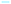Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Fluorosolvatochromism of furanyl- and thiophenyl-substituted acetophenones†
Nadine
Friebe
a,
Katja
Schreiter
a,
Joachim
Kübel
bc,
Benjamin
Dietzek
bc,
Norbert
Moszner
d,
Peter
Burtscher
d,
Alexander
Oehlke
a and
Stefan
Spange
*a
aDepartment of Polymer Chemistry, Institute of Chemistry, Technische Universität Chemnitz, 09107 Chemnitz, Germany. E-mail: stefan.spange@chemie.tu-chemnitz.de; Fax: +49 (0)371-531-21239; Tel: +49 (0)371-531-21230
bInstitute of Physical Chemistry and Abbe Center of Photonics, Friedrich-Schiller University Jena, Helmholtzweg 4, 07743 Jena, Germany
cLeibniz Institute of Photonic Technology (IPHT) Jena e.V., Albert-Einstein-Str. 9, 07745 Jena, Germany
dIvoclar Vivadent AG, Bendererstrasse 2, FL-9494 Schaan, Liechtenstein
First published on 5th May 2015
Abstract
A series of para-substituted acetophenones bearing a furanyl or a thiophenyl moiety show a large Stokes-shift, which is a function of various solvent properties. Photophysical properties such as emission lifetime of the compounds have been determined using time-correlated-single photon counting to secure the intrinsic fluorescence behaviour. The solvent dependent position of the UV/Vis emission band ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) max,em of the compounds has been measured in 26 various solvents. The influence of the solvent on
max,em of the compounds has been measured in 26 various solvents. The influence of the solvent on ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) max,em is of very complex nature and mathematically analysed by multiple square linear solvation energy (LSE)-correlation analysis using Catalán's four-solvent parameter set. Solvent acidity has a strong influence on the bathochromic shift of 2,5-disubstituted furan derivatives compared to the non-5-substituted furan and thiophene derivatives, which show a contrary behaviour. Therefore, the 5-cyanofuranyl-substituted acetophenone derivative is useful as a probe for measuring environmental properties by fluorescence spectroscopy.
max,em is of very complex nature and mathematically analysed by multiple square linear solvation energy (LSE)-correlation analysis using Catalán's four-solvent parameter set. Solvent acidity has a strong influence on the bathochromic shift of 2,5-disubstituted furan derivatives compared to the non-5-substituted furan and thiophene derivatives, which show a contrary behaviour. Therefore, the 5-cyanofuranyl-substituted acetophenone derivative is useful as a probe for measuring environmental properties by fluorescence spectroscopy.
Introduction
In recent years, there has been growing interest in organic fluorescent dyes because of their potential applications as fluorescent probes in chemistry, biochemistry and medicine, and in optoelectronics including organic light emitting diodes (OLEDs), sensors, and lasers.1–3 Such organic fluorophores can also act as environmental probes, e.g. acid–base, and solvent-sensitive indicators.4In previous work, we have studied and discussed many solvatochromic dyes, which are suitable to measure environmental effects by shift of the position of the UV/Vis absorption band as a function of polarity of the environment. For this purpose, push–pull substituted catechol dyes,5a azobenzene chromophores being linked at polyvinylamine backbones,5b and specific aminobenzofurandione dyes as basicity probes for ionic liquids5c were used.
Interactions of solvatochromic dyes with pure solvents or solvent mixtures are determined by a combination of many effects.6 In order to separate the effects of specific interactions (hydrogen-bonding capacity) from non-specific interactions including electrostatic effects (dipolarity/polarizability) on the UV/Vis band (![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) max) the concept of linear free solvation energy relationship (LSER) is used.6
max) the concept of linear free solvation energy relationship (LSER) is used.6
During the last few decades, multiple polarity effects of various environments have been considered in terms of the Kamlet–Abboud–Taft approach (eqn (1)), which has been established as a suitable tool for this purpose.
![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) max = max = ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) max,0 + a·α + b·β + s·π* max,0 + a·α + b·β + s·π* | (1) |
Kamlet, Abboud, and Taft used basically three different polarity parameters which describe the overall solvent polarity: acidity α (hydrogen-bond donor capacity), basicity β (hydrogen-bond acceptor capacity) and dipolarity/polarizability π*.6a,b,7![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) max is the longest wavelength UV/Vis maxima of the compound measured in a particular solvent;
max is the longest wavelength UV/Vis maxima of the compound measured in a particular solvent; ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) max,0 corresponds to a standard process referenced to cyclohexane as a nonpolar medium; a, b, and s are solvent-independent correlation coefficients that reflect the contribution of each parameter to the overall solute–solvent interactions.
max,0 corresponds to a standard process referenced to cyclohexane as a nonpolar medium; a, b, and s are solvent-independent correlation coefficients that reflect the contribution of each parameter to the overall solute–solvent interactions.
Unfortunately, the Kamlet–Abboud–Taft concept does not distinguish between dipolarity and polarizability effects both of which are involved in a single π*-parameter. Therefore, the improved solvent polarity parameter scales of Catalán (eqn (2)) should be used which allow dipolarity and polarizability effects of a solvent to be considered independently of each other.8
![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) max = max = ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) max,0 + a·SA + b·SB + d·SP + e·SdP max,0 + a·SA + b·SB + d·SP + e·SdP | (2) |
![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) max describes the solvent-dependent physico-chemical properties in a particular solvent and
max describes the solvent-dependent physico-chemical properties in a particular solvent and ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) max,0 the solute properties of a reference system, which is defined for the gas phase. The SA (solvent acidity) scale8a,b,e has been established as a measure of the hydrogen-bond donor (HBD) and electron pair acceptor (EPA) properties, whereas the SB (solvent basicity) scale8c,e is responsible for the hydrogen-bond acceptor (HBA) and electron pair donor (EPD) properties. The nonspecific interactions polarizability and dipolarity are reflected by the parameters SP8d,e and SdP,8e respectively. a, b, d, and e are the correlation coefficients characterising the sensitivity of the property
max,0 the solute properties of a reference system, which is defined for the gas phase. The SA (solvent acidity) scale8a,b,e has been established as a measure of the hydrogen-bond donor (HBD) and electron pair acceptor (EPA) properties, whereas the SB (solvent basicity) scale8c,e is responsible for the hydrogen-bond acceptor (HBA) and electron pair donor (EPD) properties. The nonspecific interactions polarizability and dipolarity are reflected by the parameters SP8d,e and SdP,8e respectively. a, b, d, and e are the correlation coefficients characterising the sensitivity of the property ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) max to different solute–solvent interaction mechanisms.
max to different solute–solvent interaction mechanisms.
Fluorosolvatochromism, compared to the comprehensively reported solvatochromism of push–pull substituted chromophores, is still not widely established for measuring environmental effects because only a few probes are known which show a distinct function of ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) max,em as a function of Kamlet–Abboud–Taft or Catalán solvent parameter. Allegeable solvent effects on the shift of
max,em as a function of Kamlet–Abboud–Taft or Catalán solvent parameter. Allegeable solvent effects on the shift of ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) max,em indicated as fluorosolvatochromism are reported for a few dyes and interpreted in detail concerning specific effects.8e,9 So far, multiple square correlation analyses of
max,em indicated as fluorosolvatochromism are reported for a few dyes and interpreted in detail concerning specific effects.8e,9 So far, multiple square correlation analyses of ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) max,em as a function of various solvent properties are still not established taking into account a numerous set of solvents.
max,em as a function of various solvent properties are still not established taking into account a numerous set of solvents.
In this work, we present four acetophenone derivatives bearing the heterocycles furan or thiophene in the para-position to the acetyl unit as a promising class of fluorosolvatochromic compounds. Cross-coupling reactions such as Heck, Stille, Suzuki, Sonogashira, and Negishi type reactions provide an easy route to link carbon atoms of different aryl compounds. Especially by means of the application of the nucleophilicity–electrophilicity principle, those reactions are well manageable.10 A nucleophilic (metal) aryl compound reacts with an aryl halide under transition metal catalysis. Palladium has particularly proven to be the transition metal of choice. It bears an extraordinary potential as an activation agent for oxidative addition, insertion, reductive elimination and β-hydride elimination.11
The main focus of the work concentrates on the synthesis, photophysical properties and fluorosolvatochromism12 of the acetophenone derivatives. Furan (para electrophilic substituent constant σp+ = −0.39)13 and thiophene (σp+ = −0.43)13 are well-known electron-donating heterocycles that are widely used to lower the energy band of conjugated materials. These heterocycles are indeed weaker donor substituents as the methoxy group (σp+ OCH3 = −0.78),13 but they offer the option to enhance the length of the chromophoric π-electron system. This implicates a decrease in the energetic HOMO–LUMO gap and results in a shift towards an even lower-energy absorption range.
Results and discussion
Synthesis of furanyl- or thiophenyl-substituted acetophenones
In this work, the palladium-catalysed Suzuki and Heck coupling served as a synthesis tool for the C–C bond linkage between acetophenone and a furan or thiophene moiety. By modifying the furan part with electron donor (–OMe) or acceptor (–CN) moieties it was possible to achieve a structure–property relationships.In the Suzuki reaction, an aryl halide (in the case of O1: 4-bromoacetophenone, O2: 2-bromo-5-methoxyfuran) was reacted with organoboron compounds (O1: 2-furanboronic acid, O2: 4-acetylphenylboronic acid) in the presence of base and tetrakis(triphenylphosphine) palladium(0) (Scheme 1).
However, the functionalized acetophenones O3 and S114 were prepared by Heck coupling. Analogous to ref. 14, 2-cyanofuran was converted with the electron-deficient 4-bromoacetophenone to compound O3 (Scheme 2).
 | ||
| Scheme 2 Synthesis of the functionalized acetophenones O3 and S1 using the palladium-catalysed Heck coupling. | ||
Theoretical calculations
DFT theoretical studies have been carried out to gain an insight into the electronic structure of acetophenones. All geometries have been optimized using density functional theory with the B3-LYP functional15 in combination with the def2-TZVPP basis set.16 The calculated HOMO–LUMO energy gaps of compounds O1, O2, O3, and S1 (see ESI,† Table S1) present values of the HOMO ranging between 4.045 eV for O1 and 3.737 eV for O2. A comparison of the furanyl- or thiophenyl-substituted acetophenone derivatives with acetophenones bearing electron-donating groups (e.g. –OCH3) shows a significant decrease of the energy gap between HOMO and LUMO. The linear dependency between the calculated HOMO–LUMO energy gaps and the measured UV/Vis absorption maxima![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) max (Fig. 1) indicates that UV/Vis absorption of acetophenones under investigation is mainly justified by a HOMO–LUMO transition.
max (Fig. 1) indicates that UV/Vis absorption of acetophenones under investigation is mainly justified by a HOMO–LUMO transition.
 | ||
| Fig. 1 Experimentally measured UV/Vis absorption maxima of acetophenone derivatives (in DCM) as a function of the theoretically calculated HOMO–LUMO energy differences. | ||
Solvent effects on the UV/Vis absorption spectra
Optical properties such as UV/Vis absorption and fluorescence of functionalized acetophenones O1, O2, O3, and S1 were investigated both in solution and in the solid state. UV/Vis absorption and emission maxima of the acetophenone derivatives in a variety of solvents are given in the ESI† (Table S3). Fig. 2 exemplarily shows the UV/Vis absorption and emission spectra of the respective compounds recorded in four selected solvents. | ||
| Fig. 2 Normalized UV/Vis absorption and emission spectra of O1, O2, O3, and S1 measured in toluene (A), DCM (B) DMAc (C), and DMSO (D); λexc = 310 nm (O1, O3, and S1), λexc = 350 nm (O2). | ||
All compounds show the shortest wavelength UV/Vis absorption band in n-hexane. The compounds O1 and S1 exhibit the longest wavelength UV/Vis absorption band in acetone, while O3 shows the strongest bathochromic shift in hexamethylphosphoramide (HMPA). Methoxy-functionalized acetophenone O2 presents the longest wavelength UV/Vis absorption band in alcohols such as 1-butanol or 1-propanol. These UV/Vis shifts correspond to a positive solvatochromism with a solvatochromic range of Δ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) (O1) = 2140 cm−1, Δ
(O1) = 2140 cm−1, Δ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) (O2) = 1340 cm−1, Δ
(O2) = 1340 cm−1, Δ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) (O3) = 2350 cm−1, and Δ
(O3) = 2350 cm−1, and Δ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) (S1) = 2050 cm−1. Considering the furan derivatives, it is obvious that the solvatochromic range increases with increasing electron-withdrawing character of the substituent in the 5-position (OMe < H < CN).
(S1) = 2050 cm−1. Considering the furan derivatives, it is obvious that the solvatochromic range increases with increasing electron-withdrawing character of the substituent in the 5-position (OMe < H < CN).
Furthermore, it is quite evident that an enlargement of the conjugated π-system causes a bathochromic shift of the absorption wavelength as a consequence. The UV/Vis absorption maxima of the furan- and thiophene-functionalized acetophenones O1 and S1 are each detected at 318 nm in DCM (non-HBA/HBD-solvent). A bathochromic shift of 5900 cm−1 is observed in relation to the UV/Vis absorption maximum of 4-methoxy-acetophenone (λmax = 268 nm). A stronger push–pull system is generated by the additional introduction of a methoxy group on the furan ring (O2). In comparison to the unsubstituted heterocyclic compounds (λmax = 318 nm), this result causes an enormous bathochromic shift of the UV/Vis absorption maximum at 349 nm, measured in DCM. However, the electron-withdrawing substituent –CN in O3 effects a slight hypsochromic shift (λmax = 313 nm, in DCM).
Additionally, the UV/Vis spectroscopic behaviour of O1, O2, O3, and S1 was investigated in the solid-state. The UV/Vis absorption and emission spectra of the solids are shown in Fig. 3. Generally, UV/Vis absorption bands are non-symmetric and very broad whereby the band broadening of the compounds with substituents in the 5-position of the furan ring (O2 and O3) is significantly more pronounced.
Solvent effects on the UV/Vis emission spectra
In contrast to the UV/Vis absorption maxima, the UV/Vis emission maxima of the functionalized acetophenones O1, O2, O3, and S1 show a pronounced shift as a function of solvent properties, as illustrated in Fig. 2.The shortest wavelength UV/Vis emission band was detected in 2-propanol (λmax(O1) = 405 nm), tetrachloromethane (CCl4, λmax(O2) = 398 nm, λmax(O3) = 419 nm) and triethylamine (λmax(S1) = 404 nm). The compounds O2 and O3 bearing substituents on the furan ring exhibit the longest wavelength emission band in alcohols (λmax(O2) = 479 nm in MeOH, λmax(O3) = 483 nm in 2,2,2-trifluoroethanol (TFE)), whereas the furan-/thiophene-functionalized acetophenones show the longest wavelength emission band in the solvents γ-butyrolactone and N,N-dimethylformamide (DMF, λmax(O1) = 467 nm) as well as in dimethyl sulfoxide (DMSO, λmax(S1) = 466 nm). In this context, the respective UV/Vis shifts correspond to a positive fluorosolvatochromism, but the solvatochromic range of Δ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) (O1) = 3280 cm−1, Δ
(O1) = 3280 cm−1, Δ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) (O2) = 4260 cm−1, Δ
(O2) = 4260 cm−1, Δ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) (O3) = 3170 cm−1 and Δ
(O3) = 3170 cm−1 and Δ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) (S1) = 3290 cm−1 is considerably larger.
(S1) = 3290 cm−1 is considerably larger.
The UV/Vis spectroscopic results (absorption and fluorescence) of individual compounds demonstrate that O1, O3 and S1 absorb at shorter wavelengths compared to O2, but the fluorescence is observed at similar wavelengths. Thus, an exceptionally large Stokes shift (in DCM: Δ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) (O1) = 8360 cm−1, Δ
(O1) = 8360 cm−1, Δ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) (O2) = 5700 cm−1, Δ
(O2) = 5700 cm−1, Δ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) (O3) = 8590 cm−1, Δ
(O3) = 8590 cm−1, Δ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) (S1) = 9280 cm−1) has been obtained (see ESI,† Table S3).
(S1) = 9280 cm−1) has been obtained (see ESI,† Table S3).
The Stokes shifts of these furanyl- or thiophenyl-substituted acetophenones can be evaluated as disproportionately large compared to other fluorescent dyes, e.g. coumarin derivatives (Δ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) of 4200−5300 cm−1).17 However, acetophenone bearing a pyrene function in the para-position shows a comparably large Stokes shift in alcohol solutions (Δ
of 4200−5300 cm−1).17 However, acetophenone bearing a pyrene function in the para-position shows a comparably large Stokes shift in alcohol solutions (Δ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) ∼ 10
∼ 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm−1) for S1.18 One of the reasons for the large Stokes shift is certainly that the functionalized acetophenones have a higher dipole moment in the first excited state S1 than in the ground state S0. Associated with this property a change in the dipole moment in strength and orientation is induced. The solvent molecules respond with a reorganization of the solvent shell around the excited acetophenone molecules. Hence, the state S1 of the acetophenone molecule is better solvated by polar solvents and as a result its energetical stabilization is improved. The electronic state after emission S0,emission is energetically unfavourably solvated due to the shorter timescale of photon emission in comparison to solvent molecule reorganization. After relaxation of the solvent molecules the equilibrium state S0 is reached. The difference in the energies of S0,emission and S0 adds to a strongly red-shifted fluorescence of the acetophenone systems.
000 cm−1) for S1.18 One of the reasons for the large Stokes shift is certainly that the functionalized acetophenones have a higher dipole moment in the first excited state S1 than in the ground state S0. Associated with this property a change in the dipole moment in strength and orientation is induced. The solvent molecules respond with a reorganization of the solvent shell around the excited acetophenone molecules. Hence, the state S1 of the acetophenone molecule is better solvated by polar solvents and as a result its energetical stabilization is improved. The electronic state after emission S0,emission is energetically unfavourably solvated due to the shorter timescale of photon emission in comparison to solvent molecule reorganization. After relaxation of the solvent molecules the equilibrium state S0 is reached. The difference in the energies of S0,emission and S0 adds to a strongly red-shifted fluorescence of the acetophenone systems.
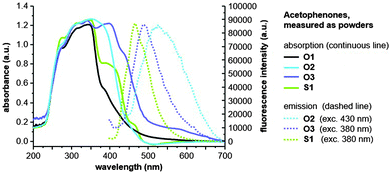
Methoxyfuran-, cyanofuran-, and thiophene-functionalized acetophenones (O2, O3, and S1) also demonstrate a pronounced solid-state fluorescence (Fig. 4). However, compound O1 shows no fluorescence in the solid-state.
 | ||
| Fig. 4 Photographs of the solid-state fluorescence of acetophenones O2, O3, and S1, excited at 380 nm (O3, S1) and 430 nm (O2). | ||
These observations indicate that the substitution in the 5-position of the furan ring or the exchange of oxygen with sulphur plays an important role in tuning the photochemical properties of these types of acetophenones. Fluorescence spectroscopic measurements of the functionalized acetophenones O2, O3, and S1 in the solid-state are also shown in Fig. 3. The UV/Vis emission maxima of methoxyfuran-substituted acetophenone (O2) is located at 524 nm which is red-shifted by Δ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) = 1500 cm−1 compared to its cyanofuran-substituted analogue O3. Compound S1 is fluorescent with a UV/Vis emission band at 465 nm. It is evident that the solid-state fluorescence of methoxyfuran-functionalized acetophenone (O2) is strongly red-shifted by Δ
= 1500 cm−1 compared to its cyanofuran-substituted analogue O3. Compound S1 is fluorescent with a UV/Vis emission band at 465 nm. It is evident that the solid-state fluorescence of methoxyfuran-functionalized acetophenone (O2) is strongly red-shifted by Δ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) = 1800 cm−1 in comparison to the lowest-energy emission band measured in polar solvents (Fig. 2 and 3). The UV/Vis emission maxima of the solids O3 and S1 are similar to those in solution (polar solvents, e.g. λmax(O3) = 483 nm in TFE, λmax(S1) = 466 nm in DMSO).
= 1800 cm−1 in comparison to the lowest-energy emission band measured in polar solvents (Fig. 2 and 3). The UV/Vis emission maxima of the solids O3 and S1 are similar to those in solution (polar solvents, e.g. λmax(O3) = 483 nm in TFE, λmax(S1) = 466 nm in DMSO).
Determination of excited-state lifetimes
The fluorescence decay profiles of the furanyl- or thiophenyl-functionalized acetophenones O1, O2, O3, and S1 were measured by time-correlated single photon counting (TCSPC, Fig. 5). The emission lifetimes are in the order of 0.2 to 2 ns (Table 1) and support the assignment of the emission to be due to fluorescence. For O2 and O3 the fluorescence decay-curves were found to be bi-exponential, while for the other substances exponential decays were observed. There is no unique relationship between solvent polarity and emission lifetime. Nevertheless, it is noted that for O1 and O2 the lifetime in DCM is longer than in ethanol (EtOH), while the opposite holds true for O3 and S1. This points to significant differences in the electronic structures of the excited states and the impact of specific solute–solvent interactions, e.g. hydrogen-bonding ability.| Compd | τ (ns) | |||
|---|---|---|---|---|
| DCM | EtOH | CCl4 | DMF | |
| O1 | 1.4 | 0.5 | — | 0.2 (65%) |
| 1.5 (35%) | ||||
| O2 | 0.4 (49%) | 0.2 (93%) | <0.2 (88%) | — |
| 1.4 (51%) | 1.2 (7%) | 0.9 (12%) | ||
| O3 | 0.6 (86%) | 1.9 | 0.2 (65%) | — |
| 2.0 (14%) | 1.3 (35%) | |||
| S1 | 0.5 | 1.8 | — | 1.3 |
Linear solvation energy correlation analyses
The influence of the solvent behaviour on the UV/Vis shift of the absorption and emission maxima has been determined by means of multiple linear regression analyses using the solvent polarity parameter set of Catalán.19 The solvatochromism of the furanyl- or thiophenyl-functionalized acetophenones was studied in 26 solvents with dipolarities/polarizabilities and hydrogen-bonding capacities.However, the HBD/HBA abilities of the environment have only a marginal influence upon the UV/Vis absorption maxima of the solvatochromic probe.
The results of multiple linear regression analysis of the solvent dependent emission band of substituted acetophenones O1, O2, O3, and S1, which are qualitatively the best according to the solvent scale of Catalán, are compiled in Table 2. The correlation coefficients r are greater than 0.9 (with the exception of O1) for the LSE relationships. These results indicate a very high validity of the obtained multiparameter equations and allow significant conclusions on the fluorosolvatochromic behaviour.
![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) max,0 gas phase, number of solvents (n), correlation coefficient (r), standard deviation (sd), and significance (f) of the fluorosolvatochromism of the acetophenone-functionalized compounds O1, O2, O3, and S1
max,0 gas phase, number of solvents (n), correlation coefficient (r), standard deviation (sd), and significance (f) of the fluorosolvatochromism of the acetophenone-functionalized compounds O1, O2, O3, and S1
| Entry | Compound |
![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) max,0 (103 cm−1)
max,0 (103 cm−1) |
a | b | d | e | n | r | sd | f |
|---|---|---|---|---|---|---|---|---|---|---|
| 2 | O1 | 26.926 | 1.198 | 1.936 | −4.204 | −2.847 | 23 | 0.841 | 0.591 | ≤1.175 × 10−4 |
| 3 | O2 | 24.787 | −2.768 | −0.993 | — | −1.887 | 25 | 0.960 | 0.351 | ≤9.269 × 10−12 |
| 4a | O3 | 23.111 | −2.808 | — | — | — | 31 | 0.920 | 0.360 | ≤2.353 × 10−13 |
| 4b | O3 | 22.984 | −2.619 | — | — | — | 12 | 0.870 | 0.258 | ≤9.735 × 10−6 |
| 5 | S1 | 25.466 | 1.958 | 1.917 | −3.322 | −2.202 | 24 | 0.929 | 0.349 | ≤6.051 × 10−8 |
In this context, the solvent independent correlation coefficient a provides information about solvent interactions with the hydrogen-bond acceptor/electron pair donor fragment (i.e. lone electron pair of the partially negatively charged oxygen from the keto group) of the respective dye. However, the coefficient b describes specific solvation of the dye by the hydrogen-bond donor/electron pair acceptor group using appropriate HBA/EPD-solvents. Fig. 6 shows, for instance, the assumed electron distribution of compounds S1 and O2 in the excited state with possible specific solute–solvent interactions which can have an influence on the fluorosolvatochromic behaviour.
A positive sign indicates that the push–pull system is decreased by the solute–solvent interactions and this results in a hypsochromic shift. Consequently, a negative sign is associated with a bathochromic shift. The appearance of the latter is associated with an enhancement of the push–pull character of the dye.
Surprisingly, the calculated LSER of the solvent dependent emissions of furan-/thiophene-functionalized acetophenones (O1, S1) show that the solvent independent correlation coefficients a and b are positive. In this case protic solvents, which can act as hydrogen-bond donors, mainly interact with the negatively charged oxygen atom of the keto group (a > 0).
As a result, the (+M)-effect of the O− group is decreased and also the push–pull character of the aromatic system is weakened.
A weakening of the push–pull system can also be observed by interactions of EPD-solvents with acetophenones O1 and S1. Thereby, EPD-solvents interact predominantly with the oxygen or sulphur of the heterocyclic ring due to its positive charge located in the excited state (b > 0). The strongest effect of the fluorosolvatochromic behaviour of O1 and S1 is exerted by the polarizability (SP) and the dipolarity (SdP) of the solvents. The respective coefficients d and e have a negative value and thus indicate positive fluorosolvatochromism. Here, a bathochromic shift of the emission maxima is observed with increasing polarizability and dipolarity of the solvent. Considering the emission spectra of the functionalized acetophenones O1, O2, O3, and S1 (Fig. 2) reveal that the compounds O1 and S1 show a smaller difference between the emission maxima of non-polar solvents (i.e. toluene) and those of polar solvents (i.e. DMSO). One possible explanation for these observation could be that the terms of the nonspecific interactions (d and e) exhibit negative values, but the coefficients a and b are positive. The sum of all solvent interactions results in a bathochromic shift, but it is not as large as the shift of acetophenone O2, as can be seen in the following interpretation.
In contrast to O1 and S1, the functionalized acetophenone O2 shows a negative sign of parameter a, which indicates that the interaction of HBD/EPA-solvents with the lone electron pairs of oxygen atoms of the furan ring is significantly stronger than those with oxygen of the keto group (a < 0). Therefore, electron density is donated by the furan ring to the respective solvent molecules. This effect induces a reinforcement of the push–pull system and finally a bathochromic shift is caused. The correlation coefficient b of O2 obtained from the LSE correlation analyses also illustrates a bathochromic shift, but it is less pronounced. Furthermore, the correlation of O2 shows that the absolute e value has a lower influence than the coefficient a. This result demonstrates that the UV/Vis emission spectrum is weakly dependent on the change in the dipolarity of the chromophore environments rather than on the capacity of the solvent to act as a hydrogen-bond donor. Surprisingly, O2 shows no influence with regard to the polarizability (coefficient d) of the solvent. Fig. 7 exemplarily shows the linear relationship between the measured and calculated wavenumbers for the solvent dependent UV/Vis emission band of acetophenone O2.
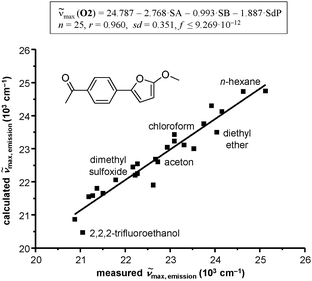 | ||
| Fig. 7 Linear solvation energy relationship for the fluorescence of O2 in 25 various solvents. Plots of measured vs. calculated emission maxima, according to the Catalán equation are shown. | ||
In comparison to the methoxyfuran-functionalized acetophenone, the result of multiple linear regression analysis of a CN analogue O3 shows that its SA sensitivity is of highest significance as shown by the equation in Fig. 8. Therefore, the UV/Vis emission maxima of O3 were described using the single-parameter equation (Table 2, entry 4a) and show an excellent correlation.
 | ||
Fig. 8 Linear solvation energy relationship between calculated and measured fluorescence ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) max,em for O3 in 12 solvents with hydrogen-bond donating ability. max,em for O3 in 12 solvents with hydrogen-bond donating ability. | ||
Fig. 8 displays the plot of the calculated UV/Vis maxima for the emission as a function of the corresponding experimental values for only classic HBD-solvents.
Conclusions
In this work, four furanyl- or thiophenyl-functionalized acetophenones have been produced by means of palladium-catalysed cross-coupling reactions such as Heck or Suzuki coupling. Optical properties (UV/Vis absorption and emission) were been studied both in solution and in the solid-state. The represented results show that the UV/Vis absorption spectra of the substituted acetophenones O1, O2, O3, and S1 do not noticeably shift as the solvent is varied. In contrast, the emission spectra exhibit a strong solvent-dependence.Due to the dependence of the emission maxima on the nature of the solvent, the fluorosolvatochromic behaviour of the furanyl- or thiophenyl-substituted acetophenones was studied in solvents of different acidity, basicity and polarity. The results of LSER correlations using the Catalán parameter set suggest that the influence of solvent dipolarity/polarizability on the long wavelength emission maximum for O1 and S1 is predominant. Furthermore, the influence on the fluorosolvatochromic behaviour of O2 is reflected by negative signs of a, b and e coefficients, whereby the strongest is exerted by the acidity of the solvent. A single-parameter equation according to Catalán was established for compound O3. Accordingly, the fluorosolvatochromic dye O3 can be used as a HBD/EPA-sensitive probe for several environments (solvent and solid-state).
Acetophenones O2, O3, and S1 fluoresce in solution as well as in the solid-state. However, the solid-state fluorescence of O1 is not observed.
Experimental section
Materials
Organic solvents were dried according to standard methods in an argon atmosphere and freshly distilled prior to use. All commercial reagents were used without further purification. They were purchased from the following suppliers: Acros: 4-bromoacetophenone (98%), 2-cyanofuran (99%), thiophene (>99%), potassium acetate (97%), and p-toluenesulfonic acid (99%); Alfa Aesar: 2-methoxyfuran (97%); ChemPur: tetrakis-(triphenylphosphine) palladium(0) (99%); Fluka: palladium(II) acetate (47% Pd); Fluorochem: 4-acetylphenylboronic acid (98%) and furan-2-boronic acid (98%); Merck: N-bromo-succinimide (99%).Instrumentation
The melting points were measured on a hot-stage microscope using a digital temperature measuring system from Wagner & Munz and are uncorrected. Differential scanning calorimetry (DSC) measurements were accomplished using a Mettler Toledo, type DSC 30. Elemental analyses were determined using a Vario-EL analyser. Attenuated total reflection infrared (ATR IR) spectra were recorded using a Golden Gate ATR accessory on a Perkin-Elmer Fourier transform 1000 spectrometer at room temperature in the wavenumber range 400 to 4000 cm−1. 1H NMR (250 MHz) and 13C NMR (69.9 MHz) spectroscopy were carried out on a Bruker Avance 250 NMR spectrometer. The residue signals of the solvents were used as internal standards. High-resolution mass spectra were recorded on a Bruker Daltonik microTOF-QII spectrometer. UV/Vis absorption spectra were obtained on a MCS 400 diode array UV/Vis spectrometer from Carl Zeiss, Jena, connected via glass-fibre optics. Fluorescence spectra were recorded on a Fluoromax®-4 Horiba Jobin Yvon spectrometer. Excitation and emission slits were set to 5 nm. The excitation wavelength was varied between 310 nm (O1, O3, and S1) and 350 nm (O2). Correlation analysis: multiple regression analysis was performed using the Origin Pro 9.0G statistics program. The emission decay curves were obtained via time-correlated-single-photon-counting. After excitation with a frequency-doubled Ti-sapphire laser adjusted to 700 nm (Tsunami, Newport Spectra-Physics GmbH, pulse-to-pulse repetition rate 800 kHz after passing a pulse selector, model 3980, Newport Spectra-Physics GmbH), i.e. at λexc = 350 nm, the luminescence of the sample was collected in a 90°-geometry and detected using a Becker & Hickl PMC-100-4 photon-counting module. Two long-pass filters (355 nm and 395 nm) are inserted into the detection beam path. The instrumental response function (IRF) is measured using a scattering sample (P25 nanoparticles in water). The measured curves are fitted mono- or bi-exponentially including the convolution with the IRF trace using a home-written algorithm in Scilab.20Methods
All geometries have been optimized using density functional theory with the B3-LYP functional15 in combination with the def2-TZVPP basis set.16 The geometries have been converged to a residual gradient norm of 10−3 a.u. or better. The energy was converged to at least 10−6 a.u., and fine quadrature grids have been used to calculate the exchange and correlation potential (size 5).21 All minima have been characterized by normal-mode analysis. All calculations have been performed using the TURBOMOLE program package (for a current version, see http://www.turbomole.de).22Syntheses
Mp: 96–98 °C. DSC (25–350 °C, 10 K min−1, N2): 100 °C (endo), 230 °C (endo). Found: C, 77.2; H, 5.4. Calc. for C12H10O2: C, 77.4; H, 5.4%. νmax/cm−1 3105 (ArCH), 1666 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1602 (C
O), 1602 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1560 (C
C), 1560 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C; furan), 1263 (def vib; 1,4-disubstituted benzene), 839 (def vib; 1,4-disubstituted benzene). δH (250 MHz; CDCl3; Me4Si) 2.61 (3 H, s, Me), 6.52 (1 H, dd, J 3.4 Hz, J 1.7 Hz, fuH), 6.81 (1 H, d, J 3.4 Hz, fuH), 7.54 (1 H, d, J 1.7 Hz, fuH), 7.75 (2 H, d, J 8.4 Hz, ArH), 7.98 (2 H, d, J 8.4 Hz, ArH). δC (62.9 MHz, CDCl3, Me4Si) 26.7, 107.6, 112.3, 123.7, 129.1, 135.0, 135.7, 143.4, 153.0, 197.6.
C; furan), 1263 (def vib; 1,4-disubstituted benzene), 839 (def vib; 1,4-disubstituted benzene). δH (250 MHz; CDCl3; Me4Si) 2.61 (3 H, s, Me), 6.52 (1 H, dd, J 3.4 Hz, J 1.7 Hz, fuH), 6.81 (1 H, d, J 3.4 Hz, fuH), 7.54 (1 H, d, J 1.7 Hz, fuH), 7.75 (2 H, d, J 8.4 Hz, ArH), 7.98 (2 H, d, J 8.4 Hz, ArH). δC (62.9 MHz, CDCl3, Me4Si) 26.7, 107.6, 112.3, 123.7, 129.1, 135.0, 135.7, 143.4, 153.0, 197.6.
Mp: 88–92 °C. DSC (25–350 °C, 10 K min−1, N2): 92 °C (endo), 181 °C (exo). νmax/cm−1 3004 (ArCH), 2838 (−OCH3), 1667 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1569 (C
O), 1569 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1551 (C
C), 1551 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C; furan), 1250 (def vib; 1,4-disubstituted benzene), 959 (CH def vib; 2,5-disubstituted furan), 816 (def vib; 1,4-disubstituted benzene). δH (250 MHz; CDCl3; Me4Si) 2.59 (3 H, s, Me), 3.93 (3 H, s, Me), 5.31 (1 H, d, J 3.5 Hz, fuH), 6.71 (1 H, d, J 3.5 Hz, fuH), 7.60 (2 H, d, J 8.8 Hz, ArH), 7.94 (2 H, d, J 8.8 Hz, ArH). δC (62.9 MHz, CDCl3, Me4Si) 26.5, 57.9, 82.5, 109.5, 122.0, 129.0, 134.5, 134.9, 143.0, 162.4, 197.3. HRMS (ESI-TOF): calc. for C13H13O3: m/z 217.0859, found: m/z 217.0864.
C; furan), 1250 (def vib; 1,4-disubstituted benzene), 959 (CH def vib; 2,5-disubstituted furan), 816 (def vib; 1,4-disubstituted benzene). δH (250 MHz; CDCl3; Me4Si) 2.59 (3 H, s, Me), 3.93 (3 H, s, Me), 5.31 (1 H, d, J 3.5 Hz, fuH), 6.71 (1 H, d, J 3.5 Hz, fuH), 7.60 (2 H, d, J 8.8 Hz, ArH), 7.94 (2 H, d, J 8.8 Hz, ArH). δC (62.9 MHz, CDCl3, Me4Si) 26.5, 57.9, 82.5, 109.5, 122.0, 129.0, 134.5, 134.9, 143.0, 162.4, 197.3. HRMS (ESI-TOF): calc. for C13H13O3: m/z 217.0859, found: m/z 217.0864.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1598 (C
O), 1598 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1559 (C
C), 1559 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C; thiophene), 1258 (def vib; 1,4-disubstituted benzene), 822 (def vib; 1,4-disubstituted benzene), 714 (def vib; monosubstituted thiophene). δH (250 MHz; CDCl3; Me4Si) 2.61 (3 H, s, Me), 7.12 (1 H, dd, J 4.9 Hz, J 3.0 Hz, thH), 7.36 (1 H, d, J 4.9 Hz, thH), 7.43 (1 H, d, J 3.0 Hz, thH), 7.69 (2 H, d, J 8.4 Hz, ArH), 7.97 (2 H, d, J 8.4 Hz, ArH). δC (62.9 MHz, CDCl3, Me4Si) 26.7, 124.7, 125.8, 126.6, 128.5, 129.3, 135.9, 138.9, 143.1, 197.5. HRMS (ESI-TOF): calc. for C12H11OS: m/z 203.0525, found: m/z 203.0532 [M + H]+.
C; thiophene), 1258 (def vib; 1,4-disubstituted benzene), 822 (def vib; 1,4-disubstituted benzene), 714 (def vib; monosubstituted thiophene). δH (250 MHz; CDCl3; Me4Si) 2.61 (3 H, s, Me), 7.12 (1 H, dd, J 4.9 Hz, J 3.0 Hz, thH), 7.36 (1 H, d, J 4.9 Hz, thH), 7.43 (1 H, d, J 3.0 Hz, thH), 7.69 (2 H, d, J 8.4 Hz, ArH), 7.97 (2 H, d, J 8.4 Hz, ArH). δC (62.9 MHz, CDCl3, Me4Si) 26.7, 124.7, 125.8, 126.6, 128.5, 129.3, 135.9, 138.9, 143.1, 197.5. HRMS (ESI-TOF): calc. for C12H11OS: m/z 203.0525, found: m/z 203.0532 [M + H]+.
Mp: 94–108 °C. DSC (25–350 °C, 10 K min−1, N2): 104 °C (endo), 230 °C (endo). νmax/cm−1 3135 (ArCH), 2226 (C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N), 1677 (C
N), 1677 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1609 (C
O), 1609 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1562 (C
C), 1562 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C; furan), 1268 (def vib; 1,4-disubstituted benzene), 961 (CH def vib; 2,5-disubstituted furan), 814 (def vib; 1,4-disubstituted benzene). δH (250 MHz; CDCl3; Me4Si) 2.64 (3 H, s, Me), 6.87 (1 H, d, J 3.6 Hz, fuH), 7.21 (1 H, d, J 3.6 Hz, fuH), 7.81 (2 H, d, J 8.2 Hz, ArH), 8.03 (2 H, d, J 8.2 Hz, ArH). δC (62.9 MHz, CDCl3, Me4Si) 26.8, 108.1, 111.7, 124.1, 124.9, 126.2, 129.3, 132.6, 137.4, 157.3, 197.7. HRMS (ESI-TOF): calc. for C13H10NO2: m/z 212.0706, found: m/z 212.0714 [M + H]+.
C; furan), 1268 (def vib; 1,4-disubstituted benzene), 961 (CH def vib; 2,5-disubstituted furan), 814 (def vib; 1,4-disubstituted benzene). δH (250 MHz; CDCl3; Me4Si) 2.64 (3 H, s, Me), 6.87 (1 H, d, J 3.6 Hz, fuH), 7.21 (1 H, d, J 3.6 Hz, fuH), 7.81 (2 H, d, J 8.2 Hz, ArH), 8.03 (2 H, d, J 8.2 Hz, ArH). δC (62.9 MHz, CDCl3, Me4Si) 26.8, 108.1, 111.7, 124.1, 124.9, 126.2, 129.3, 132.6, 137.4, 157.3, 197.7. HRMS (ESI-TOF): calc. for C13H10NO2: m/z 212.0706, found: m/z 212.0714 [M + H]+.
Acknowledgements
Financial support from the Fonds der Chemischen Industrie is gratefully acknowledged. We also thank K. Müller for elemental analyses and Dr. R. Buschbeck and B. Kempe (TU Chemnitz, Prof. Dr. H. Lang group) for mass-spectrometry measurements.Notes and references
- (a) A. Kraft, A. C. Grimsdale and A. B. Holmes, Angew. Chem., Int. Ed., 1998, 37, 402–428 CrossRef; (b) U. Mitschke and P. Bäuerle, J. Mater. Chem., 2000, 10, 1471–1507 RSC; (c) C. J. Tonzola, M. M. Alam, W. Kaminsky and S. A. Jenekhe, J. Am. Chem. Soc., 2003, 125, 13548–13558 CrossRef CAS PubMed; (d) H.-C. Yeh, L.-H. Chan, W. Wu and C.-T. Chen, J. Mater. Chem., 2004, 14, 1293–1298 RSC; (e) C.-T. Chen, Chem. Mater., 2004, 16, 4389–4400 CrossRef CAS; (f) C.-L. Chiang, M.-F. Wu, D.-C. Dai, Y.-S. Wen, J.-K. Wang and C.-T. Chen, Adv. Funct. Mater., 2005, 15, 231–238 CrossRef CAS PubMed; (g) T. M. Figueira-Duarte and K. Müllen, Chem. Rev., 2011, 111, 7260–7314 CrossRef CAS PubMed.
- (a) K. Yoshida, Y. Ooyama, S. Tanikawa and S. Watanabe, J. Chem. Soc., Perkin Trans. 2, 2002, 708–714 RSC; (b) Z. M. Hudson and S. Wang, Acc. Chem. Res., 2009, 42, 1584–1596 CrossRef CAS PubMed; (c) Z. Chi, X. Zhang, B. Xu, X. Zhou, C. Ma, Y. Zhang, S. Liu and J. Xu, Chem. Soc. Rev., 2012, 41, 3878–3896 RSC; (d) L. K. Calderón Ortiz, H. Würfel, E. Täuscher, D. Weiß, E. Birckner, H. Görls and R. Beckert, Synthesis, 2014, 126–134 Search PubMed.
- (a) H. Mizuno, U. Haku, Y. Marutani, A. Ishizumi, H. Yanagi, F. Sasaki and S. Hotta, Adv. Mater., 2012, 24, 5744–5749 CrossRef CAS PubMed; (b) O. Mhibik, T. Leang, A. Siove, S. Forget and S. Chénais, Appl. Phys. Lett., 2013, 102, 41112 CrossRef PubMed.
- (a) N. Boens, W. Qin, N. Basaric, A. Orte, E. M. Talavera and J. M. Alvarez-Pe, J. Phys. Chem. A, 2006, 110, 9334–9343 CrossRef CAS PubMed; (b) Q. A. Best, N. Sattenapally, D. J. Dyer, C. N. Scott and M. E. McCarroll, J. Am. Chem. Soc., 2013, 135, 13365–13370 CrossRef CAS PubMed; (c) N. Marcotte and A. M. Brouwer, J. Phys. Chem. B, 2005, 109, 11819–11828 CrossRef CAS PubMed.
- (a) F. Riedel and S. Spange, J. Phys. Org. Chem., 2012, 25, 1261–1268 CrossRef CAS PubMed; (b) K. Hofmann, S. Brumm, C. Mende, K. Nagel, A. Seifert, I. Roth, D. Schaarschmidt, H. Lang and S. Spange, New J. Chem., 2012, 36, 1655–1664 RSC; (c) A. Schade, N. Behme and S. Spange, Chem. – Eur. J., 2014, 20, 2232–2243 CrossRef CAS PubMed.
- (a) C. Reichardt, Solvents and Solvent Effects in Organic Chemistry, VCH, Weinheim, 2nd edn, 1988, and references therein Search PubMed; (b) C. Reichardt, Chem. Rev., 1994, 94, 2319–2358 CrossRef CAS; (c) P. Müller, Pure Appl. Chem., 1994, 66, 1077–1079 CrossRef; (d) Y. Marcus, Chem. Soc. Rev., 1993, 22, 409–416 RSC; (e) V. Gutmann, Coord. Chem. Rev., 1976, 18, 225–240 CrossRef CAS; (f) V. Gutmann, The Donor-Acceptor Approach to Molecular Interactions, Plenum Press, New York, 1978 Search PubMed.
- M. J. Kamlet, J. L. M. Abboud, M. H. Abraham and R. W. Taft, J. Org. Chem., 1983, 48, 2877–2887 CrossRef CAS.
- (a) J. Catalán and C. Díaz, Liebigs Ann., 1997, 1941–1949 CrossRef PubMed; (b) J. Catalán and C. Díaz, Eur. J. Org. Chem., 1999, 885–891 CrossRef; (c) J. Catalán, C. Díaz, V. López, P. Pérez, J.-L. G. de Paz and J.-G. Rodríguez, Liebigs Ann., 1996, 1785–1794 CrossRef PubMed; (d) J. Catalán and H. Hopf, Chem. – Eur. J., 2004, 4694–4702 CrossRef PubMed; J. Catalán, J. Phys. Chem. B, 2009, 113, 5951–5960 Search PubMed; (e) J. Catalán, J. Phys. Org. Chem., 2015, 28, 329–336 CrossRef PubMed.
- (a) V. Cavalli, D. C. da Silva, C. Machado, V. G. Machado and V. Soldi, J. Fluoresc., 2006, 16, 77–86 CrossRef CAS PubMed; (b) M. El-Sayed, T. Blaudeck, F. Cichos and S. Spange, J. Photochem. Photobiol., A, 2007, 185, 44–50 CrossRef CAS PubMed; (c) H. Akdas-Kilig, T. Roisnel, I. Ledoux and H. Le Bozec, New J. Chem., 2009, 33, 1470–1473 RSC; (d) I. Baraldi, E. Benassi, S. Ciorba, M. Šindler-Kulyk, I. Škorić and A. Spalletti, Chem. Phys., 2009, 361, 61–67 CrossRef CAS PubMed; (e) D. Jana and B. K. Ghorai, Tetrahedron, 2012, 68, 7309–7316 CrossRef CAS PubMed; (f) Z. Seferoğlu, H. Ihmels and E. Şahin, Dyes Pigm., 2015, 113, 465–473 CrossRef PubMed.
- L. Yin and J. Liebscher, Chem. Rev., 2007, 107, 133–173 CrossRef CAS PubMed.
- O. Reiser, Chem. Unserer Zeit, 2001, 2, 94–100 CrossRef.
- N. Tyutyulkov, J. Fabian, A. Mehlhorn, F. Dietz and A. Tadjer, Polymethine Dyes – Structure and Properties, St. Kliment Ohridski University Press, Sofia, 1991 Search PubMed.
- C. Hansch, A. Leo and R. W. Taft, Chem. Rev., 1991, 91, 165–195 CrossRef CAS.
- J. Roger, F. Požgan and H. Doucet, Green Chem., 2009, 11, 425–432 RSC.
- (a) A. Köhn and C. Hättig, J. Chem. Phys., 2003, 119, 5021–5036 CrossRef PubMed; (b) C. Hättig, J. Chem. Phys., 2003, 118, 7751–7761 CrossRef PubMed; (c) C. Hättig and F. Weigand, J. Chem. Phys., 2000, 113, 5154–5161 CrossRef PubMed.
- F. Weigend and R. Ahlrichs, Phys. Chem. Chem. Phys., 2005, 7, 3297–3305 RSC.
- F. Riedel, A. Oehlke and S. Spange, Z. Anorg. Allg. Chem., 2009, 635, 1335–1340 CrossRef CAS PubMed.
- J. Dobkowski, Z. R. Grabowski, J. Waluk, W. Kühnle, W. Rettig, C. Rullière, W. Yang, J. Adamus and J. Gebicki, Proc. Indiana Acad. Sci., 1992, 104, 143–152 CAS.
- A. Filarowski, M. Kluba, K. Cieślik-Boczula, A. Koll, A. Kochel, L. Pandey, W. M. De Borggraeve, M. Van der Auweraer, J. Catalán and N. Boens, Photochem. Photobiol. Sci., 2010, 9, 996–1008 CAS.
- Scilab Enterprises, Scilab: Free and Open Source software for numerical computation (OS, Version 5.4.1) [Software], 2012, Available from: http://www.scilab.org.
- R. Ahlrichs, M. Bär, M. Häser, H. Horn and C. Kölmel, Chem. Phys. Lett., 1989, 162, 165–169 CrossRef CAS.
- O. Treutler and R. Ahlrichs, J. Chem. Phys., 1995, 102, 346–354 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: DFT theoretical calculations, Catalán parameter set, UV/Vis absorption and emission maxima of all compounds, and linear solvation energy relationships. See DOI: 10.1039/c5nj00256g |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2015 |