A novel calix[4]arene-based dimeric-cholesteryl derivative: synthesis, gelation and unusual properties†
Ying
Wu
,
Kaiqiang
Liu
,
Xiangli
Chen
,
Yongping
Chen
,
Shaofei
Zhang
,
Junxia
Peng
and
Yu
Fang
*
Key Laboratory of Applied Surface and Colloid Chemistry, Ministry of Education, School of Chemistry and Chemical Engineering, Shaanxi Normal University, Xi'an 710062, P. R. China. E-mail: yfang@snnu.edu.cn; Fax: +86-29-815310787; Tel: +86-29-81530788
First published on 3rd November 2014
Abstract
A calix[4]arene-based dimeric-cholesteryl derivative with naphthalene in the linkers (C2N2C) was designed and synthesized. The gelation behaviors of the compound in 36 liquids were evaluated. It was demonstrated that C2N2C could gel 16 of the liquids tested, which include both polar and apolar liquids. SEM and AFM studies revealed that the morphologies of the gel networks are dependent on the concentrations of C2N2C and the nature of the liquids under study. Importantly, rheological studies revealed that the gel of the compound in benzene possesses sensitive, fast and fully reversible thixotropic property. More importantly, the Tgel of the C2N2C/benzene gel could be at least more than 60 degrees higher than the boiling point of benzene when the gelator concentration is greater than 6% (w/v), a result never reported before. CD measurements revealed the chiral nature of the assemblies of the gel networks. Further investigation by AFM measurements confirmed the right-hand helical structures of the gel networks of C2N2C/benzene gel. As anticipated, hydrogen bonding and π–π stacking are the two main driving forces for the formation of the gels.
1. Introduction
As a typical class of soft materials, molecular/supramolecular gels based on low molecular mass gelators (LMMGs) have experienced a rapid development during the last few decades.1–3 The efforts expended have been focused on the development of new types of LMMGs not only for probing the structure–property relationships of the gelators and the relevant gels, but also for exploiting their potential applications in various technological areas such as oil recovery, controlled release, tissue engineering, cultivation of crystals, template preparation of micro-/nano-materials, mild separation, etc.4–10 It is known that LMMGs self-assemble into one-dimensional assemblies (fibers, tapes, rods, etc.) as a first step in their gelation process,11,12 then leading to three-dimensional networks through non-covalent intermolecular interactions that can be any (or a combination of some) of hydrogen bonding, π–π interaction, van der Waals interaction, electrostatic interaction, coordination interaction, host–guest interaction, etc. in suitable liquids.13,14 It is the weakness of the interactions that makes the formation and de-formation of the molecular gels controllable by various external stimuli such as heating–cooling, pH, light, sound, host–guest interaction, chemicals, redox, magnetic field, shear force, etc.15–22During the past few decades, a great deal of research about LMMGs has been done and it is revealed that a wide variety of chemicals can be employed as LMMGs, which include alkanes, sugars, fatty acids, polycyclic aromatics, cholesterol, amino acids, and their relevant derivatives.1,23–27 However, research studies on macrocyclic compounds and their derivative-based LMMGs are relatively few.28–31 It is to be noted that this kind of compounds have been widely studied in host–guest chemistry for their recognition and inclusion characteristics,32 and the gels with them as LMMGs reported till now show thixotropic and some other unusual properties.33,34
Calixarenes are named as the third generation of supramolecular host compounds and have been extensively used as basic scaffolds for the preparation of functionalized host molecules due to their easiness in synthesis and modification at both the upper and lower rims and their conformational adaptability, as well as the hydrophobicity of their cavities.35,36 Recently, calixarenes have also gained significant attention for developing them into LMMGs with superior performances, the main concerns of which are their multiple binding sites and conformational adaptability which are crucial for promoting the formation of 3D networks, a necessity for gelation. Moreover, the cavities of calixarenes may be functioning as pockets to ‘hold’ solvent molecules, which is favorable for formation of gels. All these guarantee calixarenes and their derivatives as a group of remarkable molecules in the design of cavity-containing LMMGs.
To the best of our knowledge, the first finding on LMMGs based on calixarenes comes from Shinkai and co-workers.37 In the report, the authors discovered that calix[n]arenes (n = 4, 6, 8) having aliphatic chains at the lower rims could act as excellent gelators of some apolar organic liquids (e.g., toluene, carbon tetrachloride, hexane, etc.), and as expected the sol–gel phase transition of the gels is thermal reversible. About ten years later, Xu and co-workers built a coordination-induced organogel based on 3-pyridine-azo-calix[4]arenes and [Pd(en)(H2O)2]2+.38 The gel displayed a great stability in the aqueous phase over a wide pH range (1–13) even at 100 °C. The unique stability of the gel allowed it to efficiently “uptake” neutral organic molecules at low concentrations from the aqueous phases. Zheng and co-workers reported that chiral calix[4]arenes bearing long tertiary alkyl groups at the upper rim and S-1-phenylethylamine groups at the lower rim can form a heat-set gel enantio-selectively with D-2,3-dibenzoyltartaric acid in cyclohexane.39 It was revealed that the gel contains egg-like vesicle internal structures, and the size of the vesicles could be tuned by changing the length of the alkyl chains. The authors also found for the first time that chiral calix[4]arenes bearing L-2,3-dibenzoyltartaric acid groups at the lower rim could form gel only with one enantiomer of chiral amines, and showed excellent chiral recognition.40 Ogden and co-workers are the first to report a hydrogelator, a proline-functionalised calix[4]arene, the gel formation of which is critically dependent on the presence of specific anions, with efficacy linked to the Hofmeister series. Furthermore, variation of the associated cations can be also used to tune the properties of the gel but the effect is not as significant as changes in the anions.41 Very recently, Park and co-workers prepared, in the presence of K+ or Rb+, some supramolecular gels with calix[4]arene tetra-acetate as the LMMGs. The Rb gel showed higher mechanical stability than the one containing K+. Intermolecular hydrogen bonding is the main driving force for the formation of the K gel, while intermolecular hydrogen bonding and coordination bonds mainly reinforced the stability of the Rb gel.42 Chung and co-workers reported that a bis-calix[4]arene without long alkyl chains could form organogels in various alcoholic liquids.43 Furthermore, the compound exhibited an excellent phase selective gelation property, a property potentially useful in oil spill recovery. They also reported a bis-calix[4]arene which assembled into nano-particles and micro-spheres in CH3CN, and eventually formed a transparent blue-light emitting molecular organogel.44 More recently, our group synthesized a calix[4]arene-based dimeric-cholesteryl derivative, and found that it could not gel any of the pure liquids tested.45 However, it could efficiently gel a mixture of n-decane and acetonitrile. Interestingly, the gel exhibits super-smart and fully reversible thixotropic property. We also prepared another two cholesteryl derivatives of calix[4]arene with the L- or D-phenylalanine residue in the linkers, and found that the compounds could gel some pure organic liquids and act as good gelators.46
The related studies have shown that aromatic groups may play a crucial role in the formation of 3D networks that are important for gelation, which can finely adjust the arrangement of self-assembled supramolecular structures, and thereby alter the properties of the materials. Naphthalene as a kind of polycyclic aromatic hydrocarbons has been widely adopted as an important building block for designing and creating molecular materials due to its π–π stacking capability.47
As a continuation of our efforts on the studies of LMMGs with calix[4]arene and cholesterol as the main building blocks, a naphthyl structure was employed and embedded in the linkers connecting a calix[4]arene unit and a cholesteryl structure in order to alter the self-assembly behavior of the calix[4]arene derivative of cholesterol. As expected, gelation tests demonstrated that the compound (C2N2C) as created could gel 16 of the 36 liquids tested, and is a remarkable organogelator. Rheological studies demonstrated that its benzene gel possesses sensitive, fast and fully reversible thixotropic property. This paper reports the details.
2. Experimental section
2.1. Reagents and materials
4-tert-Butylphenol (>98.0%), cholesteryl chloroformate (98%), and thionyl chloride (99%) are products of Tokyo Chemical Industry Co., Ltd, Alfa Aesar, and Aladdin Chemistry Co., Ltd, respectively. Ethyl chloroacetate (99%), 1,5-diaminonaphthalene (97%) and diphenyl ether (99%) were purchased from J&K Technology Co., Ltd. The reagents were used directly without further purification. All organic liquids were of analytical grade and used as received or dried to eliminate any water residue before the experiment.2.2. Synthesis of C2N2C
The synthetic route towards C2N2C is schematically shown in Scheme 1. The details of the synthesis and characterization are described below.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, –O), 1628 (C
O, –O), 1628 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, –NH), 1534 (NH, bending vibration), and 1233 (–C–O). MS: m/z calcd for [(M + H)+]: 571.4258, found: 571.4257.
O, –NH), 1534 (NH, bending vibration), and 1233 (–C–O). MS: m/z calcd for [(M + H)+]: 571.4258, found: 571.4257.
Compound 5 (0.8907 g, 1.56 mmol) and purified triethylamine (1 mL) were both dissolved in 20 mL of purified toluene, and then to this mixture 20 mL of purified toluene containing all the obtained residues (compound 4) was added dropwise under stirring in a nitrogen atmosphere. The resulting mixture was stirred and refluxed for 12 h. Thin layer chromatography (TLC) was adopted to monitor the reaction process. After the reaction, the solvent was evaporated under reduced pressure, and then the resulting crude product was dried in vacuum. The product as obtained was further purified by column chromatography (silicone gel, 200–300 mesh; CH2Cl2–acetone, v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v = 40
v = 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and finally the desired product was obtained as a white powder with a yield of 45%. For 6: m.p.: 205.2–205.9 °C. 1H NMR (CDCl3/Me4Si, 400 MHz) δ (ppm): 0.666–2.522 (m, 122H, –C(CH3)3, cholesteryl protons), 3.50 (d, J = 13.6 Hz, 4H, –ArCH2Ar–), 4.31 (d, J = 13.6 Hz, 4H, –ArCH2Ar–), 4.60 (s, 4H, ArOCH2), 4.68 (dd, J = 16.0 Hz, 11.2 Hz, 2H, oxcyclohexyl), 5.45 (s, 2H, alkenyl), 6.47 (s, 2H, –OH), 6.701–6.901 (m, 6H, –ArH, naphthyl protons), 7.061–7.237 (m, 8H, –ArH, naphthyl protons), 7.41 (d, J = 8.4 Hz, 2H, naphthyl protons), 7.58 (d, J = 7.2 Hz, 2H, naphthyl protons), 7.76 (s, 2H, –NHCOO–), 7.83 (d, J = 8.0 Hz, 2.0 Hz, naphthyl protons), 9.47 (s, 2H, –NHCO–). FTIR, νmax/cm−1: 3408 (OH, NH), 2953 (CH3), 2903 (CH2), 2868 (CH), 1706 (C
1) and finally the desired product was obtained as a white powder with a yield of 45%. For 6: m.p.: 205.2–205.9 °C. 1H NMR (CDCl3/Me4Si, 400 MHz) δ (ppm): 0.666–2.522 (m, 122H, –C(CH3)3, cholesteryl protons), 3.50 (d, J = 13.6 Hz, 4H, –ArCH2Ar–), 4.31 (d, J = 13.6 Hz, 4H, –ArCH2Ar–), 4.60 (s, 4H, ArOCH2), 4.68 (dd, J = 16.0 Hz, 11.2 Hz, 2H, oxcyclohexyl), 5.45 (s, 2H, alkenyl), 6.47 (s, 2H, –OH), 6.701–6.901 (m, 6H, –ArH, naphthyl protons), 7.061–7.237 (m, 8H, –ArH, naphthyl protons), 7.41 (d, J = 8.4 Hz, 2H, naphthyl protons), 7.58 (d, J = 7.2 Hz, 2H, naphthyl protons), 7.76 (s, 2H, –NHCOO–), 7.83 (d, J = 8.0 Hz, 2.0 Hz, naphthyl protons), 9.47 (s, 2H, –NHCO–). FTIR, νmax/cm−1: 3408 (OH, NH), 2953 (CH3), 2903 (CH2), 2868 (CH), 1706 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, –O), 1601 (C
O, –O), 1601 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, –NH), 1485 (NH, bending vibration), and 1206 (–C–O). MS: m/z calcd for [(M + H + Na)+]: 1893.2424, found: 1893.2354.
O, –NH), 1485 (NH, bending vibration), and 1206 (–C–O). MS: m/z calcd for [(M + H + Na)+]: 1893.2424, found: 1893.2354.
2.3. Gelation tests
A known weight (0.025 g) of C2N2C and a measured volume (1 mL) of a chosen pure liquid were placed into a sealed test tube, followed by shaking for a while and then the system was placed at room temperature for one day. Then the test tube was inversed to observe if a gel had formed. Gels acquired under this condition were denoted as “G*”, and when transparent gels formed they were referred to as “TG*”. The systems in which only homogeneous solution was left were named as “S”. The remaining test tubes were heated until the solid was dissolved completely, and then the system was cooled to room temperature in air and finally the test tube was inversed to examine the state of the sample inside. A system in which a turbid gel formed was denoted as “G”. When a transparent gel formed, it was referred to as “TG”. A system in which gel and solution coexisted was denoted as “PG”. A system in which the gelator was substantially insoluble even at the boiling point of a pure liquid was denoted as “I”. In some cases, clear solutions were obtained during heating but precipitation occurred during cooling, and this kind of system was labeled “P”. Systems where viscous solutions remained after cooling were denoted as “VS”.2.4. T gel of gel systems
The temperatures of sol–gel or gel–sol transitions were obtained by employing a conventional falling ball method. The gel of 1 mL was prepared in a sample bottle of 5 mL. A small glass ball with a smooth and regular shape (diameter ∼ 3 mm) was gently put onto the center top of the gel. The sample bottle was slowly heated (0.5 °C min−1) in a thermo-stated oil bath. The temperatures of a glass ball beginning to fall and a ball just sinking to the bottom of the sample bottle were recorded, respectively. The average value of the two temperatures was acquired as the Tgel of the gel under test. The thermo-stability of a specific gel (C2N2C/benzene, 6%, w/v) was also determined by employing a DSC technique. The instrument used was NETZSCH DSC204HP (GER), and the test was conducted in a closed cell under a nitrogen atmosphere with a pressure of 1 MPa.2.5. Morphological studies
2.6. Rheological tests
The rheological properties of the gel were measured by employing a stress-controlled rheometer (TA Instrument, AR-G2) equipped with a steel-coated parallel-plate geometry (20 mm diameter). The gap distance was fixed at 1000 mm. A solvent-trapping device was placed above the plate, and the temperature of all measurements was controlled at 15 °C to minimize evaporation of the solvent. All samples were placed on the sample holder for 30 min to form a stable gel before each measurement.Stress sweep: for rheological measurements, the first step was to determine the linear visco-elastic range of the gel sample through the stress sweep. The storage modulus G′ and the loss modulus G′′ of the gel sample were monitored as functions of the shear stress at a fixed frequency, which were used to display the mechanical strength of the gel tested.
Frequency sweep: the frequency sweep was conducted from 0.1 to 628.0 rad s−1 at a constant shear stress of 200 Pa, which is well within the linear visco-elastic region of traces from shear stress sweep. The storage modulus G′ and the loss modulus G′′ of the gel sample were recorded as functions of the angle frequency, which were used to evaluate the tolerance of the gel to external forces.
Time sweep: the time sweep was conducted to examine the thixotropic behavior of a supramolecular gel. The test procedure consisted of the following three steps: (1) initial state: the gel was subjected to a very small shear stress of 10 Pa well within the linear region of the stress sweep trace of the gel sample for a short time (2 min). It is believed that the gel is strong enough to bear this small force; (2) deformation process: a high shear force of 1500 Pa, which is far beyond the linear range of the gel system, was exerted to destroy the gel for 2 min; (3) recovery process: the high shear force was removed and a very small monitoring shear stress of 10 Pa was exerted on the destroyed gel. The storage modulus G′ and the loss modulus G′′ of the system were monitored as functions of time.
2.7. FTIR measurements
The FT-IR spectra of the samples were recorded by using a Bruker VERTEX70 V infrared spectrometer in an attenuated total reflection (ATR) mode. The KBr wafer was obtained by mixing a small quantity of the sample and anhydrous KBr powder which was measured in the transparent mode.2.8. 1H NMR measurements
1H NMR data of samples were obtained by employing a Bruker AVANCE 400 MHz spectrometer (Me4Si as an internal standard). During characterization, the 1H NMR spectra of the compounds were collected in CDCl3 as a solvent. The temperature-dependent and concentration-dependent 1H NMR spectra of the gelator were measured in C6D6.2.9. UV-vis and CD measurements
The concentration-dependent or temperature-dependent UV-vis and CD spectra of the gel were measured by employing a Chirascan circular dichroism spectrometer. The gel sample was prepared by heating a mixture of the gelator and a solvent, then immediately transferring the hot solution to a quartz cell of 0.1 mm which was carefully sealed to avoid evaporation of the solvent, and then cooling to room temperature to form a stable gel. The temperature-dependent UV-vis and CD spectra were recorded from lower temperature to higher temperature. The heating rate was 5 °C min−1.2.10. X-ray diffraction (XRD) measurements
The XRD pattern of the xerogel sample was measured on a D/Max-2550/PC with Cu Kα X-ray radiation generated under a voltage of 40 kV and a current of 40 mA. The scan rate was 0.5° min−1. The scan range of 2θ was from 1.5° to 10°. The xerogel was prepared by freeze-drying the fresh gel.3. Results and discussion
3.1. Gelation behaviors
The results from the gelation test are collectively shown in Table 1. Reference to the table reveals that C2N2C gels 16 of the 36 organic liquids tested, showing much more efficient gelation ability than the calix[4]arene-based compounds reported in the literature.45,46 With further inspection of the results shown in the table, it is seen that the compound gels not only protic liquids such as n-pentanol and n-heptanol, but also aprotic liquids such as benzene, toluene, o-xylene, etc. However, the formation of these gels requires heating–cooling treatment. For the opaque gels, however, they formed spontaneously at room temperature. It is noteworthy that these gels not only exhibit thermal reversibility, but also a very smart thixotropic property. Whereas the gelator could gel DMF at room temperature, but the system was not homogeneous, and phase transition would occur a few days later. The same phenomenon was observed in kerosene, DMSO, and CCl4, and the difference between them is that these gels could only form via a heating–cooling cycle. Therefore, the temperatures of sol–gel phase transition (Tgel) and the critical gelation concentrations (CGCs) were not tested for these gels. These results clearly indicate that the nature of the solvent has a great effect on the formation and property of the gels.| Liquids | Result | T gel (°C) | Boiling point (°C) | Liquids | Result | T gel (°C) | Boiling point (°C) |
|---|---|---|---|---|---|---|---|
| a I: insoluble; S: soluble; VS: viscous solution; P: precipitation; G: turbid gel; G*: gelation at room temperature; PG: partial gel; the figures in parentheses are the CGCs of the corresponding gels (%). | |||||||
| Methanol | I | Ethylacetate | I | ||||
| Ethanol | I | Petroleumether | I | ||||
| 1-Propanol | I | Kerosene | G (—) | — | |||
| Isopropanol | I | Benzene | G (0.5) | 102 | 80.1 | ||
| 1-Butanol | VS | Toluene | G (0.33) | 117.5 | 110.6 | ||
| 1-Pentanol | G (0.075) | 127.5 | 137.3 | o-Xylene | G (0.5) | 113.5 | 144.4 |
| 1-Hexanol | G (0.1) | 159.5 | 157 | m-Xylene | G (0.25) | 129.5 | 139 |
| Cyclohexanol | G (0.2) | 121.8 | 160.84 | p-Xylene | G (0.2) | 137 | 138.5 |
| 1-Heptanol | G (0.1) | 158 | 175.8 | 2,6-Dimethylaniline | PG (—) | — | |
| 1-Octanol | G (0.1) | 156.5 | 196 | THF | S | ||
| 1-Nonanol | G (0.1) | 156.5 | 214 | DMF | G* (—) | — | |
| 1-Decanol | G (0.1) | 156.5 | 232.9 | DMSO | G (—) | — | |
| Cyclohexane | I | TEA | I | ||||
| 1-Hexane | I | Pyridine | S | ||||
| 1-Heptane | I | Ethyl ether | I | ||||
| 1-Nonane | I | CH2Cl2 | S | ||||
| 1-Decane | I | CHCl3 | S | ||||
| Acetonitrile | I | CCl4 | G (—) | — | |||
It is known that CGC is one of the two main parameters to judge the gelling ability of a compound,48 and thereby the CGCs of the compound in the liquids were determined and the results are also listed in Table 1. Reference to the table demonstrates that the CGCs of C2N2C in benzene, toluene, o-xylene, m-xylene and p-xylene are 0.5%, 0.33%, 0.5%, 0.25% and 0.2%, respectively, and in particular the CGCs of C2N2C in higher alcohols are around 0.1%, indicating that the compound is a super-gelator of the alcohols.
As a matter of fact, the formation process of the gel is a subtle balance between precipitation and dissolution of the gelator molecules in the relevant solvent. In a given solvent, the aggregation ability of the gelator molecules is too strong leading to phase separation of the system, so does the strong solubility affecting the stability of the 3D gel networks. Only when the two reach equilibrium, the formation of a gel could be possible. Compared with the gelation behaviors of a calix[4]arene-based dimeric-cholesteryl derivative with hydrazine or chiral phenylalanine as connecting arms,45,46 the gelation ability of a calix[4]arene-based dimeric-cholesteryl derivative with naphthalene in the linkers is much more efficient. Apparently, the introduction of a naphthyl unit not only strengthens the rigidity of the gelator molecules, but also boosts the intermolecular π–π stacking interactions. Thus, the gelator molecules have a stronger tendency to form aggregates in solution, and thereby greatly reinforce the gelation ability of the gelator.
3.2. Stability of the gel
The stability of a gel is crucial to its practical applications. Generally, the stability of a gel is often measured through its storage stability and thermal stability. It has been shown that the C2N2C gels from the benzene series and higher alkyl alcohols are stable for at least one year when they are sealed and placed in a dark place at room temperature, suggesting a very good stability with time. However, the gels from several other solvents are unstable because their lifetimes are not longer than a few days when they are placed in a routine way at room temperature. The thermal stability of a gel can be expressed by its sol–gel phase transition temperature (Tgel). A larger Tgel value means a better thermal stability. Taking the benzene gel as an example, its Tgel was studied with the change in the concentration of the gelator. The results are shown as Fig. 1. It is seen that the value of the Tgel of this gel significantly increases with increasing gelator concentration. It is to be noted that the Tgel of the gel was greater than the boiling point of benzene (80.1 °C). Surprisingly, the value of Tgel could be 60 degrees higher than the boiling point of pure benzene when C2N2C concentration reaches 6 wt%.The finding is further confirmed by the result from DSC measurements (cf. Fig. S1, ESI†). It is seen that there are three significant endothermic peaks around 190 °C within the temperature region studied, which may be correlated to the phase transition of the solvent from liquid to gas, the collapse of the gel networks, and the melting of the gelator. The unusual thermo-stability could be attributed, at least partially, to the strong interaction between neighboring molecules of the gelator, such as π–π stacking between naphthyl structures and van der Waals interaction among cholesteryl units, which is the basis for liquid crystal formation of cholesteryl derivatives. Furthermore, the conformational adaptability of calix[4]arene may endow the above mentioned structural units with more opportunities to arrange in comfortable positions, which must be beneficial for enhancing the thermo-stability of the gel networks. To the best of our knowledge, this exceptional thermo-stability is so unique and has never been reported before.
Further inspection of Fig. 1 shows that the increasing rate and trend are still there, suggesting that a further increase in the gelator concentration would result in even higher Tgel values. This is highly possible because the solubility of C2N2C in benzene is good enough to encompass more gelator in the system. The test was stopped because of safety reasons. Similarly, the Tgel of C2N2C/toluene gel (2.5%, w/v) is also greater than the boiling point of the solvent (111 °C). However, the Tgel values of the C2N2C gels of o-xylene, m-xylene, p-xylene and higher alcohols were lower than the boiling point of the corresponding pure solvent. The differences in the Tgel values of the gels of different solvents may be attributed to the differences in the structures of the gel networks, which will be studied in the following section, and the interactions of the gel networks with the solvents.
3.3. Gel network structures
In order to investigate the aggregation behaviors of the gelator in different solvents, the microstructures of the xerogels were measured by employing SEM. The results are shown in Fig. S2 (ESI†). As seen from the figure, the morphologies of the xerogels change with variation of the solvents. For example, the aggregate of the compound in benzene adopts continuous, flexible lamellar structures (Fig. S2a, ESI†). In p-xylene it is also characterized by flexible, continuous and fibrous structures, but possesses no lamellar feature (Fig. S2f, ESI†). For the toluene system, the molecules of the gelator aggregated into coarse fibers (Fig. S2b, ESI†). As for the morphologies of the networks of C2N2C in n-pentanol, o-xylene and m-xylene, they are similar to each other and feature as dense fibrous aggregates. Clearly, as expected, the morphological structures of the xerogels are not only dependent on the structure of the molecule of the gelator itself, but are also strongly influenced by the interactions between the gelator molecules and the solvents. That is to say, the nature of the solvent has a significant impact on the morphologies of the xerogels.To have a detailed understanding of the evolution of the morphologies of the gel networks, the concentration dependence of the morphological structure of the gel of C2N2C/benzene was studied by recording the AFM and/or SEM images of the gel networks at different gelator concentrations, and the results are shown in Fig. 2. From the AFM pictures, it can be observed that C2N2C starts to aggregate at concentrations much lower than its CGC in benzene, and is aggregated into spherulite-like structures at the concentration of 0.005 wt% studied (Fig. 2a). Increasing the concentration to 0.01 wt% resulted in the formation of helical fibrous aggregates with an average diameter of 0.5 μm (Fig. 2b). A further increase in the concentration favors the formation of continuous structures, either fibrous networks (Fig. 2c), mixtures of the primary structures (fibers, belts, flakes and even particles, etc.) (Fig. 2d), and 3D continuous sheets or networks (Fig. 2e and f). These results demonstrate that the formation of the gel networks is a progressive evolution process; that is the molecules of C2N2C first aggregate into spherulite-like structures, then helical fibers, and then sheets or flakes, finally networked structures. It is the networked structures of C2N2C that immobilize the solvent molecules, leading to the formation of molecular gels.
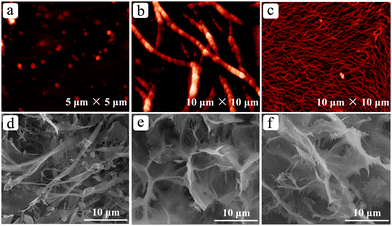 | ||
| Fig. 2 AFM images (a, 0.005%; b, 0.01%; c, 0.05%, w/v) and SEM images (d, 0.1%; e, 0.5%; f, 3.0%, w/v) of the C2N2C/benzene gel system. | ||
3.4. Thixotropic properties
Generally speaking, good mechanical strength is a pre-requirement for soft materials to find real-life applications.49 In the studies of the gelation behavior of the compound, it was found that the gels may possess sensitive thixotropic property, that is, the gels become solutions or suspensions after vigorous shaking, but lose their fluidity immediately when the shaking is stopped. In order to explore the property in detail, the rheological behaviors of the gels were studied.To investigate the effects of different solvents on the mechanical properties of the gels, the storage modulus G′, associated with the energy storage, and loss modulus G′′, associated with the loss of energy, of the gel systems (2.5%, w/v) in benzene, toluene, and p-xylene were measured as functions of shear stress at a constant frequency of 1.0 Hz at 15 °C. The results are shown in Fig. 3a. It can be seen that the value of G′ is much larger than the value of G′′ with the small shear force at the beginning, suggesting the stability and the dominant elastic property of the gel.50 The values of G′ and G′′ remain almost unchanged with the increase of the shear force. Finally, the G′′ value is greater than G′ after a critical shear stress, revealing the collapse of the 3D gel networks and the dominant fluid character of the gel. Reference to the figure reveals that the value of the storage modulus G′ and the yield stress of the gel increase gradually when the compound is respectively in benzene, toluene and p-xylene. All these demonstrate that the nature of the solvent has a significant influence on the mechanical property of the corresponding gel. In order to explore the effect of gelator concentration on the rheological properties of the gels, G′ and G′′ of the gels at different concentrations were measured as functions of shear stress. The results are shown in Fig. 3b. With reference to the figure, it is seen that the value of G′ increased from 8.3 × 104 Pa to 8.6 × 105 Pa with the increase in the concentration of the gelator from 1.5% to 3.5% (w/v), and the corresponding yield stress increased from 398 Pa to 3548 Pa, suggesting that the concentration of the gelator has a great effect on the mechanical strength and the elasticity of the gels. These results suggest that the rheological properties of the gel system are largely dependent on the concentration of the gelator, and the stability of the 3D network and visco-elastic behavior of the gel are also enhanced with increasing gelator concentration.
Frequency sweep is an important method for evaluating the tolerance of the gel to external forces.51 Thus, G′ and G′′ of the gels (2.5%, w/v) in benzene, toluene, and p-xylene were measured as functions of the angle frequency at a shear stress of 200 Pa within the linear region of the gel sample. The results are shown in Fig. S3 (ESI†). As can be seen from the figure, the storage modulus G′ of the three gels is always greater than the loss modulus G′′ when the angular frequency is gradually increased from 0.1 rad s−1 to 628.0 rad s−1, indicating that there has been no phase transition during the test and the system is a true gel.52 Additionally, the values of G′ and G′′ do not change significantly and remain substantially stable within the whole frequency region swept. This demonstrates that the gel systems exhibit typical visco-elastic properties and have an excellent tolerance to external forces.
It is to be noted that the preliminary test showed that the gels under study possess sensitive and reversible thixotropic property. In order to have a detailed understanding of the important property, the gel in benzene (2.5%, w/v) was taken as an example and the measurement was conducted at a constant frequency of 1.0 Hz at 15 °C. The results are shown in Fig. 4. The tests were conducted as follows: (1) initial state: the gel was subjected to a very small shear stress of 10 Pa well within the linear region of the gel sample for a short time (2 min), (2) deformation process: the gel was subjected to a high shear force of 1500 Pa far beyond the linear range of the gel system for 2 min, as a result of which the gel was destroyed and acquired a fluidity character, and (3) recovery process: the high shear force was removed and a very small monitoring shear stress of 10 Pa was exerted on the destroyed gel for 2 min. The storage modulus G′ and the loss modulus G′′ of the system were monitored with the change of time. As can be seen from Fig. 4, when t < 120 s, the values of G′ are always greater than those of G′′, indicating that the system is a true gel. When 120 s < t < 240 s, however, the values of G′′ are always higher than those of G′, revealing that the gel had been destroyed presenting a fluidity character. When 240 s < t < 360 s, the values of G′ are always greater than those of G′′, suggesting that the system recovered promptly and completely after removing the shear force. The shear-flowing and standing-gelling processes could be at least repeated four times. It is believed that the test would be repeated many more times if the solvent within the gel was not evaporative. Anyway, the results already demonstrated that the gel of the compound in benzene possesses sensitive, fast and fully reversible thixotropic property.
 | ||
| Fig. 4 Thixotropic behaviors of the C2N2C/benzene gel system (formation: 10 Pa, deformation: 1500 Pa; 2.5%, w/v). | ||
Beyond doubt, the smart thixotropic property of the C2N2C/benzene gel has laid a foundation for its potential application in some special fields, such as injection molding, 3D printing, drug delivery, etc. Similarly, the thixotropic properties of the gels in toluene and p-xylene were also tested, and the results are shown in Fig. S4 and S5 (ESI†), respectively. Reference to the figures reveals that the two gel systems also exhibit very sensitive and fully reversible thixotropic property. As revealed by others, the rheological properties of molecular gels are reflections of their internal structures.53–55 With careful inspection of the micro-structures of the aggregates of the gelator in the gel system as shown in Fig. 2, it is not difficult to find that the morphologies of the aggregates gradually transform from small spherulite-like aggregates to fibrous aggregates and eventually to the lamellar structures. The easy sliding between these aggregates might be the reason why the gels possess the so smart and fully reversible thixotropic property, and suggests again that the rheological properties of the gels are closely related to their internal network structures. Based on the above discussion, it is not difficult to come to a conclusion that the geometrical configuration of the aggregated structure of a gel has a very significant impact on its thixotropic property.
3.5. Aggregation and chirality properties
The building blocks in the gelators ensure that self-assembly can be facilitated by the synergistic effect of several non-covalent forces, such as π–π interactions and hydrogen bonding during the formation of the 3D gel networks. The existence of π–π stacking among the naphthalene functionality may play a crucial role in the self-assembly of the gelator. The changes of UV-vis absorption spectra can be used to not only probe the interactions between π-conjugated structures, but also explore the possible assembling mode.56,57 The red shift in absorption upon aggregation indicates that most probably the so-called J-type aggregates are formed. In contrast, H-type aggregates are formed. The UV-vis absorption spectrum of the gelator in benzene was recorded at different concentrations. The results are shown in Fig. S6a (ESI†). It is revealed that the absorption intensity increases with a gradual increase in the concentration of the gelator. The absorption spectrum of the gelator at low concentration shows three main absorption bands. However, at high concentration it shows two main absorption bands. In addition, when analyzing carefully the UV-vis data, a blue shift (about 11 nm) of the position of the maximum absorption and the longer wavelength side of the absorption spectrum was observed with increasing gelator concentration. These changes are consistent with an enhancement in the aggregation and π–π interaction of the gelator molecules, suggesting the formation of H-type face-to-face π stacking in the aggregates. The conclusion is further supported by the results from temperature-dependent UV-vis absorption spectrum measurements (Fig. S6b, ESI†).It is known that the formation of the gel is a self-assembling process of the gelator molecules. In particular, chiral architectures may be formed if the gelator molecules are arranged in an appropriate orientation.58–60 Therefore, CD measurements may play a role in monitoring the assembly process and revealing the chiral structures of the assemblies. Accordingly, the concentration-/temperature-dependent CD spectroscopy measurements were conducted with the C2N2C/benzene gel as an example system. The results are shown in Fig. 5a and b, respectively. Reference to the spectra shown in Fig. 5a reveals that coinciding with the changes in the UV-vis spectrum, the CD intensity increases along with a gradual increase in the concentration of the gelator. It is apparent that in the sol state (before gelation), the CD spectrum is totally silent; in the gel state (after gelation), strong CD signals are detected, the zero-crossing of which corresponds to the absorption maximum. The three bands of the CD spectrum do not have the same intensity showing a non-conservative exciton pattern corresponding to the absorption of naphthalene chromophore. In addition, a strong Cotton effect is observed at the position of the π–π* band with a positive and negative sign. Temperature-dependent CD spectroscopy measurements indicate that the CD intensity decreases with increasing temperature from 50 to 80 °C (Fig. 5b). All the findings suggest that the CD signals originate from the network structures of the gelator rather than from the molecule itself, and furthermore, the molecules of the gelator are assembled in a helical manner, which explains the helical structure shown in Fig. 2b.
 | ||
| Fig. 5 (a) Concentration-dependent CD spectra of C2N2C in benzene; (b) temperature-dependent CD spectra of C2N2C in benzene (1.3%, w/v). | ||
3.6. Bonding nature of the gel networks
In order to get further information about the formation of the gel networks, the aggregation behaviors of the gelator molecules in a solvent were studied in detail by temperature-/concentration-dependent 1H NMR spectroscopy measurements.61,62 The measurements were conducted in C6D6, and the results are shown in Fig. 6. It is apparent that variation in temperature or concentration resulted in a profound change in the chemical shifts of the O–H protons, the N–H protons and part of the naphthalene protons. With reference to Fig. 6a, it is seen that the chemical shifts of the above mentioned protons shifted remarkably to up-field with increasing temperature from 298 K to 338 K. Specifically, the chemical shift of one of the N–H protons changed from 9.72 to 9.42 ppm, and another from 7.71 to 7.02 ppm. Furthermore, the chemical shifts of the O–H protons, which belong to the calix[4]arene skeleton, shifted from 6.81 to 6.64 ppm, and at the same time, the partial signals of the naphthalene structure changed from 8.21–8.11 to 8.15–8.06 ppm with increasing temperature from 298 K to 338 K. | ||
| Fig. 6 (a) Temperature-dependent 1H NMR spectra of C2N2C in benzene (0.5%, w/v); (b) concentration-dependent 1H NMR spectra of C2N2C in benzene (298 K). | ||
Based on the above discussion, it is obvious that hydrogen bonding and π–π stacking may have played a crucial role in the gel formation process. It is known that only intermolecular weak interactions between gelator molecules can promote gel network formation. To confirm if the hydrogen bonding and π–π stacking formed inter-molecularly, the 1H NMR measurements were performed at different gelator concentrations at a temperature of 298 K. It was demonstrated that the chemical shifts of the O–H protons and the N–H protons shifted remarkably to down-field with a gradual increase in the concentration of the gelator from 0.1 wt% to 0.9 wt% (Fig. 6b), indicating the intermolecular interaction nature. Furthermore, the π–π stacking between the naphthyl structures was also formed inter-molecularly.
3.7. Orderliness and packing mode of gel networks
As mentioned already, aggregation of gelator molecules to form 3D networks is a necessity for the formation of molecular gels. However, the aggregation herein is not as simple as formation of a precipitate, and in fact, it is a process in between crystallization (a process to form highly ordered structures) and precipitation (a process to form mostly amorphous structures). In other words, the gel networks may possess quite a high degree of order that makes it possible to explore the structure with the X-ray diffraction technique.63 Accordingly, in the present work, the structure of the xerogel of C2N2C/benzene gel was investigated, and the XRD trace is depicted in Fig. S7 (ESI†). It can be seen that the XRD pattern of the xerogel is characterized by three obvious reflection peaks in the low-angle region, revealing that the sample is highly ordered or possesses a crystal-like structure. The d values of the three peaks are 2.91, 1.69 and 1.09 nm, respectively, and the ratio of them follows 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (1/√3)
(1/√3)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (1/√7), satisfying the requirement of hexagonal packing that is 1
(1/√7), satisfying the requirement of hexagonal packing that is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/√3
1/√3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/2
1/2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/√7
1/√7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/3
1/3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/√12.64,65 Though some important peaks were missed in the traces, it can be still inferred that the molecules of the gelator in the gel may have self-assembled into a hexagonal structure. This is because the gel networks are not real crystals and the densities of them are quite low which weakens the signals. The repeat distance of the aggregate is 2.91 nm, which almost equals the length of the gelator molecule as modeled by molecular dynamics simulation, suggesting that the hexagonal stacking of the gelator molecules might be the primary structure of the gel networks under study. In other words, the structure might be further assembled into a spherulite-like structure, then with a further increase in the concentration of the gelator, the spherulites aggregate gradually into fiber-like structures, and eventually entangle into gel networks as revealed by AFM and SEM studies. The tentative formation process of the gel networks may be represented by a cartoon schematically shown in Scheme 2.
1/√12.64,65 Though some important peaks were missed in the traces, it can be still inferred that the molecules of the gelator in the gel may have self-assembled into a hexagonal structure. This is because the gel networks are not real crystals and the densities of them are quite low which weakens the signals. The repeat distance of the aggregate is 2.91 nm, which almost equals the length of the gelator molecule as modeled by molecular dynamics simulation, suggesting that the hexagonal stacking of the gelator molecules might be the primary structure of the gel networks under study. In other words, the structure might be further assembled into a spherulite-like structure, then with a further increase in the concentration of the gelator, the spherulites aggregate gradually into fiber-like structures, and eventually entangle into gel networks as revealed by AFM and SEM studies. The tentative formation process of the gel networks may be represented by a cartoon schematically shown in Scheme 2.
 | ||
| Scheme 2 Schematic representation of a plausible formation process of the helical aggregates of C2N2C in benzene. | ||
4. Conclusions
In the present study, a novel calix[4]arene-based dimeric-cholesteryl derivative with naphthalene in the linkers has been designed and synthesized. The compound was capable of transforming a variety of organic solvents into gels and exhibited a remarkable gelling ability, especially in solvents of the benzene series and higher alkyl alcohols. AFM and SEM measurements revealed that the compound self-assembled into hierarchical structures in benzene, specifically, starting from hexagonal stacking, then micro-/nano-particles, and then fibers, and finally networked structures with a gradual increase in the concentration of the gelator. CD and AFM analyses demonstrated that the molecules of the gelator self-assembled into chiral aggregates with a right-hand helical feature. Interestingly and importantly, the gels of the benzene series possess sensitive, fast and fully reversible thixotropic property. Furthermore, the Tgel of the C2N2C/benzene gel could be more than, at least, 60 degrees higher than the boiling point of benzene when the gelator concentration is greater than 6% (w/v), a result never reported before. From a practical viewpoint, the thermo-responsive, superior thixotropic property and the exceptional thermo-stability of the gels make them potentially useful in controlled release, injection molding, and 3D printing. The corresponding possibilities of the applications are currently under exploration.Author contributions
The manuscript was written through discussion and contributions of all authors. All authors have given approval to the final version of the manuscript. Miss Ying Wu and Professor Yu Fang have made main contributions through design, experimental work, writing, etc. Other authors contributed equally.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
The authors thank the Natural Science Foundation of China (21273141 and 21206089), the “Program for Changjiang Scholars and Innovative Research Team in University” of China (IRT1070) and the 111 Project. This work is also supported by the Fundamental Research Funds for the Central Universities (GK201301006) and the Research Funds of Shaanxi Normal University (X2012YB02). Mr Honglin Lu working at the Xi'an Modern Chemistry Research Institute is acknowledged for his help in conducting the DSC test.Notes and references
- D. J. Abdallah and R. G. Weiss, Adv. Mater., 2000, 12, 1237 CrossRef CAS.
- P. Dastidar, Chem. Soc. Rev., 2008, 37, 2699 RSC.
- G. C. Yu, X. Z. Yan, C. Y. Han and F. H. Huang, Chem. Soc. Rev., 2013, 42, 6697 RSC.
- D. Khatua and J. Dey, Langmuir, 2005, 21, 109 CrossRef CAS PubMed.
- K. J. Skilling, F. Citossi, T. D. Bradshaw, M. Ashford, B. Kellama and M. Marlow, Soft Matter, 2014, 10, 237 RSC.
- D. K. Kumar and J. W. Steed, Chem. Soc. Rev., 2014, 43, 2080 RSC.
- X. J. Liao, G. S. Chen and M. Jiang, Polym. Chem., 2013, 4, 1733 RSC.
- M. Llusar and C. Sanchez, Chem. Mater., 2008, 20, 782 CrossRef CAS.
- S. Yamamichi, Y. Jinno, N. Haraya, T. Oyoshi, H. Tomitori, K. Kashiwagi and M. Yamanaka, Chem. Commun., 2011, 47, 10344 RSC.
- A. R. Hirst, B. Escuder, J. F. Miravet and D. K. Smith, Angew. Chem., Int. Ed., 2008, 47, 8002 CrossRef CAS PubMed.
- M. George and R. G. Weiss, Acc. Chem. Res., 2006, 39, 489 CrossRef CAS PubMed.
- A. Ajayaghosh and V. K. Praveen, Acc. Chem. Res., 2007, 40, 644 CrossRef CAS PubMed.
- X. Z. Yan, F. Wang, B. Zheng and F. H. Huang, Chem. Soc. Rev., 2012, 41, 6042 RSC.
- R. M. Yang, S. H. Peng and T. C. Hughes, Soft Matter, 2014, 10, 2188 RSC.
- X. Ran, H. T. Wang, P. Zhang, B. L. Bai, C. X. Zhao, Z. X. Yu and M. Li, Soft Matter, 2011, 7, 8561 RSC.
- G. Cravotto and P. Cintas, Chem. Soc. Rev., 2009, 38, 2684 RSC.
- Y. Suzaki, T. Taira and K. Osakada, J. Mater. Chem., 2011, 21, 930 RSC.
- J. R. Lu, J. Hu, Y. Song and Y. Ju, Org. Lett., 2011, 13, 3372 CrossRef CAS PubMed.
- S. I. Kawano, N. Fujita and S. Shinkai, J. Am. Chem. Soc., 2004, 126, 8592 CrossRef CAS PubMed.
- Y. Zhao, F. Sakai, L. Su, Y. J. Liu, K. C. Wei, G. S. Chen and M. Jiang, Adv. Mater., 2013, 25, 5215 CrossRef CAS PubMed.
- A. Dawn, T. Shiraki, H. Ichikawa, A. Takada, Y. Takahashi, Y. Tsuchiya, L. T. N. Lien and S. Shinkai, J. Am. Chem. Soc., 2012, 134, 2161 CrossRef CAS PubMed.
- Z. F. Sun, Z. Y. Li, Y. H. He, R. J. Shen, L. Deng, M. H. Yang, Y. Z. Liang and Y. Zhang, J. Am. Chem. Soc., 2013, 135, 13379 CrossRef CAS PubMed.
- D. J. Abdallah, S. A. Sirchio and R. G. Weiss, Langmuir, 2000, 16, 7558 CrossRef CAS.
- A. Friggeri, O. Gronwald, K. J. C. van Bommel, S. Shinkai and D. N. Reinhoudt, J. Am. Chem. Soc., 2002, 124, 10754 CrossRef CAS PubMed.
- V. A. Mallia, P. D. Butler, B. Sarkar, K. T. Holman and R. G. Weiss, J. Am. Chem. Soc., 2011, 133, 15045 CrossRef CAS PubMed.
- A. D. Guerzo, A. G. L. Olive, J. Reichwagen, H. Hopf and J. P. Desvergne, J. Am. Chem. Soc., 2005, 127, 17984 CrossRef PubMed.
- M. Suzuki, M. Yumoto, M. Kimura, H. Shirai and K. Hanabusa, Chem. – Eur. J., 2003, 9, 348 CrossRef CAS PubMed.
- X. Z. Yan, D. H. Xu, X. D. Chi, J. Z. Chen, S. Y. Dong, X. Ding, Y. H. Yu and F. H. Huang, Adv. Mater., 2012, 24, 362 CrossRef CAS PubMed.
- K. L. Liu, Z. X. Zhang and J. Li, Soft Matter, 2011, 7, 11290 RSC.
- Y. W. Lin, L. F. Li and G. W. Li, Carbohydr. Polym., 2013, 92, 429 CrossRef CAS PubMed.
- J. A. Foster and J. W. Steed, Angew. Chem., Int. Ed., 2010, 49, 6718 CrossRef CAS PubMed.
- M. Megyesi, L. Biczók and I. Jablonkai, J. Phys. Chem. C, 2008, 112, 3410 CAS.
- Z. B. Qin, D. S. Guo, X. N. Guo and Y. Liu, Soft Matter, 2014, 10, 2253 RSC.
- L. Mutihac, J. H. Lee, J. S. Kim and J. Vicens, Chem. Soc. Rev., 2011, 40, 2777 RSC.
- J. Zhang, D. S. Guo, L. H. Wang, Z. Wang and Y. Liu, Soft Matter, 2011, 7, 1756 RSC.
- C. Hippius, I. H. M. van Stokkum, E. Zangrando, R. Williams and F. Würthner, J. Phys. Chem. C, 2007, 111, 13988 CAS.
- H. Kawabata, M. Aoki, K. Murata and S. Shinkai, Supramol. Chem., 1993, 2, 33 CrossRef CAS.
- B. G. Xing, M. F. Choi and B. Xu, Chem. Commun., 2002, 362 RSC.
- J. L. Zhou, X. J. Chen and Y. S. Zheng, Chem. Commun., 2007, 5200 RSC.
- Y. S. Zheng, S. Y. Ran, Y. J. Hu and X. X. Liu, Chem. Commun., 2009, 1121 RSC.
- T. Becker, C. Y. Goh, F. Jones, M. J. McIldowie, M. Mocerino and M. I. Ogden, Chem. Commun., 2008, 3900 RSC.
- D. Hwang, E. Lee, J. H. Jung, S. S. Lee and K. M. Park, Cryst. Growth Des., 2013, 13, 4177 CAS.
- C. C. Tsai, Y. T. Cheng, L. C. Shen, K. C. Chang, I. T. Ho, J. H. Chu and W. S. Chung, Org. Lett., 2013, 15, 5830 CrossRef CAS PubMed.
- C. C. Tsai, K. C. Chang, I. T. Ho, J. H. Chu, Y. T. Cheng, L. C. Shen and W. S. Chung, Chem. Commun., 2013, 49, 3037 RSC.
- X. Q. Cai, K. Q. Liu, J. L. Yan, H. L. Zhang, X. Y. Hou, Z. Liu and Y. Fang, Soft Matter, 2012, 8, 3756 RSC.
- X. Q. Cai, Y. Wu, L. Y. Wang, N. Yan, J. Liu, X. H. Fang and Y. Fang, Soft Matter, 2013, 9, 5807 RSC.
- X. Huang, P. Terech, S. R. Raghavan and R. G. Weiss, J. Am. Chem. Soc., 2005, 127, 4336 CrossRef CAS PubMed.
- S. Boothroyd, A. F. Miller and A. Saiani, Faraday Discuss., 2013, 166, 195 RSC.
- M. Lescanne, P. Grondin, A. d'Aléo, F. Fages, J. L. Pozzo, O. M. Monval, P. Reinheimer and A. Colin, Langmuir, 2004, 20, 3032 CrossRef CAS.
- S. Fateixa, A. L. Daniel-da-Silva, H. I. S. Nogueira and T. Trindade, J. Phys. Chem. C, 2014, 118, 10384 CAS.
- T. Kar, S. Mukherjee and P. K. Das, New J. Chem., 2014, 38, 1158 RSC.
- S. Boothroyd, A. Saiani and A. F. Miller, Biopolymers, 2013, 101, 669 CrossRef.
- Z. Y. Xu, J. X. Peng, N. Yan, H. Yu, S. S. Zhang, K. Q. Liu and Y. Fang, Soft Matter, 2013, 9, 1091 RSC.
- J. H. Shi, X. Y. Liu, J. L. Li, C. S. Strom and H. Y. Xu, J. Phys. Chem. B, 2009, 113, 4549 CrossRef CAS PubMed.
- P. Terech, D. Pasquier, V. Bordas and C. Rossat, Langmuir, 2000, 16, 4485 CrossRef CAS.
- S. I. Kawano, N. Fujita and S. Shinkai, Chem. – Eur. J., 2005, 11, 4735 CrossRef CAS PubMed.
- P. Pratihar, S. Ghosh, V. Stepanenko, S. Patwardhan, F. C. Grozema, L. D. A. Siebbeles and F. Würthner, Beilstein J. Org. Chem., 2010, 6, 1070 CrossRef CAS PubMed.
- A. P. H. J. Schenning, P. Jonkheijm, E. Peeters and E. W. Meijer, J. Am. Chem. Soc., 2001, 123, 409 CrossRef CAS.
- A. Ajayaghosh, P. Chithra and R. Varghese, Angew. Chem., Int. Ed., 2007, 46, 230 CrossRef CAS PubMed.
- G. Gottarelli, S. Lena, S. Masiero, S. Pieraccini and G. P. Spada, Chirality, 2008, 20, 471 CrossRef CAS PubMed.
- K. Q. Liu and J. W. Steed, Soft Matter, 2013, 9, 11699 RSC.
- J. Hu, M. Zhang and Y. Ju, Soft Matter, 2009, 5, 4971 RSC.
- E. Ostuni, P. Kamaras and R. G. Weiss, Angew. Chem., Int. Ed., 1996, 35, 1324 CrossRef CAS.
- N. A. Melosh, P. Davidson and B. F. Chmelka, J. Am. Chem. Soc., 2000, 122, 823 CrossRef CAS.
- C. M. Yu, M. Xue, K. Liu, G. Wang and Y. Fang, Langmuir, 2014, 30, 1257 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Microscopy studies of the xerogels; rheological studies of the C2N2C gels; UV-vis absorption spectra of C2N2C in benzene; XRD study of the C2N2C/benzene xerogel. See DOI: 10.1039/c4nj01517g |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2015 |



