Routes to 3D conformal solid-state dielectric polymers: electrodeposition versus initiated chemical vapor deposition†
Megan B.
Sassin
*a,
Jeffrey W.
Long
a,
Jean Marie
Wallace
b and
Debra R.
Rolison
a
aU.S. Naval Research Laboratory, 4555 Overlook Avenue SW, Code 6170, Washington, DC 20375, USA. E-mail: megan.sassin@nrl.navy.mil; jeffrey.long@nrl.navy.mil; rolison@nrl.navy.mil
bNova Research Inc., 1900 Elkin Street, Suite 230, Alexandria, VA 22308, USA. E-mail: jean.wallace.ctr@nrl.navy.mil
First published on 29th June 2015
Abstract
We show that two distinct methods, electropolymerization and initiated chemical vapour deposition (iCVD), can be adapted to generate ultrathin polymers (30–50 nm thick) at three dimensionally (3D) porous conductive substrates comprising ∼300 μm-thick carbon-coated silica fiber paper (C@SiO2). We selected 1,3,5-trivinyl-1,3,5-trimethylcyclotrisiloxane (“V3D3”) as a common monomer amenable to polymerization by either approach. Electroanalytical and electrical measurements confirm that all carbon surfaces are passivated with electronically insulating poly(V3D3) coatings.
Conceptual insightsThe Achilles heel that trips up all-solid-state 3D energy-storage devices resides in the inability to form ultrathin dielectrics with high fidelity when conformally coating non-line-of-sight substrates such as ultraporous scaffolds and architectures. Of the limited fabrication protocols available to paint polymers throughout macroscopically thick structures while “blind,” electrodeposition and initiated chemical vapour deposition are the most compelling. Both methods offer conditions under which growth can be limited thus ensuring that monomer can access all of the internal surfaces. The critical chemical, physical, and electrical properties of nanoscale polymers generated by such methods—well-studied on planar substrates—must also be validated for the polymers when expressed in 3D. These data are necessary to demonstrate performance as a solid-state dielectric in 3D batteries and capacitors. |
Introduction
Advances in solid-state dielectrics and electrolytes are critical in the effort to transition from the simple coplanar configuration of active materials in present batteries and capacitors to next-generation designs in which the three critical components (anode, cathode, and dielectric/electrolyte) are reconstructed in three dimensions (3D) as interpenetrating networks.1–4 The 3D-integrated design simultaneously minimizes the separation distance and maximizes the interface between the two opposing electrodes, mitigating power limitations present in conventional 2D designs. Although substantial progress has been made in 3D solid-state energy-storage devices, a common roadblock to achieving full functionality is the dielectric/electrolyte component. This piece of the 3D energy-storage puzzle must be: (i) conformal to the supporting electrode architecture; (ii) ultrathin, typically tens of nanometers to a few micrometers; (iii) pinhole-free; (iv) electronically insulating; (v) chemically and electrochemically stable; and (vi) in the case of a battery or electrochemical capacitor, ionically conducting.1,5,6 These requirements coupled with the complex geometry of the 3D energy-storage architectures of interest, render most dielectric/electrolyte fabrication methods ineffective, as they typically require line-of-sight conditions.Electrodeposition is a non-line-of-sight fabrication method that is readily adapted to modify conductive 2D and 3D substrates with nanoscale, conformal-to-the-surface, pinhole-free, electronically insulating polymer coatings.5–7 We previously demonstrated that electro-oxidative polymerization of phenol-based monomers generates ultrathin (tens of nanometers), conformal polymer coatings on both planar substrates8,9 and 3D architectures.10–12 The resulting phenylene-oxide polymer coatings are highly electronically insulating, with dielectric strengths comparable to those measured for the corresponding bulk polymer. Ionic conductivity was imparted by impregnation of the polymer film with electrolyte salts8,12,13 or by copolymerizing with monomers that have pendant ionic functionalities.9
While these earlier findings demonstrated the prospective functionality of nanoscale polymers for 3D all-solid-state battery architectures, polymer formation via electro-oxidation may not always be desirable, particularly when the underlying architecture on which the polymer is deposited is designed to serve as the negative electrode. Electroreductive polymerization via vinyl-functionalized monomers14 is thus an attractive alternative. Electropolymerization from acrylate-type monomers en route to solid-state electrolyte coatings was reported by Owen and co-workers; they deposited micrometres-thick polyacrylonitrile coatings on nickel foam and vitreous carbon followed by incorporation of electrolyte salts into the film to achieve ionic conduction.15,16 Brandell and co-workers took the formation of a solid-state electrolyte on a 3D substrate (arrays of 4–8 μm-tall Cu pillars) a step further by demonstrating that Li salts were incorporated into poly(propylene glycol) acrylate films during the electropolymerization process.17 Prieto and co-workers electrodeposited polyethyleneoxide-based films through the electroreduction of a diazonium group; the resulting films were grafted to the electrode surface and displayed electronic conductivity of 10−10 S cm−1, but the <10 nm thickness will limit the operating voltage when functioning as a solid-state electrolyte.18
Chemical vapour deposition (CVD) offers a potential route to polymer coatings,19 but often is ill-suited for non-line-of-sight-deposition conditions. One exception is initiated chemical vapour deposition (iCVD),20–22 a technique based on free-radical polymerization in which the desired monomer, typically containing vinyl moieties, and an initiating species (e.g., tert-butylperoxide) are introduced in vapour form to the desired substrate. Heated filaments above the substrate induce radical formation in the initiator but not the monomer, such that polymerization proceeds only as the monomer and activated initiator co-adsorb on the cool substrate. Gleason and coworkers have demonstrated that iCVD provides excellent control of thickness and uniformity in vapour-deposited polymers, and that such coatings exhibit high electronic resistivity (>1014 Ω cm).23,24 Early work with iCVD focused on polymer deposition at planar substrates, but this technique has also been extended to coat more complex 3D objects, including fiber mats25 and trenched substrates.26,27 Gleason and Dunn recently explored the use of Li+-impregnated iCVD-derived polymer coatings at 2D indium-tin oxide substrates as nanoscale solid-state electrolytes.28
In the present report, we contrast these two distinct protocols, electroreductive polymerization (EP) and iCVD, as the means to generate ultrathin, electronically insulating polymer coatings, using carbon-coated silica fibre paper29 as our test-bed 3D architecture (designated “C@SiO2 FP”; see scanning electron micrographs, SEMs, in Fig. 1). We chose 1,3,5-trivinyl-1,3,5-trimethylcyclotrisiloxane (“V3D3”, Scheme 1) as the common monomer for both deposition methods, based in part on its established use for iCVD20,23 and its ability to solvate Li+ salts (introduced after deposition) via coordination at the cyclotrisiloxyl moieties in the polymer.28
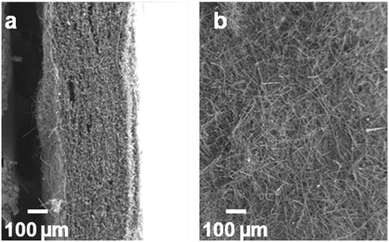 | ||
| Fig. 1 Low magnification scanning electron micrograph of (a) cross section and (b) exterior surface of C@SiO2 fibre paper. | ||
While deposition of poly(V3D3) with iCVD closely followed the methods described by Gleason and co-workers, electropolymerization of V3D3 has not been previously reported, requiring us to develop and optimize electrodeposition protocols. We show that under the appropriate conditions, both deposition techniques can be adapted to generate ultrathin (<60 nm), conformal poly(V3D3) coatings that passivate all surfaces of the C@SiO2 FP. Poly(V3D3) derived from iCVD exhibits a more cross-linked and solid-like structure, in contrast to electropolymerized V3D3, which has a gel-like consistency due to incomplete reaction of vinyl groups in the polymer.
Results and discussion
Formation of conductive carbon coatings on macroscopically thick silica fibre paper via pyrolytic CVD from a benzene precursor was recently reported by Lytle et al.29 This deposition protocol yields conformal carbon coatings wrapped around the fibres; for the present study we chose deposition conditions that produce carbon coatings with thickness of ∼75–100 nm (Fig. 2, top row). The lack of charging when the C@SiO2 FP is examined by SEM confirms that the electronically insulating SiO2 FP has been thoroughly coated with conductive carbon. The ∼300 μm-thick SiO2 FP comprises fibres ranging from ∼100 nanometres to several micrometres in diameter, interconnected by void spaces ranging from 0.5–10 μm that facilitate transport of monomer to the interior of this complex architecture. Before proceeding to polymer coating steps, we first confirmed that C@SiO2 FP functions as an effective electrode by performing cyclic voltammetry in an acetonitrile-based electrolyte; based on measurements of double-layer capacitance we calculate an electroactive surface area of 300 cm2, as expressed in C@SiO2 FP with 1 × 2 cm macroscale dimensions (see ESI†).Electropolymerization of V3D3 in 3D
Electroreductive polymerization of V3D3 was performed by infiltrating the C@SiO2 FP electrode with a monomer-containing electrolyte solution and applying linear-sweep voltammetry (LSV) to scan the electrode potential through the reduction peak (Fig. 3a); this feature was previously assigned to chemical grafting at the electrode surface.14 To thicken the electrodeposited polymer, the electrode was held at the grafting potential for a specified duration (Fig. 3b). A large current is initially observed, followed by a decay that indicates a diminishing supply of monomer at the surfaces of the C@SiO2 FP (Fig. 3b). Returning the electrode to a rest potential positive of the grafting potential for a short period allows an influx of fresh monomer to the C@SiO2 FP for subsequent grafting cycles (Fig. 3a and b). The magnitude of the current decreases with each cycle, confirming that a passivating polymer film is formed at the carbon surfaces in C@SiO2 FP.Electrodeposition at the grafting potential leads to formation of a covalently bonded nanoscale polymer film (<10 nm thick)14,30 that is not necessarily thick enough to serve as the dielectric/solid-state electrolyte for the 3D energy-storage architectures of interest.1 Unlike our previous reports for electro-oxidative polymerization of phenol monomers,8,9 the electrodeposition of poly(V3D3) is not truly self-limiting. Scanning the “grafted” poly(V3D3)–C@SiO2 FP electrode to potentials negative of the grafting potential yields LSV deposition curves and chronoamperometric transients with current magnitudes similar to the first scan of the grafting potential, revealing that additional polymer deposition proceeds at the “grafted” poly(V3D3)–C@SiO2 FP surfaces (Fig. 3c and d).
Production of additional radical anions at potentials negative of the grafting potential drives polymerization in the bulk solution near the surfaces of the electrode, the products of which become entangled within the network of the grafted poly(V3D3), thereby increasing the thickness.14,30 The thickening polymer film, coupled with exhaustion of the monomer supply in the voids of the C@SiO2 FP electrode, impede further deposition, as evidenced by decreasing current for subsequent cycles (Fig. 3c and d). If the poly(V3D3)–C@SiO2 FP is poised at even more negative potentials, but prior to electrolyte breakdown, radical anions can still be produced, resulting in more extensive solution polymerization, further increasing the thickness of the polymer film (Fig. 3e and f).
Characterization of EP vs. iCVD poly(V3D3)–C@SiO2 FP
The electronically insulating nature of poly(V3D3)23,24 presents challenges for direct imaging via SEM; however, maintaining identical brightness and contrast settings for all samples and using a low accelerating voltage allows us to qualitatively assess the nature of the polymer coating at the C@SiO2 FP for EP-poly(V3D3) samples described above, and also for poly(V3D3) deposited by established iCVD protocols (see ESI† for more details). The increase in brightness observed for both types of poly(V3D3)–C@SiO2 FP samples relative to the unmodified C@SiO2 FP confirms the presence of an electronically insulating polymer coating. Although electropolymerization of V3D3 is not strictly self-limiting, we observe conformal polymer coatings throughout the C@SiO2 FP substrates, similar to that for iCVD-generated poly(V3D3) (Fig. 2). The texture of the EP-poly(V3D3) coating is smoother than that derived from iCVD, which more closely resembles the particulate morphology of the underlying carbon surface. Cross-sectional images show that poly(V3D3) generated by either route uniformly coats the fibres that comprise the C@SiO2 FP. The average thickness of the poly(V3D3) coating is estimated from SEM to be ∼33 nm for the electropolymerized sample (“EP1”) and ∼45 nm for a 25 min iCVD-generated coating (“iCVD1”; Table 1 and Fig. 2).‡ By adding two more LSV and potentiostatic cycles at −1.8 V vs. Ag, thickness can be increased to ∼51 nm (“EP2”, Fig. S1, ESI†). Increasing the deposition time for iCVD to 40 min (“iCVD2”) increases the thickness modestly, also to ∼51 nm (Table 1, Fig. S2, ESI†).‡| Resistance (Ω) | Poly(V3D3) thickness (nm) | |
|---|---|---|
| C@SiO2 FP | 340 | — |
| EP1 poly(V3D3)–C@SiO2 | 670 | 33 ± 6 |
| EP2 poly(V3D3)–C@SiO2 | 2800 | 51 ± 16 |
| iCVD1 poly(V3D3)–C@SiO2 | 740 | 45 ± 13 |
| iCVD2 poly(V3D3)–C@SiO2 | 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
51 ± 12 |
We used specular-reflectance FTIR spectroscopy to assess the chemical structure of EP- and iCVD-derived poly(V3D3) coatings, but with Au-coated glass slides as our test substrate; poly(V3D3) deposition was performed at Au-coated glass slides under the same conditions as for C@SiO2 FP substrates. The IR spectra for poly(V3D3) prepared by iCVD (Fig. 4) are consistent with those previously published.20,31 Electropolymerization of V3D3 results in more complex IR spectra that include bands common with iCVD-poly(V3D3), but also multiple distinct peaks (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretch, 1596 cm−1;
C stretch, 1596 cm−1; ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 scissor, 1443 cm−1; C–H stretch, 3050–2950 cm−1) that are ascribed to unreacted vinyl groups that originate from the V3D3 monomer. The persistence of vinyl groups in EP-derived poly(V3D3) films indicates a lower degree of cross-linking as compared to the iCVD-derived analogues, a finding consistent with qualitative visual/tactile observations of the gel-like nature of EP-derived films.17
CH2 scissor, 1443 cm−1; C–H stretch, 3050–2950 cm−1) that are ascribed to unreacted vinyl groups that originate from the V3D3 monomer. The persistence of vinyl groups in EP-derived poly(V3D3) films indicates a lower degree of cross-linking as compared to the iCVD-derived analogues, a finding consistent with qualitative visual/tactile observations of the gel-like nature of EP-derived films.17
Electrical and electrochemical properties of EP vs. iCVD poly(V3D3)–C@SiO2 FP
To assess the passivating nature of the poly(V3D3) coatings on the fibres in C@SiO2 FP, we performed cyclic voltammetry in the presence of a well-known aqueous-electrolyte redox probe, K3Fe(CN)6; aqueous electrolytes were used anticipating that the poly(V3D3) coating would exhibit minimal swelling in H2O.32 Voltammetry at uncoated C@SiO2 FP shows the expected redox peaks for the reversible FeII(CN)64−/FeIII(CN)63− reaction, further confirming that this substrate serves as an effective electrode (Fig. 5a). When viewed on the same current scale, voltammograms for all poly(V3D3)–C@SiO2 FP electrodes show minimal response to the redox probe, demonstrating that the shell of poly(V3D3) has passivated the ∼300 cm2 of electrochemical surface area in the 1 × 2 cm piece of C@SiO2 FP. At much lower current scales, we do observe reduction features that may indicate a modest degree of partition/permeation of the redox probe into/through these nanometers-thick films (Fig. 5b),14,17 with the EP-poly(V3D3)–C@SiO2 FPs showing greater apparent permeability than the iCVD-derived analogues, a trend that is consistent with the gel-like nature of the EP-derived polymer.Solid-state AC and DC electrical measurements provide information on the critical properties of nanoscale polymer systems under consideration as electrolyte or dielectric phases.1 Making reproducible and non-damaging electrical contact to nanoscale polymers for such measurements can be challenging; we previously established that liquid metal probes (Hg or GaIn) are effective for this purpose.8,9,12 Herein we used Hg, dispensed via syringe and gently lowered onto the C@SiO2 FPs with a micromanipulator to make contact (Fig. S3, ESI†). Due to the porous nature of the C@SiO2 FP, it was difficult to assess the actual contact area, but the extruded Hg droplet size was relatively constant in all cases.
As a first test, we applied DC voltage sweeps between ±0.1 V; in all cases we observe linear i–V curves that indicate Ohmic behaviour, but with varying resistance (Fig. 6). The lowest resistance is observed for unmodified C@SiO2 FP, as expected because the Hg probe directly contacts the conductive carbon surfaces (Fig. 6a). Resistance increases thereafter for all poly(V3D3)–C@SiO2 FPs, with the final value depending on the thickness of the polymer and the method used for deposition (Fig. 6b and Table 1). When comparing polymers of similar thickness, the resistance of EP-derived coatings are lower, which we attribute to the more gel-like consistency of said coatings that may deform when contacted by the Hg probe. The presence of residual unreacted vinyl groups in the EP-derived polymer may also provide pathways for current leakage.24 The iCVD-derived poly(V3D3)–C@SiO2 FP exhibits the highest resistance at ∼25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Ω, an ∼70-fold increase over the unmodified C@SiO2 FP, a notable achievement for an ∼50 nm-thick polymer film deposited on a complex 3D substrate.
000 Ω, an ∼70-fold increase over the unmodified C@SiO2 FP, a notable achievement for an ∼50 nm-thick polymer film deposited on a complex 3D substrate.
To assess the impact of the thickness and deposition method on the frequency response of poly(V3D3)–C@SiO2 FPs, we performed solid-state impedance spectroscopy. As expected, unmodified C@SiO2 FP behaves as a resistor from 1 to 500 kHz, with a real impedance of 340 Ω (Fig. 7a). Incorporating a thin coating of poly(V3D3) via electropolymerization (EP1) or iCVD (iCVD1) increases the impedance to 670 Ω and 730 Ω, respectively, but does not change the overall character of the response from that of a resistor. Increasing the thickness of the poly(V3D3) coating via electropolymerization to ∼51 nm (EP2) increases the real impedance by a factor of 4 compared to the ∼33 nm coating (EP1), while still retaining the resistor-like frequency response. The 51 nm-thick poly(V3D3) film deposited via iCVD (iCVD2) displays the most distinct frequency response, showing the highest real impedance at low frequency coupled with a departure to capacitive behaviour at high frequency (>10 kHz), as evidenced by increasing phase angle (Fig. 7b).
These preliminary findings demonstrate the feasibility of using both EP and iCVD to generate conformal, nanoscale, passivating polymer coatings at complex 3D architectures. Future studies will focus on further optimizing deposition conditions to produce thicker polymer films (up to 200 nm), establishing the relationship between poly(V3D3) thickness and composite electrical properties, incorporating electrolyte salts for ionic conductivity,28 and assessing performance of iCVD- and EP-derived siloxane phases in a fully integrated 3D battery or capacitor configuration.
Acknowledgements
This work was supported by the U.S. Office of Naval Research.Notes and references
- J. W. Long, B. Dunn, D. R. Rolison and H. S. White, Chem. Rev., 2004, 104, 4463 CrossRef CAS.
- L. Baggetto, F. Roozeboom, R. A. H. Niessen and P. H. L. Notten, Solid State Technol., 2008, 51, 30 CAS.
- L. Baggetto, R. A. H. Niessen, F. Roozeboom and P. H. L. Notten, Adv. Funct. Mater., 2008, 18, 1057 CrossRef CAS PubMed.
- S. Ferrari, M. Loveridge, S. D. Beattie, M. Jahn, R. J. Dashwood and R. Bhagat, J. Power Sources, 2015, 286, 25 CrossRef CAS PubMed.
- D. R. Rolison, J. W. Long, J. C. Lytle, A. E. Fischer, C. P. Rhodes, T. M. McEvoy, M. E. Bourg and A. M. Lubers, Chem. Soc. Rev., 2009, 38, 226 RSC.
- T. S. Arthur, D. J. Bates, N. Cirigliano, D. C. Johnson, P. Malati, J. M. Mosby, E. Perre, M. T. Rawls, A. L. Prieto and B. Dunn, MRS Bull., 2011, 36, 523 CrossRef CAS.
- J. F. M. Oudenhoven, L. Baggetto and P. H. L. Notten, Adv. Energy Mater., 2011, 1, 10 CrossRef CAS PubMed.
- C. P. Rhodes, J. W. Long, M. S. Doescher, J. J. Fontanella and D. R. Rolison, J. Phys. Chem. B, 2004, 108, 13079–13087 CrossRef CAS.
- C. P. Rhodes, J. W. Long and D. R. Rolison, Electrochem. Solid-State Lett., 2005, 8, A579 CrossRef CAS PubMed.
- C. P. Rhodes, J. W. Long, M. S. Doescher, B. M. Dening and D. R. Rolison, J. Non-Cryst. Solids, 2004, 350, 73 CrossRef CAS PubMed.
- J. C. Lytle, J. W. Long, C. N. Chervin, M. B. Sassin and D. R. Rolison, SPIE: Micro- and Nanotechnology Sensors, Systems, and Applications III, 2011, 8031 Search PubMed.
- C. P. Rhodes, J. W. Long, K. A. Pettigrew, R. M. Stroud and D. R. Rolison, Nanoscale, 2011, 3, 1731 RSC.
- N. S. Ergang, J. C. Lytle, K. T. Lee, S. M. Oh, W. H. Smyrl and A. Stein, Adv. Mater., 2006, 18, 1750 CrossRef CAS PubMed.
- D. Bélanger and J. Pinson, Chem. Soc. Rev., 2011, 40, 3995 RSC.
- G. El-Enany, M. J. Lacey, P. A. Johns and J. R. Owen, Electrochem. Commun., 2009, 11, 2320 CrossRef CAS PubMed.
- M. J. Lacey, M. Sosna and J. R. Owen, Electrochim. Acta, 2013, 96, 23 CrossRef CAS PubMed.
- B. Sun, D. Rehnlund, M. J. Lacey and D. Brandell, Electrochim. Acta, 2014, 137, 320 CrossRef CAS PubMed.
- D. J. Bates, C. M. Elliot and A. L. Prieto, Chem. Mater., 2014, 26, 5514 CrossRef CAS.
- A. M. Coclite, R. M. Howden, D. C. Borrelli, C. D. Petruczak, R. Yang, J. L. Yagüe, A. Ugur, N. Chen, S. Lee, W. J. Jo, A. Liu, X. Wang and K. K. Gleason, Adv. Mater., 2013, 25, 5392 CrossRef CAS PubMed.
- S. K. Murthy, B. D. Olsen and K. K. Gleason, Langmuir, 2002, 18, 6424 CrossRef CAS.
- W. E. Tenhaeff and K. K. Gleason, Adv. Funct. Mater., 2008, 18, 979 CrossRef CAS PubMed.
- S. H. Baxamusa, S. G. Im and K. K. Gleason, Phys. Chem. Chem. Phys., 2009, 11, 5227 RSC.
- W. S. O'Shaughnessy, S. K. Murthy, D. J. Edsell and K. K. Gleason, Biomacromolecules, 2007, 8, 2564 CrossRef PubMed.
- A. K. H. Achyuta, A. J. White, H. G. Pryce Lewis and S. K. Murthy, Macromolecules, 2009, 42, 1970 CrossRef CAS PubMed.
- M. L. Ma, M. Gupta, Z. Li, L. Zhai, K. K. Gleason, R. E. Cohen, M. F. Rubner and G. C. Rutledge, Adv. Mater., 2007, 19, 255 CrossRef CAS PubMed.
- S. H. Baxamusa and K. K. Gleason, Thin Solid Films, 2009, 517, 3536 CrossRef CAS PubMed.
- W. E. Tenhaeff, L. D. McIntosh and K. K. Gleason, Adv. Funct. Mater., 2010, 20, 1144 CrossRef CAS PubMed.
- N. Chen, B. Reeja-Jayan, J. Lau, P. Moni, A. Liu, B. Dunn and K. K. Gleason, Mater. Horiz., 2015, 2, 309 RSC.
- J. C. Lytle, T. N. Zimmerman, K. A. Pettigrew and D. R. Rolison, ECS J. Solid State Sci. Technol., 2013, 2, M3078 CrossRef CAS PubMed.
- G. Deniau, P. Viel, C. Bureau, G. Zalczer, P. Lixon and S. Palacin, J. Electroanal. Chem., 2001, 505, 33 CrossRef CAS.
- G. Aresta, J. Palmans, M. C. M. van de Sanden and M. Creatore, J. Vac. Sci. Technol., A, 2012, 30, 041503 Search PubMed.
- J. Ng Lee, C. Park and G. M. Whitesides, Anal. Chem., 2003, 75, 6544 CrossRef PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental, SEM, FTIR. See DOI: 10.1039/c5mh00057b |
| ‡ Thickness measurements of the poly(V3D3) film were made on at least 5 different fibres in multiple samples. |
| This journal is © The Royal Society of Chemistry 2015 |







