773–777 CrossRef CAS.
- J.-Q. Liu, C.-F. Wang, J.-C. Chen and M.-H. Qiu, Nat. Prod. Res., 2012, 26, 1887–1891 CrossRef CAS.
- W. Yang, L.-M. Kong, S.-F. Li, Y. Li, Y. Zhang, H.-P. He and X.-J. Hao, Nat. Prod. Bioprospect., 2012, 2, 145–149 CrossRef CAS.
- J. Li, M.-Y. Li, G. Feng, J. Zhang, M. Karonen, J. Sinkkonen, T. Satyanandamurty and J. Wu, J. Nat. Prod., 2012, 75, 1277–1283 CrossRef CAS.
- M. Chen, R. Chen, S. Wang, W. Tan, Y. Hu, X. Peng and Y. Wang, Int. J. Nanomed., 2012, 8, 85–92 CAS.
- J.-H. Liu, N. Zhao, G.-J. Zhang, S.-S. Yu, L.-J. Wu, J. Qu, S.-G. Ma, X.-G. Chen, T.-Q. Zhang, J. Bai, H. Chen, Z.-F. Fang, F. Zhao and W.-B. Tang, J. Nat. Prod., 2012, 75, 683–688 CrossRef CAS.
- N. Cachet, F. Ho-A-Kwie, M. Rivaud, E. Houel, E. Deharo, G. Bourdy and V. Jullian, Phytochem. Lett., 2012, 5, 162–164 CrossRef CAS.
- S. M. M. Donkwe, E. N. Happi, J. D. Wansi, B. N. Lenta, K. P. Devkota, B. Neumann, H.-G. Stammler and N. Sewald, Planta Med., 2012, 78, 1949–1956 CrossRef CAS.
- M. G. Moghaddam and F. B. H. Ahmad, Asian J. Chem., 2012, 24, 4843–4846 CAS.
- L. Zhang and Y.-c. Zhang, Guoji Zhongliuxue Zazhi, 2012, 39, 113–116 CAS.
- D.-Y. Lee, K.-H. Seo, D.-S. Lee, Y.-C. Kim, I.-S. Chung, G.-W. Kim, D.-S. Cheoi and N.-I. Baek, J. Nat. Prod., 2012, 75, 1138–1144 CrossRef CAS.
- T. Warashina, K. Umehara and T. Miyase, Chem. Pharm. Bull., 2012, 60, 205–212 CrossRef CAS.
- Y.-C. Lai, C.-K. Chen, S.-F. Tsai and S.-S. Lee, Phytochemistry, 2012, 74, 206–211 CrossRef CAS.
- C.-x. Yang, Z.-k. Zhang, N. Liu, B. Wei and X.-l. Su, Zhongcaoyao, 2012, 43, 1471–1474 CAS.
- N. Riaz, M. A. Naveed, M. Saleem, B. Jabeen, M. Ashraf, S. A. Ejaz, A. Jabbar and I. Ahmed, J. Asian Nat. Prod. Res., 2012, 14, 1149–1155 CrossRef CAS.
- H. Zhang, H.-H. Xu, Z.-J. Song, L.-Y. Chen and H.-J. Wen, Fitoterapia, 2012, 83, 1081–1086 CrossRef CAS.
- L.-F. Peng, W.-J. Xia, L. He and T. Cui, Chin. J. Nat. Med., 2012, 10, 81–83 CrossRef CAS.
- W. Zuo, Q. Wang, W. Li, Y. Sha, X. Li and J. Wang, Magn. Reson. Chem., 2012, 50, 325–328 CrossRef CAS.
- K. Kakuta, T. Koike, K. Kinoshita, S. Ito, K. Koyama and K. Takahashi, Heterocycles, 2012, 85, 1377–1392 CrossRef CAS.
- J. Yu, S. Liu and L. Xuan, Nat. Prod. Res., 2012, 26, 630–636 CrossRef CAS.
- R. Kumari, M. Ali and V. Aeri, J. Asian Nat. Prod. Res., 2012, 14, 7–13 CrossRef CAS.
- B. S. Siddiqui, N. Khatoon, S. Begum, A. D. Farooq, K. Qamar, H. A. Bhatti and S. K. Ali, Phytochemistry, 2012, 77, 238–244 CrossRef CAS.
- H. M. P. Poumale, K. P. Awoussong, R. Randrianasolo, C. C. F. Simo, B. T. Ngadjui and Y. Shiono, Nat. Prod. Res., 2012, 26, 749–755 CrossRef CAS.
- J. Hussain, N. Ur Rehman, H. Hussain, A. Al-Harrasi, L. Ali, T. S. Rizvi, M. Ahmad and Mehjabeen, Fitoterapia, 2012, 83, 593–598 CrossRef CAS.
- X. Zhang, J. Chen and K. Gao, Biochem. Syst. Ecol., 2012, 45, 7–11 CrossRef CAS.
- L. Xiong, M. Zhu, C. Zhu, S. Lin, Y. Yang and J. Shi, J. Nat. Prod., 2012, 75, 1160–1166 CrossRef CAS.
- J. A. Kim, J. H. Son, S. Y. Yang, S. B. Song, G. Y. Song and Y. H. Kim, Arch. Pharmacal Res., 2012, 35, 647–651 CrossRef CAS.
- M. Zaffer Ahmad, M. Ali and S. R. Mir, J. Pharm. Res., 2012, 5, 548–550 Search PubMed.
- J. Pollier and A. Goossens, Phytochemistry, 2012, 77, 10–15 CrossRef CAS.
- P. Manna and P. C. Sil, Free Radical Res., 2012, 46, 815–830 CrossRef CAS.
- M. Efdi, M. Ninomiya, E. Suryani, K. Tanaka, S. Ibrahim, K. Watanabe and M. Koketsu, Bioorg. Med. Chem. Lett., 2012, 22, 4242–4245 CrossRef CAS.
- J. J. Omolo, V. Maharaj, D. Naidoo, T. Klimkait, H. M. Malebo, S. Mtullu, H. V. M. Lyaruu and C. B. de Koning, J. Nat. Prod., 2012, 75, 1712–1716 CrossRef CAS.
- C.-J. Ji, G.-Z. Zeng, J. Han, W.-J. He, Y.-M. Zhang and N.-H. Tan, Bioorg. Med. Chem. Lett., 2012, 22, 6377–6380 CrossRef CAS.
- S. Sutthivaiyakit, C. Seeka, T. Kritwinyu, S. Pisutchareonpong and N. Chimnoi, Tetrahedron Lett., 2012, 53, 1713–1716 CrossRef CAS.
- Q. Zhan, F. Zhang, L. Sun, Z. Wu and W. Chen, Molecules, 2012, 17, 14899–14907 CrossRef CAS.
- L.-J. Zhang, J.-J. Cheng, C.-C. Liao, H.-L. Cheng, H.-T. Huang, L.-M. Y. Kuo and Y.-H. Kuo, Planta Med., 2012, 78, 1584–1590 CrossRef CAS.
- I. Khlif, K. Hamden, M. Damak and N. Allouche, Chem. Nat. Compd., 2012, 48, 799–802 CrossRef CAS.
- M. A. Tantry, J. A. Dar, M. A. Khuroo and A. S. Shawl, Phytochem. Lett., 2012, 5, 253–257 CrossRef CAS.
- H.-C. Ren, R.-D. Qin, Q. Wang, W. Cheng, Q.-Y. Zhang and H. Liang, J. Asian Nat. Prod. Res., 2012, 14, 1032–1038 CrossRef CAS.
- G. Schnell, P. Schaeffer, E. Motsch and P. Adam, Org. Biomol. Chem., 2012, 10, 8276–8282 CAS.
- Z. Habibi, Z. Cheraghi, S. Ghasemi and M. Yousefi, Nat. Prod. Res., 2012, 26, 1910–1913 CrossRef CAS.
- A. A. Osorio, A. Munoz, D. Torres-Romero, L. M. Bedoya, N. R. Perestelo, I. A. Jimenez, J. Alcami and I. L. Bazzocchi, Eur. J. Med. Chem., 2012, 52, 295–303 CrossRef CAS.
- H. Yang, H.-J. Cho, S. H. Sim, Y. K. Chung, D.-D. Kim, S. H. Sung, J. Kim and Y. C. Kim, Bioorg. Med. Chem. Lett., 2012, 22, 2079–2083 CrossRef CAS.
- J. Li, H. Xu, W. Tang and Z. Song, Fitoterapia, 2012, 83, 383–387 CrossRef CAS.
- R. A. Kaskoos, M. Ali and K. J. Naquvi, J. Pharm. Res., 2012, 5, 2368–2372 Search PubMed , 2365 pp.
- T. N. Vo, P. L. Nguyen, L. T. Tuong, L. M. Pratt, P. N. Vo, K. P. P. Nguyen and N. S. Nguyen, Chem. Pharm. Bull., 2012, 60, 1125–1133 CrossRef CAS.
- H.-Z. Jiang, Q.-Y. Ma, H.-J. Fan, W.-J. Liang, S.-Z. Huang, H.-F. Dai, P.-C. Wang, X.-F. Ma and Y.-X. Zhao, J. Braz. Chem. Soc., 2012, 23, 889–893 CrossRef CAS.
- H.-C. Huang, C.-T. Chiou, P.-C. Hsiao, C.-C. Liaw, L.-J. Zhang, C.-L. Chang, I.-S. Chen, W.-C. Chen, K.-H. Lee and Y.-H. Kuo, Molecules, 2012, 17, 1837–1851 CrossRef CAS.
- Q. Jin, H.-G. Jin, A. R. Kim and E.-R. Woo, Helv. Chim. Acta, 2012, 95, 1455–1460 CrossRef CAS.
- T. K. L. Giang, T. T. Nguyen, T. H. A. Nguyen, V. S. Tran and H. C. Dao, Tap Chi Hoa Hoc, 2011, 49, 738–742 CAS.
- S. Gandomkar, M. Yousefi, Z. Habibi and M. A. As'habi, Nat. Prod. Res., 2012, 26, 648–653 CrossRef CAS.
- M. F. de Araujo, R. Braz-Filho, M. G. de Carvalho and I. J. C. Vieira, Quim. Nova, 2012, 35, 2202–2204 CrossRef CAS.
- J.-J. Li, J. Yang, F. Lu, Y.-T. Qi, Y.-Q. Liu, Y. Sun and Q. Wang, Chin. J. Nat. Med., 2012, 10, 279–283 CrossRef CAS.
- S.-A. Tang, L.-L. Ding, H.-Y. Zhai, N. Qin and H.-Q. Duan, J. Asian Nat. Prod. Res., 2012, 14, 838–843 CrossRef CAS.
- B. Kuang, J. Han, G.-Z. Zeng, X.-Q. Chen, W.-J. He and N.-H. Tan, Nat. Prod. Bioprospect., 2012, 2, 166–169 CrossRef CAS.
- C.-C. Zhao, J.-H. Shao and J.-D. Fan, Chem. Nat. Compd., 2012, 48, 803–805 CrossRef CAS.
- C. Y. Ragasa, D. L. Espineli and C.-C. Shen, Nat. Prod. Res., 2012, 26, 1869–1875 CrossRef CAS.
- J. Sun, X. Wang, H. Zhang and J. Yang, Chem. Nat. Compd., 2012, 48, 416–418 CrossRef CAS.
- Y. Liu, M. Kubo and Y. Fukuyama, J. Nat. Prod., 2012, 75, 1353–1358 CrossRef CAS.
- A. A. Gohar, G. T. Maatooq, E. M. Mrawan, A. A. Zaki and Y. Takaya, Nat. Prod. Res., 2012, 26, 1328–1333 CrossRef CAS.
- A. Alabdul Magid, N. Lalun, C. Long, N. Borie, H. Bobichon, C. Moretti and C. Lavaud, Phytochemistry, 2012, 77, 268–274 CrossRef CAS.
- L. Yu, X. Tang, L. Chen, M. Wang, J. Jian, S. Cao, X. Wang, N. Kang and F. Qiu, Fitoterapia, 2012, 83, 1636–1642 CrossRef CAS.
- S. Iwanaga, T. Warashina and T. Miyase, Chem. Pharm. Bull., 2012, 60, 1264–1274 CrossRef CAS.
- S. Nakamura, T. Moriura, S. Park, K. Fujimoto, T. Matsumoto, T. Ohta, H. Matsuda and M. Yoshikawa, J. Nat. Prod., 2012, 75, 1425–1430 CrossRef CAS.
- W. Yuan, P. Wang, G. Deng and S. Li, Phytochemistry, 2012, 75, 67–77 CrossRef CAS.
- S. Bencharif-Betina, T. Miyamoto, C. Tanaka, Z. Kabouche, A.-C. Mitaine-Offer and M. A. Lacaille-Dubois, Helv. Chim. Acta, 2012, 95, 1573–1580 CrossRef CAS.
- C. T. A. Minh, M. K. Nguyen, P. T. Thuong, I. H. Hwang, D. W. Kim and M. K. Na, Biochem. Syst. Ecol., 2012, 44, 270–274 CrossRef CAS.
- D. Ji, Y. Wu, B. Zhang, C.-F. Zhang and Z.-L. Yang, Fitoterapia, 2012, 83, 843–848 CrossRef CAS.
- M. Scognamiglio, B. D'Abrosca, V. Fiumano, A. Chambery, V. Severino, N. Tsafantakis, S. Pacifico, A. Esposito and A. Fiorentino, Phytochemistry, 2012, 84, 125–134 CrossRef CAS.
- Q.-z. Wang, X.-f. Liu, Y. Shan, F.-q. Guan, Y. Chen, X.-y. Wang, M. Wang and X. Feng, Fitoterapia, 2012, 83, 742–749 CrossRef CAS.
- H.-Z. Li, L.-Z. Fu, H.-M. Li, R.-T. Li and X.-L. Deng, Phytochem. Lett., 2012, 5, 572–575 CrossRef CAS.
- T. K. Tabopda, A.-C. Mitaine-Offer, T. Miyamoto, C. Tanaka, J.-F. Mirjolet, O. Duchamp, B. T. Ngadjui and M.-A. Lacaille-Dubois, Phytochemistry, 2012, 73, 142–147 CrossRef CAS.
- L. Yu, X. Wang, X. Wei, M. Wang, L. Chen, S. Cao, N. Kang and F. Qiu, Bioorg. Med. Chem. Lett., 2012, 22, 5232–5238 CrossRef CAS.
- X.-X. Weng, J. Zhang, W. Gao, L. Cheng, Y. Shao and D.-Y. Kong, Helv. Chim. Acta, 2012, 95, 255–260 CrossRef CAS.
- H. Matsuda, M. Hamao, S. Nakamura, H. Kon'i, M. Murata and M. Yoshikawa, Chem. Pharm. Bull., 2012, 60, 674–680 CAS.
- K. Kakuta, M. Baba, S. Ito, K. Kinoshita, K. Koyama and K. Takahashi, Bioorg. Med. Chem. Lett., 2012, 22, 4793–4800 CrossRef CAS.
- N. B. Sankahya and S. Kirmizigul, Planta Med., 2012, 78, 828–833 CrossRef CAS.
- Y. Iwamoto, S. Sugimoto, L. Harinantenaina, K. Matsunami and H. Otsuka, J. Nat. Med., 2012, 66, 321–328 CrossRef CAS.
- J. Wang, J. Lu, C. Lv, T. Xu and L. Jia, Fitoterapia, 2012, 83, 1396–1401 CrossRef CAS.
- N. Saba, R. Khatoon, Z. Ali and V. U. Ahmad, J. Chem. Soc. Pak., 2012, 34, 448–450 CAS.
- H. Altunkeyik, D. Gulcemal, M. Masullo, O. Alankus-Caliskan, S. Piacente and T. Karayildirim, Phytochemistry, 2012, 73, 127–133 CrossRef CAS.
- L. Li, Y.-X. He, M.-L. Gou and C. Dai, J. Asian Nat. Prod. Res., 2012, 14, 1169–1174 CrossRef CAS.
- Z.-x. Zhao, R.-x. Zeng, J. Jin, C.-z. Lin, T.-q. Xiong, J.-y. Cai and C.-c. Zhu, Zhongcaoyao, 2012, 43, 1267–1269 CAS.
- T. H. Quang, T. T. N. Nguyen, C. V. Minh, P. V. Kiem, H.-J. Boo, J.-W. Hyun, H.-K. Kang and Y. H. Kim, Phytochem. Lett., 2012, 5, 177–182 CrossRef CAS.
- W. Li, J. Cao, Y. Tang, L. Zhang, Q. Xie, H. Shen and Y. Zhao, Fitoterapia, 2012, 83, 147–152 CrossRef CAS.
- Y. Chen, Y. Shan, Y. Y. Zhao, Q. Z. Wang, M. Wang, X. Feng and J. Y. Liang, Chin. Chem. Lett., 2012, 23, 325–328 CrossRef CAS.
- D.-L. Wu, Y.-K. Wang, J.-S. Liu, X.-C. Wang and W. Zhang, J. Asian Nat. Prod. Res., 2012, 14, 342–347 CrossRef CAS.
- X.-F. Zhang, Y.-Y. Han, G.-H. Bao, T.-J. Ling, L. Zhang, L.-P. Gao and T. Xia, Molecules, 2012, 17, 11721–11728 CrossRef CAS.
- V. Lanzotti, P. Termolino, M. Dolci and P. Curir, Bioorg. Med. Chem., 2012, 20, 3280–3286 CrossRef CAS.
- I. K. Lee, S. U. Choi and K. R. Lee, Chem. Pharm. Bull., 2012, 60, 1011–1018 CrossRef CAS.
- C. Li, J. Fu, J. Yang, D. Zhang, Y. Yuan and N. Chen, Fitoterapia, 2012, 83, 1184–1190 CrossRef CAS.
- F. Li, C.-R. Sun, B. Chen, L.-S. Ding and M.-K. Wang, Phytochem. Lett., 2012, 5, 258–261 CrossRef CAS.
- K. Fujimoto, S. Nakamura, S. Nakashima, T. Matsumoto, K. Uno, T. Ohta, T. Miura, H. Matsuda and M. Yoshikawa, Chem. Pharm. Bull., 2012, 60, 1188–1194 CrossRef CAS.
- S. Nakamura, K. Fujimoto, S. Nakashima, T. Matsumoto, T. Miura, K. Uno, H. Matsuda and M. Yoshikawa, Chem. Pharm. Bull., 2012, 60, 752–758 CrossRef CAS.
- Z. Z. Ibraheim, W. M. Abdel-Mageed and M. Jaspars, Biochem. Syst. Ecol., 2012, 40, 86–90 CrossRef CAS.
- J. Linnek, A.-C. Mitaine-Offer, T. Paululat and M.-A. Lacaille-Dubois, Magn. Reson. Chem., 2012, 50, 798–802 CrossRef CAS.
- W. Xu, J. Fang, Z. Zhu, J. Wu and Y. Li, Nat. Prod. Res., 2012, 26, 2002–2007 CrossRef CAS.
- M. Myose, T. Warashina and T. Miyase, Chem. Pharm. Bull., 2012, 60, 612–623 CAS.
- Z.-H. Xiao, F.-Z. Wang, A.-J. Sun, C.-R. Li, C.-G. Huang and S. Zhang, Molecules, 2012, 17, 295–302 CrossRef CAS.
- C. Sayagh, C. Long, C. Moretti and C. Lavaud, Phytochem. Lett., 2012, 5, 188–193 CrossRef CAS.
- Z.-B. Wang, H. Jiang, Y.-G. Xia, B.-Y. Yang and H.-X. Kuang, Molecules, 2012, 17, 6269–6276 CrossRef CAS.
- E. A. Khatuntseva, V. M. Men'shov, A. S. Shashkov, Y. E. Tsvetkov, R. N. Stepanenko, R. Y. Vlasenko, E. E. Shults, G. A. Tolstikov, T. G. Tolstikova, D. S. Baev, V. A. Kaledin, N. A. Popova, V. P. Nikolin, P. P. Laktionov, A. V. Cherepanova, T. V. Kulakovskaya, E. V. Kulakovskaya and N. E. Nifantiev, Beilstein J. Org. Chem., 2012, 8, 763–775 CrossRef CAS , no. 787.
- A. Hamed, A. Perrone, U. Mahalel, W. Oleszek, A. Stochmal and S. Piacente, Phytochem. Lett., 2012, 5, 782–787 CrossRef CAS.
- Y. Zhang, X. Huang, L. Wang, Y. Wang, Y. Wang and W. Ye, Chin. J. Chem., 2012, 30, 1249–1254 CrossRef CAS.
- Y. Zhang, Z. Ma, C. Hu, L. Wang, L. Li and S. Song, Fitoterapia, 2012, 83, 806–811 CrossRef CAS.
- L. Bi, X. Tian, F. Dou, L. Hong, H. Tang and S. Wang, Fitoterapia, 2012, 83, 234–240 CrossRef CAS.
- L.-H. Mu, N.-Y. Wei and P. Liu, Planta Med., 2012, 78, 617–621 CrossRef CAS.
- C. Zhu, L. Gao, Z. Zhao and C. Lin, Yaoxue Xuebao, 2012, 47, 77–83 CAS.
- P. T. Nedialkov, Z. Kokanova-Nedialkova, D. Bucherl, G. Momekov, J. Heilmann and S. Nikolov, Nat. Prod. Commun., 2012, 7, 1419–1422 CAS.
- W. Hai, H. Cheng, M. Zhao, Y. Wang, L. Hong, H. Tang and X. Tian, Fitoterapia, 2012, 83, 759–764 CrossRef CAS.
- M. Zhao, H.-F. Tang, F. Qiu, X.-R. Tian, Y. Ding, X.-Y. Wang and X.-M. Zhou, Biochem. Syst. Ecol., 2012, 40, 49–52 CrossRef CAS.
- P. Alam, M. Ali and V. Ari, J. Nat. Prod. Plant Resour., 2012, 2, 272–280 CAS.
- A. Vassallo, M. Pesca, L. Ambrosio, N. Malafronte, N. D. Melle, F. Dal Piaz and L. Severino, Nat. Prod. Commun., 2012, 7, 1427–1430 CAS.
- T. S. El-Alfy, S. M. Ezzat and A. A. Sleem, Nat. Prod. Res., 2012, 26, 619–629 CrossRef CAS.
- S. Badal, Int. J. Chem. Sci., 2012, 10, 1271–1276 CAS.
- M.-Q. Zhang, Y. Liu, S.-X. Xie, T.-H. Xu, T.-H. Liu, Y.-J. Xu and D.-M. Xu, J. Asian Nat. Prod. Res., 2012, 14, 1186–1190 CrossRef CAS.
- W. Qi, D. Yuan, L.-M. Yang, K.-H. Xie, T.-Z. Cai, R. Yang and H.-Z. Fu, Nat. Prod. Res., 2012, 26, 1436–1441 CrossRef CAS.
- M. Yotova, I. Krasteva, K. Jenett-Siems, P. Zdraveva and S. Nikolov, Phytochem. Lett., 2012, 5, 752–755 CrossRef CAS.
- H. Yao, J. Duan, J. Wang and Y. Li, Biochem. Syst. Ecol., 2012, 42, 14–17 CrossRef CAS.
- L. O. A. Manguro, P. Lemmen, P. Hao and K.-C. Wong, J. Asian Nat. Prod. Res., 2012, 14, 987–1001 CrossRef CAS.
- L. Gao, L. Zhang, L.-M. Wang, J.-Y. Liu, P.-L. Cai and S.-L. Yang, J. Asian Nat. Prod. Res., 2012, 14, 333–341 CrossRef CAS.
- F. V. Mohammad, M. Noorwala, V. U. Ahmad, A. Zahoor and N. H. J. Lajis, Nat. Prod. Commun., 2012, 7, 1423–1426 CAS.
- M. Yin, X. Wang, M. Wang, Y. Chen, Y. Dong, Y. Zhao and X. Feng, Chem. Nat. Compd., 2012, 48, 258–261 CrossRef CAS.
- Y. A. Kim, C.-S. Kong, J. I. Lee, H. Kim, H. Y. Park, H.-S. Lee, C. Lee and Y. Seo, Bioorg. Med. Chem. Lett., 2012, 22, 4318–4322 CrossRef CAS.
- A. d. P. Barbosa, B. Pereira da Silva and J. P. Parente, Phytochem. Lett., 2012, 5, 626–631 CrossRef CAS.
- A. P. Oliveira, M. Raith, R. M. Kuster, L. M. Rocha, M. Hamburger and O. Potterat, Planta Med., 2012, 78, 703–710 CrossRef CAS.
- L. Harinantenaina, P. J. Brodie, M. W. Callmander, L. J. Razafitsalama, V. E. Rasamison, E. Rakotobe and D. G. I. Kingston, Nat. Prod. Commun., 2012, 7, 705–708 CAS.
- X. Li, Y.-F. Wang, Q.-W. Shi and F. Sauriol, Helv. Chim. Acta, 2012, 95, 1395–1400 CrossRef CAS.
- H. Cui, H. Xiao, X.-K. Ran, Y.-Y. Li, D.-Q. Dou and T.-G. Kang, J. Asian Nat. Prod. Res., 2012, 14, 216–223 CrossRef CAS.
- A. V. B. Djoumessi, L. P. Sandjo, J. C. Liermann, D. Schollmeyer, V. Kuete, V. Rincheval, A. M. Berhanu, S. O. Yeboah, P. Wafo, B. T. Ngadjui and T. Opatz, Tetrahedron, 2012, 68, 4621–4627 CrossRef CAS.
- C.-Q. Wang, L. Wang, C.-L. Fan, D.-M. Zhang, X.-J. Huang, R.-W. Jiang, L.-L. Bai, J.-M. Shi, Y. Wang and W.-C. Ye, Org. Lett., 2012, 14, 4102–4105 CrossRef CAS.
- J. Hu, X. Shi, J. Chen, H. Huang and C. Zhao, Fitoterapia, 2012, 83, 55–59 CrossRef CAS.
- M. J. Núñez, A. E. Ardiles, M. L. Martínez, D. Torres-Romero, I. A. Jiménez and I. L. Bazzocchi, Phytochem. Lett., 2012, 5, 244–248 CrossRef.
- G. F. Sousa, L. P. Duarte, A. F. C. Alcantara, G. D. F. Silva, S. A. Vieira-Filho, R. R. Silva, D. M. Oliveira and J. A. Takahashi, Molecules, 2012, 17, 13439–13456 CrossRef CAS.
- G. F. Sousa, F. L. Ferreira, L. P. Duarte, G. D. F. Silva, M. C. T. B. Messias and S. A. Vieira Filho, J. Chem. Res., 2012, 36, 203–205 CrossRef CAS.
- A. Patra, S. Ghosh, S. K. Bandyopadhyay, P. K. Bag, P. Bhowmik and E. Sukumar, J. Indian Chem. Soc., 2012, 89, 805–810 CAS.
- A. E. Ardiles, A. Gonzalez-Rodriguez, M. J. Nunez, N. R. Perestelo, V. Pardo, I. A. Jimenez, A. M. Valverde and I. L. Bazzocchi, Phytochemistry, 2012, 84, 116–124 CrossRef CAS.
- H.-P. Ding, X.-P. Li, W. Zhang, N. Ding, P. Wang, G.-Q. Li and Y.-X. Li, Zhongguo Yaolixue Yu Dulixue Zazhi, 2012, 26, 570–576 CAS.
- C.-J. Li, F.-G. Xie, J.-Z. Yang, Y.-M. Luo, X.-G. Chen and D.-M. Zhang, J. Asian Nat. Prod. Res., 2012, 14, 973–980 CrossRef CAS.
- J. Wu, Y. Zhou, L. Wang, J. Zuo and W. Zhao, Phytochemistry, 2012, 75, 159–168 CrossRef CAS.
- J. A. R. Salvador, V. M. Moreira, B. M. F. Goncalves, A. S. Leal and Y. Jing, Nat. Prod. Rep., 2012, 29, 1463–1479 RSC.
- L. M. Bershtein, Vopr. Onkol., 2012, 58, 744–747 CAS.
- Y.-B. Lu, M.-F. He and J.-H. Wang, Zhongliu Yanjiu Yu Linchuang, 2012, 24, 565–566 CAS.
- H. I. Al-Jaber, K. K. Abrouni, M. A. Al-Qudah and M. H. Abu Zarga, J. Asian Nat. Prod. Res., 2012, 14, 618–625 CrossRef CAS.
- C.-J. Zheng, J. Pu, H. Zhang, T. Han, K. Rahman and L.-P. Qin, Fitoterapia, 2012, 83, 49–54 CrossRef CAS.
- C. Li, L. Li, C. Wang, J. Yang, F. Ye, J. Tian, Y. Si and D. Zhang, Molecules, 2012, 17, 13960–13968 CrossRef CAS.
- Y. Saito, Y. Takashima, Y. Okamoto, T. Komiyama, A. Ohsaki, X. Gong and M. Tori, Chem. Lett., 2012, 41, 372–373 CrossRef CAS.
- A. H. Laghari, S. Memon, A. Nelofar and K. M. Khan, Helv. Chim. Acta, 2012, 95, 1556–1560 CrossRef CAS.
- G. Venkateswara Rao, M. R. Sahoo, G. D. Rajesh and T. Mukhopadhyay, J. Pharm. Res., 2012, 5, 1946–1948 Search PubMed , 1943 pp.
- J.-Y. Tao, S.-J. Dai, F. Zhao, J.-F. Liu, W.-S. Fang and K. Liu, J. Asian Nat. Prod. Res., 2012, 14, 97–104 CrossRef CAS.
- Y. H. Choi, W. Zhou, J. Oh, S. Choe, D. W. Kim, S. H. Lee and M. Na, Bioorg. Med. Chem. Lett., 2012, 22, 6116–6119 CrossRef CAS.
- G. Sun, X. Zhang, X. Xu, J. Yang, M. Zhong and J. Yuan, Molecules, 2012, 17, 504–510 CrossRef CAS.
- H. Wang, X. Wu, T. Zhou, X. Deng and D. Wang, Zhongyaocai, 2012, 35, 396–399 CAS.
- M. Shao, Y. Wang, X.-J. Huang, C.-L. Fan, Q.-W. Zhang, X.-Q. Zhang and W.-C. Ye, J. Asian Nat. Prod. Res., 2012, 14, 348–354 CrossRef CAS.
- K. H. Kim, S. U. Choi and K. R. Lee, Planta Med., 2012, 78, 86–89 CrossRef CAS.
- B.-b. Zhang, X.-l. Han, Q. Jiang, Z.-x. Liao, C. Liu and Y.-b. Qu, Fitoterapia, 2012, 83, 1242–1247 CrossRef CAS.
- M. M. Hussain, M. A. Rashid, C. M. Hasan and A. Jabbar, Int. J. Pharma Sci. Res., 2012, 3, 1826–1828 CAS.
- J. Sidana, S. Singh, S. K. Arora, W. J. Foley and I. P. Singh, Pharm. Biol., 2012, 50, 823–827 CrossRef CAS.
- J. Triana, M. Lopez, F. J. Perez, M. Rico, A. Lopez, F. Estevez, M. T. Marrero, I. Brouard and F. Leon, Molecules, 2012, 17, 12895–12909 CrossRef CAS.
- Y.-C. Chien, C.-H. Lin, M. Y. Chiang, H.-S. Chang, C.-H. Liao, I.-S. Chen, C.-F. Peng and I.-L. Tsai, Phytochemistry, 2012, 80, 50–57 CrossRef CAS.
- Q.-M. Xu, Y.-L. Liu, Y.-L. Feng, L.-H. Tang and S.-L. Yang, Chem. Nat. Compd., 2012, 48, 597–600 CrossRef CAS.
- P.-Y. Zhang, S.-H. Qin, H.-X. Zhao, F.-L. Wang, H.-J. Guo and H. Bai, J. Asian Nat. Prod. Res., 2012, 14, 607–611 CrossRef CAS.
- L.-P. Qu, G.-Y. Zheng, Y.-H. Su, H.-Q. Zhang, Y.-L. Yang, H.-L. Xin and C.-Q. Ling, Int. J. Mol. Sci., 2012, 13, 14865–14870 CrossRef CAS.
- M. Safder, R. Mehmood, B. Ali, U. R. Mughal, A. Malik and A. Jabbar, Helv. Chim. Acta, 2012, 95, 144–151 CrossRef CAS.
- M. Zhou, M. Xu, X.-X. Ma, K. Zheng, K. Yang, C.-R. Yang, Y.-F. Wang and Y.-J. Zhang, Planta Med., 2012, 78, 1702–1705 CrossRef CAS.
- E. J. T. Mbosso, J. C. A. Nguedia, F. Meyer, B. N. Lenta, S. Ngouela, B. Lallemand, V. Mathieu, P. Van Antwerpen, A. L. Njunda, D. Adiogo, E. Tsamo, Y. Looze, R. Kiss and R. Wintjens, Phytochemistry, 2012, 83, 95–103 CrossRef CAS.
- W.-J. Zuo, H.-F. Dai, Y.-B. Zeng, H. Wang, H.-Q. Chen and J.-H. Wang, J. Asian Nat. Prod. Res., 2012, 14, 308–313 CrossRef CAS.
- Y.-Y. Che, Z. Liang, N. Li, K.-W. Zeng and P.-F. Tu, Nat. Prod. Res., 2012, 26, 1991–1995 CrossRef CAS.
- L. Wang, Y. Cai, X.-Q. Zhang, C.-L. Fan, Q.-W. Zhang, X.-P. Lai and W.-C. Ye, Carbohydr. Res., 2012, 349, 39–43 CrossRef CAS.
- I. Kazmi, M. Rahman, M. Afzal, G. Gupta, S. Saleem, O. Afzal, M. A. Shaharyar, U. Nautiyal, S. Ahmed and F. Anwar, Fitoterapia, 2012, 83, 142–146 CrossRef CAS.
- J. C. Shu, J. Q. Liu, G. X. Chou and Z. T. Wang, Chin. Chem. Lett., 2012, 23, 827–830 CrossRef CAS.
- Z. Y. Babaamer, L. Sakhri, H. I. Al-Jaber, M. A. Al-Qudah and M. H. Abu Zarga, J. Asian Nat. Prod. Res., 2012, 14, 1137–1143 CrossRef CAS.
- S. R. M. Ibrahim, G. A. Mohamed, L. A. Shaala, L. M. Y. Banuls, G. Van Goietsenoven, R. Kiss and D. T. A. Youssef, Phytochem. Lett., 2012, 5, 490–495 CrossRef CAS.
- T. Wu, Q.-W. Zhang, X.-Q. Zhang, G. Liu, L. Wang, M.-M. Jiang, Y.-F. Feng and W.-C. Ye, Nat. Prod. Res., 2012, 26, 1408–1412 CrossRef CAS.
- M. Isaka, P. Chinthanom, S. Supothina and S. Mongkolsamrit, Phytochem. Lett., 2012, 5, 734–737 CrossRef CAS.
- M. Monnier, C. Lavaud, M. Litaudon and V. Dumontet, Biochem. Syst. Ecol., 2012, 42, 10–13 CrossRef CAS.
- V. Costantino, G. Della Sala, A. Mangoni, C. Perinu and R. Teta, Eur. J. Org. Chem., 2012, 2012, 5171–5176 CrossRef CAS.
- C. Y. Ragasa, D. L. Espineli, E. H. Mandia, M.-J. Don and C.-C. Shen, Chin. J. Nat. Med., 2012, 10, 284–286 CrossRef CAS.
- X. Chai, Y.-F. Su, J. Zhang, S.-L. Yan, Y.-H. Gao and X.-M. Gao, Helv. Chim. Acta, 2012, 95, 127–133 CrossRef CAS.
- C. Y. Ragasa, D. L. Espineli, E. H. Mandia, D. D. Raga, M.-J. Don and C.-C. Shen, Z. Naturforsch., B: J. Chem. Sci., 2012, 67, 426–432 CrossRef CAS.
- X.-H. Yuan, G.-B. Xu, W.-L. Wu, T. Yang and G.-Y. Li, Arch. Pharmacal Res., 2012, 35, 311–314 CrossRef CAS.
- Ö. B. Acıkara, G. S. Çitoğlu, S. Dall'Acqua, K. Šmejkal, J. Cvačka and M. Žemlička, Nat. Prod. Res., 2012, 26, 1892–1897 CrossRef.
- Z. Muhammad, S. Ahmad, R. Ullah, F. Ullah and S. Jan, Biomed. Pharmacol. J., 2012, 5, 65–70 CrossRef CAS.
- S. Wittayalai, S. Sathalalai, S. Thorroad, P. Worawittayanon, S. Ruchirawat and N. Thasana, Phytochemistry, 2012, 76, 117–123 CrossRef CAS.
- J. Yan, Z.-Y. Zhou, M. Zhang, J. Wang, H.-F. Dai and J.-W. Tan, Planta Med., 2012, 78, 1387–1391 CrossRef CAS.
- J. Li, H. Zhu, J. Ren, Z. Deng, N. J. de Voogd, P. Proksch and W. Lin, Tetrahedron, 2012, 68, 559–565 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2015 |